Introduction
The Nonalcoholic fatty liver disease (NAFLD) Activity Score (NAS) has been applied as a method for evaluating treatment response, and a ≥ 2-point improvement in NAS has been commonly utilized as an accepted as endpoint in Phase 2b clinical trials in nonalcoholic steatohepatitis (NASH)[1, 2]. Although liver fibrosis is the strongest histological predictor of liver-related outcome and all-cause mortality in NAFLD[3, 4], the association between change in NAS and change in fibrosis stage has not been fully verified. Therefore, we aimed to examine the association between change in NAS and change in fibrosis stage in well-characterized patients with NAFLD who had a paired liver biopsy assessment.
Methods
This is a longitudinal prospective study that includes a well-characterized cohort with biopsy-proven NAFLD. This study includes 123 uniquely phenotyped patients who underwent standardized research visit that included history, physical examination, biochemical testing, and paired liver biopsy assessment (using NASH CRN Histologic Scoring System[5]) at the NAFLD Research Center, University of California San Diego (UCSD) from 2006 through 2019. All patients completed written informed consent prior to enrollment. The study was approved by the UCSD Institutional Review Board. The association between change in NAS and change in fibrosis between two biopsies and factors associated with fibrosis progression and regression were investigated. Supplementary Methods shows additional methods.
Results
A total of 123 patients (62.6% female) who underwent two liver biopsies were enrolled in this study. The median (interquartile range) age was 54 (44–60) years, and body mass index was 31.8 (29–36) kg/m2. The interval between paired biopsies was 1.5 (0.7–2.7) years. Ninety-six patients (78.0%) were enrolled in clinical trials between the two biopsies and other patients were treated with standard of care. The number of patients with fibrosis stage 0, 1, 2, 3, and 4 at the baseline assessment was 34, 44, 15, 19, and 11, respectively. Thirty (24.4%) patients had fibrosis progression and 24 patients (19.5%) had fibrosis regression. Supplemental Table.1 shows the patient characteristics and no significant difference was observed in biochemical data and histological findings between patients with no change in fibrosis, fibrosis progression, and fibrosis regression.
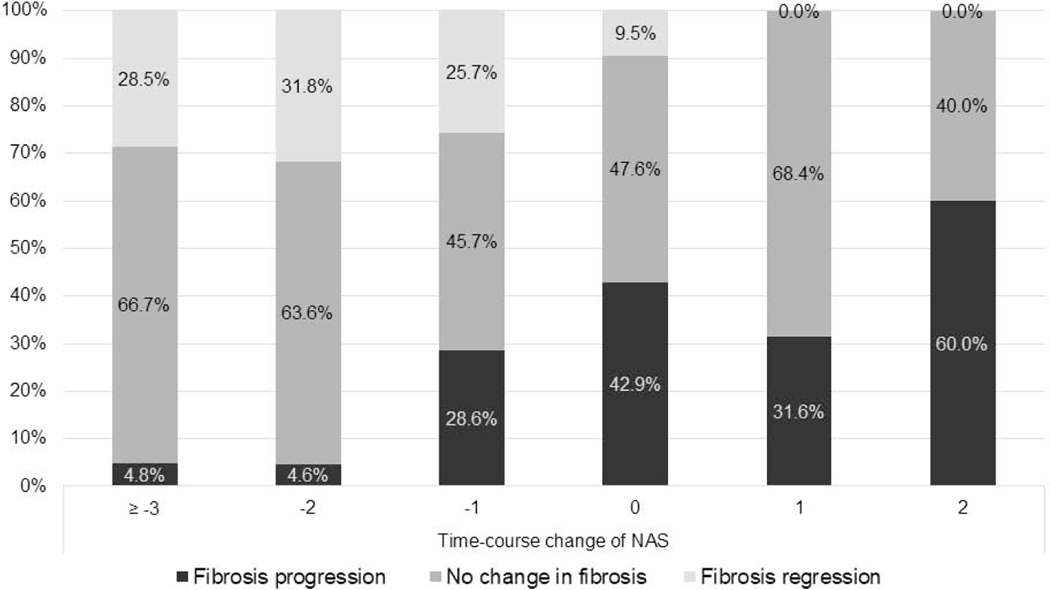
The relationship between changes in NAS and changes in liver fibrosis was investigated (Figure.1). The proportion of patients with fibrosis progression with change in NAS ≥−3, −2, −1, 0, 1, and 2 was 4.8%, 4.6%, 28.6%, 42.9%, 31.6%, and 60.0%, respectively. Similarly, the proportion of patients with fibrosis regression with change in NAS ≥−3, −2, −1, 0, 1, and 2 was 28.5%, 31.8%, 25.7%, 9.5%, 0%, and 0%, respectively. The proportion of patients with fibrosis progression and regression changed dose-dependently with a greater increase and decrease in NAS (p < 0.001).
Figure.1.
The proportion of patients with fibrosis progression and regression with change in NAS
Fibrosis stage and NAS were evaluated using NASH CRN scoring system. Fibrosis progression was defined as ≥1 stage increase in fibrosis and fibrosis regression was defined as ≥1 stage decrease in fibrosis, respectively.
NAS, Nonalcoholic fatty liver disease Activity Score
Baseline characteristics and changes in clinical and histological factors were examined for their association with fibrosis progression and regression. In multivariable-adjusted logistic regression analysis (adjusted for age, gender, diabetes status, race/ethnicity, and change in aspartate aminotransferase), an increase in NAS (per one point) was an independent predictor of fibrosis progression with multivariable-adjusted odds ratio (aOR) of 1.85 (95% confidence interval [CI]: 1.2–2.8, p = 0.003, Supplemental Figure.1). Similarly, a decrease in NAS (per one point) was an independent predictor of fibrosis regression with aOR of 2.09 (adjusted for age, gender, diabetes status, race/ethnicity, gamma-glutamyl transferase at baseline, and change in platelet counts, 95% CI: 1.2–3.6, p = 0.006).
Discussion
This study demonstrated that change in NAS is associated with change in fibrosis stage in a dose-dependent manner. Moreover, a one-point increase and decrease in NAS were associated with two times higher odds of fibrosis progression and regression. In addition to this study, a recent study examined patients who received standard of care and underwent paired biopsies showed the significant association between changes in NAS and changes in fibrosis[6]. In our study, majority of patients were enrolled in clinical trials, hence, the significant association between change in NAS and change in fibrosis was demonstrated in these patients. These results support the findings of this seminal study and demonstrate the validity of using improvement in NAS as an endpoint in clinical trials. Although identifying noninvasive surrogate markers for evaluating changes in NAS is necessary for clinical practice in NAFLD[7], changes in NAS is a reasonable surrogate endpoint to evaluate the efficacy of therapeutic agent. In conclusion, change in NAS over time is associated with change in liver fibrosis, and these data provide justification of using improvement in NAS to assess treatment response in Phase 2b trials in NASH and may be used allow for sample-size estimation for fibrosis improvement response in Phase 3 trials.
Supplementary Material
Acknowledgments
Funding: Rohit Loomba receives funding support from NIEHS (5P42ES010337), NIDDK (U01DK61734, R01DK121378, P30DK120515, R01DK124318, and R01DK106419), and DOD PRCRP (CA170674P2). Veeral Ajmera receives funding support from NIDDK (K23DK119460). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Nobuharu Tamaki receives funding support from the Uehara Memorial Foundation.
Abbreviations:
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NAS
NAFLD Activity Score
- UCSD
University of California San Diego
- aOR
adjusted odds ratio
- CI
confidence interval
Footnotes
Conflict of interests: Rohit Loomba serves as a consultant or advisory board member for Anylam/Regeneron, Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly, Galmed, Gemphire, Gilead, Glympse bio, GNI, GRI Bio, Inipharm, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prometheus, Promethera, Sanofi, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Grail, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, pH Pharma, Prometheus, and Siemens. He is also co-founder of Liponexus, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cheung A, Neuschwander-Tetri BA, Kleiner DE, et al. Defining Improvement in Nonalcoholic Steatohepatitis for Treatment Trial Endpoints: Recommendations From the Liver Forum. Hepatology 2019;70:1841–1855. [DOI] [PubMed] [Google Scholar]
- 2.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385:956–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65:1557–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor RS, Taylor RJ, Bayliss S, et al. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020;158:1611–1625.e1612. [DOI] [PubMed] [Google Scholar]
- 5.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 6.Kleiner DE, Brunt EM, Wilson LA, et al. Association of Histologic Disease Activity With Progression of Nonalcoholic Fatty Liver Disease. JAMA Netw Open 2019;2:e1912565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loomba R, Neuschwander-Tetri BA, Sanyal A, et al. Multicenter validation of association between decline in MRI-PDFF and histologic response in nonalcoholic steatohepatitis. Hepatology 2020;21:31121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.