Abstract
Cardiovascular wellbeing has been dramatically affected by severe acute respiratory syndrome coronavirus (SARS-CoV-2), the reason for the coronavirus disease pandemic 2019 (COVID-19) pandemic. There is a greater risk of morbidity and death in individuals with preexisting heart diseases. Clinical syndromes of the acute coronary syndrome, acute myocardial injury, myocarditis, arrhythmias, heart failure, and venous thromboembolism can, directly and indirectly, affect the heart. There may also be adverse heart effects of specific therapeutics under review for COVID-19. The renin-angiotensin-aldosterone system (RAAS) mechanism in virus replication makes it essential to understand the consequences of the system-modulating medications. For optimum patient care, detailed knowledge of specific cardiovascular symptoms of COVID-19 and the role of RAAS in the prognosis of COVID-19 disease is necessary.
Introduction
A global tumult has been produced for the coronavirus disease pandemic in 2019 (COVID-19).1 According to current reports, ∼20%-36% of patients with COVID-19 infection are affected by severe myocardial injury with a higher prevalence than those without cardiac injury, commensurate with the elevation of cardiac troponin (cTn).2, 3, 4 Furthermore, ∼6%-17% of patients experience cardiac arrhythmias, such as malignant ventricular arrhythmias (VAs),5 , 6 with a higher incidence (∼44%) recorded in patients admitted to the intensive care unit (ICU).6 Notably, there can be a low prevalence of arrhythmias in clinically healthy patients7, but seriously ill patients are at more serious risk of cardiac arrhythmias.8 Patients with preexisting comorbidities are at an elevated risk of severe acute respiratory syndrome coronavirus (SARS-CoV2) infection and appear to have poorer health outcomes. High side effects are postulated in patients with cardiovascular diseases, diabetes, and hypertension.
In cardiac patients, death rates of 10.5% and death rates of 7.3% and 6.0% for diabetes and hypertension have been recorded.9
Epidemiology
Sex
Men have more potentiate for hospital admission, morbidity, and mortality during the COVID-19 outbreak. Data from the COVID-19 net dataset (May 9, 2020) from the United States reported that males accounted for 52.9% of the hospitalized population, compared to 47.1% of females. A study of 5700 patients from a hospital system in New York showed that 60.3% of the admitted patients were males. In this research, COVID-19 mortality was higher in males than females at all ages.10 The pathophysiology and importance of male predominance of COVID-19 disease remain unknown. In this field, further research is underway.
Age
There is a direct relationship between age and the risk of contracting significant COVID-19 disease. Centers for Disease Control and Prevention (CDC) research in the United States indicates that patients younger than 20 years old with COVID-19 disease have a hospitalization rate of 2% to 3% relative to a hospitalization risk that is 2% to 3%. Over 31% of patients over the age of 85.11 In comparison, no patients under the age of 20 in this cohort need. The hospitalization rate was 2% to 4% in the 20-45 age range, and the hospitalization rate rose in the 75-84-year cohort.
Increased mortality with age in the US with case fatality rates of 0.1% to 0.2% in patients younger than 44 years of age and 10.4% to 27.3% in patients 85 years old and up being estimated to be 11% to 31% .12 There have been studies of a rare multisystem lately, COVID-19-related inflammatory syndrome resembling Kawasaki disease in children.13 Much is uncertain as to how often this happens and what the risk factors might be.
Socio-Economic Agents
In addition to ethnicity and race, other socio-economic influences have been found to exacerbate COVID-19 disease outcomes. With high resulting morbidity and mortality, nursing homes and assisted living facilities have shown to be places of quick spread. It has been reported that all prisoners and corrections personnel in US jails are at risk. Homeless shelters were also shown to be at risk of COVID-19 illness.11
Ethnicity / Race
Encounter from the United States and the UK have proposed that race/ ethnicity may play a part in the severity of COVID-19 disease. Information from the CDC found that in patients hospitalized for COVID-19 illness, dark patients have spoken to 33% of the COVID-19 inpatient populace whereas speaking to 18% of the community in common, individually.14 This indicates that the African American community has a disproportionate influence. It has also been mentioned that aboriginal groups are at elevated risk of COVID-19 disease. In the US, as opposed to the world as a whole, the COVID-19 occurs four times as high on reservations.15
Covid-19 and Cardiac-Related Manifestations Myocarditis
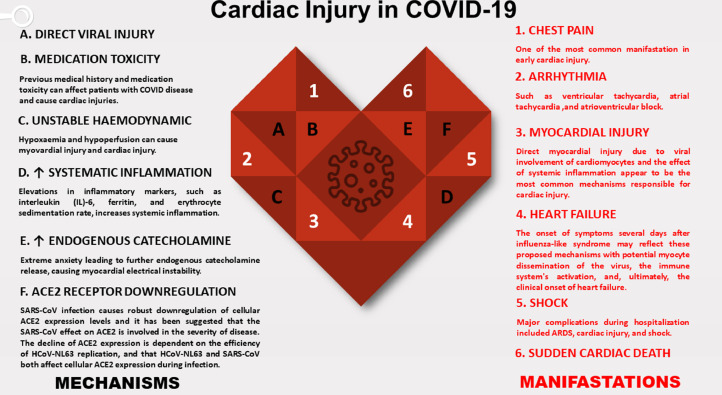
As far as previous reports,7% of COVID-19-related deaths are presumably associated with myocardial infection with COVID-19; however, its actual occurrence is still indeterminate. The range of symptoms can differ from minor symptoms like minor chest pain, weakness, and dyspnea to the more extreme left and right ventricular failure, arrhythmias, cardiogenic shock, and abrupt cardiac death with fulminant myocarditis.16 Figure 1 illustrated the most critical cardiac manifestations and mechanisms.17, 18, 19, 20, 21, 22, 23, 24 While there is no clear current evidence promoting direct COVID-19 viral myocarditis, MERS-CoV and SARS-CoV viral RNAs, which are closely linked to SARS-CoV-2, have been identified in the cardiac tissue of infected animals. COVID-19 myocarditis is possibly usually a combination of direct cell damage and cytotoxicity mediated by T lymphocytes that can be enhanced by cytokine storm syndrome.16 Myocarditis caused by COVID-19 can mimic a coronary artery disease with an elevation of the ST segment and cardiac enzymes due to injury.25
FIG 1.
Cardiac Injury in COVID-19. (Color version of figure is available online.)
Arrhythmias
Symptomatic/asymptomatic tachycardia is the most often diagnosed arrhythmia in COVID-19 disease. Bradycardia has also been noted. Arrhythmia may happen within the setting of myocarditis, myocardial ischemia, and in basically sick patients with shock and hypoxia.26 Different types of arrhythmias have been accounted for due to the COVID-19 disease. A few instruments might trigger or disturb arrhythmias in patients with COVID-19. Potential causes incorporate unsettling electrolyte influence (for the most part hypokalemia), antagonistic impacts of treatments (Eg, chloroquine, Azithromycin, and Hydroxychloroquine) that draw out QT interval [measured from the beginning of the QRS complex to the end of the T wave] span with expected advancement of polymorphic ventricular tachycardia (VT)27 and fever, which may expose instances of heart channelopathies, for example, Brugada disorder and long QT condition.9
Acute Coronary Syndrome
In the case of COVID 19 disease, there are no precise data on ST-segment elevation myocardial infarction (STEMI) from intracoronary plaque rupture or obstruction is possibly flawed. Plaque collapse and coronary thrombosis may cause acute coronary events due to inflammation/increased shear stress in high-risk patients. Tam et al. detailed a considerable decrease in the number of patients with STEMI looking for clinical consideration at their institute.28 They ascribed this to the hesitance of patients to go to an emergency clinic during the COVID-19 episode, delays in assessing patients with STEMI after medical clinic appearance because of preventive measures, for example, definite travel and contact history, symptomatology, and chest X-ray. The transfer of patients to the catheterization center consequently postpone. Further preventive measures taken in the catheterization laboratory, like the time necessary to wear protective clothing, can further prolong the operation .28
Heart Failure
Data on the occurrence of left ventricular systolic malfunction, acute left ventricular collapse, and cardiogenic shock are inadequate. Fifty-two percent of dead patients and 12% of discharged patients reported heart disease in one report.29 Many critically ill patients may develop reversible sepsis-related cardiomyopathy with left ventricular dilatation, impaired systolic function, and recovery within 7-10 days.16 COVID-19 infections can cause decompensation of underlying heart failure and may lead to mixed shock syndrome. If possible, intrusive hemodynamic monitoring can help to control the cardiac portion of shock in such circumstances.30
Long-Term Cardiovascular Outcome
It is too early to estimate long-term cardiovascular sequels in patients emerging from COVID-19 infection (Table 1 ).6 , 25 , 29 , 31 , 32 Nevertheless, the likely results could be close to that observed in SARS-CoV virus-induced severe acute respiratory syndrome (SARS). Outcomes of patients suffering from SARS for 12 years found that 40% had cardiovascular problems, 60% had impaired glucose metabolism, and 68% had irregular lipid metabolism.28
Table 1.
Cardiovascular complications in patients with COVID-19
| Study | Patients | Outcomes | |
|---|---|---|---|
|
Arrhythmia |
- Wang 20206 retrospective, single center case series - Liu 202031 retrospective, nine- tertiary hospitals (cohort) |
-138 hospitalized patients - 137 hospitalized patients |
-Total events: 23 (16.7%) ICU Vs non-ICU patients: 16 (44.4%) Vs 7 (6.9%), P < 0.001) - Total events: 10 (7.3%) * |
|
Myocardial injury (elevated cTnI) |
- Huang 202025 retrospective, cohort Study - Wang 20206 retrospective, single-center case series - Zhou 202029 retrospective, multicenter cohort study |
- 41 hospitalized patients - 138 hospitalized patients - 191 hospitalized patients |
- Overall: 5 (12%) ICU patients: 4 (31%) Vs non-ICU patients: 1 (4%), p ¼ 0.017 - Overall: 10 (7.2%) ICU patients: 8 (22.2%) Vs non-ICU patients 2 (2.0%), P < 0.001 - Overall: 33 (17%) Survivors: 1 (1%) Vs non survivors: 32 (59%), P < 0.0001 |
|
Myocarditis |
- Ruan 202032 retrospective, multicenter Study |
- 68 deaths from 150 hospitalized patients | - 5 (7%) deaths from myocardial damage and circulatory failure 22 (33%) deaths from myocardial damage and respiratory failure⁎⁎ |
|
Heart Failure |
- Zhou 202029 retrospective, multicenter cohort study |
- 191 hospitalized patients | - Overall: 44 (23%) Survivors: 16 (12%) Vs non-survivors 28 (52%), P < 0.0001 |
Data are presented as n (%).
Abbreviations: cTnI, cardiac Troponin I; ICU, intensive care unit.
Patients presented heart palpitations as an initial symptom.
Some patients died of myocarditis.
Cerebrovascular Disease
A prothrombotic condition that induces venous and arterial thrombosis and elevated D-dimer is consistent with COVID-19 infection.33 The documented occurrence of cerebrovascular disorder in drastic patients with COVID-19 ranged from 2.3% to 22%.34 As a potential cause of ischaemic stroke, increased production of antiphospholipid antibodies has been indicated. The correlation of stroke with COVID-19 illustrated with a 2.5-fold rise in disease incidence.34
Hemostasis and Thrombosis
The laboratory and autopsy reports detected a hypercoagulable condition with severe COVID-19 disease. Coagulation factors and platelets are implicated in host immune response regulation, demonstrating proinflammatory roles separate from their immune response. Hemostatic effects and higher D-dimer correlates with bad outcomes.35
COVID-19 and the RAAS System
The renin-angiotensin-aldosterone system (RAAS) is a complex hormonal system that is involved in the contraction of renin, angiotensinogen, angiotensin-converting enzyme (ACE), angiotensin-converting enzyme 2 (ACE2), angiotensin II, and aldosterone. In summary, RAAS is a framework that reacts to a physiologic condition of hypovolemia, hyponatremia, adrenergic, and hypotension and propels a vasoconstriction reaction and liquid maintenance.36 The ACE2 receptor is located at the top respiratory tract cells and lung alveoli and is also the leading site of the virus's entrance into the body.37 It is often found in various concentrations in other tissues, such as the gastrointestinal tract (which is presumably the cause for a typical diarrhea symptom) and the heart muscle (which may describe the cardiac symptoms of COVID-19).
The RAAS mechanism in viral entry makes it essential to understand the consequences of the system-modulating medications. Inhibitors of angiotensin transmitting enzymes (ACEi) and antagonists of angiotensin receptors (ARBs) are popular medicines used to treat hypertension. In an extensive cohort survey of patients with COVID-19, an excess incidence of hypertension cases with unfavorable performance, indicating that these drugs could play a role in the morphology of the disease.38 165 [15%] of those cases had hypertension in that cohort of 1099 participants, but 24 [35.8%] of the 67 with poor results (defined as ICU admission, utilization mechanical ventilation, or fatal) were hypertensive. ARB and ACEi induce ACE2 up-regulation and probably enhance the viral entry.39 So, plasma tests of SARS-CoV-2 contaminated patients displayed a significantly elevated angiotensin II amount and were linearly correlated with viral load and liver disease. Even so, increased ACE activity and reduced ACE2 activity in mouse models are shown to lead to lung injury.36 This recommends that meds like ACEi and ARBs, which diminishes ACE action and increment upregulation of ACE2, may benefit significantly. Subsequently, from a conceptual view, ACEi and ARBs may simplify viral passage into respiratory cells prompting viral interceded cell harm. However, these equivalent drugs may upregulate ACE2 and improve acute lung injury. Which of these behaviors are prevalent in people with COVID-19 is not apparent and more study is needed. In the meantime, most existing recommendations for cardiology say that sufferers with ACEi and ARBs should continue to take their drugs as usual and should not avoid using CoVID-19 disorder.40
Treatment
At present, there is no approved treatment for COVID-19. Because of the vast arising ideas about COVID-19, the urgent nature of diagnosis and management, and the executives' direness, no randomized controlled preliminaries indicate the adequacy of a particular treatment 2019-nCOV. The main aims of using various strategies in the care of individuals are supportive and symptomatic care. Non-steroidal anti-inflammatory drugs (NSAIDs), including Ibuprofen, have been accounted for clinically worsening in individual patients with drastic COVID-19.
The momentum suggestion is to beware of NSAIDs use in patients with COVID-19 illness.41 Using corticosteroids in patients with COVID-19 can be destructive, and steroids can cause liquid maintenance, electrolyte disturbance, and hypertension. Moreover, SARS pieces of information dedicated to an expansion of viral shedding after steroid use.42 However, treatment with methylprednisolone might be helpful if acute respiratory distress syndrome (ARDS) add to other disorders .43
New investigations assess potential strange coagulation courses in extreme COVID-19 cases that may prompt microthrombi in many end organs, particularly the lungs. In those patients, a high D-dimer leads to a guarded prognosis and a high death rate. After prescription of 40-60 mg, enoxaparin/d, or unfractionated heparin 10,000-15,000 U/d, Tang, N. et al. reported a decrease of 28-day mortality in patients with high sepsis-induced coagulopathy (SIC) score more than 4, or D-dimer over 6-fold of the standard upper limit.44 Recently realized antiviral and different medications have been taken a stab at patients without suitable preliminaries constrained by extreme ill patients and demise in affirmed cases with COVID-19 sickness. Numerous examinations have at present been led researching the potential impact of the combination of Hydroxychloroquine and Azithromycin, immune therapy, Remdesivir, distinctive antiviral meds, and utilization of the relieved patients' plasma of COVID-19.9 , 45, 46, 47, 48, 49, 50, 51, 52, 53, 54 It should be borne in mind that some of these medications have many cardiac side effects. According to a brief French trial in COVID-19, Chloroquine and Hydroxychloroquine, a more potent engineered type of chloroquine, is utilized alongside Azithromycin, a macrolide anti-toxin, for experimental administration.45 , 46 Chloroquine can cause atrioventricular (AV) block, and alongside Azithromycin, increased QT distance. Hydroxychloroquine can also produce difficulty in cardiac conductions. Adverse effects of the simultaneous utilization of beta-blockers or calcium channel blockers can prompt bradycardia prompting cerebral hypoperfusion with syncope.50 The current suggestion is to evaluate QT before beginning treatment and close checking in patients with extra danger components or patients utilizing different meds to upgrade the QT prolongation.9 Moreover, electrolytes were observing, mainly hypokalemia result from the interactivity of 2019-nCoV with the RAAS.9 A new multicenter, randomized controlled trial (RCT) of Hydroxychloroquine including 150 adults admitted to the emergency clinic for COVID-19, announced no critical impact of the medication on quickening viral clearance.53
Lopinavir/ritonavir are protease inhibitors that are affirmed for HIV-1 contamination treatment. Prolongation of PR interval [the time from the beginning of the P wave (atrial depolarization) to the beginning of the QRS complex (ventricular depolarization)] and QT interval prompting high-grade AV blocks and seldom torsade de Pointes are reported adverse effects. They can diminish serum convergence of the dynamic metabolites of clopidogrel, prasugrel while expanding that of ticagrelor and statins levels with the danger of rhabdomyolysis. Lopinavir/ritonavir potentiates the impacts of factor Xa inhibitors, for instance, apixaban and rivaroxaban, by the prohibition of CYP3A4, increased risk of bleeding.47 Cao B et al. led a randomized, controlled, open-mark preliminary, including hospitalized adult patients with affirmed extreme COVID-19 sickness. Analysts noticed no advantage following treatment with lopinavir/ritonavir in hospitalized adult patients with severe COVID-19 superior to the standard. Future studies in patients with severe disease may assist in affirming or prohibit the chance of a treatment profit.53
Remdesivir is a mono-phosphor amidite prodrug of an adenosine analog that hinders viral RNA synthesis with a wide antiviral range, including paramyxoviruses, filoviruses, coronaviruses, and pneumo-viruses. There is restricted data on antagonistic impacts. Nonetheless, hypokalemia is a typical adverse effect54, and there was a patient who created hypotension and bradycardia when this drug was utilized to treat Ebola.48 The security and adequacy of Remdesivir for the treatment of COVID-19 are being assessed in numerous progressing Phase 3 clinical preliminaries. In a new randomized, double-blind, multicenter preliminary study at ten medical clinics in Hubei, China, directed on adult admitted patients to an emergency clinic for extreme COVID-19 illness, Remdesivir did not show a significant profit clinically. Further studies need to justify a mathematical decrease to an approved clinical improvement.54
Tocilizumab, a counter-acting agent for IL-6R, is known for its possible viability in decreasing inflammatory reactions, including the cytokine storm adding to ARDS and even death.25 Reports illustrated the increment of cholesterol level notwithstanding, contradictory reports indicated its impact on morbidity and mortality during an extended period .55
Vaccination
According to WHO, COVID-19 mass vaccination campaign first started in early December 2020. More than 13 different vaccines (across four platforms) have been administered.
On 31 December 2020, the Pfizer/BioNtech Comirnaty vaccine was listed for WHO Emergency Use Listing (EUL). The SII/Covishield and AstraZeneca/AZD1222 vaccines (developed by AstraZeneca/Oxford and manufactured by the State Institute of India and SK Bio, respectively) were given EUL on 16 February. The Janssen/Ad26.COV 2.S developed by Johnson & Johnson was approved for EUL on 12 March 2021. On 30 April 2021, the Moderna COVID-19 vaccine (mRNA 1273) was certified for EUL, and the Sinopharm COVID-19 vaccine was certified on 7 May 2021. Beijing Bio-Institute of Biological Products Co Ltd, as a subsidiary of China National Biotec Group (CNBG), produced the Sinopharm vaccine. The Sinovac-CoronaVac was listed for EUL on 1 June 2021.
Several types of possible COVID-19 vaccines are being developed, including:
-
•
Virus vaccines that use a form of the virus that has been inactivated or weakened so that it does not cause disease but still triggers an immune response.
-
•
Protein-based vaccines, which safely produce an immune response by using harmless protein fragments or protein shells that resemble the COVID-19 virus.
-
•
Viral vector vaccines, which use a non-pathogenic virus as a platform for producing coronavirus proteins and triggering an immune response.
-
•
RNA and DNA vaccines, a cutting-edge method that generates a protein from genetically modified RNA or DNA, safely trigger an immune response.
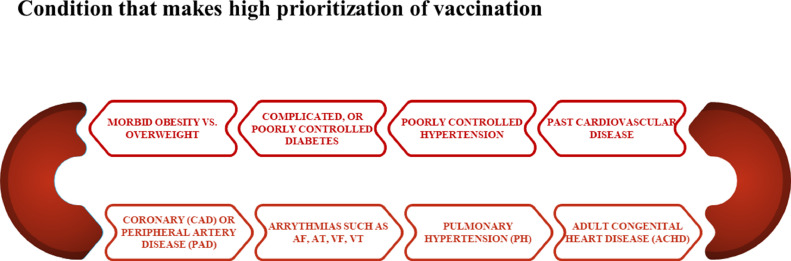
The prioritization of people who are eligible for the Covid vaccine can be seen in Figure 2 below.
FIG 2.
Prioritization of vaccination. (Color version of figure is available online.)
As you can see, the prioritization of COVID vaccination is given to people who have a history of cardiovascular disease or related conditions. Therefore, it is difficult to evaluate the safety of the COVID vaccine regarding cardiovascular complications. Table 2 provides a comprehensive set of studies related to cardiac complications of the corona vaccine. In general, the most common complication was myocarditis, followed by cardiac arrhythmias.
Table 2.
Comprehensive set of studies related to cardiac complications of the corona vaccine
| Author (Date) |
Patient(s) information | Present illness | Past medical history | Vaccine history | Cardiac markers and imaging | The main Side Effect |
|---|---|---|---|---|---|---|
| Montgomery et al. 2021/6 |
23 males between 20 to 51 y old | chest pain | - | second vaccination Seven: the BNT162b2-mRNA and 16 the mRNA-1273 vaccine |
elevated c-Trop, Evidence in CMR of 8 patients. |
myocarditis |
| Park et al. 2021/6 |
Two adolescent males | chest pain | - | 3 days after the first and second dose BNT162b2 vaccine | troponin levels, ST segment elevation, and enhancement of the myocardium in CMR | myocarditis |
| Berto et al. 2021/6 |
63 y old woman | retronasal severe acute respiratory syndrome | - | 1 d after receiving the first dose of mRNA-1273 (Moderna, Cambridge, MA, USA) COVID-19 vaccination. |
Elevation of High-sensitivity troponin, N-terminal pro-B-type natriuretic peptide and CRP, a moderately impaired left ventricular ejection fraction of 40%. |
Takotsubo cardiomyopathy (TCM) was made. |
| Lee et al. 2021/6 |
1. 70 y old female 2. 44-y-old female 3. 73-y-old female |
1. Chest pain, diaphoresis and vomiting 2. chest discomfort and palpitations 3. palpitations and shortness of breath |
1. DM2 , HTN, HLP, and previous stroke. 2. mitral valve prolapse and mild mitral regurgitation. 3. HTN |
1. Six hours after receiving first dose of Pfizer-BioNTech COVID-19 vaccination. 2. Fifteen minutes after receiving her first dose of Pfizer vaccination. 3. First dose of Pfizer vaccination two hours prior. |
1. ECG: widespread ST-segment Depressions with ST-segment elevation in aVR. 2. ECG showed ST-segment elevations in the inferolateral leads. Troponin I was elevated. Transthoracic echocardiogram: mildly depressed left ventricular ejection fraction of 50% with apical ballooning. 3. ECG: normal, Troponin I was elevated. Transthoracic echocardiogram showed left ventricular ejection fraction of 60% with no regional wall motion abnormalities. |
myocardial injury |
| Boivin and Martin et al. 2021/2 |
96 y old female | Chest discomfort | Poorly controlled hypertension and a DNR/DNI code status | Approximately one hour after receiving the first dose of Moderna vaccine. | ECG: ST segment elevation in the inferior leads with reciprocal ST segment depression in the lateral leads. ultrasound: an anterior wall motion abnormality. Initial troponin was 0.07 ng/mL, with a peak of 2.63 ng/mL. |
unfortunate MI |
| Albert et al. 2021/5 |
24 y old man | Acute substernal chest pain | - | 4 d after second vaccination COVID-19 Moderna | elevated troponins, cMRI: edema and delayed gadolinium enhancement of the left ventricle in a midmyocardial and epicardial distribution | myocarditis |
| Schauer et al. 2021/6 |
13 patients Median age was 15 ye (range, 12-17 ye). Most patients were male (N=12, 92%). |
Sudden onset, intense and persistent chest pain. Shortness of breath (N=6, 46.2%) Tactile temperature (N=5, 38.5%) Myalgias (N=4, 30.7%). |
- | Median time to presentation from the second dose of the Pfizer COVID-19 mRNA vaccine was 3 d (range 2-4 d). |
CMR: myocardial inflammation and edema, elevated serum troponin levels (median 9.18 ng/mL, range 0.65-18.5) reduced LV systolic function in two patients, left ventricular wall motion abnormalities. |
|
| Mohamed et al. 2021/6 |
70 y old male | Malaise, fever, and upper respiratory tract symptoms |
HTN, polycythaemia vera, gout, with no known cardiac disease or prior exercise limitations. |
Fully vaccinated (Pfizer) |
His troponin was 57 ng/L (normal 3-15) | developed bradycardia and asystole, which were refractory to resuscitation. |
| Isaak et al. |
15 y old boy | Fever, myalgia, and intermittent tachycardia | - | After second vaccination dose (BNT162b2-mRNA SARS-CoV2, Biontech/Pfizer) | elevation of ST-segment and CRP. CMR: a small pericardial effusion. T2-weighted short TI inversion myocardial edema of the lateral wall, Late gadolinium enhancement: inflammatory necrosis | Vaccine associated myocarditis is rare, but more common in the young male population, Clinical course is typically self-limited. |
| Vidula et al. 2021/6 |
18, 19 y old men 61, 60, 21 y old women |
Acute substernal chest pressure and/or dyspnea | - | 19 y old man and 60 y old woman: The second dose of the Pfizer-BioNTech vaccine. 18 y old man: The second dose of the Moderna vaccine. 21 y old woman: The first dose of the Pfizer-BioNTech vaccine. 61 y old woman: 4 wk after her second dose of the Pfizer- BioNTech vaccine. |
ECG: Diffuse ST elevations, elevated cardiac biomarkers and inflammatory markers, and mildly reduced LV function on echocardiography |
|
| Patrignani et al. 2021/6 |
56-y-old man | Episode of acute epigastric pain, profuse sweating, tachycardia, hypotension |
- | 4 d of the first dose of BNT162b2 mRNA vaccine, |
Troponin I level was elevated. Cardiac Magnetic Resonance showed a pattern of acute myocarditis. |
|
| McLean and Johnson 2021/6 |
16-y old male |
sharp/stabbing chest pain | - | second dose of his Pfizer-BioNTech COVID-19 |
Computed tomography angiogram of the heart: minimal lateral wall subepicardial hyperenhancement, suggestive of myocarditis. | Myopericarditis |
| Muthukumar, et al. 2021/6 |
a 52-y-old male | substernal chest pain, high fevers, shaking chills, myalgias and a headache |
HTN, hypercholesterolemia, obstructive sleep apnea | mRNA-1273 (Moderna) vaccine for COVID-19 | ECG: sinus rhythm with left axis deviation and incomplete right bundle branch block. hs-cTnI: 2768 ng/L, CAD |
myocarditis |
| Nevet 2021/6 |
Three previously healthy men, 20, 29, and 24 y old, | acute fever and chest pain | - | BNT162b2. | Elevation of myocardial enzymes and ST in ECG. Myocardial edema, gadolinium enhancement of the myocardium and MRI confirming the diagnosis of myocarditis | acute myocarditis |
| Habib et al. 2021/6 |
A 37-y-old Filipino man | severe chest pain, generalized body aches, fever, chills, and headache | ex-smoker, drinking alcohol occasionally, HTN | BNT162b2 | ECG: mild ST-segment elevation in anterior leads and high level of troponin T. | myocarditis |
| Tate et al. 2021/7 |
A 29-y-old woman | palpitations | - | Pfizer-BioNTech COVID vaccination | Sinus Tachycardia in ECG with no ST or T-wave abnormalities |
CAD, coronary artery disease; CMR, Cardiac Magnetic Resonance; cMRI, cardiac magnetic resonance imaging; CRP, C-reactive protein; ECG, Electrocardiography; LV, left ventricular ; TCM, Takotsubo cardiomyopathy; hs-cTnI, cardiac troponin I .
Regardless of the origin, post-COVID-19 vaccine-associated cardiac diagnoses necessitate timely and thorough professional clinical management, as demonstrated by the few cardiovascular and cardiac-related deaths that have occurred following COVID-19 vaccination.
As a result, informed authorization for mRNA COVID-19 vaccines should include information about the rare but possible occurrence of myocarditis or pericarditis in the week after immunization, and subjects should be advised of potential symptoms and the need to seek immediate clinical care.
Although age (mostly, 30 years) and gender (mainly, male) may influence general perceptions of the rare risk of myocarditis following COVID-19 vaccination, an individual's personal risk should be considered based on their current health status, the impact of severe COVID-19 disease, and the risk of infection from locally circulating SARS-CoV-2 variants.
To this date, global safety monitoring data suggests that life-threatening serious adverse events are uncommon after COVID-19 vaccination, but they do necessitate close clinical vigilance, early detailed investigation, and clinical management because they can occur after all COVID-19 vaccine type, at first or second dose.
Significantly, at this early point, evaluations of these data and discussions by North American and European health organizations indicate that the advantages of COVID-19 vaccination outweigh the hazards in all demographics, including the rare risk of myocarditis, for all recommended age groups.
Additional research data may be valuable in determining a direct causal link between the host and the vaccine. Until then, no definite contraindication of COVID-19 immunization can be issued to the general public.
Vaccination is widely accepted as necessary for the general population since the benefits outweigh the hazards.
Any adverse event that arises from immunization must be handled the same way as any other conventional vehicle. There have been no specific post-vaccination cardiac care regimens proposed so far. Treatment procedures suited to each patient are recommended for the time being.
Conclusion
This review explained the cardiac problems of COVID-19 and discussed the crucial concepts of the virus and its interaction with cardiac therapies, particularly ACEi and ARBs. Further testing on different facets of this emerging coronavirus is underway, and physicians observe to understand as best as they are in the process.
Footnotes
None of the authors have financial or proprietary interests in any of the materials or methods mentioned.
Reference
- 1.Manolis A.S., Manolis T.A. Cardiovascular complications of the coronavirus (COVID-19) infection: COVID-19 and the heart. Rhythmos. 2020;15:23–28. [Google Scholar]
- 2.Parohan M., Yaghoubi S., Seraji A. Cardiac injury is associated with severe outcome and death in patients with Coronavirus disease 2019 (COVID-19) infection: a systematic review and meta-analysis of observational studies. Eur Heart J. 2020;9:665–677. doi: 10.1177/2048872620937165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lala A., Johnson K.W., Januzzi J.L., et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Y., Xu D., Fu S., et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: a cross-sectional study. Crit Care. 2020;24:1–10. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17:1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sala S., Peretto G., De Luca G., et al. Low prevalence of arrhythmias in clinically stable COVID-19 patients. Pacing Clin Electrophysiol. 2020;43:891–893. doi: 10.1111/pace.13987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazaridis C., Vlachogiannis N.I., Bakogiannis C., et al. Involvement of cardiovascular system as the critical point in coronavirus disease 2019 (COVID-19) prognosis and recovery. Hellenic J Cardiol. 2020;61:381. doi: 10.1016/j.hjc.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong T.-Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. European heart journal. 2020 doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baggett T.P., Keyes H., Sporn N., Gaeta J.M. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323:2191–2192. doi: 10.1001/jama.2020.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Team C.C.-R., Team C.C.-R., Team C.C.-R., et al. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:343–346. doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verdoni L., Mazza A., Gervasoni A., et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garg S., Kim L., Whitaker M., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. Morbidity and mortality weekly report. 2020;69:458. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez-Lonebear D., Barceló N.E., Akee R., Carroll S.R. American Indian Reservations and COVID-19: Correlates of Early Infection Rates in the Pandemic. J Public Health Manag Pract. 2020;26:371–377. doi: 10.1097/PHH.0000000000001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19–related myocarditis: The possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai C.C., Ko W.C., Lee P.I., et al. Extra-respiratory manifestations of COVID-19. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atri D., Siddiqi H.K., Lang J.P., et al. COVID-19 for the Cardiologist: basic virology, epidemiology, cardiac manifestations, and potential therapeutic strategies. JACC. 2020;5:518–536. doi: 10.1016/j.jacbts.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kochi A.N., Tagliari A.P., Forleo G.B., et al. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31:1003–1008. doi: 10.1111/jce.14479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dijkman R., Jebbink M.F., Deijs M., et al. Replication-dependent downregulation of cellular angiotensin-converting enzyme 2 protein expression by human coronavirus NL63. J Gen Virol. 2012;93:1924–1929. doi: 10.1099/vir.0.043919-0. [DOI] [PubMed] [Google Scholar]
- 21.Zeng J.-H., Liu Y.-X., Yuan J., et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metabo Syndr. 2020;14:247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inciardi R.M., Lupi L., Zaccone G., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shirazi S., Mami S., Mohtadi N., et al. Sudden cardiac death in COVID-19 patients, a report of three cases. Future Cardiol. 2021;17:113–118. doi: 10.2217/fca-2020-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li R., Pei S., Chen B., et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV-2) Science. 2020;368:489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clerkin K.J., Fried J.A., Raikhelkar J., et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 28.Tam C.-C.F., Cheung K.-S., Lam S., et al. Impact of coronavirus disease 2019 (COVID-19) outbreak on ST-segment–elevation myocardial infarction care in Hong Kong, China. Circulation. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barlow A., Landolf K.M., Barlow B., et al. Review of emerging pharmacotherapy for the treatment of coronavirus disease 2019. Pharmacotherapy. 2020;40:416–437. doi: 10.1002/phar.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu K., Fang Y.Y., Deng Y., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan Q., Yang K., Wang W., et al. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beyrouti R., Adams M.E., Benjamin L., et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol, Neurosurg Psychiatry. 2020;91:889–891. doi: 10.1136/jnnp-2020-323586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aggarwal G., Lippi G., Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. 2020;15:385–389. doi: 10.1177/1747493020921664. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L., Yan X., Fan Q., et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wösten-van Asperen R.M., Lutter R., Specht P.A., et al. Acute respiratory distress syndrome leads to reduced ratio of ACE/ACE2 activities and is prevented by angiotensin-(1–7) or an angiotensin II receptor antagonist. J Pathol. 2011;225:618–627. doi: 10.1002/path.2987. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann M., Kleine-Weber H., Schroeder S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan W.-j., Ni Z.-y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrario C.M., Jessup J., Chappell M.C., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 40.Guo J., Huang Z., Lin L., Lv J. Coronavirus disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Day M. Covid-19: Ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]
- 42.Lee N., Chan K.A., Hui D.S., et al. Effects of early corticosteroid treatment on plasma SARS-associated Coronavirus RNA concentrations in adult patients. J Clin Virol. 2004;31:304–309. doi: 10.1016/j.jcv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Inter Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang N., Bai H., Chen X., et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao X., Ye F., Zhang M., et al. In vitro antiviral activity and projection of optimized dosing design of Hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gautret P., Lagier J.-C., Parola P., et al. Hydroxychloroquine and Azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan J.F.-W., Yao Y., Yeung M.-L., et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulangu S., Dodd L.E., Davey R.T., Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen C., Wang Z., Zhao F., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Page R.L., O'Bryant C.L., Cheng D., et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134:e32–e69. doi: 10.1161/CIR.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 51.Svanström H., Pasternak B., Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704–1712. doi: 10.1056/NEJMoa1300799. [DOI] [PubMed] [Google Scholar]
- 52.Tang W., Cao Z., Han M., et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369 doi: 10.1136/bmj.m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao B., Wang Y., Wen D., et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Y., Zhang D., Du G., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soubrier M., Pei J., Durand F., et al. Concomitant use of statins in tocilizumab-treated patients with rheumatoid arthritis: a post hoc analysis. Rheumatol Ther. 2017;4:133–149. doi: 10.1007/s40744-016-0049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]