Abstract
Introduction
Despite safety and benefits of physical activity during treatment of localised breast cancer, successful exercise strategies remain to be determined. The primary objective of the ‘dispositif connecté’, that is, connected device in English trial is to evaluate the efficacy of two 6-month exercise interventions, either single or combined, concomitant to adjuvant treatments, on the physical activity level of patients with breast cancer, compared with usual care: an exercise programme using a connected device (activity tracker, smartphone application, website) and a therapeutic patient education intervention. Secondary objectives are to evaluate adherence to interventions, their impact at 6 and 12 months, representations and acceptability of interventions, and to assess the cost-effectiveness of the interventions using quality-adjusted life-years.
Methods and analysis
This is a 2×2 factorial, multicentre, phase III randomised controlled trial. The study population (with written informed consent) will consist of 432 women diagnosed with primary localised invasive breast carcinoma and eligible for adjuvant chemotherapy, hormonotherapy and/or radiotherapy. They will be randomly allocated between one of four arms: (1) web-based connected device (evolving target number of daily steps and an individualised, semisupervised, adaptive programme of two walking and one muscle strengthening sessions per week in autonomy), (2) therapeutic patient education (one educational diagnosis, two collective educational sessions, one evaluation), (3) combination of both interventions and (4) control. All participants will receive the international physical activity recommendations. Assessments (baseline, 6 and 12 months) will include physical fitness tests, anthropometrics measures, body composition (CT scan, bioelectrical impedance), self-administered questionnaires (physical activity profile (Recent Physical Activity Questionnaire), quality of life (European Organization for Research and Treatment of Cancer Quality-Of-Life Questionnaire-30, EQ-5D-5L), fatigue (Piper Fatigue Scale-12), social deprivation (Evaluation of Deprivation and Inequalities in Health Examination Centres), lifestyle, physical activity barriers, occupational status) and biological parameters (blood draw).
Ethics and dissemination
This study was reviewed and approved by the French Ethics Committee. The findings will be disseminated to the scientific and medical community via publications in peer-reviewed journals and conference presentations.
Trial registration number
Keywords: breast tumours, sports medicine, medical education & training
Strengths and limitations of this study.
This randomised clinical trial with four arms has the advantage to evaluate the efficacy of two interventions, either single or their combination, using a 2×2 factorial design, ensuring a higher statistical power than a classic trial with three arms, for a similar sample size.
While the connected device intervention is semisupervised, the exercise programme has been designed according to the preferences of women with breast cancer so as not to leave patients in total autonomy and to provide organisational flexibility to patients to facilitate adherence.
Despite the potential benefits of connected devices in cancer care, their use may face important issues, such as ethical challenges related to the security of sensitive data storage, technical challenges related to technological robustness and reliability, exacerbating access disparities and self-assessment of the participant’s fatigue or health condition.
The primary outcome measure is based on a declarative evaluation of physical activity that confers methodological limits to the study, but the validated questionnaire was chosen according for its easy implementation for patients with cancer compared with accelerometer monitoring and its relevance for the primary outcome.
Introduction
Breast cancer is the leading cause of cancer in women worldwide with 1.6 million new cases diagnosed each year,1 representing more than one-third of all new cancer cases in women. In France, breast cancer also represents the leading cause of cancer incidence and mortality among women, with approximately 58 000 new cases and 12 000 breast cancer deaths estimated in 2018.2 Despite a very good prognosis worldwide with overall survival of 85% at 5 years (87% in France) and 71% at 10 years (78% in France) for all stages combined,3–5 a large number of patients with breast cancer experience adverse effects of cancer and its treatments such as fatigue, impaired quality of life, anxiety or weight gain.6–8
In women with breast cancer, deteriorations of physical activity level and cardiorespiratory fitness are frequent.9 10 Physical activity is defined as any bodily movement produced by skeletal muscles that requires energy expenditure, including any daily life activity of household, occupation, recreation (eg, sports) or transportation. Exercise is a subset of physical activity that is planned, structured and repetitive, in the purpose of improving or maintaining physical fitness.11 After a breast cancer diagnosis, lack of physical activity, obesity and weight gain have been shown to increase the risk of cancer-related comorbidities and treatment adverse effects, to worsen long-term health and to cause poor prognosis.12–14 The benefits of physical activity have been well recognised in primary cancer prevention.15 Numerous studies have shown the safety16 and benefits of physical activity performed concomitantly with breast cancer treatments. These benefits include reduced fatigue17–19 and comorbidities,20 improved quality of life21 22 and physical functioning,10 17 19 22 as well as possibly reduced risk of recurrence23 and improved overall and specific survival with a positive dose–response relationship.14 23 24 Despite these benefits and international evidence-based guidelines of physical activity prescription for clinicians and their patients, accessibility to exercise programmes and implementing the guidelines in the cancer care process remain a challenge for patients and healthcare providers.25–27 While a growing number of facilities offer exercise programmes to patients with cancer, distance from home constitutes a barrier to regular exercise during cancer treatments.26 Successful exercise strategies during and beyond cancer treatment remain to be determined in clinical trials.28
The recent development of connected devices such as activity trackers offers a real opportunity in oncology to promote and monitor patients’ physical activity.29 While adherence to lifestyle interventions is a major challenge, connected activity trackers and smartphone applications enable structured monitoring of health parameters and provide feedback to patients. A systematic review of randomised controlled trials of physical activity interventions using new technologies such as activity trackers in patients with cancer (including five studies in breast cancer) has shown that patients significantly increased their number of steps per day in the majority of the studies.30 Recent reviews of intervention studies conducted among patients with breast cancer have also shown that patients increased their physical activity when they used activity trackers.31 32 Overall, connected activity trackers receive increasing interest for being systematically integrated into clinical oncology practice.33 34 Yet, more research is needed, especially clinical trials, to demonstrate the effectiveness of these tools and to respond to the preferences of patients with breast cancer.35–37
Therapeutic patient education has emerged in the 1990s in response to the recognition of the need to support patients in the self-management of their chronic diseases, such as diabetes and asthma.38 39 According to WHO, therapeutic patient education aims to ‘help patients acquire or maintain the skills they need to best manage their lives with a chronic disease’.40 In the cancer field, several cancer-specific programmes of therapeutic patient education have been set up to manage pain, fatigue, side effects of treatment (chemotherapy, surgery) or compliance to treatment.41–44 By enhancing relevant knowledge and skills, therapeutic patient education may greatly contribute to increasing patients’ autonomy in their disease management. Despite the performance in modifying long-term individual behaviours and adherence to cancer treatments,44 the benefits of therapeutic patient education on physical activity levels in patients with cancer early after diagnosis has been poorly investigated.45 46 The research on therapeutic patient education in the breast cancer and exercise context is limited to date and warrants further research.
Several biological mechanisms have been proposed to explain the effects of physical activity on breast cancer risk and outcome. Preclinical and human studies have shown the influence of physical activity on several signalling pathways involved in tumour development, growth and progression, including the insulin signalling pathway (IGF-1, insulin), chronic inflammation (involving inflammatory cytokines such as interleukin 6 (IL-6), tumour necrosis factor alpha (TNFα), c-reactive protein (CRP)) and endocrine hormone regulation (oestrogens, adipokines).47–49 By affecting the endogenous systemic milieu, physical activity is believed to influence cellular processes and tumour growth, and therefore reduce the risk of recurrence, increase treatment efficacy and improve survival.50 Also, because vitamin D alters mechanisms implicated in cellular growth and proliferation, accumulating evidence suggests that normal-to-high ranges of serum vitamin D levels improve breast cancer prognosis and outcome.51 Based on the data in the literature, it is not possible to conclude a causal relationship between the metabolic effects of physical activity and the impact on breast cancer risk and survival. Biological effects of physical activity on these biomarkers of endogenous mechanisms interfering in cancer suppression or proliferation remain to be elucidated in order to better understand the benefit of physical activity during adjuvant treatment.49
In this context, given the accumulating evidence for the benefits and safety of regular exercise during treatments of localised breast cancer, it is necessary to systematically encourage patients to remain or become physically active from the time of diagnosis and to implement and assess the most appropriate strategies of physical activity in clinical practice. The aim of the ‘dispositif connecté', that is, connected device in English (DISCO) trial is to encourage engagement in exercise during breast cancer treatment through two innovative types of interventions, that is, to say a web-based connected device and therapeutic patient education, which aim to develop patients’ autonomy in their practice of physical activity. The primary objective of the DISCO trial is to evaluate the efficacy of two interventions, either single or combined, concomitant to adjuvant treatments, on the physical activity level of patients with breast cancer at the end of the 6-month interventions, compared with usual care: one is an exercise programme using a connected device (comprising an activity tracker linked to a smartphone application and a website and providing an individualised, semisupervised, technology-based exercise programme) and the other is a therapeutic patient education intervention. The research hypothesis is that patients participating in the 6-month exercise programme using the connected device or therapeutic education intervention are more likely to achieve the international physical activity recommendations, compared with women receiving physical activity recommendations only (usual care). The WHO recommendations to maintain or improve health, which applied when the study protocol was developed, are to do at least 150 min of moderate-intensity or 75 min of vigorous-intensity aerobic physical activity or an equivalent combination each week, and muscle-strengthening activities at least 2 days a week.11 Secondary objectives are: (1) to evaluate the adherence to the interventions; the impact of the interventions on physical fitness, physical activity profile, anthropometrics, quality of life, fatigue, biological parameters, occupational status and lifestyle factors; the efficacy of the 6-month interventions on physical activity level at 12 months; the representations and acceptability of activity tracker and therapeutic patient education and (2) to assess the cost-effectiveness of the interventions. If one of the interventions is individually effective, the efficacy of the combination of both interventions at 6 and 12 months will be evaluated.
Methods and design
Trial design
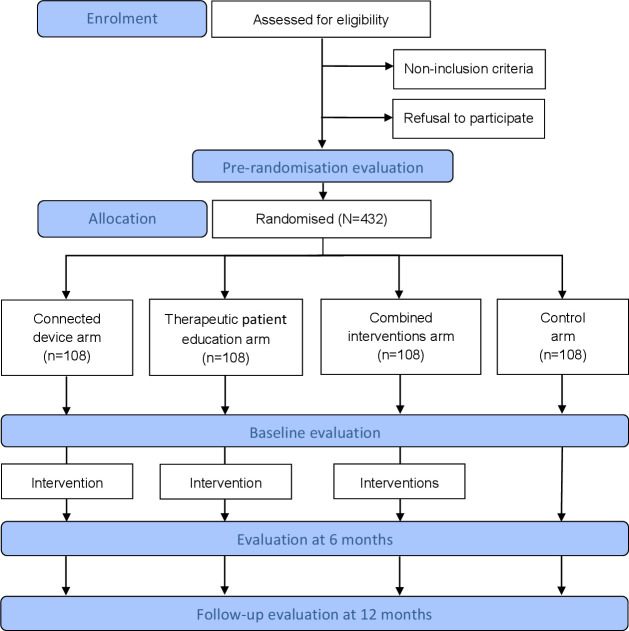
The DISCO trial is a 2×2 prospective, multicentre, factorial, randomised, controlled and open-label study (phase III), conducted by the Léon Bérard comprehensive cancer centre (Lyon, France) among women receiving treatment for localised breast cancer. The clinical protocol was designed and written according to the Standard Protocol Items: Recommendations for Interventional Trials guidelines (see online supplemental file 1). The flow chart of the study is presented in figure 1. Patients will be randomly assigned to one of the four arms of the study according to the 2×2 factorial design (1:1:1:1 ratio). They will all receive international recommendations on physical activity,11 and: (1) women allocated to the ‘connected device’ arm will benefit from a 6-month individualised, semisupervised exercise programme carried out autonomously. The programme consists of an evolving goal of daily numbers of steps using an activity tracker and two sessions of brisk walking and one session of muscle strengthening per week, using dedicated smartphone application and website; (2) women allocated to the ‘therapeutic patient education’ arm will benefit from four therapeutic education sessions on exercise; (3) women allocated to the ‘combined’ arm will benefit from both interventions in parallel and (4) women allocated to the ‘control’ arm will receive usual care.
Figure 1.
Flow chart of participants through the DISCO trial. DISCO, ‘dispositif connecté', that is, connected device in English.
bmjopen-2020-045448supp001.pdf (73.5KB, pdf)
Eligibility criteria for participants
Inclusion criteria include: being a female 18–75 years old; diagnosed with a first primary non-metastatic invasive breast carcinoma histologically confirmed; treated with curative surgery and requiring adjuvant treatment (chemotherapy, hormonotherapy and/or radiotherapy) that present at one of the investigating centres; providing a medical certificate of no contraindication to exercise; being available and willing to participate in the study for the duration of the interventions and follow-up; using a personal smartphone compatible with an application used for the intervention (iOS operating system from V.9.3, Android operating system from V.5.0, no Microsoft operating system) and having a computer with internet access; being able to understand, read and write French; and being affiliated with a social security scheme.
Non-inclusion criteria include: recurrent, metastatic or inflammatory breast cancer; personal history or coexistence of other primary cancer (except for in situ cancer regardless of the site, basal cell skin cancer and non-mammary cancer in complete remission for more than 5 years); presenting a contraindication to exercise according to the investigator (such as cardiorespiratory or bone pathologies, non-stabilised chronic diseases such as diabetes, malnutrition, etc); presenting severe malnutrition according to the criteria of the French National Health Authority (ie, for women≤70 years: weight loss ≥15% in 6 months or ≥10% in 1 month; for women >70 years: weight loss ≥15% in 6 months or ≥10% in 1 month and body mass index (BMI) <18 kg/m²)52; being unable to be followed for medical, social, family, geographical or psychological reasons for the duration of the study; pregnant or breast feeding or of childbearing age without effective contraception for the duration of the study.
Recruitment
Recruitment started in May 2018. Participants will be recruited at several national comprehensive cancer centres, clinics or hospitals located in France, which will ensure adequate participant enrolment to reach the target sample size in a timely manner. Inclusion of patients will be carried out after surgery and confirmation of the indication of adjuvant treatment. The study will be proposed to patients at the postoperative, prechemotherapy or preradiotherapy consultation (by the surgeon, oncologist or radiotherapist investigator, respectively) depending on the patient’s treatment plan. At this visit, the investigator will check all eligibility criteria and propose to the eligible patients to participate in the study, explain the objectives and study process and give them an information notice. After sufficient time for reflection, eligible patients who agree to participate will date and sign an informed consent (see online supplemental file 2) and will be included prior to the onset of adjuvant therapy (or within 1 month thereafter). The number of eligible patients refusing to participate in the study and the reason for non-participation will be recorded.
bmjopen-2020-045448supp002.pdf (74.2KB, pdf)
Randomisation
Prior to randomisation, participants will be asked to complete the Recent Physical Activity Questionnaire (RPAQ) to assess their level of physical activity.53 Their weight, body size and prescribed adjuvant treatments will be collected from the patient’s medical record.
Participants will be randomised using EnnovClinical software (V.7.5.710.4, Ennov, Paris, France) into one of the four arms of the trial, by using the following minimisation criteria54 55: BMI (<25 kg/m², ≥25 and<30 kg/m², ≥30 kg/m²), baseline physical activity level from RPAQ (<150 min/week, ≥150 min/week of moderate-to-vigorous physical activity) and prescribed adjuvant treatments at inclusion (ie, chemotherapy+hormone therapy±radiotherapy, hormone therapy±radiotherapy, chemotherapy±radiotherapy, radiotherapy only).
Interventions
At baseline, all participants will receive the international recommendations in terms of physical activity for promoting health in the general population,11 which will be delivered orally by a certified exercise instructor with the help of a leaflet.
Intervention with a connected device
Participants randomised to the ‘connected device’ arm will benefit from a 6-month exercise programme. The connected device consists of an activity tracker (connected wristband, LS417-F model, CARE Fitness, Bobigny, France) that participants will wear daily, a dedicated smartphone application and a dedicated website proposing an individualised, semisupervised exercise programme adapted to patients with cancer (developed by BIOMOUV, Paris, France). This automated web-based and mobile-based exercise programme will aim to support participants to enhance physical activity in two ways: doing structured exercise sessions and increasing daily physical activity (number of steps). Exercise sessions will be automatically generated by an algorithm based on the patient profile (described below). The participants will receive notifications informing them of a new structured exercise session available on the website or mobile application or alerting them when a session was not carried out, and inviting them to execute it when possible. Participants will receive a free 6-month subscription to the programme.
Setting up the connected device
At the end of the baseline assessment, the certified exercise instructor will introduce the customised exercise programme to the participants and will give them the activity tracker and a user guide for the connected device. Then, the certified exercise instructor will explain the functioning of the activity tracker, the dedicated smartphone application and the dedicated website, as well as assist the participants to instal the application on their smartphone. The participants will be registered in the customised exercise programme by the certified exercise instructor. The registration will consist of completing a web-based questionnaire about personal and health data to determine the participant profile (age, weight, height, level of aerobic and muscular strength, treatment, symptoms, availabilities for exercise sessions and sports materials).
Baseline level of aerobic and muscular strength for the individualised exercise programme
The physical fitness tests performed at baseline will be used to classify the participants at the start of the exercise programme according to their aerobic level (for the walking sessions) and their muscular strength level (for the strengthening sessions). The aerobic level categories will be determined by the distance performed during the 6 min walk test (6MWT): aerobic group 1 (<460 m), aerobic group 2 (460–580 m) and aerobic group 3 (>580 m). The muscular strength level categories will be determined by the number of sit-ups performed on a chair in 30 s during the Sit-to-stand test: muscular strength group 1 (≤10 repetitions), muscular strength group 2 (11–14 repetitions) and muscular strength group 3 (≥15 repetitions). Thresholds were based on average values reached by women receiving breast cancer treatments for the 6MWT (pooled mean value, 523 m) and the Sit-to-stand test (pooled mean value, 13 repetitions) from a previous study;56 these values were checked for consistency with percentile scores obtained at the 6MWT and Sit-to-stand test in community-dwelling older women,57 then the IQR was used to determine the thresholds for the three groups of this study. The level categories assigned will be entered by the exercise instructor in the baseline patient profile and will be used by the automated algorithm to set up the level of the first walking and muscle strengthening sessions.
Exercise programme
The 6-month exercise programme will be semisupervised by the certified exercise instructor through an individual follow-up of participants (see ‘Participant follow-up’ part and ‘Continuous monitoring’ part). It will be carried out autonomously by the participants at home by using the smartphone application and the website. The programme is based on three structured unsupervised sessions per week alternating two types of exercise: two walking sessions (by following oral instructions given via the smartphone application) and one muscle strengthening session (by using videos accessible on the website). The levels of the first walking and muscle strengthening sessions will be determined by the fitness tests performed at baseline (see ‘Baseline level’ part). Then, subsequent sessions will be planned according to the available days of the participant. Strengthening exercises will be adapted according to sports materials available at their home (eg, Swiss ball, sports mat, stick, weight, etc). Each session will include: (1) a warm-up period of 5 min; (2) a body session of 10–35 min of strengthening exercises or 10–50 min of walking (mixing continuous and/or intermittent effort); (3) a 5 min recovery period, consisting of stretching and relaxation during strengthening sessions or a cool down during walking sessions. Sessions will be of moderate-to-high intensity (≥3 and ≤9 METs).
The three structured unsupervised exercise sessions per week are configured by a unique algorithm hosted by an accredited personal healthcare data host (Orange Business Services, Paris, France), to plan the exercise sessions and determine the exercise level in an adapted and progressive manner by increasing the duration and then intensity in accordance with principles of exercise training and progression.58 59 At the beginning of each session, the duration and intensity of the session will be determined according to the perceived difficulties (evaluated by a Borg scale) and emotional state (recorded by an emoji) of the participant in the previous session, and will be modified or postponed according to the level of fatigue (evaluated by a Borg scale), the level of dyspnoea (evaluated by a Borg scale), the presence or absence of unusual muscle pain and the presence or absence of unusual nausea/diarrhoea. In case of a severe adverse event related to disease or treatment (ie, joint disability, osteoarthritis, cachexia, hand-foot syndrome, aplasia, diuretic, axillary node dissection, pace-maker, chemotherapy, targeted therapy, hormone therapy, radiotherapy, chronic obstructive pulmonary disease (COPD), diabetes) or temporary contraindication to exercise, declared by the participant on her device, the programme and sessions will be adapted or suspended until the participant’s health improves.
In addition, participants will have the opportunity to perform additional exercise sessions according to their preferences and lifestyle, outside the programme. Participants will be asked to record these sessions through the smartphone application or the website: type of activity (eg, walking, hiking, cycling) from a list adapted from Ainsworth’s Compendium,60 and its duration and intensity.
Number of daily steps
Participants will be advised to wear the activity tracker daily and to launch the application regularly (preferably daily), which will automatically synchronise with the activity tracker via Bluetooth connection and will collect the number of steps. The target number of steps will be 3000 steps per day at the programme onset, and then will be re-set based on the average number of daily steps during the first week after inclusion. The target number of daily steps will evolve automatically every 3 weeks based on the average number of daily steps achieved during the previous 3 weeks, and will be updated automatically in the application. Consistent with principles of exercise training and progression,58 59 after each 3-week cycle, if the goal of steps per day is reached by the participant, the target goal will increase by 15% during the following 3-week cycle, within a maximum target of 10 000 daily steps. If the average number of daily steps does not meet the goal, the target will remain unchanged in the next cycle.
Participant follow-up
Telephone follow-ups will be carried out by the certified exercise instructor at 10 days, 2 months and 4 months after the intervention onset to ensure the proper functioning of the connected device, review the use of the connected device, review the conduct of the sessions and answer the participants’ questions if they may have. Participants will be orally encouraged to remain physically active on a daily basis (reminder of the benefits and recommendations of physical activity, success and satisfaction during the exercise sessions). During the 6-month intervention, the participants will have the opportunity to contact the certified exercise instructor or the clinical research assistant at any time, by email (directly through the website) or by telephone for any question or assistance with the connected device.
Continuous monitoring
The certified exercise instructor will monitor the use of the connected device by the participants and their progress in the programme through a dedicated professional website that provides real-time access to the participants’ data. On this website, an automatically generated daily event table will inform the certified exercise instructor of the occurrence of disabilities reported by the participants that may lead to modifying their programme (eg, severe fatigue, dyspnoea, unusual muscle pain) or if participants have not performed their planned sessions or used their activity tracker for seven consecutive days. On these alerts, the certified exercise instructor will contact the participants to precisely analyse the reported disabilities, advise participants, identify the causes of non-use of the connected device, solve possible technical problems or reinforce participant’s motivation if necessary.
End of the intervention
At the end of the 6-month programme, participants will keep their activity tracker to be encouraged to continue regularly exercising in autonomy. On their request, continued subscription to the dedicated application and website will be offered for another 6 months, with no individual follow-up anymore.
Intervention of therapeutic patient education
Participants randomised to the therapeutic patient education arm will benefit from a therapeutic patient education intervention, in addition to receiving the international physical activity recommendations. The intervention is part of the therapeutic patient education programme set up at the Léon Bérard cancer centre and validated by the Regional Health Agency (‘Agence Régionale de Santé Rhône-Alpes’). It will be disseminated in the investigating centres according to the criteria of the Regional Health Agency. The therapeutic patient education intervention consists of four sessions that will be scheduled according to participants’ availability during their follow-up visits as part of their usual clinical management over a 6-month period.
First, participants will be invited to an initial 1-hour individual face-to-face session of educational diagnosis with a health professional trained in therapeutic patient education. This session will assess their needs and establish a contract of objectives to reach. Then, participants will be invited to participate in two collective educational sessions (1h30 each with a group of 10 patients maximum per session). These sessions will be composed of theoretical and practical workshops to help them understand their physical activity in their daily life and implement the necessary means to practice regular exercise in autonomy. Finally, participants will be invited to another 1-hour individual session, where an educational evaluation will be conducted to identify whether they achieve their individual objectives set at the time of the educational diagnosis.
Combined interventions
Participants randomised to the ‘combined intervention’ arm will benefit from a combination of the connected device intervention and the therapeutic patient education intervention in parallel for 6 months.
Evaluations
The initial assessment (T0) will be performed prior to randomisation for minimisation purposes. The other three evaluations will then be conducted at baseline (T1), 6 months (T2) and 12 months (T3). All study participants will then be followed at 6 months±1 month postrandomisation (corresponding to the end of participation in the interventions for women in the connected device, therapeutic patient education and combined arms) and at 12 months±1 month postrandomisation (corresponding to a follow-up period of 6 months postinterventions). Assessments will be carried out by a clinical research assistant and a certified exercise instructor. The clinical research assistant will contact participants by phone to invite them to follow-up visits and to promote participant retention and complete follow-up. Participants will have no compensation for participation and all study visits will be scheduled on days of their medical or health-related appointments.
All evaluations (baseline, 6 and 12 months) will include physical fitness tests, anthropometric measures, self-administered questionnaires and a non-fasting blood draw (baseline and 6 months only). Data will be recorded using an electronic case report form (eCRF).
Data collection
The study outcome measures and their schedule are summarised in table 1.
Table 1.
Summary of outcome measures and data collection schedule for the DISCO trial
| Assessments | Tools | Baseline±1 month | 6 months±1 month | 12 months±1 month |
| Demographic and clinical data | Patient’s medical record | |||
| Month/year of birth | X | |||
| Age at diagnosis | X | |||
| Employment status | X | X | X | |
| Personal history of breast cancer | X | |||
| Current treatment | X | X | X | |
| Hormonal receptor status | X | |||
| Tumour histology | X | |||
| Disease progression | X | X | ||
| Anthropometrics | ||||
| Height | Gauge | X | ||
| Weight | Scale | X | X | X |
| Waist-to-hip circumference | Measuring tape | X | X | X |
| Body composition: fat mass, lean mass, dry lean mass, body water | Bioelectrical impedance analysis | X | X | X |
| Physical fitness | X | X | X | |
| Walking endurance with perceived difficulty | 6MWT and Borg scale | |||
| Lower limb muscle strength | Sit-to-stand test | |||
| Hand prehensile strength | Hand-grip test | |||
| Flexibility of lower limbs | Sit-and-reach flexibility test | |||
| Balance | Single-leg stance test | |||
| Physical activity level, sitting time and achievement of physical activity recommendations | RPAQ Questionnaire | X | X | X |
| Patient-reported outcomes | ||||
| Quality of life | EORTC QLQ-C30 questionnaire and BR-23 module | X | X | X |
| Health-related quality of life | EQ-5D-5L Questionnaire | X | X | X |
| Fatigue | PFS-12 questionnaire | X | X | X |
| Social vulnerability | EPICES questionnaire | X | X | |
| Determinants of physical activity | ||||
| Barriers to regular physical activity; lifestyle | Self-administered questionnaire | X | X | X |
| Uses, representations and motivation of physical activity; acceptability of activity trackers (only for patients in the ‘connected device’ and ‘combined’ arms); acceptability of therapeutic patient education (only for patients in the ‘therapeutic patient education’ and ‘combined’ arms) | Online self-administered questionnaire | X | X | X |
| Biological data | Blood sample | X | X | |
| Serum endocrine factors (IGF-1, insulin, estradiol) | ||||
| Plasmatic inflammatory cytokines (IL-6, TNFα, CRP) | ||||
| Plasmatic adipokines (adiponectin, leptin) | ||||
| Vitamin D status | ||||
| Compliance with each intervention (only for patients in the ‘connected device’, ‘therapeutic patient education’ and ‘combined’ arms) | Connected device and/or patient’s record | X | ||
| Adverse events (neuropathies, joint pain) | Patient’s diary, CTCAE V.5 | X | X |
BR-23, Breast Cancer Questionnaire; CRP, c-reactive protein; CTCAE, Common Terminology Criteria for Adverse Events; DISCO, dispositif connecté', that is, connected device in English; EORTC, European Organization for Research and Treatment of Cancer; EPICES, Evaluation of Deprivation and Inequalities in Health Examination Centres; EQ-5D-5L, European Quality of Life-5 dimensions-5 levels; IGF-1, insulin signalling pathway-1; IL-6, interleukin 6; 6MWT, 6 min walk test; PFS-12, Piper Fatigue Scale-12; QLQ-C30, Quality-Of-Life Questionnaire; RPAQ, Recent Physical Activity Questionnaire; TNFα, tumour necrosis factor alpha.
Sociodemographic and clinical data
Sociodemographic and clinical data, including month/year of birth, age at diagnosis of breast cancer, family status, level of education, hormonal status, tumour histology and personal history of breast cancer will be collected at baseline. Family status, potential cancer progression and all treatments received for cancer will be collected at 6 and 12 months. All data will be extracted from patients’ electronic medical records, except family status and level of education that will be self-reported in a questionnaire.
The occupational status will be assessed using a self-administered questionnaire asking employment status, occupation, size of the company, the perceived intensity of the physical effort at work, the evolution of employment status at return to work in case of sick leave.61
Anthropometrics and body composition
The standing height (cm), body weight (kg) and waist (cm) and hip (cm) circumferences will be measured using standardised procedures and BMI will be calculated as the body weight in kilograms divided by the square of the height in metres (kg/m²). The waist circumference will be measured midway between the last floating rib and the iliac crest. The hip circumference will be measured at the tip of the pubis. Body composition will be measured by a bioelectrical impedance metre (Biody XPert ZM II, eBiody, eBIODY SAS, La Ciotat, France) to assess fat mass (in kg), lean body mass (in kg), muscle mass (in kg), dry lean mass (in kg), total body water (in L), intracellular fluid (in L) and extracellular fluid (in L).
Physical fitness
Cardiorespiratory fitness will be evaluated by the walking endurance during the 6MWT (distance covered in metres) with perceived difficulty using the Borg scale.62 During this test, participants will be asked to perform the maximum walk shuttle distance on a 30 m long flat corridor in 6 min.
The lower limb muscle strength will be measured using the sit-to-stand test (number of sit-ups on a chair in 30 s). During this test, participants will be asked to sit down on a chair and get up as many times as possible during 30 s.63
Hand prehensile strength will be measured by the handgrip test using hand dynamometry (Jamar Plus Digital Hand Dynamometer, Patterson Medical, Huthwaite, UK), which is a validated index of the isometric strength of the hand and forearm muscles.64 During this hand-grip test, participants will be asked to squeeze the handgrip as strongly as possible to obtain the maximal force (in kg). Two measures will be performed on each hand and the best performance will be registered.
The flexibility of lower limbs will be measured using the sit-and-reach flexibility test (Deluxe Baseline flexibility test, 3B Scientific, Bartenheim, France).65 In this test, participants will sit on the floor on a mat with their legs stretched out straight ahead. They will be asked to lean forward as far as possible and the distance between fingertips and toes will be measured (in cm) (ie, by considering the level of the feet as recording zero, any measure that does not reach the toes is negative and any measure beyond the toes is positive).
The balance will be measured using the bilateral single-leg stance test.66 The participants will stand and be asked to lift a foot and hold the position for a maximum of 60 s, then to do the same exercise on the other foot (duration held in equilibrium, 2 times 60 s).
Physical activity level, sitting time and achievement of physical activity recommendations
The validated self-administered questionnaire RPAQ will be used to measure the self-reported physical activity.53 67 The RPAQ was designed to assess usual physical activity in the last 4 weeks, covering three activity domains: domestic physical activity, including sitting time that is a good proxy of sedentary behaviour; occupational physical activity, including transportation to and from work; and recreational physical activity. The RPAQ gives specific scores in the metabolic equivalent of task (MET) unit for activities of very low intensity (<1.5 METs, ie, sedentary activities), low intensity (1.5 to <3 METs), moderate intensity (3 to <6 METs) and high intensity (≥6 METs, ie, vigorous activities) within each domain during the past 4 weeks. Questions will be coded and converted in MET-minute per 4 weeks according to the Compendium of Physical Activities60 by multiplying the number of METs by the duration and frequency of each activity. Then, the global score of physical activity will be obtained by adding the number of MET-minutes per 4 weeks in each intensity and each domain. The physical activity profile will be defined as the time spent in physical activities of low, moderate and high intensities. The physical activity level will be defined by the overall weekly physical activity (average expressed in MET-hour/week).
Achievement of international physical activity guidelines will be computed for each individual by dividing the time spent in moderate-to-vigorous physical activity (ie, ≥3 METs) into two categories11: <150 min/week of moderate-to-vigorous physical activity (ie, under physical activity guidelines); ≥150 min/week of moderate-to-vigorous physical activity (ie, reaching physical activity guidelines).
Patient-reported outcomes
The quality of life will be measured using the European Organization for Research and Treatment of Cancer (EORTC) Quality-Of-Life Questionnaire (QLQ-C30) and its specific module for breast cancer (BR-23).68 The QLQ-C30 is a 30-item validated self-administered questionnaire that evaluates five functioning domains (ie, physical, role, emotional, cognitive and social), a global quality-of-life domain, three symptom domains (ie, pain, fatigue and nausea) and six single items (ie, dyspnoea, insomnia, anorexia, diarrhoea, constipation and financial impact). Each item is associated with a score ranging from 0 to 100. For the functioning and global quality-of-life scales, a higher score corresponds to a better functioning level. For scales related to symptoms, a lower score corresponds to a better functioning level. The BR-23 module gathers data about perceived body image, sexual functioning, sex enjoyment, arm symptoms, breast symptoms and systemic therapy side effects.
The health-related quality of life will be assessed using the European Quality of Life-5 dimensions-5 levels (EQ-5D-5L) questionnaire.69 This standardised self-administered questionnaire describes five dimensions (ie, mobility, self-care, usual activities, pain/discomfort and anxiety/depression) being rated using five levels (ie, no, slight, moderate, severe and extreme problems), and comprises a 0–100 Visual Analogue Scale recording the self-rated health (where the endpoints are labelled ‘The best health you can imagine’ and ‘The worst health you can imagine’).
Fatigue will be assessed using the Piper Fatigue Scale-12 (PFS-12), a 12-item self-reported questionnaire with four subscales (ie, behavioural, affective, sensory and cognitive/mood aspects of fatigue)70: the higher the score, the worse the fatigue. All items together will produce a total score for fatigue that will be used to define categories as follows: no fatigue (score=0), mild fatigue (score 1–3), moderate fatigue (score 4–6) and severe fatigue (score 7–10).
Social deprivation will be assessed using the EPICES (Evaluation of Deprivation and Inequalities in Health Examination Centres) score.71 The score will be computed by adding each question coefficient to the intercept whenever the answer is ‘yes’. The score ranges from 0 to 100 (ie, the higher the score, the greater the deprivation level) with the threshold for deprivation at 30.
Lifestyle factors, assessed using a self-administered questionnaire, include tobacco status (ie, never, former, current smoker), lifetime and current tobacco use (expressed in pack-years) and alcohol intake over the past 6 months (usual frequency of consumption (ie, never, less than 1/month, 1–3 times/month, 1–6 times/week, daily) of different categories of alcoholic beverages (ie, wine, beer, cider, aperitif wine, cocktail/punch, aniseed alcohol, spirits) as well as the usual number of glasses). The amount of alcohol will be computed by multiplying the frequency of consumption by the number of glasses and alcohol content of each type of alcoholic beverage. The average daily alcohol intake over the past 6 months (in g/day) will be computed by summing the amount of alcohol from each beverage.
Determinants of physical activity
The 21-item self-administered questionnaire ‘Barriers to Being Active Quiz’ will be used to qualitatively assess barriers to the regular practice of physical activity.72
Uses, representations and motivation towards physical activity will be assessed within the study population using a self-administered questionnaire available online. Acceptability of connected devices and acceptability of therapeutic patient education will be assessed among participants randomised to the corresponding arms using a paper-based self-administered questionnaire. These questionnaires will be developed following the Unified Theory of Acceptance and Use of Technology,73 which is a specification of the Theory of Planned Behaviour74 designed to explain and predict the probability of behaviour change among individuals faced with new technologies. The Theory of Planned Behaviour has been massively used during the last two decades to promote health behaviours such as physical activity. Besides, item wording will be based on the results of individual and collective interviews conducted for that purpose and designed to identify social representations75 of health protection and physical activity incentive devices.
Compliance with interventions
Compliance with each intervention will be assessed at the 6-month evaluation only for patients randomised to the ‘connected device’, ‘therapeutic patient education’ and ‘combined’ arms. Compliance will be assessed by the number of days of use of the activity tracker, the participation rate in scheduled exercise sessions, the participation rate in scheduled therapeutic education sessions and the proportion of compliant patients, depending on the intervention allocated, following the recommendations of the protocol. Patients’ compliance and reasons for non-compliance during the intervention period (6 months) will be described for each arm.
Biological assessments
A non-fasting blood sample (one 10 mL EDTA tube and one 10 mL dry tube) will be collected at baseline and 6 months. In particular, blood will be drawn at baseline before the onset of adjuvant treatments, otherwise no blood samples will be collected. The following biological factors will be assessed in the blood samples: circulating serum levels of endocrine factors (IGF-1, insulin, estradiol), circulating plasma levels of inflammatory cytokines (IL-6, TNFα, CRP), circulating plasma levels of adipokines (adiponectin, leptin) and vitamin D status.
Study outcomes
The primary endpoint will be the proportion of women who achieve at 6 months the internationally recommended level of physical activity (at least 150 min/week of moderate-to-vigorous physical activity, ie, intensity ≥3 METs) assessed by the RPAQ self-administered questionnaire.
Secondary endpoints will be:
Assessment of the efficacy of the programmes at 12 months (ie, the proportion of women who achieve the internationally recommended level of physical activity).
Assessment of the adherence to the interventions at 6 months (the proportion of participants who are compliant to the programme, participation rate in planned sessions).
Assessment of the impact between baseline and 6 months and between 6 and 12 months of the interventions on physical activity profile (changes in time spent in different intensities of physical activity and time spent in sedentary activities), physical fitness (changes in results to the 6 MWT, hand-grip test, sit-to-stand test, sit-and-reach flexibility test and single-leg stance test), anthropometrics (changes in weight, waist and hip circumferences, BMI, fat mass, lean body mass, muscle mass, dry lean mass and body water), quality of life (changes in scores obtained from the EORTC QLQ-C30 questionnaire and its BR-23 module), fatigue condition (changes in scores obtained from the PFS-12 questionnaire), health-related quality of life (changes in scores obtained from the EQ-5D-5L questionnaire), social deprivation (changes in scores obtained from the EPICES self-administered questionnaire), occupational status (the proportion of participants who changed their employment status, with return to work and who perceived difficulty at work obtained from a self-administered questionnaire) and lifestyle factors (the proportion of participants who change their tobacco use and alcohol intake obtained from a self-administered questionnaire).
Assessment of the impact of the interventions on biological parameters between baseline and 6 months (changes in serum circulating levels of endocrine factors (insulin, IGF1, estradiol), changes in plasma circulating levels of cytokines (inflammatory cytokines: IL-6, TNF and CRP; adipokines: adiponectin and leptin), the proportion of participants with a modification on vitamin D status).
Assessment of the representations and acceptability of activity tracker and therapeutic patient education, at baseline, 6 and 12 months (proportions of participants who accept the connected device and who accept the therapeutic programme, according to scores obtained from a self-administered qualitative questionnaire used in social psychology science).
Assessment of refusal rate among eligible patients (the proportion of patients who refuse to participate).
Assessment of the cost–utility and the cost-effectiveness of implementing each intervention at 12 months, using clinical data (treatments received, patients’ diary on medical consultations), hospital costs (national data) and benefit in physical activity level.
Statistical analysis
Sample size determination
The efficacy rate assumptions are μ=40 %, μ+μA=55% and μ+μB=65% for the ‘control’, ‘therapeutic patient education’ and ‘connected device’ arm modalities, respectively. The expected benefit in the ‘therapeutic patient education’ arm compared with the ‘control’ arm is 15% (40% efficacy in the ‘control’ arm vs 55% efficacy in the ‘therapeutic patient education’ arm). The expected benefit in the ‘connected device’ arm compared with the ‘control’ arm is 25% (40% efficacy in the ‘control’ arm vs 65% efficacy in the ‘connected device’ arm).23
The sample size is calculated to allow the two comparisons of interest to be tested bilaterally at the threshold of 0.025. Assuming that the ‘therapeutic patient education’ intervention and the ‘connected device’ intervention act independently (additive model), the sample size required to compare therapeutic patient education (ie, participants assigned to the ‘therapeutic patient education’ and ‘combined’ arms) vs no therapeutic patient education (ie, participants assigned to the ‘control’ and ‘connected device’ arms) is given by the following formula:
[μ + (μ+μB)]/2, vs [(μ+μA) + (μ + μA + μB)/2], that is, (40%+65%)/2=52.5 %, versus (55%+80)/2=67.5%
With a first species risk α=0.025 and a power of 80% in the bilateral situation, the number of patients to include per treatment arm to demonstrate the efficacy of the therapeutic patient education will be 108 (or 432 for the four treatment arms) (nQuery V.6.0, χ2 test with continuity correction). This number of patients will also allow a power greater than 95% to evaluate the efficacy of the ‘connected device’ intervention, always with a risk α=0.025 in the bilateral situation.
Data analysis plan
The following populations will be defined for statistical analyses: (1) the intention-to-treat (ITT) population, which includes all randomised participants in the study; (2) the per-protocol population, which consists of a subgroup of participants from the ITT population, who has no major protocol violations and who follows the procedure for the duration of the study. Analyses in the ITT population will be performed for all the study endpoints; analyses in the per-protocol population will be performed for exploratory purposes. The randomisation date will be considered as the reference date in all delay calculations, unless any other way is specified.
Baseline data will be described in the ITT population and presented by randomised arms. For the primary outcome, proportions will be estimated for the two targeted comparisons: (1) participants who received the connected device vs participants who did not; (2) participants who benefited from the therapeutic patient education intervention vs participants who did not. Results will be presented with their 95% CI. The use of a 2×2 factorial design will allow to test, respectively: the efficacy of the intervention with a connected device (compared with without a connected device); the efficacy of the therapeutic patient education intervention (compared with no therapeutic patient education); and the interest of two combined intervention modalities (ie, connected device and therapeutic patient education) compared with the single intervention with the connected device only or with therapeutic patient education only. The analysis strategy will, therefore, be as follows76: (1) searching first for an interaction by a specific interaction test, performed at the significance level of 0.05 (χ2 test or use of an interaction term in a logistic model); (2) in the absence of interaction, testing each of the two bilateral interest comparisons at the threshold of 0.025, namely the efficacy of the intervention with the connected device and the efficacy of the therapeutic patient education intervention; (3) in case of the efficacy of either one of the intervention modalities, evaluating the interest of the combination of the two interventions compared with the single intervention with the connected device only or with therapeutic patient education only.
For secondary outcome variables, the efficacy of the programme at 12 months, as well as according to stratification criteria, will be analysed similarly to the primary outcome. The adherence to the interventions will be evaluated by the proportion of compliant participants and participation rate in planned sessions. Changes in physical activity profile, physical fitness, anthropometrics, quality of life, fatigue, social deprivation and biological parameters will be analysed by the absolute and/or relative variations in each of these endpoints; these variations will be compared between with and without each intervention, for each intervention, and between combined interventions and the single one, using a parametric test. Occupational status and lifestyle factors will be analysed by comparing the proportion of participants between interventions or their combination. Representations and acceptability of activity tracker and therapeutic patient education will be analysed by comparing the proportion of participants between randomisation and follow-up assessments. A method for imputing missing data will be considered if necessary.
Statistical analyses will be performed using SAS software V.9.4 or later.
Medico-economic analysis
The cost-effectiveness analysis will be conducted alongside the trial using the French national health insurance perspective. Quantities of resources used (external consultations, hospital stays including diagnosis-related groups, drugs with extra payments and other healthcare-related costs) will be collected on the eCRF and multiplied by the respective unit costs. The intervention with therapeutic patient education and the intervention with connected device will be evaluated using a bottom-up microcosting approach.77 78 Using the diagnosis-related group, hospital stays will be evaluated based on the French National hospital costs study database. External consultations and wider examinations, community care (general practitioner visits, nurse visits, etc) will be valued on the basis of the general nomenclature of professional treatments (‘Nomenclature Générale des Actes Professionnels’). The cost of biological treatments will be estimated using the nomenclature of biological medical treatments (‘Nomenclature des Actes de Biologie Médicale’). The cost of technical treatments (eg, imaging) will be estimated using the common classification of medical treatments (‘Classification Commune des Actes Médicaux’). Acquisition costs for the most expansive drugs will be based on the list of common units of dispensation for supplementary medicines (‘liste des unités communes de dispensation prise en charge en sus’). Finally, costs of medical transport will be derived from the French Court of Audit’s report on medical transport expenses covered by the French National Health insurance. The time horizon will be 12 months. Hence, neither costs nor effectiveness will be discounted. Mean costs and effectiveness will be derived for all four strategies under consideration: connected device, therapeutic patient education, combined and control arms. Incremental cost-effectiveness ratios (ICERs) will be expressed in cost per quality-adjusted life-year gained using EQ-5D-5L to estimate utility, cost per life-year gained, cost per BMI unit lost and cost per centimetre of waist-to-hip circumference lost. One-way sensitivity analyses will be conducted by varying resource consumption and unit cost parameters and graphically illustrated in a Tornado diagram. The uncertainty surrounding the ICERs will be also captured by a probabilistic analysis using non-parametric bootstrap methods as recommended by the French National Authority for Health.79
Adverse events
All participants will continuously report the occurrence of adverse events regarding neuropathies and joint pain in their patient’s notebook, which will be collected at 6 and 12 months. Those equipped with the connected device will also report potential adverse events before and after each session of their exercise programme (see Connected device). The reported adverse events will then be graduated according the Common Terminology Criteria for Adverse Events (CTCAE) V.5. Due to the low risks associated with the interventions,16 this study is part of the so-called ‘intervention research with minimal risks and constraints’ in the French legislation and therefore only these adverse events arising within the framework of the study will be reported.
In the occurrence of an adverse event regarding neuropathies and joint pain, the principal investigator will report it to the health authorities responsible for vigilance without delay. The promotor will also report the adverse events, as well as any safety measures to be proposed, to the French Ethics Committee and the investigators without delay.
Data management
The database for clinical data and randomisation will be created using EnnovClinical software. Its access will be secured (personal identification and password protection) for maintaining confidentiality at all times. Individual participants will not be identified in any reports of this trial. All data from the connected device will be merged to the clinical database at the end of the study. Investigators and data analysts will have access to the final dataset.
Data monitoring will be provided by the trial steering committee, including overall project supervision, progress monitoring, advice on scientific credibility and ensuring the integrity and appropriate running of the project. The clinical research assistant will verify all consent forms, compliance with established protocol and procedures, and data quality in the eCRF. The research team will make biannual reports to the trial steering committee.
Patient and public involvement
An association of breast cancer patients’ representatives (Europa Donna France, http://www.europadonna.fr/) was involved in preparing the conduct of interventions and evaluations, in particular by considering patients' expectations, experience and desire for global care. The association will be involved in plans to disseminate the study results to patients with breast cancer, study participants and wider patient communities concerned.
Ethics and dissemination
The study protocol was approved by the French ethics committee (Comité de Protection des Personnes Est I, ID RCB 2017-A03360-53, 1 February 2018) and its database was reported to the French National Commission for Data Protection and Liberties (CNIL, ref. MR-001 no. 2016177, 13 December 2016). Substantial protocol modifications will be submitted to the ethics committee for approval and protocol amendment.
The study findings will be widely disseminated through the clinical community by publications in international, peer-reviewed journals and by presentations at national and international conferences. They will also be communicated to patients through associations of patients’ representatives and science-based information websites. They will be useful for improving the clinical care of patients with cancer and providing useful information for implementing exercise programmes for patients with cancer to health professionals, institutions and public authorities. The study sponsors will disseminate the study findings to their stakeholders.
Discussion
This article presents the protocol for the DISCO trial, which aims to evaluate the efficacy of a web-based and mobile-based connected device intervention and of a therapeutic patient education intervention, either single or combined, on the physical activity levels of patients with breast cancer undergoing adjuvant treatment, as well as to assess the cost-effectiveness of the interventions. This multicentre study opened in May 2018 and recruitment is expected to end in Summer 2021. In the short term, the expected results are to develop the autonomy of patients with breast cancer in their practice of physical activity, as well as to identify the best strategies of physical activity during breast cancer adjuvant treatments to increase and sustain physical activity levels in patients, overall or in specific subgroups according to BMI, baseline physical activity level and type of adjuvant treatment. In the medium term, the goal of the DISCO trial is to disseminate innovative programmes in supportive cancer care, based on scientific evidence, to systematically integrate exercise in breast cancer cares.
While an increasing number of studies have demonstrated the benefits of exercise in patients with breast cancer, the routine implementation in the cancer care process lacks behind evidence and practice guidelines.80–82 While the prescription of physical activity in supervised programmes has been shown superior compared with non-supervised programmes,22 83 semisupervised interventions seem to yield comparable or superior benefits to supervised programmes.84 Therefore, the semisupervised exercise programme of the DISCO trial through continuous follow-up has been designed according to the preferences of women with breast cancer so as not to leave patients in total autonomy.36 85 Connected devices are tools developed over the last 10 years that are very promising for promoting physical activity in the general population and in patients with chronic diseases such as cancer86 87 and for developing distance-based physical activity interventions.88
The semisupervised home-based physical activity programme of the DISCO trial using the connected device provides flexibility to patients that may facilitate adherence and to overcome barriers due to distance of facilities from women’s home and spatial inequalities of access.27 Connected devices allow proposing a tailored physical activity programme to patients regardless of their place of residence, and enable patients to practice physical activities of their choice, at any time that suits them. Therefore, they may reduce geographical and organisational barriers in the access of patients to exercise, a key issue to improve their engagement in regular and sustained physical activity.27 Previous studies in oncology have reported that the use of mobile devices has benefits to overcome motivational barriers to physical activity, which can help patients staying physically active over the medium and long term.89 90 Moreover, some studies have shown that patients with breast cancer achieved higher fitness levels during supervised training compared with unsupervised training, even low and medium levels of supervision have been effective, as less resource-intensive options for effective and longer-term behavioural change strategies based on exercises in patients with cancer and survivors.84 91
Activity trackers have become increasingly popular in recent years. Patients have reported positive feedback on using activity trackers such as pleasant to wear, easy to use and a strong motivational role through the real-time display of daily number of steps.92 Also, walking is an inexpensive activity that can be performed anywhere and does not require specific skills. A study on preferences for technology-supported interventions in breast cancer survivors has reported that 63% would like to use a physical activity mobile application and 90% would find a physical activity tracker useful to monitor and increase physical activity.35
Despite the potential benefits of connected devices in cancer care, their use may face several important issues. First, ethical challenges related to the security of sensitive data storage have been raised.93 To ensure that data transfer and storage guarantee informational privacy and patient safety,94 an activity tracker made in France (ie, allowing storing health data only in France) and an accredited national health data host were chosen for the DISCO trial. Particularly, ensuring medical data security is a reassuring choice for patients to participate in this new kind of medical research. Second, technical challenges have been raised, related to technological robustness, reliability of data collection and processing, and ease of use. Therefore, an activity tracker with a step display on the screen, a user-friendly interface, good reliability and a good price-performance ratio was chosen in the DISCO trial. Third, connected devices may create or exacerbate access disparities related to technological literacy and economic means, as well as reliable access to the internet in rural or isolated areas.93 Fourth, medical reasons are usually not easy to control in patients’ adherence to exercise programmes. Reliance on self-assessment of the participant’s fatigue, evaluation of the participant before and after each session on the remote monitoring, up as the source of information about the participant’s health, can result in the ignorance of aspects of the participant’s health that cannot easily be monitored.93
Therapeutic patient education has been suggested to increase physical activity level in patients with chronic diseases46 and to improve multiple health outcomes, together with behavioural interventions including physical activity.95 Therapeutic patient education interventions might be promising for promoting a physically active lifestyle in patients with cancer as it helps patients establish lifestyle changes and reinforce self-management.95 Therapeutic patient education differs from traditional patient education in its intrinsic structure. Traditional patient education is directed towards informing and teaching patients how to manage their condition or disease. In contrast, therapeutic patient education differs from traditional patient education in the self-management conferred on the patient.40 Therefore, therapeutic patient education is more broadly directed towards how the patient accepts his/her condition and manages his/her problems on a daily basis and the impact of the disease on personal, family, professional and social life. Yet, in oncology, few therapeutic patient education studies targeting pain, fatigue, toxicities or treatment adherence are ongoing, and evaluations are rarely conducted.41 To our knowledge, only one programme of therapeutic patient education specific to physical activity has been evaluated in patients with cancer.45 However, a recent qualitative study has shown the value of therapeutic patient education on the attitudes towards the physical activity of women with breast cancer to promote regular exercise, which is a guarantee of a better quality of life.96
In order to evaluate the efficacy of two interventions in the DISCO trial, the primary outcome measure will be based on the physical activity level of the participants with or without interventions compared with international recommendations. The RPAQ questionnaire will be used for the primary outcome measure on account of its easy implementation. The authors acknowledge that this declarative evaluation confers methodological limits to the study. But the RPAQ questionnaire has been validated against objective methods (ie, combined accelerometry and heart rate monitoring)67 to evaluate moderate-to-vigorous physical activities, which is relevant for the primary outcome. No objective measures of physical activity have been planned because of organisational and logistic difficulties to equip and follow participants for 1 week (ie, the usual duration of monitoring with an accelerometer such as Actigraph).97 Such a test would even be particularly overwhelming for patients with cancer during the demanding period of adjuvant treatment onset. Additionally, the number of daily steps reported by the activity tracker was not chosen as the primary outcome because the activity tracker used in the study was not validated for monitoring physical activity in research or for medical purposes when the study was designed, although its reliability was evaluated against other devices (data not shown). However, recently the performance and reliability of smart devices tend to be increasingly validated.98
To understand the results of the DISCO clinical study, it is essential to study beliefs about connected devices and their appropriation by the patients, particularly to understand why behaviours of the patients tend to fade over time. In therapeutic education, beliefs and representations are essential to the success of the intervention. Moreover, with the connected devices, only technical dimensions are not sufficient to understand and highlight why individuals adopt or misuse the connected devices.73 74
There is still limited evidence or contrasting conclusions surrounding the cost-effectiveness of interventions promoting physical activity among women with breast cancer from studies conducted in France, the Netherland and Australia.99–104 In various chronic conditions other than cancer, there is now clear evidence in favour of exercise-based programmes for the treatment of various chronic conditions such as musculoskeletal, rheumatological disorders and cardiovascular diseases.105 As more research is needed to evaluate the cost-effectiveness of physical activity in the treatment of cancers, particularly breast cancer, the economic evaluation planned in the DISCO trial will fill in the gap by adding useful information.
In conclusion, the study findings will provide valuable information on the efficacy of exercise interventions during breast cancer treatments, overcoming current barriers of access to facilities. They will further guide the development of evidence-based innovative interventions, to systematically include physical activity in the breast cancer care process. Finally, the economic evaluation planned in the DISCO trial will provide useful information for decision-makers.
Supplementary Material
Acknowledgments
The authors are thanksful to Romain Buono, Meyssane Djebali, Carmen Dupuis, Stephany Fiore, Emilie Rémir, Cécile Dalban, Antonin Poullet, Renaud Meyrand, Rodolf Mongondry, Magali Dubois, Solène Poirey, Valentine Bertollin, Amélie Dupré, Apolline Michel, Axel Lion, Magalie Hureau and Morgane Sejallon for their contribution to the conduct of the DISCO trial at the Léon Bérard cancer centre. The authors are also grateful to Mrs Françoise Picard from Europa Donna France, association of breast cancer patients’ representatives, for her active participation, advice and support in the DISCO trial, and to Dr Hwayoung Noh for English editing.
Footnotes
Contributors: BFe (principal investigator), MT, LD and TD conceived the study. BFe and MT obtained funding for the research. BFe, MT, BFo and OP designed the protocol. DP and OP conceived the methodological aspects of the trial. BFe, MT, BFo, LD, FF, SP and TD conceived the connected device and exercise training. LP, MT, OP, AM and EB designed the medico-economic study and eCRF. MP, TL, MT, OP and AM designed the part on uses and representations. J-BF, MT and OP designed the part on occupational status. All authors were involved in in planning the methods and measurement and a priori analysis planning. MT and BFo wrote the initial draft of the manuscript. All authors reviewed and provided comprehensive contribution to the manuscript and approved the final manuscript.
Funding: The DISCO trial is financially supported by the ‘Fondation ARC pour la Recherche sur le Cancer’ and the French National Cancer Institute (‘Institut National du Cancer’) (grant no. PREV201601260), the Foundation for Medical Research (‘Fondation pour la Recherche Médicale’, grant no. DOC20161136212), ‘Métropole de Lyon/Cancéropôle Lyon Auvergne Rhône-Alpes’ (CLARA) (grant no. 2016 Projet Structurant) and AG2R La Mondiale.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Defossez G, Le Guyader-Peyrou S, Uhry Z. Estimations nationales de l’incidence et de la mortalité par cancer en France métropolitaine entre 1990 et 2018. Étude partir des registres des cancers du réseau Francim. Synthèse. Santé publique France, 2019: 20. https://www.e-cancer.fr/content/download/266450/3759432/file/Synthese_Estimations%20nationales%20incidence%20et%20mortalite%20par%20cancer_juillet_2019.pdf [Google Scholar]
- 3.Cowppli-Bony A, Uhry Z, Remontet L, et al. Survival of solid cancer patients in France, 1989-2013: a population-based study. Eur J Cancer Prev 2017;26:461–8. 10.1097/CEJ.0000000000000372 [DOI] [PubMed] [Google Scholar]
- 4.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018;391:1023–75. 10.1016/S0140-6736(17)33326-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allemani C, Minicozzi P, Berrino F, et al. Predictions of survival up to 10 years after diagnosis for European women with breast cancer in 2000-2002. Int J Cancer 2013;132:2404–12. 10.1002/ijc.27895 [DOI] [PubMed] [Google Scholar]
- 6.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer 2012;118:2261–9. 10.1002/cncr.27475 [DOI] [PubMed] [Google Scholar]
- 7.Makari-Judson G, Braun B, Jerry DJ, et al. Weight gain following breast cancer diagnosis: implication and proposed mechanisms. World J Clin Oncol 2014;5:272–82. 10.5306/wjco.v5.i3.272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galizia D, Milani A, Geuna E, et al. Self-evaluation of duration of adjuvant chemotherapy side effects in breast cancer patients: a prospective study. Cancer Med 2018;7:4339–44. 10.1002/cam4.1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foucaut A-M, Berthouze SE, Touillaud M, et al. Deterioration of physical activity level and metabolic risk factors after early-stage breast cancer diagnosis. Cancer Nurs 2015;38:E1–9. 10.1097/NCC.0000000000000187 [DOI] [PubMed] [Google Scholar]
- 10.Møller T, Andersen C, Lillelund C, et al. Physical deterioration and adaptive recovery in physically inactive breast cancer patients during adjuvant chemotherapy: a randomised controlled trial. Sci Rep 2020;10:9710. 10.1038/s41598-020-66513-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization . Global recommendations on physical activity for health. World Health Organization, 2011: 60. http://apps.who.int/iris/bitstream/10665/44399/1/9789241599979_eng.pdf [PubMed] [Google Scholar]
- 12.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol 2014;25:1901–14. 10.1093/annonc/mdu042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Playdon MC, Bracken MB, Sanft TB, et al. Weight gain after breast cancer diagnosis and all-cause mortality: systematic review and meta-analysis. J Natl Cancer Inst 2015;107:djv275. 10.1093/jnci/djv275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc 2019;51:1252–61. 10.1249/MSS.0000000000001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Cancer Research Fund International/American Institute for Cancer Research . WCRF/AICR. diet, nutrition, physical activity and cancer: a global perspective. The third expert report, 2018. Available: https://www.wcrf.org/dietandcancer
- 16.Heywood R, McCarthy AL, Skinner TL. Safety and feasibility of exercise interventions in patients with advanced cancer: a systematic review. Support Care Cancer 2017;25:3031–50. 10.1007/s00520-017-3827-0 [DOI] [PubMed] [Google Scholar]
- 17.Juvet LK, Thune I, Elvsaas I K Ø, et al. The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast 2017;33:166–77. 10.1016/j.breast.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 18.Lipsett A, Barrett S, Haruna F, et al. The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. Breast 2017;32:144–55. 10.1016/j.breast.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 19.Coughlin SS, Caplan LS, Williams V. Home-Based physical activity interventions for breast cancer patients receiving primary therapy: a systematic review. Breast Cancer Res Treat 2019;178:513–22. 10.1007/s10549-019-05424-4 [DOI] [PubMed] [Google Scholar]
- 20.Kang D-W, Lee E-Y, An KY, et al. Associations between physical activity and comorbidities in Korean cancer survivors. J Cancer Surviv 2018;12:441–9. 10.1007/s11764-018-0683-y [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y, Huang M, Cheng ASK, et al. Meta-Analysis of the effects of exercise intervention on quality of life in breast cancer survivors. Breast Cancer 2014;21:262–74. 10.1007/s12282-014-0521-7 [DOI] [PubMed] [Google Scholar]
- 22.Buffart LM, Kalter J, Sweegers MG, et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: an individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev 2017;52:91–104. 10.1016/j.ctrv.2016.11.010 [DOI] [PubMed] [Google Scholar]
- 23.Lahart IM, Metsios GS, Nevill AM, et al. Physical activity, risk of death and recurrence in breast cancer survivors: a systematic review and meta-analysis of epidemiological studies. Acta Oncol 2015;54:635–54. 10.3109/0284186X.2014.998275 [DOI] [PubMed] [Google Scholar]
- 24.Friedenreich CM, Stone CR, Cheung WY, et al. Physical activity and mortality in cancer survivors: a systematic review and meta-analysis. JNCI Cancer Spectr 2020;4:pkz080. 10.1093/jncics/pkz080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedenreich CM, Neilson HK, Farris MS, et al. Physical activity and cancer outcomes: a precision medicine approach. Clin Cancer Res 2016;22:4766–75. 10.1158/1078-0432.CCR-16-0067 [DOI] [PubMed] [Google Scholar]
- 26.Demark-Wahnefried W, Schmitz KH, Alfano CM, et al. Weight management and physical activity throughout the cancer care continuum. CA Cancer J Clin 2018;68:64–89. 10.3322/caac.21441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foucaut A-M, Morelle M, Kempf-Lépine A-S, et al. Feasibility of an exercise and nutritional intervention for weight management during adjuvant treatment for localized breast cancer: the PASAPAS randomized controlled trial. Support Care Cancer 2019;27:3449–61. 10.1007/s00520-019-4658-y [DOI] [PubMed] [Google Scholar]
- 28.Turner RR, Steed L, Quirk H, et al. Interventions for promoting habitual exercise in people living with and beyond cancer. Cochrane Database Syst Rev 2018;9:CD010192. 10.1002/14651858.CD010192.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunde P, Nilsson BB, Bergland A, et al. The effectiveness of smartphone Apps for lifestyle improvement in noncommunicable diseases: systematic review and meta-analyses. J Med Internet Res 2018;20:e162. 10.2196/jmir.9751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaffer K, Panneerselvam N, Loh KP, et al. Systematic review of randomized controlled trials of exercise interventions using digital activity Trackers in patients with cancer. J Natl Compr Canc Netw 2019;17:57–63. 10.6004/jnccn.2018.7082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coughlin SS, Caplan LS, Stone R. Use of consumer wearable devices to promote physical activity among breast, prostate, and colorectal cancer survivors: a review of health intervention studies. J Cancer Surviv 2020;14:386–92. 10.1007/s11764-020-00855-1 [DOI] [PubMed] [Google Scholar]
- 32.Dorri S, Asadi F, Olfatbakhsh A, et al. A systematic review of electronic health (eHealth) interventions to improve physical activity in patients with breast cancer. Breast Cancer 2020;27:25–46. 10.1007/s12282-019-00982-3 [DOI] [PubMed] [Google Scholar]
- 33.Beg MS, Gupta A, Stewart T, et al. Promise of wearable physical activity monitors in oncology practice. J Oncol Pract 2017;13:82–9. 10.1200/JOP.2016.016857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schinköthe T. Individualized eHealth support for oncological therapy management. Breast Care 2019;14:130–4. 10.1159/000500900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillips SM, Conroy DE, Keadle SK, et al. Breast cancer survivors' preferences for technology-supported exercise interventions. Support Care Cancer 2017;25:3243–52. 10.1007/s00520-017-3735-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Phillips SM, Courneya KS, Welch WA, et al. Breast cancer survivors' preferences for mHealth physical activity interventions: findings from a mixed methods study. J Cancer Surviv 2019;13:292–305. 10.1007/s11764-019-00751-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen AM, Welch WA, Gavin KL, et al. Preferences for mHealth physical activity interventions during chemotherapy for breast cancer: a qualitative evaluation. Support Care Cancer 2020;28:1919–28. 10.1007/s00520-019-05002-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lacroix A, Assal J-P. L’éducation thérapeutique des patients: accompagner les patients avec une maladie chronique : nouvelles approches. 3rd ed, 2011. [Google Scholar]
- 39.Fabre S. Evaluating therapeutic patient education: objectives and criteria. Joint Bone Spine 2017;84:121–3. 10.1016/j.jbspin.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization . Therapeutic patient education: continuing education programmes for health care providers in the field of prevention of chronic diseases. Report of a who Working group. World Health Organization, Regional Office for Europe, 1998: 76. https://apps.who.int/iris/handle/10665/108151 [Google Scholar]
- 41.Buiret G, Chidiac F, Pujo K, et al. [How to reconcile therapeutic patient education and care pathology in oncology: Application in head and neck neoplams]. Bull Cancer 2019;106:468–78. 10.1016/j.bulcan.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 42.Bourmaud A, Anota A, Moncharmont C, et al. Cancer-Related fatigue management: evaluation of a patient education program with a large-scale randomised controlled trial, the PEPs fatigue study. Br J Cancer 2017;116:849–58. 10.1038/bjc.2017.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pérol D, Toutenu P, Lefranc A, et al. [Therapeutic education in oncology: involving patient in the management of cancer]. Bull Cancer 2007;94:267–74. [PubMed] [Google Scholar]
- 44.Arthurs G, Simpson J, Brown A, et al. The effectiveness of therapeutic patient education on adherence to oral anti-cancer medicines in adult cancer patients in ambulatory care settings: a systematic review. JBI Database System Rev Implement Rep 2015;13:244–92. 10.11124/01938924-201513050-00014 [DOI] [PubMed] [Google Scholar]
- 45.Carayol M, Delpierre C, Bernard P, et al. Population-, intervention- and methodology-related characteristics of clinical trials impact exercise efficacy during adjuvant therapy for breast cancer: a meta-regression analysis. Psychooncology 2015;24:737–47. 10.1002/pon.3727 [DOI] [PubMed] [Google Scholar]
- 46.Conn VS, Hafdahl AR, Brown SA, et al. Meta-Analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns 2008;70:157–72. 10.1016/j.pec.2007.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meneses-Echávez JF, Correa-Bautista JE, González-Jiménez E, et al. The effect of exercise training on mediators of inflammation in breast cancer survivors: a systematic review with meta-analysis. Cancer Epidemiol Biomarkers Prev 2016;25:1009–17. 10.1158/1055-9965.EPI-15-1061 [DOI] [PubMed] [Google Scholar]
- 48.Kang D-W, Lee J, Suh S-H, et al. Effects of exercise on insulin, IGF axis, adipocytokines, and inflammatory markers in breast cancer survivors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2017;26:355–65. 10.1158/1055-9965.EPI-16-0602 [DOI] [PubMed] [Google Scholar]
- 49.Ballard-Barbash R, Friedenreich CM, Courneya KS, et al. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst 2012;104:815–40. 10.1093/jnci/djs207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patel AV, Friedenreich CM, Moore SC, et al. American College of sports medicine roundtable report on physical activity, sedentary behavior, and cancer prevention and control. Med Sci Sports Exerc 2019;51:2391–402. 10.1249/MSS.0000000000002117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer 2014;14:342–57. 10.1038/nrc3691 [DOI] [PubMed] [Google Scholar]
- 52.Haute Autorité de Santé . Recommandations professionnelles - Stratégie de prise en charge en cas de dénutrition protéino-énergétique chez la personne âgée [Professional recommendations - Management strategy in case of protein-energy malnutrition in the Elderly. Haute Autorité de Santé, 2007: 25. https://www.has-sante.fr/upload/docs/application/pdf/denutrition_personne_agee_2007_-_recommandations.pdf [Google Scholar]
- 53.Besson H, Brage S, Jakes RW, et al. Estimating physical activity energy expenditure, sedentary time, and physical activity intensity by self-report in adults. Am J Clin Nutr 2010;91:106–14. 10.3945/ajcn.2009.28432 [DOI] [PubMed] [Google Scholar]
- 54.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–15. 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 55.Scott NW, McPherson GC, Ramsay CR, et al. The method of minimization for allocation to clinical trials. A review. Control Clin Trials 2002;23:662–74. 10.1016/S0197-2456(02)00242-8 [DOI] [PubMed] [Google Scholar]
- 56.Neil-Sztramko SE, Kirkham AA, Hung SH, et al. Aerobic capacity and upper limb strength are reduced in women diagnosed with breast cancer: a systematic review. J Physiother 2014;60:189–200. 10.1016/j.jphys.2014.09.005 [DOI] [PubMed] [Google Scholar]
- 57.Rikli RE, Jones CJ. Functional fitness normative scores for Community-Residing older adults, ages 60-94. J Aging Phys Act 1999;7:162–81. 10.1123/japa.7.2.162 [DOI] [Google Scholar]
- 58.Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med 2012;46:909–16. 10.1136/bjsports-2010-082719 [DOI] [PubMed] [Google Scholar]
- 59.Sasso JP, Eves ND, Christensen JF, et al. A framework for prescription in exercise-oncology research. J Cachexia Sarcopenia Muscle 2015;6:115–24. 10.1002/jcsm.12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and Met values. Med Sci Sports Exerc 2011;43:1575–81. 10.1249/MSS.0b013e31821ece12 [DOI] [PubMed] [Google Scholar]
- 61.Fassier J-B, Durand M-J, Caillard J-F, et al. Results of a feasibility study: barriers and facilitators in implementing the Sherbrooke model in France. Scand J Work Environ Health 2015;41:223–33. 10.5271/sjweh.3489 [DOI] [PubMed] [Google Scholar]
- 62.Schmidt K, Vogt L, Thiel C, et al. Validity of the six-minute walk test in cancer patients. Int J Sports Med 2013;34:631–6. 10.1055/s-0032-1323746 [DOI] [PubMed] [Google Scholar]
- 63.Jones CJ, Rikli RE, Beam WC. A 30-S chair-stand test as a measure of lower body strength in community-residing older adults. Res Q Exerc Sport 1999;70:113–9. 10.1080/02701367.1999.10608028 [DOI] [PubMed] [Google Scholar]
- 64.Cantarero-Villanueva I, Fernández-Lao C, Díaz-Rodríguez L, et al. The handgrip strength test as a measure of function in breast cancer survivors: relationship to cancer-related symptoms and physical and physiologic parameters. Am J Phys Med Rehabil 2012;91:774–82. 10.1097/PHM.0b013e31825f1538 [DOI] [PubMed] [Google Scholar]
- 65.Wells KF, Dillon EK. The sit and Reach—A test of back and leg flexibility. Research Quarterly. American Association for Health, Physical Education and Recreation 1952;23:115–8. 10.1080/10671188.1952.10761965 [DOI] [Google Scholar]
- 66.Bohannon RW. Single limb stance times: a descriptive meta-analysis of data from individuals at least 60 years of age. Topics in Geriatric Rehabilitation 2006;22:70–7. [Google Scholar]
- 67.Golubic R, May AM, Benjaminsen Borch K, et al. Validity of electronically administered recent physical activity questionnaire (RPAQ) in ten European countries. PLoS One 2014;9:e92829. 10.1371/journal.pone.0092829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hjermstad MJ, Fossa SD, Bjordal K, et al. Test/retest study of the European organization for research and treatment of cancer core quality-of-life questionnaire. J Clin Oncol 1995;13:1249–54. 10.1200/JCO.1995.13.5.1249 [DOI] [PubMed] [Google Scholar]
- 69.Janssen MF, Pickard AS, Golicki D, et al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res 2013;22:1717–27. 10.1007/s11136-012-0322-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reeve BB, Stover AM, Alfano CM, et al. The Piper fatigue Scale-12 (PFS-12): psychometric findings and item reduction in a cohort of breast cancer survivors. Breast Cancer Res Treat 2012;136:9–20. 10.1007/s10549-012-2212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Labbe E, Blanquet M, Gerbaud L, et al. A new reliable index to measure individual deprivation: the EPICES score. Eur J Public Health 2015;25:604–9. 10.1093/eurpub/cku231 [DOI] [PubMed] [Google Scholar]
- 72.Centers for Disease Control and Prevention . Overcoming barriers to physical activity: barriers to being active Quiz-What keeps you from being more active? 2007Published online. Available: https://www.cdc.gov/diabetes/ndep/pdfs/8-road-to-health-barriers-quiz-508.pdf
- 73.Venkatesh, Morris, Davis . User acceptance of information technology: toward a unified view. Mis Quarterly 2003;27:425. 10.2307/30036540 [DOI] [Google Scholar]
- 74.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179–211. 10.1016/0749-5978(91)90020-T [DOI] [Google Scholar]
- 75.Jodelet D. Interconnections between social representations and intervention. In: Social Representations in the “Social Arena.”. A. De Rosa: Routledge, 2012: 77–88. https://www.academia.edu/36160041/Jodelet_D._2012_._Interconnections_between_social_representations_and_intervention [Google Scholar]
- 76.Green S, Liu P-Y, O'Sullivan J. Factorial design considerations. J Clin Oncol 2002;20:3424–30. 10.1200/JCO.2002.03.003 [DOI] [PubMed] [Google Scholar]
- 77.Frick KD. Microcosting quantity data collection methods. Med Care 2009;47:S76–81. 10.1097/MLR.0b013e31819bc064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Khal M, Perrier L, Carretier J, et al. [The cost of the therapeutic education program "Eat better, move more through nutrition education" in patients with breast cancer]. Bull Cancer 2020;107:1252–9. 10.1016/j.bulcan.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 79.Haute Autorité de Santé . Guide méthodologique - Choix méthodologique pour l’évaluation économique la HAS [Methodological guide - Methodological choices for economic evaluation at the National Health Authority]. Haute Autorité de Santé, 2011. https://www.has-sante.fr/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf [Google Scholar]
- 80.Rock CL, Doyle C, Demark-Wahnefried W, et al. Nutrition and physical activity guidelines for cancer survivors. CA Cancer J Clin 2012;62:242–74. 10.3322/caac.21142 [DOI] [PubMed] [Google Scholar]
- 81.Institut National du Cancer . Plan cancer 2014-2019 : priorités et objectifs. Institut National du Cancer, 2015: 210. http://www.e-cancer.fr/Plan-cancer/Plan-cancer-2014-2019-priorites-et-objectifs [Google Scholar]
- 82.Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr 2017;36:11–48. 10.1016/j.clnu.2016.07.015 [DOI] [PubMed] [Google Scholar]
- 83.Sweegers MG, Altenburg TM, Chinapaw MJ, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 2018;52:505–13. 10.1136/bjsports-2017-097891 [DOI] [PubMed] [Google Scholar]
- 84.Bluethmann SM, Vernon SW, Gabriel KP, et al. Taking the next step: a systematic review and meta-analysis of physical activity and behavior change interventions in recent post-treatment breast cancer survivors. Breast Cancer Res Treat 2015;149:331–42. 10.1007/s10549-014-3255-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Delrieu L, Vallance JK, Morelle M, et al. Physical activity preferences before and after participation in a 6-month physical activity intervention among women with metastatic breast cancer. Eur J Cancer Care 2020;29:e13169. 10.1111/ecc.13169 [DOI] [PubMed] [Google Scholar]
- 86.Quintiliani LM, Mann DM, Puputti M, et al. Pilot and feasibility test of a mobile Health-Supported behavioral counseling intervention for weight management among breast cancer survivors. JMIR Cancer 2016;2:e4. 10.2196/cancer.5305 [DOI] [PubMed] [Google Scholar]
- 87.Delrieu L, Pialoux V, Pérol O, et al. Feasibility and health benefits of an individualized physical activity intervention in women with metastatic breast cancer: intervention study. JMIR Mhealth Uhealth 2020;8:e12306. 10.2196/12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Groen WG, van Harten WH, Vallance JK. Systematic review and meta-analysis of distance-based physical activity interventions for cancer survivors (2013-2018): We still haven't found what we're looking for. Cancer Treat Rev 2018;69:188–203. 10.1016/j.ctrv.2018.07.012 [DOI] [PubMed] [Google Scholar]
- 89.Cadmus-Bertram LA, Marcus BH, Patterson RE, et al. Randomized trial of a Fitbit-Based physical activity intervention for women. Am J Prev Med 2015;49:414–8. 10.1016/j.amepre.2015.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lavallée JF, Abdin S, Faulkner J, et al. Barriers and facilitators to participating in physical activity for adults with breast cancer receiving adjuvant treatment: a qualitative metasynthesis. Psychooncology 2019;28:468–76. 10.1002/pon.4980 [DOI] [PubMed] [Google Scholar]
- 91.Westphal T, Rinnerthaler G, Gampenrieder SP, et al. Supervised versus autonomous exercise training in breast cancer patients: a multicenter randomized clinical trial. Cancer Med 2018;7:5962–72. 10.1002/cam4.1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Brien T, Troutman-Jordan M, Hathaway D, et al. Acceptability of wristband activity trackers among community dwelling older adults. Geriatr Nurs 2015;36:S21–5. 10.1016/j.gerinurse.2015.02.019 [DOI] [PubMed] [Google Scholar]
- 93.Ho A, Quick O. Leaving patients to their own devices? Smart technology, safety and therapeutic relationships. BMC Med Ethics 2018;19:18. 10.1186/s12910-018-0255-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.European Union (EU), European Economic Area (EEA) . General data protection regulation (EU), 2016. Available: https://ec.europa.eu/info/law/law-topic/data-protection/eu-data-protection-rules_en
- 95.Lagger G, Pataky Z, Golay A. Efficacy of therapeutic patient education in chronic diseases and obesity. Patient Educ Couns 2010;79:283–6. 10.1016/j.pec.2010.03.015 [DOI] [PubMed] [Google Scholar]
- 96.Mino J-C LC. Improved understanding of the experience of physical activity after a cancer from a philosophy of health perspective [Mieux comprendre l’expérience de l’activité physique après un cancer grâce la philosophie de la santé]. Santé Publique 2016;28:S101–7. [PubMed] [Google Scholar]
- 97.Peddle-McIntyre CJ, Cavalheri V, Boyle T, et al. A review of Accelerometer-based activity monitoring in cancer survivorship research. Med Sci Sports Exerc 2018;50:1790–801. 10.1249/MSS.0000000000001644 [DOI] [PubMed] [Google Scholar]
- 98.Degroote L, De Bourdeaudhuij I, Verloigne M, et al. The accuracy of smart devices for measuring physical activity in daily life: validation study. JMIR Mhealth Uhealth 2018;6:e10972. 10.2196/10972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mourgues C, Gerbaud L, Leger S, et al. Positive and cost-effectiveness effect of spa therapy on the resumption of occupational and non-occupational activities in women in breast cancer remission: a French multicentre randomised controlled trial. Eur J Oncol Nurs 2014;18:505–11. 10.1016/j.ejon.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 100.Mewes JC, Steuten LMG, Duijts SFA, et al. Cost-Effectiveness of cognitive behavioral therapy and physical exercise for alleviating treatment-induced menopausal symptoms in breast cancer patients. J Cancer Surviv 2015;9:126–35. 10.1007/s11764-014-0396-9 [DOI] [PubMed] [Google Scholar]
- 101.Gordon LG, DiSipio T, Battistutta D, et al. Cost-Effectiveness of a pragmatic exercise intervention for women with breast cancer: results from a randomized controlled trial. Psychooncology 2017;26:649–55. 10.1002/pon.4201 [DOI] [PubMed] [Google Scholar]
- 102.van Waart H, van Dongen JM, van Harten WH, et al. Cost-Utility and cost-effectiveness of physical exercise during adjuvant chemotherapy. Eur J Health Econ 2018;19:893–904. 10.1007/s10198-017-0936-0 [DOI] [PubMed] [Google Scholar]
- 103.Perrier L, Foucaut A-M, Morelle M, et al. Cost-Effectiveness of an exercise and nutritional intervention versus usual nutritional care during adjuvant treatment for localized breast cancer: the PASAPAS randomized controlled trial. Support Care Cancer 2020;28:2829–42. 10.1007/s00520-019-05078-4 [DOI] [PubMed] [Google Scholar]
- 104.Khan KA, Mazuquin B, Canaway A, et al. Systematic review of economic evaluations of exercise and physiotherapy for patients treated for breast cancer. Breast Cancer Res Treat 2019;176:37–52. 10.1007/s10549-019-05235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guillon M, Rochaix L, Dupont J-CK. Cost-Effectiveness of interventions based on physical activity in the treatment of chronic conditions: a systematic literature review. Int J Technol Assess Health Care 2018;34:481–97. 10.1017/S0266462318000533 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-045448supp001.pdf (73.5KB, pdf)
bmjopen-2020-045448supp002.pdf (74.2KB, pdf)