Abstract
Objective:
To assess thermal-sensory thresholds and psychosocial factors in children with Complex Regional Pain Syndrome Type 1 (CRPS-I) compared to healthy children.
Methods:
We conducted quantitative sensory testing on 34 children with CRPS-I and 56 pain-free children. Warm, cool, heat, and cold stimuli were applied to the forearm. Children with CRPS-I had the protocol administered to the pain site and the contralateral-pain site. Participants completed the self-report Behavior Assessment System for Children.
Results:
Longer pain durations (>5.1 months) were associated with decreased sensitivity to cold pain on the pain site (P = .04). Higher pain-intensity ratings were associated with elevated anxiety scores (P = .03). Anxiety and social stress were associated with warmth sensitivity (both P < .05) on the contralateral-pain site.
Conclusions:
Pain duration is an important factor in assessing pediatric CRPS-I. Hyposensitivity in the affected limb may emerge due to degeneration of nociceptive nerves. Anxiety may contribute to thermal-sensory perception in childhood CRPS-I.
Keywords: pain, CRPS, children, adolescents, QST, anxiety, depression
Chronic pain poses a significant burden for children and adolescents, causing suffering, disability, anxiety, and emotional distress.1 Like adults, children experience many different types of chronic pain caused by disease, injury, psychological factors, or by factors currently unknown and yet to be identified.2-7
Complex regional pain syndrome (CRPS) is a chronic pain condition, which can be induced by surgery, fractures, trauma, ischemia, or nerve lesion.8,9 CRPS type 1 (CRPS-I) occurs in the absence of nerve injury, but it may be a result of tissue and/or bone damage.10 CRPS-I is usually initiated after an initial noxious event and is accompanied with edema, changes in skin blood flow, as well as thermal and mechanical hyperalgesia/allodynia in the affected area.11
The causes and clinical presentation of CRPS-I in children and adolescents differs to those seen in adults, and this discrepancy can delay diagnosis.12,13 In adults, the diagnostic criteria for CRPS-I includes the presence of several of the following symptoms: allodynia, hyperesthesia, edema, vasomotor changes, sudomotor changes, joint stiffness, or temperature differences between extremities. In adults, the duration of CRPS is associated with alterations in sensory perception and/or clinical presentation of symptoms.14,15 Yet, clinical features differ in pediatric CRPS-I, who are also affected by the disorder, but at much lesser rates.16,17 Pediatric CRPS-I is more likely to present in the lower limb18 and the presentation of dystonia is more common in children compared to adults.17
Additionally, CRPS-I in children is more likely to improve or resolve compared to adults.19 However, without better characterization of the features of pediatric CRPS-I, developing treatment options or preventative interventions is challenging. Although previous pediatric quantitative sensory testing studies have examined pain sensitivity in child and adolescent CRPS populations,20,21 findings have been largely inconclusive and have varied widely based on pain symptomatology, making it challenging to quantitative-sensory testing findings into clinical risk factors.
Although pediatric CRPS-I is characterized by an increased risk of experiencing somatic symptoms, CRPS-I can be associated with subsequent psychosocial problems, particularly anxiety.22 Children with CRPS are statistically more likely to have experienced stressful life events, have difficulties at school, and can have familial stressors. In turn, psychosocial factors are often considered when treating pediatric CRPS patients and may unduly influence the resulting somatosensory symptoms of the disease.5,23,24
The objectives of the current study were to characterize thermal sensory processing through obtaining quantitative sensory testing data on thermal detection and pain thresholds in children and adolescents with CRPS-I compared to pain-free adolescents. We also sought to determine whether alterations in thermal-sensory processing were associated with psychosocial factors in children with and without chronic pain.
Patients and Methods
Participants
Patients were recruited from the Chronic Pain Clinic at the Hospital for Sick Children, Toronto, Ontario. Inclusion criteria were a diagnosis of CRPS-I affecting the lower limb, aged <17 years, fluent in English, and no other comorbid conditions or learning disabilities.
Pain-free participants were recruited during the same time period by advertisements posted in local hospitals and in a community newspaper. Inclusion criteria included age <17 years, able to read and speak English, no chronic pain (pain lasting greater than 3 months) or illnesses, no known learning disabilities or psychiatric conditions, or a risk for such conditions.
All participants gave written informed consent. The study was approved by the Research Ethics Board at the Hospital for Sick Children. Participants were compensated for their travel expenses and received a gift certificate of a CAD$30 value.
Quantitative Sensory Testing Experimental Procedure
For patients and typically developing children and adolescents, thermal sensory stimuli were applied to a site 10 cm above the participants’ wrists on the volar surfaces of their dominant forearms. Five patients (14.7%) and 7 (12.5%) typically developing participants were left handed. Participants rested their arms on a padded surface during testing. Participants’ skin temperatures were measured at both test sites prior to testing. In addition to the testing of detection and pain thresholds on the dominant forearms, patients had the pain site and the contralateral pain site tested.
Thermal Stimulation
Thermal stimulation was accomplished using a Medoc Neuro Sensory Analyzer, Model TSA-II (Medoc Ltd, Ramat Yishai, Israel). Stimuli were delivered using a Peltier thermode (3 × 3-cm). The thermode rested on the skin with constant pressure by use of a support stand. The thermode was held on the skin, and participants were told that they could withdraw their arm at any time during testing.25 Prior to testing, participants were given ample time to adapt to the room temperature. A baseline temperature of 32°C was used for all thermal testing.
Thermal Detection Thresholds
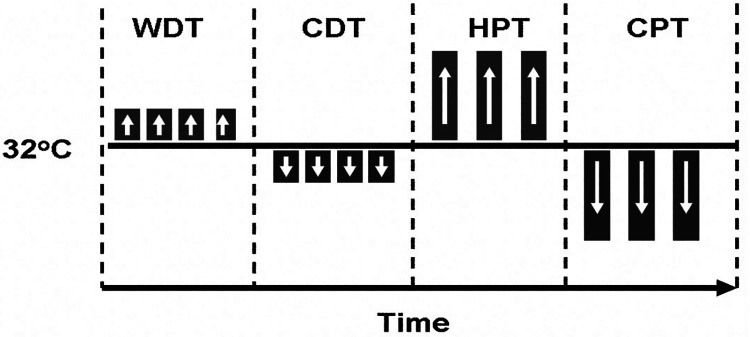
Thermal detection thresholds were determined by a method of limits (Figure 1). The temperature of the thermode moved away from the baseline at a rate of 0.5°C /s. Participants were trained to press a button when they first felt a warm (warm detection threshold) or cool (cold detection threshold) sensation, which automatically returned the probe to the baseline temperature (32°C). Four consecutive warm trials followed by 4 consecutive cold trials were completed with an intertrial duration of 6 seconds. Warm and cold detection thresholds were defined as the mean of the 4 trials.
Figure 1.
Stimulation protocol for innocuous and noxious stimuli. Thermal thresholds were assessed using a Medoc Neuro Sensory Analyzer TSA-II with a 3 × 3-cm probe. The stimuli were applied to the pain site (thigh, calf, ankle, or foot) to the contralateral nonpainful site and to the volar surface of the dominant forearm. Measures were determined in a structured sequence using the method of limits. The baseline temperature was 32°C, and noxious and innocuous temperatures were increased or decreased. Participants were trained to detect the following: warm detection threshold (WDT), cold detection threshold (CDT), heat pain threshold (HPT), and cold pain threshold (CPT).
Thermal Pain Thresholds
Thermal pain thresholds were determined by a method of limits (Figure 1). The temperature of the thermode moved away from the baseline at a rate of 1.0°C /s. Participants were trained to press a button with the contralateral hand when the hot or cold sensation changed to a ‘hurt feeling’ to determine their heat and cold pain thresholds, respectively. Following the participant’s response, the thermode immediately returned to baseline temperature (32°C). To ensure participant safety, upper and lower limits of 50°C and 0°C were used for heat and cold pain thresholds, respectively. Three consecutive heat and cold trials each were completed with an intertrial duration of 20 seconds. The heat and cold pain thresholds were defined as the mean of the 3 trials. The same testing procedures were administered to the patients and typically developing children, with the exception of the testing of the pain site and contralateral pain site in the patients.
Psychosocial Measures
The Behavioral Assessment System for Children (BASC, version 1) was used for the psychosocial assessment measure. In 5 children with CRPS-I, version 2 of the Behavioral Assessment System for Children was administered. The assessment is a validated self-report questionnaire, frequently used to evaluate the emotional and personality factors associated with chronic pain, including problem behaviors and emotions.26 The Behavioral Assessment System for Children has been shown to have good construct validity and good reliability, with internal consistency ratings ranging from 0.85 to 0.97, and test-retest reliability ranging from 0.78 to 0.86.26,27 For this study, T scores for anxiety, depression, social stress, and somatization were collected. T scores have a mean of 50 and a standard deviation of 10, where higher scores indicate higher levels of the characteristic, and lower scores indicate lower levels of the characteristic.
Statistical Analysis
Statistical analyses were computed using SPSS (Statistics for the Social Sciences, v.26, IBM, Armonk, NY). Descriptive measures were determined for all thermal sensory and psychosocial data collected. The thermal threshold data were tested for normality using the Shapiro-Wilk test. Log transformations of the thermal thresholds were applied to non-normally distributed data, in order to standardize the data.
Within the patient group, a repeated measures analysis of variance was used to examine the quantitative sensory testing thresholds obtained from the pain site and the control pain site adjusting for pain intensity and duration. Models were also adjusted for biological sex and age.
Thermal thresholds on the dominant forearm were examined between the patient and control groups using multivariate models, adjusting for age, biological sex, and handedness.
Psychosocial measures were assessed in relation to thermal thresholds using multivariate models. The Behavioral Assessment System for Children subscales of anxiety, depression, and social stress were entered as the dependent variables in separate models. The thermal detection and threshold data were entered as covariates, adjusting for biological sex and age. A P value of <.05 was considered significant for all statistical tests.
Results
Participant Characteristics
A total of 34 children and adolescents (28 [82%] female participants, mean age 12.03 years, standard deviation=2.4) with lower limb CRPS-I were recruited for the study. The distribution of pain sites ranged from the hip to the ankle and foot. The majority of patients had chronic pain in the foot (10, 29%), followed by the ankle (7, 21%), leg (5, 15%), and knee (5, 15%). The other 7 patients had pain in various locations of the lower limbs. The pain sites were unilateral in all patients. More than half of the patients had pain on the left side of the body (19, 56%). The mean pain duration was 8.79 months (SD = 11.55). The mean pain site temperature was 30.5°C. The majority of the patients were right-handed (29, 85%). Our resulting sample of pain-free adolescents was composed of 56 participants (28 [50%] female participants, mean age 15.7 years, SD = 1.1, 49 [88%] right-handed). One typically developing participant did not complete the quantitative sensory testing protocol.
Quantitative Sensory Testing
The means and standard deviations of the raw thermal detection and threshold data are in Table 1.
Table 1.
Thermal Detection and Threshold Data for Typically Developing Children and Children With CRPS-I.
| Dominant arm | WDT | CDT | HPT | CPT |
|---|---|---|---|---|
| Typically developing | 33.76 (1.02) | 30.9 (.82) | 39.95 (4.05) | 17.98 (10.3) |
| CRPS-I | 34.3 (2.2) | 29.8 (4.3) | 40.2 (4.3) | 18.2 (9.4) |
| Pain Site | 37.5 (3.6) | 28.5 (2.9) | 41.3 (4.1) | 18.9 (10.3) |
| Contralateral pain site | 36.5 (2.8) | 29.1 (2.12) | 41.3 (3.7) | 18.2 (10.2) |
Abbreviations: CDT, cold detection threshold; CPT, cold pain threshold; CRPS-I, complex regional pain syndrome type 1; HPT, heat pain threshold; WDT, warm detection threshold.
We examined thermal detection and pain thresholds in the pain site compared to the contralateral site in children with CRPS-I, using a repeated measures analysis of variance. The data were adjusted for pain duration and intensity. The effect of pain sites (pain site vs contralateral pain site) was not significant in the warm detection threshold, cold detection threshold, cold pain threshold, or heat pain threshold (all P > .05) data; however, pain duration was associated with cold pain thresholds (F = 9.5, P = .005). A subsequent interaction analysis examined the cold pain thresholds on the pain site and the contralateral pain site in relation to pain duration (separated by groups based on a median split of the years of chronic pain duration, 5.0 months). A significant effect of site and duration [group] was evident (F = 7.9, P = .01). Children with longer pain durations (>5.1 months) were less sensitive to cold pain on the pain site compared to the contralateral pain site (F = 5.0, P = .04, Figure 2), based on a post hoc analysis and correcting for multiple comparisons. An opposite pattern was seen in the children with shorter pain durations; however, this association was not significant (F = 3.5, P = .09), based on a post hoc analysis.
Figure 2.
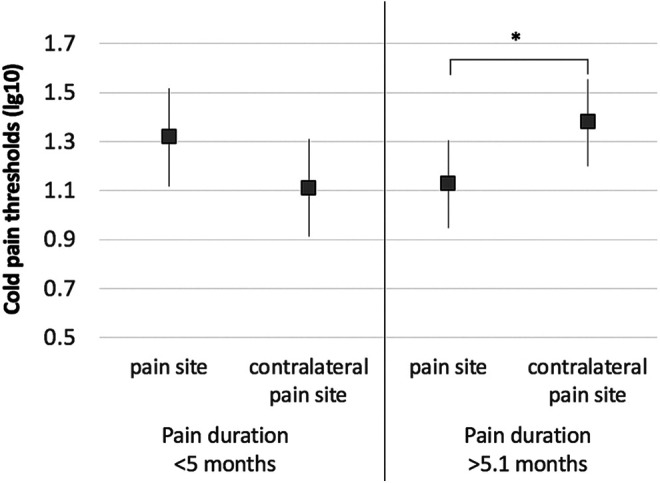
Cold pain thresholds (lg10) on the pain site compared to the contralateral pain site in children with lower limb CRPS-I who had short durations of chronic pain (left, <5 months) and those who had longer pain durations (right, >5.1 months). Values represent the estimated marginal means from repeated measures analysis of variance conducted for short and long pain durations separately. Children with longer pain durations had detected cold pain at lower temperatures, and were less sensitive to cold pain, on the pain site compared to the control pain site (F = 5.0, P = .04), Bonferroni corrected for multiple comparisons. *P < .05.
Adolescents with CRPS-I had comparable thermal detection and threshold levels to that of pain-free adolescents on the dominant forearm (all, P > .06). Age effects were evident in the cold detection threshold (F = 7.5, P = .007) and warm detection threshold (F = 4.3, P = .04) data. Biological sex or handedness effects were not evident in the data.
Psychosocial Measures
The self-report version of the Behavioral Assessment System for Children was completed by all patients. Thirty-nine (70%) typically developing children completed the Behavioral Assessment System for Children. The measures were not obtained in the full sample of typically developing children due to limited resources. Anxiety scores for both groups of children were largely in the typical range, and the mean scores for patients (49.3, SD = 9.67) and controls (48.7, SD = 9.97) were not significantly different (P = .8). More than a third of the patients (33%) had anxiety scores that were elevated (i.e., more than 0.5 SD above average; scores > 56), and a quarter (26%) of the typically developing children also had anxiety scores in the elevated range. Similarly, depression and social stress scores were comparable between groups (both P > .5). Few depression scores for the patients (4, 13%) and the controls (3, 8%) were in the clinically significant range.
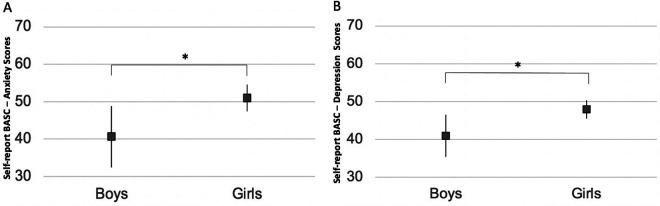
Within the patient group, increased pain intensity was associated with higher anxiety scores (F = 5.2, P = .03; Figure 3) in a model adjusting for pain duration and age. Additionally, girls with chronic pain had higher anxiety (F = 5.6, P = .03; Figure 4A) and depression scores (F = 5.4, P = .03; Figure 4B) compared with boys. None of the Behavioral Assessment System for Children–Self-Report scores were associated with pain duration (all, P > .05). Depression and social stress scores were not associated with pain intensity ratings (all, P > .05).
Figure 3.
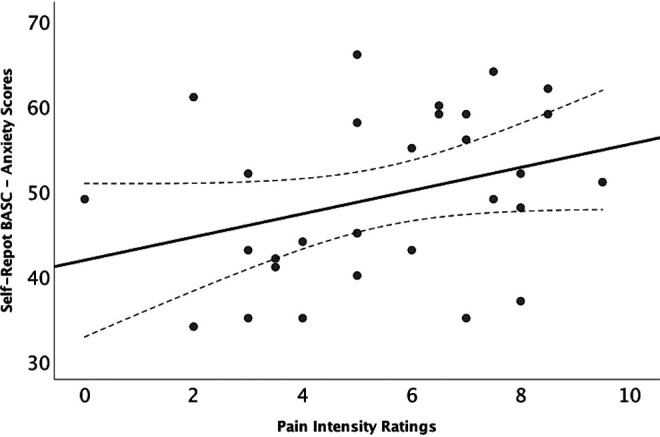
Elevated anxiety scores on the BASC-SR were associated with higher pain intensity ratings in children with CRPS-I (F = 5.2, P = .03). BASC scores are T scores that have a mean of 50 and a standard deviation of 10. Higher scores indicate more severe anxiety symptoms. BASC-SR, Behavioral Assessment System for Children–Self-Report; CRPS-I, complex regional pain syndrome type 1.
Figure 4.
(A) Girls with CRPS-I had higher anxiety scores on the BASC-SR compared to boys (F = 5.6, P = .03). (B) Girls with CRPS-I had significantly had higher depression scores compared to boys (F = 5.4, P = .03). BASC scores are T scores that have a mean of 50 and a standard deviation of 10. Higher scores indicate more severe anxiety or depressive symptoms. Values represent the estimated marginal means from univariate models conducted for anxiety and depression scores on the BASC-SR separately. Results are Bonferroni corrected for multiple comparisons. *P < .05. BASC-SR, Behavioral Assessment System for Children–Self-Report; CRPS-I, complex regional pain syndrome type 1.
The somatization scale is only available for children ≥12 years of age. Somatization scores were available in patients aged ≥12 years (n = 14) and were significantly higher for patients in comparison to controls (t = 2.96, P = .009). As only data from less than half of the sample were available, the somatization scores were excluded from the subsequent analyses.
The Behavioral Assessment System for Children–Self-Report scores were examined in relation to thermal thresholds obtained on the dominant forearm in patients and controls in 3 separate models, 1 for each Behavioral Assessment System for Children subscale (anxiety, depression, social stress), adjusted for biological sex and age. None of the Behavioral Assessment System for Children scores were associated with the thermal detection or pain threshold data on the dominant arm (P > .1). Group and biological sex effects were not significantly associated with anxiety, depression, or social stress scores (all P > .05).
Lastly, the models were repeated to examine within the patient group the association of the Behavioral Assessment System for Children–Self-Report scores with the thermal detection and pain threshold data obtained from the pain site and the contralateral pain site. Anxiety, depression and social stress scores were not associated with threshold data from the pain site (all, P > .05). However, higher anxiety scores on the Behavioral Assessment System for Children–Self-Report were negatively associated with warm detection thresholds (F = 6.9, P = .02; Figure 5) on the contralateral pain site, whereby higher scores were associated with increased sensitivity to warm stimuli. Higher anxiety scores were also negatively associated with cold detection thresholds (F = 5.0, P = .04), on the contralateral pain site in patients. Additionally, biological sex (F = 5.6, P = .03) was significant in the model. A subsequent interaction analysis examining biological sex and cold detection thresholds revealed no statistically significant association (F = 1.8, P = .2). Social stress was also associated with warm detection thresholds on the contralateral pain site (F = 5.0, P = .04).
Figure 5.
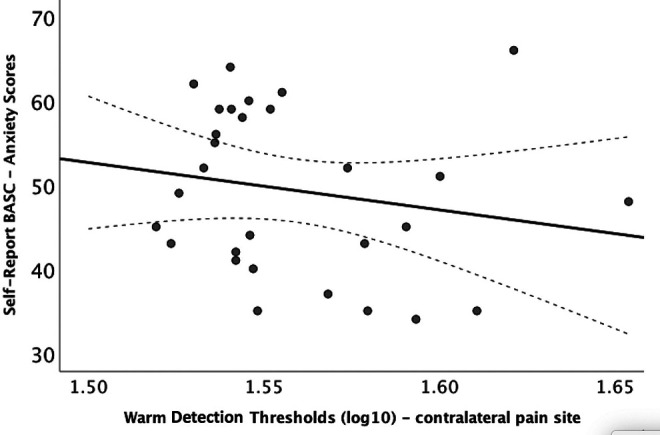
Anxiety scores and warm detection thresholds (lg10) in patients with lower limb CRPS-I. BASC scores are T scores that have a mean of 50 and a standard deviation of 10. Higher scores indicate more severe anxiety symptoms. BASC-SR, Behavioral Assessment System for Children–Self-Report; CRPS-I, complex regional pain syndrome type 1.
Discussion
In a group of children with CRPS-I impacting the lower limb, using quantitative sensory testing to assess sensory functioning, longer pain durations were associated with decreased sensitivity to cold pain on the pain site compared to the contralateral pain site. An opposite pattern was observed for patients with shorter pain durations in participants with chronic pain who had increased sensitivity to cold pain on the pain site relative to the control pain site, athough this relationship was not significant. No deficits in thermal processing on the dominant (unaffected) arm were evident in patients with CRPS-I compared with pain-free typically developing adolescents. Examination of psychological factors involved in the maintenance of chronic pain, namely, anxiety, indicated that children with chronic pain with higher levels of anxiety were more sensitive to warm stimuli but less sensitive to cool stimuli on the contralateral pain site. Findings suggest that anxiety may be an important risk factor when evaluating somatosensory processing in children with CRPS-I.
Chronic pain can have nociceptive and neuropathic components. Clinically relevant nociceptive pain is usually caused by injury-induced activation of peripheral nociceptors (e.g., tissue damage, bone damage, inflammation) with pain lessening as the injury heals.28 In contrast, neuropathic pain, although initiated or caused by a primary lesion or disease of the somatosensory system, persists or intensifies despite an absence of evidence of continuing injury.29 Neuropathic pain is attributable to multiple mechanisms. Chronic pain is often characterized by spontaneous pain, referred pain, hyperalgesia, and allodynia. Hyperalgesia is pain that is abnormally intense, such as severe pain evoked by mild- to moderate-intensity noxious stimuli.30 Allodynia is pain evoked by a normally innocuous (nonpainful) stimulus, such as light touch, cooling, or warmth.30 Both hyperalgesia and allodynia can be thermal or tactile. Hyperalgesia and allodynia are thought to occur following injury-induced sensitization of nociceptive primary afferents, alongside altered central processing that includes increased responsivity at spinal and supraspinal levels as well as abnormal responses.30-32
A previous study with a pediatric sample used quantitative sensory testing to evaluate thermal perception (warm and cold detection thresholds) in 74 children aged 3-7 years.33 Stimuli applied to the volar surface of the forearm yielded a mean cold detection threshold of 29.4°C and a mean warm detection threshold of 34.5°C. Small peripheral nerve fiber functions were evaluated at different body sites in a cohort of healthy children and adolescents, 8-17 years of age. Mean cold and warm thresholds on the volar distal forearm were 30.5°C and 33.7°C, respectively, for the children, 8-9 years old, and 31.2°C and 33.1°, respectively for the adolescents, 14-17 years old.34 In turn, these previously reported values are comparable to the threshold data obtained in the current study from the nonaffected dominant forearm in children with CRPS-I. Additionally no differences in dominant-arm thermal threshold data was evident between the patients and controls.
A previous study found primarily cold allodynia in children with CRPS types 1 and 2 with varying pain durations (0.5-72 months).20 In the current study, our results would indicate sensitivity to cold pain stimuli on the pain site in children with longer pain durations and a trend towards increased sensitivity to cold pain on the pain site in patients with shorter pain durations. This finding may reflect peripheral or central sensitization that occurs during the preliminary stages of the disease. Loss of cold pain sensitivity may occur in more chronic cases of CRPS-I in children. Decreased sensitivity may emerge over time as a function of chronicity because of the degeneration of A-delta and C-fibers, previously reported in neuropathic pain and inflammatory pain conditions.35,36 Future studies with larger samples of pediatric patients with CRPS-I should be conducted to address the issue of chronic pain chronicity and sensory sensitivity to cold pain stimuli.
Previous research has indicated that children with anxiety symptoms had heightened perceived pain experiences, particularly related to higher ratings of pain intensity.37 A previous study reported in a sample of 66 adult patients with CRPS-I that anxiety was strongly associated with ratings of pain intensity.38 Changes in the sympathetic nervous system may reflect this association, given the role that the sympathetic nervous system plays in CRPS-I and anxiety. Results are consistent with findings in the current study, which determined a similar association between higher pain intensity ratings and increased anxiety in a pediatric population.
Furthermore, in our study, female participants with CRPS were found to experience a greater number of psychological symptoms, including depression and anxiety, compared with their male counterparts; however the majority of the sample was female. The present study’s findings have important clinical implications and warrants the need for an increased understanding between the association of pain intensity and anxiety, and should be an area further investigated in relation to the development of early physical and psychological treatment plans for pediatric patients with CRPS-I.
Conclusions
In a sample of children and adolescents with CRPS-I affecting the lower limb, we examined thermal thresholds to warm, cool, heat pain and cold pain stimuli. We further examined the association of the thermal threshold data in relation to psychosocial variables assessed with the Behavioral Assessment System for Children. Findings indicated that pain duration was associated with decreased sensitivity to cold pain on the affected lower limb in children with CRPS-I. Examination of the psychosocial data indicated that chronic pain intensity was associated with higher anxiety scores. Furthermore, higher anxiety and social stress scores were associated with increased sensitivity to warmth, whereas higher anxiety scores also predicted decreased sensitivity to cool stimuli on the contralateral pain site. Findings highlight the importance of assessing psychological contributors to alterations in sensory processing in pediatric chronic pain patients. Results indicating that longer pain durations are associated with alterations in pain sensitivity highlight the importance of early assessment and treatment of CRPS-I in children.
Acknowledgments
We thank Dr James O’Leary for his input on the study design. We also thank Ms Erika Kewley, a Research Assistant, at the Divisional Center for Pain Management and Pain Research, at the Hospital for Sick Children in Toronto, Ontario, Canada for aiding with the quantitative sensory testing.
Footnotes
Author Contributions: SCB and DR conceived the study. Data acquisition and quality control were done by SCB and DR. EET, MM, EGD conducted the statistical analyses. EET and EGD wrote the first draft of the manuscript and prepared the tables and figures. All authors substantially contributed to the interpretation of results, revised the manuscript, and approved the final version of the manuscript. DR and EGD contributed equally to the work described in this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Emma E. Truffyn  https://orcid.org/0000-0001-5614-9494
https://orcid.org/0000-0001-5614-9494
Emma G. Duerden  https://orcid.org/0000-0002-9734-7865
https://orcid.org/0000-0002-9734-7865
Ethical Approval: The study was conducted according to the criteria set by the declaration of Helsinki and was approved by the Research Ethics Board at the Hospital for Sick Children. Informed consent was obtained from the parents/guardians and assent was obtained in the children.
References
- 1.Hechler T, Kanstrup M, Holley AL, et al. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain. Pediatrics. 2015;136(1):115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landry BW, Fischer PR, Driscoll SW, et al. Managing chronic pain in children and adolescents: a clinical review. PM R. 2015;7(11)(suppl):S295–S315. [DOI] [PubMed] [Google Scholar]
- 3.McGrath PA, Hiller LM. Modifying the psychological factors that intensify children’s pain and prolong disability. In: Schechter NL, Berde CB, Yaster M, eds. Pain in Infants, Children, and Adolescents. Philadelphia, PA: Lippincott Williams & Wilkins; 2003:85–104. [Google Scholar]
- 4.McGrath PA. Pain in Children: Nature, Assessment and Treatment. New York: Guilford; 1990. [Google Scholar]
- 5.Wilder RT, Berde CB, Wolohan M, Vieyra MA, Masek BJ, Micheli LJ. Reflex sympathetic dystrophy in children. Clinical characteristics and follow-up of seventy patients. J Bone Joint Surg Am. 1992;74(6):910–919. [PubMed] [Google Scholar]
- 6.McGrath PJ, Finley GA, eds. Chronic and Recurrent Pain in Children and Adolescents. IASP Press; 1999. Progress in Pain Research and Management. [Google Scholar]
- 7.Asmundson GJ, Noel M, Petter M, Parkerson HA. Pediatric fear-avoidance model of chronic pain: foundation, application and future directions. Pain Res Manag. 2012;17(6):397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goh EL, Chidambaram S, Ma D. Complex regional pain syndrome: a recent update. Burns Trauma. 2017;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birklein F, Ajit SK, Goebel A, Perez RSGM, Sommer C. Complex regional pain syndrome—phenotypic characteristics and potential biomarkers. Nat Rev Neurol. 2018;14(5):272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urits I, Shen AH, Jones MR, Viswanath O, Kaye AD. Complex regional pain syndrome, current concepts and treatment options. Curr Pain Headache Rep. 2018;22(2):10. [DOI] [PubMed] [Google Scholar]
- 11.Shah A, Kirchner JS. Complex regional pain syndrome. Foot Ankle Clin. 2011;16(2):351–366. [DOI] [PubMed] [Google Scholar]
- 12.Goldschneider KR. Complex regional pain syndrome in children: asking the right questions. Pain Res Manag. 2012;17(6):386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesaroli G, Ruskin D, Campbell F, et al. Clinical features of pediatric complex regional pain syndrome: a 5-year retrospective chart review. Clin J Pain. 2019;35(12):933–940. [DOI] [PubMed] [Google Scholar]
- 14.Bruehl S, Harden RN, Galer BS, Saltz S, Backonja M, Stanton-Hicks M. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002;95(1-2):119–124. [DOI] [PubMed] [Google Scholar]
- 15.Eberle T, Doganci B, Krämer HH, et al. Warm and cold complex regional pain syndromes: differences beyond skin temperature? Neurology. 2009;72(6):505–512. [DOI] [PubMed] [Google Scholar]
- 16.de Mos M, de Bruijn AG, Huygen FJ, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129(1-2):12–20. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Arafeh H, Abu-Arafeh I. Complex regional pain syndrome in children: incidence and clinical characteristics. Arch Dis Child. 2016;101(8):719–723. [DOI] [PubMed] [Google Scholar]
- 18.Murray CS, Cohen A, Perkins T, Davidson JE, Sills JA. Morbidity in reflex sympathetic dystrophy. Arch Dis Child. 2000;82(3):231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernstein BH, Singsen BH, Kent JT, et al. Reflex neurovascular dystrophy in childhood. J Pediatr. 1978;93(2):211–215. [DOI] [PubMed] [Google Scholar]
- 20.Sethna NF, Meier PM, Zurakowski D, Berde CB. Cutaneous sensory abnormalities in children and adolescents with complex regional pain syndromes. Pain. 2007;131(1-2):153–161. [DOI] [PubMed] [Google Scholar]
- 21.Becerra L, Sava S, Simons LE, et al. Intrinsic brain networks normalize with treatment in pediatric complex regional pain syndrome. Neuroimage Clin. 2014;6:347–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weissmann R, Uziel Y. Pediatric complex regional pain syndrome: a review. Pediatr Rheumatol Online J. 2016;14(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geertzen JH, de Bruijn-Kofman AT, de Bruijn HP, van de Wiel HB, Dijkstra PU. Stressful life events and psychological dysfunction in complex regional pain syndrome type I. Clin J Pain. 1998;14(2):143–147. [DOI] [PubMed] [Google Scholar]
- 24.Aasland A, Flatö B, Vandvik IH. Psychosocial factors in children with idiopathic musculoskeletal pain: a prospective, longitudinal study. Acta Paediatr. 1997;86(7):740–746. [DOI] [PubMed] [Google Scholar]
- 25.McGrath PA, Brown SC. Quantitative sensory testing in children: practical considerations for research and clinical practice. Pain. 2006;123(1-2):1–2. [DOI] [PubMed] [Google Scholar]
- 26.Matazow GS, Kamphaus R. Behavior Assessment System for Children (BASC): toward accurate diagnosis and effective treatment. In: Andrews JJW, Saklofske DH, Janzen HL, eds. Handbook of Psychoeducational Assessment, chap 9. San Diego, CA: Academic Press; 2001:257–288. [Google Scholar]
- 27.Wilder L, Sudweeks R. Reliability of ratings across studies of the BASC. Educ Treat Child. 2003;26(4):382–399. [Google Scholar]
- 28.Loeser JD, Treede RD. The Kyoto protocol of IASP basic pain terminology. Pain. 2008;137(3):473–477. [DOI] [PubMed] [Google Scholar]
- 29.Jensen TS, Baron R, Haanpää M, et al. A new definition of neuropathic pain. Pain. 2011;152(10):2204–2205. [DOI] [PubMed] [Google Scholar]
- 30.Jensen TS, Finnerup NB. Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–935. [DOI] [PubMed] [Google Scholar]
- 31.Dubner R, Ruda MA. Activity-dependent neuronal plasticity following tissue injury and inflammation. Trends Neurosci. 1992;15(3):96–103. [DOI] [PubMed] [Google Scholar]
- 32.Price DD. Psychological Mechanisms of Pain and Analgesia, vol 15. Seattle, WA: IASP Press; 1999. [Google Scholar]
- 33.Hilz MJ, Glorius SE, Schweibold G, Neuner I, Stemper B, Axelrod FB. Quantitative thermal perception testing in preschool children. Muscle Nerve. 1996;19(3):381–383. [DOI] [PubMed] [Google Scholar]
- 34.van den Bosch GE, van Dijk M, Tibboel D, Valkenburg AJ. Thermal quantitative sensory testing in healthy Dutch children and adolescents standardized test paradigm and Dutch reference values. BMC Pediatr. 2017;17(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes JP, Chessell I, Malamut R, et al. Understanding chronic inflammatory and neuropathic pain. Ann N Y Acad Sci. 2012;1255:30–44. [DOI] [PubMed] [Google Scholar]
- 37.Kain ZN, Mayes LC, Caldwell-Andrews AA, Karas DE, McClain BC. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. 2006;118(2):651–658. [DOI] [PubMed] [Google Scholar]
- 38.Bean DJ, Johnson MH, Heiss-Dunlop W, Lee AC, Kydd RR. Do psychological factors influence recovery from complex regional pain syndrome type 1? A prospective study. Pain. 2015;156(11):2310–2318. [DOI] [PubMed] [Google Scholar]