Abstract
Objectives
To understand the survival in a cohort of children living with HIV/AIDS (CLHAs) and to study the factors associated with survival in CLHAs in government antiretroviral therapy (ART) centres in Mumbai, India.
Design
This is a retrospective cohort analysis.
Setting
Data from electronic ART records of children from 15 ART centres in Mumbai, Maharashtra, India.
Participants
2224 CLHAs registered in one of these ART centres from 2004 until October 2019. CLHAs up to the age of 18 at the time of registration were considered for these analyses.
Primary and secondary outcomes
We accessed the following data: date of test, date of initiation of ART, date of last follow-up, age at the time of registration, gender, potential route of infection, baseline CD4 counts, ART regimen, adherence and presence of co-infection (TB). We estimated the survival probabilities, plotted the Kaplan-Meier survival graphs and estimated HRs for mortality.
Results
The mortality rate in our population was 22.75 (95% CI 20.02 to 25.85) per 1000 person-years. The 1-year survival was 0.92 (95% CI 0.91 to 0.93), 0.89 (95% CI 0.88 to 0.91) at 5 years and 0.85 (95% CI 0.83 to 0.87) at 10 years after initiation of ART. Children with adherence less than 80% had lowest survival in the first year (0.54, 95% CI 0.46 to 0.61). It reduced drastically at 5 and 10 years. After adjusting for demographic and clinical parameters, mortality was associated with poor adherence (<80%) (HR 11.70, 95% CI 8.82 to 15.53; p<0.001). However, CD4 counts of greater than 200 and age more than 1 year were protective.
Conclusions
Poor adherence to ART and low CD4 counts were significantly associated with higher mortality. Adherence counselling should be an important component of CLHA monitoring in all ART centres. It is also important to identify children early in the infection and start ART medications appropriately.
Keywords: epidemiology, HIV & AIDS, public health
Strengths and limitations of this study.
We have analysed data from a large cohort of 2224 children living with HIV/AIDS.
We used demographic, clinical and treatment data from electronic records in the administrative database.
We did not have information on multiple CD4 counts and other opportunistic infections in these children.
We did not have anthropometric measures for these children in the first year of birth.
We only had clinical information on tuberculosis (co-infection) in these children.
Introduction
According to recent data, India has about 2.1 million people living with HIV/AIDS; about 61 000 are children living with HIV/AIDS (CLHAs).1 It is estimated that about 7% of total new HIV infections in India are in children.2 Prevention of parent-to-child transmission (PPTCT) programme was introduced in 2002 to reduce perinatal transmission in children; even though the proportion of cases has reduced over the past two decades, perinatal transmission is still the most important route of transmission of HIV in children in India.2 3 India initiated free antiretroviral therapy (ART) for adults in government centres in 2004; however, treatment for children living with HIV/AIDS (CLHAs) was started under the National Paediatric HIV/AIDS initiative in 2006.3 Under this initiative, paediatric formulations of ART, first-line ART, second-line ART, CD4 testing services, early infant diagnosis, counselling, and care and support services are available at government ART centres.3 Furthermore, seven Pediatric Centres of Excellence for management of complicated cases have also been established.3
It has been shown that early diagnosis and initiation of treatment reduces the progression of the disease and mortality in CLHAs.4 Even though the PPTCT programme was initiated before treatment for the paediatric population in India, it was observed that nearly half of the children who are perinatally infected present late to the ART centres.5 Some of the other factors that are associated with survival outcomes in children on ART are age and gender of the child, type of setting (such as resource rich or resource limited), nutritional status of the child and presence of AIDS-defining illness.6–9 Another important factor that needs to be considered is adherence to medications. Adherence in children may depend on the type of regimen, demographic and clinical characteristics of children, and even the knowledge about ART and characteristics of their caregivers.10 11
It has been more 10 years since the initiation of free ART to CLHAs in government ART centres in India. Thus, it will be important to understand the survival in these children over this period of one and half decade. Though few Indian studies12–14 have studied the outcomes in CLHAs, the sample has been small and restricted to a single centre. We propose to estimate the survival in a cohort of CLHAs and to study the factors associated with survival in CLHAs in government ART centres in Mumbai, India.
Methods
This is a retrospective cohort analysis of 2224 children who were registered with the government ART centres in Mumbai.
Data source and variables
We accessed data from the ART records of these children from 15 ART centres in Mumbai, Maharashtra, India. These records were accessed from an electronic administrative database. These children were registered in one of these 15 ART centres from 2004 onward until October 2019. CLHAs who were up to 18 years of age at the time of registration were included for the present analysis. We used the following variables from these records: age at the time of registration, gender, potential route of infection, baseline CD4 counts, ART regimen, adherence (assessed as pill count) and presence of co-infection (TB). We also recorded date of test, date of initiation of ART, and the date of last follow-up. The following outcomes were recorded: alive, dead or lost to follow-up.
The main time factor for our analysis was time since initiation of ART to the occurrence of the aforementioned outcomes. It was the date of death in children who had died during follow-up. It was the last date of visit to the ART centre in children who were classified as lost-to-follow up. In the rest of the children (who were alive), we considered their visit up to October 2019 as the last visit. The other time variable included in the analysis was time from HIV test to initiation of the ART. The age at registration was categorised in the following groups: 0–1 years, >1 to 5 years, >5–12 years and >12–18 years. The adherence was classified as follows: Good >95%, Average 80%–95% and Poor<80%. The adherence of every child is assessed by a trained counsellor when the child visits the ART centre for regular check-ups and taking medications. This includes discussion with the care-taker as well as the examining the residual drugs. The staging of the HIV infection was according to the WHO staging.
Statistical methods
We estimated the means and SD, and median and interquartile (IQR) range for continuous variables. We estimated proportions for categorical variables.
The data were set for survival analysis. The outcome for survival analysis was death and the time factor for the analysis was the time from initiation of ART to the date of the event as described previously. We estimated the mortality rates and their 95% CI in these CLHAs. The mortality rates were estimated for the whole cohort and according to various demographic and clinical characteristics.
We used Kaplan-Meier curves to examine the survival in these children. The survival curves were plotted for various demographic and clinical characteristics. The equality of the survivor functions was assessed using the log-rank test. We estimated the survival and it 95% CI at years 1, 5 and 10 after initiation of the ART according to the demographic and clinical criteria. We also estimated the incidence rate ratios according to clinical and demographic characteristics. We then built models to estimate the HRs for mortality in these children. We started by using the Cox proportional-hazards model. We tested the proportional-hazards assumption. Since the proportional-hazards assumption was not met in our cohort, we used the Weibull model. The model was chosen based on the distribution and fit of residuals, and the Akaike Information Criteria.15 We built unifactorial models and multifactorial models. The following variables were used in the models: age, gender, baseline CD4 counts, adherence, diagnosis of a TB infection, type of ART regimen, and time between the test and start of ART. In our dataset, we had missing values for ‘baseline CD4 counts’. Only eight missing values were detected in the whole dataset (0.36%). A p value of<0.05 was considered statistically significant. Data were analysed using Stata V.15.1 (StataCorp, College Station, Texas, USA).
Patient and public involvement
Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Results
The mean (SD) age at registration of these 2224 CLHAs was 9.68 (5.58) years. The proportion of males and females in the cohort was 54% and 46%, respectively. About 11% (224) children had died in our cohort and about 8% were categorised as lost to follow-up. Majority of the children were perinatal transmission (82%), 7% were sexual transmission, 2% were infected blood/unsafe injections and in 9% of the children the route of transmission could not be identified. In our cohort, the route of transmission as infected blood/unsafe injections in only two children who were in the first year of life. The median (IQR) duration of follow-up after ART was 4.01 (1.62–7.32) years. The median time from diagnosis of HIV to initiation of ART was 0.62 (0.08–4.17) years. The baseline CD4 count was >500/mm3 in majority of CLHAs (52%); it was less than 200 in 18% of CLHAs. As indicated earlier, only eight CLHAs have missing values for baseline CD4 counts (0.36%). The CLHAs were registered in the ART clinic from 2004 onward. Although the proportion of CLHAs registered was low in the initial 3 years (<3%), after 2007 the proportion was 6% to 8% every year. The proportion of CLHAs was highest in 2018 (13%). The total follow-up time of the whole cohort after initiation of ART was 10 372 years.
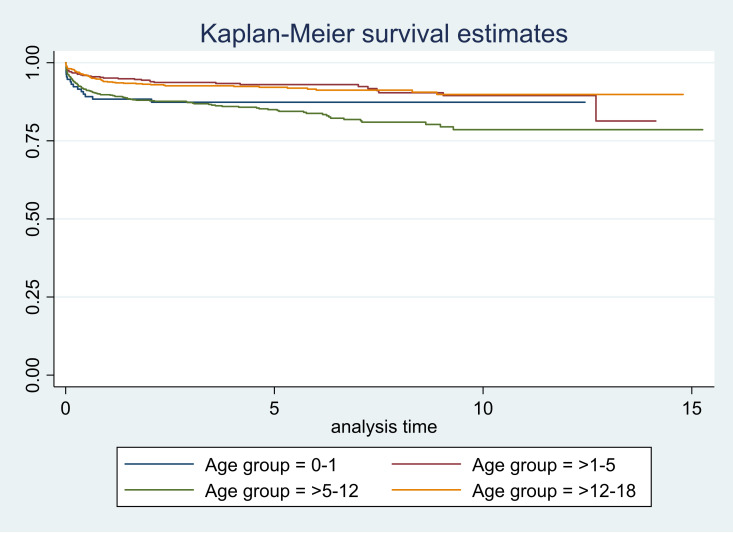
The mortality rate in our population was 22.75 (95% CI 20.02 to 25.85) per 1000 person-years (PY). The mortality was relatively high in CHLAs up to 1 year of age (32.13, 95% CI 20.73 to 49.79) and those aged 5 to 12 years (35.27, 95% CI 29.49 to 42.18). The mortality was highest in those who had poor adherence (307.83, 95% CI 253.77 to 373.41) (table 1). Additional details of the mortality rates and incidence rate ratios have been shown in table 1. The 1-year survival was 0.92 (95% CI 0.91 to 0.93); it reduced to 0.89 (95% CI 0.88 to 0.91) at 5 years and 0.85 (95% CI 0.83 to 0.87) at 10 years after initiation of ART. In children up to 1 year of age, the survival dropped in the first year of initiation of ART; however, it remained nearly same until 10 years. The lowest survival in the first year was in children with poor adherence (0.54, 95% CI 0.46 to 0.61) and it reduced sharply at 5 and 10 years (table 2). We have presented the survival estimates in table 2.
Table 1.
Mortality rates and incidence rates ratios in 2224 children living with HIV/AIDS, Mumbai, India
| Mortality rates (MR) /1000 PY |
95% CI for MR | Incidence rate ratios Estimate (95% CI) |
P value | |
| Total | 22.75 | 20.02 to 25.85 | ||
| Age groups | ||||
| 0–1 | 32.13 | 20.73 to 49.79 | Reference | |
| >1–5 | 14.42 | 10.44 to 19.90 | 0.45 (0.26 to 0.77) | 0.004 |
| >5–12 | 35.27 | 29.49 to 42.18 | 1.10 (0.68 to 1.76) | 0.70 |
| >12–18 | 15.60 | 12.09 to 20.14 | 0.49 (0.29 to 0.81) | 0.005 |
| Gender | ||||
| Male | 22.43 | 18.84 to 26.69 | Reference | |
| Female | 23.15 | 19.18 to 27.92 | 1.03 (0.80 to 1.33) | 0.81 |
| Adherence | ||||
| Good >95% | 13.16 | 11.06 to 15.66 | Reference | |
| Average 80%–95% | 15.49 | 6.96 to 34.48 | 1.18 (0.52 to 2.67) | 0.70 |
| Poor<80% | 307.83 | 253.77 to 373.41 | 23.4 (18.0 to 30.3) | <0.001 |
| Baseline CD4 counts | ||||
| 0–200 | 58.58 | 48.25 to 71.12 | Reference | |
| 201–350 | 26.65 | 20.14 to 35.27 | 0.45 (0.32 to 0.64) | <0.001 |
| 351–500 | 11.94 | 7.52 to 18.95 | 0.20 (0.12 to 0.34) | <0.001 |
| >500 | 11.96 | 9.34 to 15.30 | 0.20 (0.15 to 0.28) | <0.001 |
| TB | ||||
| No | 24.33 | 21.10 to 28.06 | Reference | |
| Yes | 18.05 | 13.56 to 24.03 | 0.74 (0.54 to 1.02) | 0.067 |
PY, person-years.
Table 2.
Survival probabilities and their 95% CIs in 2224 children, Mumbai, India
| 1 year | 5 years | 10 years | |
| Probability (95% CIs) | Probability (95% CIs) | Probability (95% CIs) | |
| Total | 0.92 (0.91 to 0.93) | 0.89 (0.88 to 0.91) | 0.85 (0.83 to 0.87) |
| Age groups | |||
| 0–1 | 0.85 (0.78 to 0.90) | 0.84 (0.76 to 0.89) | 0.84 (0.76 to 0.89) |
| >1–5 | 0.95 (0.93 to 0.97) | 0.93 (0.90 to 0.95) | 0.89 (0.85 to 0.93) |
| >5–12 | 0.89 (0.87 to 0.91) | 0.84 (0.81 to 0.87) | 0.78 (0.73 to 0.82) |
| >12–18 | 0.94 (0.92 to 0.96) | 0.92 (0.90 to 0.94) | 0.90 (0.87 to 0.93) |
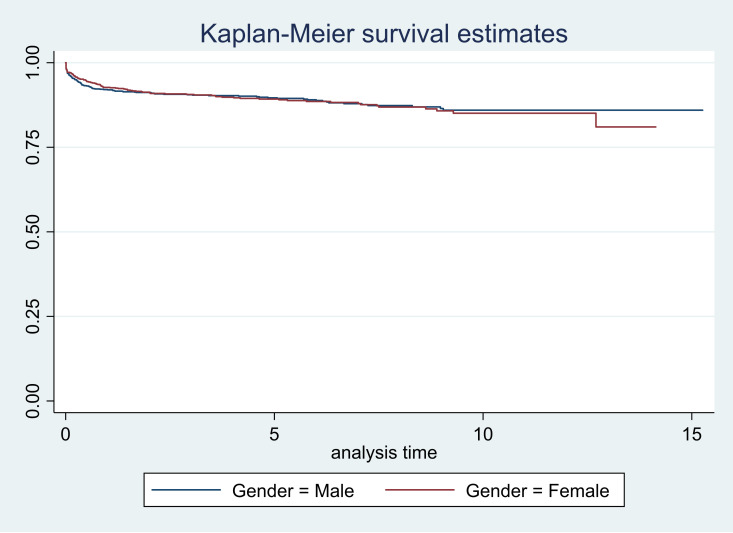
| Gender | |||
| Male | 0.92 (0.90 to 0.93) | 0.89 (0.87 to 0.91) | 0.86 (0.83 to 0.88) |
| Female | 0.93 (0.91 to 0.94) | 0.89 (0.87 to 0.91) | 0.85 (0.81 to 0.88) |
| Adherence | |||
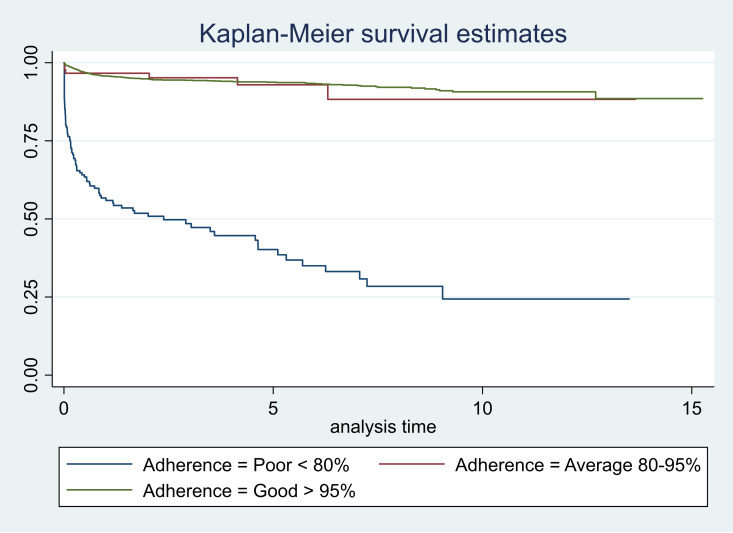
| Good >95% | 0.96 (0.95 to 0.96) | 0.94 (0.92 to 0.95) | 0.91 (0.88 to 0.92) |
| Average 80%–95% | 0.97 (0.90 to 0.99) | 0.92 (0.82 to 0.97) | 0.88 (0.71 to 0.95) |
| Poor | 0.54 (0.46 to 0.61) | 0.38 (0.29 to 0.47) | 0.22 (0.12 to 0.34) |
| Baseline CD4 counts | |||
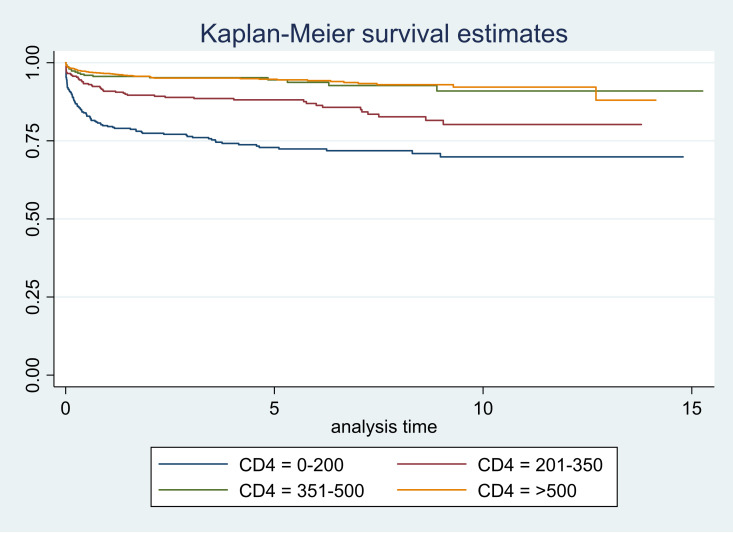
| 0–200 | 0.80 (075 to 0.83) | 0.73 (0.68 to 0.77) | 0.70 (0.64 to 0.75) |
| 201–350 | 0.91 (0.87 to 0.93) | 0.88 (0.84 to 0.91) | 0.79 (0.72 to 0.85) |
| 351–500 | 0.96 (0.92 to 0.97) | 0.94 (0.91 to 0.97) | 0.91 (0.85 to 0.95) |
| >500 | 0.96 (0.95 to 0.97) | 0.95 (0.93 to 0.96) | 0.92 (0.89 to 0.94) |
| TB | |||
| No | 0.91 (0.90 to 0.93) | 0.89 (0.87 to 0.90) | 0.86 (0.83 to 0.88) |
| Yes | 0.95 (0.93 to 0.97) | 0.91 (0.88 to 0.94) | 0.85 (0.79 to 0.89) |
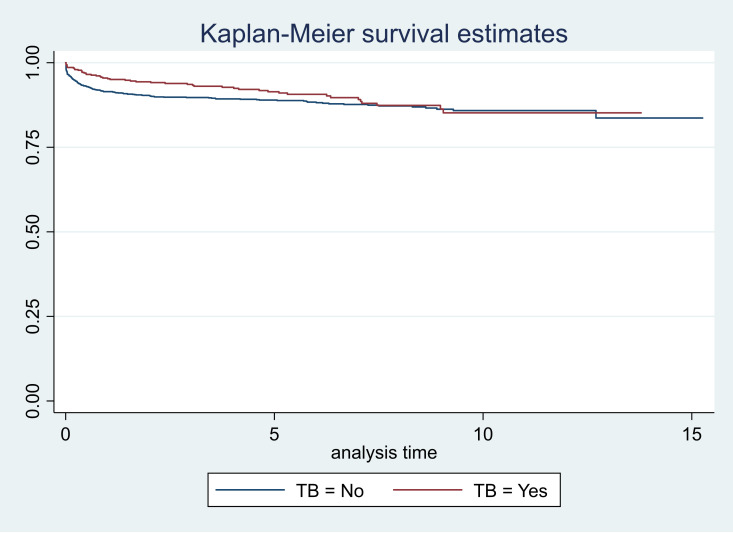
The survival graphs are shown in figures 1–5. As seen in figure 3, the survival started reducing in the first year and continued over the follow-up period in CLHAs with poor adherence; there was a significant difference in the survivor function across these three categories of adherence (p<0.001). The survival was low for CLHAs with baseline CD4 count of <200/mm3; it was slightly better in those with a baseline CD4 count of 201–350/mm3. However, there did not appear to be any difference in survival of CLHAs with CD4 count of 351–500/mm3 or >500/ mm3 (figure 4). There was no significant difference in the survivor function of CLHAs with and without tuberculosis (p=0.16) (figure 5).
Figure 1.
Survival curves according to age groups.
Figure 2.
Survival curves according to gender.
Figure 3.
Survival curves according to adherence.
Figure 4.
Survival curves according to CD4 counts.
Figure 5.
Survival curves according to TB co-infection.
After adjusting for demographic and clinical parameters, we found that hazard for mortality was highest for CLHAs with poor adherence (<80%) (HR 11.70, 95% CI 8.82 to 15.53; p<0.001) compared with those who had an adherence of >95%. The HR reduced with an increase in the baseline CD4 counts (table 3). Similarly, the HR for mortality was lower in children older than 1 year of age compared with those who registered with the ART centre in the first year of life. We also found that CLHAs with TB had lower hazard for mortality compared with those without TB (HR 0.66, 95% CI 0.48 to 0.92; p<0.015). The time to initiation to ART after detection and gender of the CLHA was not significantly associated with mortality. We have provided the HRs and their 95% CIs in table 3.
Table 3.
Unadjusted and adjusted estimates of HRs and their 95% CIs in 2224 children in Mumbai, India
| Variables | HR (95% CIs) | P value | HR (95% CIs) | P value |
| Unadjusted estimates | Adjusted estimates | |||
| Age | ||||
| 0–1 | Reference | Reference | ||
| >1–5 | 0.45 (0.26 to 0.78) | 0.005 | 0.42 (0.24 to 0.74) | 0.003 |
| >5–12 | 1.05 (0.65 to 1.68) | 0.852 | 0.52 (0.30 to 0.89) | 0.017 |
| >12–18 | 0.47 (0.29 to 0.79) | 0.004 | 0.28 (0.15 to 0.50) | <0.001 |
| Gender | ||||
| Male | Reference | Reference | ||
| Female | 1.02 (0.79 to 1.31) | 0.905 | 0.98 (0.75 to 1.27) | 0.857 |
| Baseline CD4 counts | ||||
| 0–200 | Reference | Reference | ||
| 201–350 | 0.48 (0.34 to 0.68) | <0.001 | 0.55 (0.39 to 0.77) | 0.001 |
| 351–500 | 0.21 (0.13 to 0.34) | <0.001 | 0.24 (0.15 to 0.41) | <0.001 |
| >500 | 0.19 (0.14 to 0.27) | <0.001 | 0.21 (0.14 to 0.30) | <0.001 |
| Adherence | ||||
| Good >95% | Reference | Reference | ||
| Average 80%–95% | 1.09 (0.48 to 2.47) | 0.837 | 1.12 (0.49 to 2.54) | 0.792 |
| Poor | 15.97 (12.26 to 20.81) | <0.001 | 11.70 (8.82 to 15.53) | <0.001 |
| TB | ||||
| No | Reference | Reference | ||
| Yes | 0.79 (0.57 to 1.08) | 0.139 | 0.66 (0.48 to 0.92) | 0.015 |
| Regimen | ||||
| First-line ART | Reference | Reference | ||
| Second-line ART | 0.41 (0.26 to 0.67) | <0.001 | 0.44 (0.27 to 0.72) | 0.001 |
| Third-line ART | 0.56 (0.14 to 2.27) | 0.419 | 0.77 (0.19 to 3.15) | 0.718 |
| Time between test and start of ART | 0.85 (0.81 to 0.91) | <0.001 | 0.96 (0.91 to 1.02) | 0.171 |
ART, antiretroviral therapy.
Discussion
Thus, in the present study, we found that the overall mortality in CLHAs in Mumbai was 22.75/1000 PY. Mortality rates were higher in CLHAs with an adherence of less than 95% (particularly in those with an adherence of less than 80%), those with a CD4 count of less than 200/mm3 at baseline, and in neonates and infants (up to 1 year of age) and those in the age group of 5 to 12 years. Interestingly, we found that CLHAs without a diagnosis of TB had a relatively better survival compared with those children with a diagnosis of TB.
Adherence to ART in CLHAs was one of the most important factors associated with mortality. Adherence in younger CLHAs will depend on the child and also on the caregiver. Some of the common barriers reported by children are taste and formulation of the medicine, difficulty in sticking to regular time due to school and other activities, type of regimen and toxicity of the medications, and stigma associated with HIV.16–19 Furthermore, caregiver characteristics such as financial issues, psychological stress, poor understanding of the role of ART in management of HIV, existing support systems in the family and health system issues (delays in receiving ART, long waiting periods at ART centres) may be additional barriers for adherence.17 18 20 21 It was also reported that concerns related to disclosure and multiple caregivers (as may be the case in many children) was also an important barrier.19 Improve adherence was observed after disclosure, and in whom the parent–child relationship or social support systems were strong.22 23 Thus, it is important to develop adherence counselling interventions for CLHAs in India. Unlike in adults, in whom the primary responsibility of care is with the individual, in children and adolescents the adherence counselling should be for children (who are able to comprehend) as well as their care providers (parents and/or grandparents). Indeed, a review of adherence counselling interventions identified these models of interventions: fewer clinic visits by providing ART for more than a month in stable patients; group-based care in the form of support groups; home-based care that involved visits by healthcare workers and adherence support.24 The first two are useful for CLHAs who are ART experienced and virally suppressed whereas the third one will be for ART-naïve children.24 Thus, such interventions should be a part of the National Programme in India. The present manuscript will provide evidence for advocacy of special adherence programmes for children and adolescents in India.
In general, it has been reported that low CD4 counts at baseline in CLHAs is associated with an increased mortality.25 26 As seen in our study, children who had CD4 counts of less than 200 at baseline had the highest mortality. Delay in presentation to the ART centre is a common hindrance to starting ART on time, even in children that are perinatally infected.5 With the introduction of ‘test and treat’, it is important that we diagnose children and adolescents early in the infection–both in perinatally infected children and in whom the route of transmission is other than the perinatal route. We did not have anthropometric measures for these children in the first year of birth; however, we did find that the mortality is higher in the first year in CLHAs. Malnutrition and infections in infants may be associated with mortality27 28; thus, along with ART, it is important to monitor children regularly to identify early signs of undernutrition. Management of undernutrition and counselling about appropriate feeding practices are important interventions to reduce mortality in CLHAs.29 Another interesting feature that we found in our study was that children who were diagnosed with TB had lower mortality compared with those who did not. It is likely that some cases of TB were missed in the population; it has been reported that diagnosis of TB may be difficult in children.30 Microbiological diagnosis may be difficult due to difficulty in sample collection (sputum) in children; even in those in whom we have been able to collect the sample, the sensitivity of culture is poor due to the paucibacillary nature of the infection.31 32 In HIV-infected children, the diagnosis may become more difficult because of clinical and radiological features that are not very specific in the diagnosis of TB.32 Some of the new diagnostic tests such as nucleic acid amplification tests have helped improve the diagnosis and shortened the time to diagnosis of TB in children.31 33–35 Since diagnosis in children in now being done (2017 onward) using the cartridge-based nucleic acid amplification tests, it will be worthwhile to follow up the cohort and reanalyse the data subsequently.
Since we used demographic and clinical data from electronic records, we could only use the available data. This was a limitation of the study. For instance, we did not have information on multiple CD4 counts and other opportunistic infections in these children. We have used absolute CD4 values rather than proportion in younger children; however, the cut-off for the absolute CD4 count was low (200 cells/cumm). Since we did not have information on other opportunistic infections, we did not include this variable in the model. Thus, the lower mortality observed in the TB group may be attributed to residual confounding due to non-inclusion of this variable in the model. Data were from the government ART centres; generally, people from middle, lower middle and lower socioeconomic strata access these centres. Thus, it is likely that some of the experiences may differ in private centres, usually accessed by the upper middle and upper class. Nonetheless, despite these limitations, the present study provides useful information on the survival of CLHAs in India.
We have analysed data from a large cohort of CLHAs. We found that poor adherence to ART and low CD4 counts at baseline were significantly associated with higher mortality in these CLHAs. It will be important to develop adherence counselling modules specifically for children and their caregivers. Furthermore, adherence counselling should be an important component of CLHA monitoring and should form a part in all ART centres. As we noticed, CLHAs who had a lower CD4 count at baseline had higher mortality. Thus, it is also important to identify children early in the infection and start ART medications appropriately. Finally, CLHAs in the first year of life had high mortality. Thus, CLHAs should be closely monitored in the first year of life; policies about feeding, malnutrition, immunisation, and secondary infections should be emphasised in all ART centres.
Supplementary Material
Acknowledgments
We would like to acknowledge the cooperation from all the ART centres and support from UW International Training and Education Center for Health, India. We would like to thank PEPFAR India for their support.
Footnotes
Contributors: SA was responsible for conceptualising the study, data interpretation, and writing and reviewing the manuscript. AP was responsible for collecting data and help with analysis. APS was responsible for providing feedback on the study, reviewing the manuscript and securing funds for publication charges. MSS was responsible for data analysis, interpretation and writing the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. Data access is limited due to ethical and legal considerations; however, request may be considered after data request submission and approval from relevant authorities and Ethics Committee.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The study was approved by the Institutional Ethics Committee of Mumbai Districts AIDS Control Society (Ref No. 006/2019).
References
- 1.National AIDS Control Organization & ICMR-National Institute of Medical Statistics . India HIV estimations 2017: technical report. New Delhi, India: NACO, Ministry of Health and Family Welfare, Government of India, 2018. [Google Scholar]
- 2.Nath A. Pediatric HIV in India: current scenario and the way forward. Indian J Public Health 2017;61:124–30. 10.4103/ijph.IJPH_314_15 [DOI] [PubMed] [Google Scholar]
- 3.Department of Health & Family Welfare . Annual report 2015–16. New Delhi, India: Ministry of Health & Family Welfare, Government of India, 2016. [Google Scholar]
- 4.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 2008;359:2233–44. 10.1056/NEJMoa0800971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nimkar S, Kinikar A, Chavan A, et al. High prevalence of late presentation of ART-naïve perinatally infected children for care in Pune, India. AIDS Care 2020;32:1415–20. 10.1080/09540121.2020.1727407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Y, Zhou O, Zheng Z, et al. Effect of AIDS-defining events at initiation of antiretroviral therapy on long-term mortality of HIV/AIDS patients in southwestern China: a retrospective cohort study. AIDS Res Ther 2020;17:44. 10.1186/s12981-020-00300-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callens SFJ, Shabani N, Lusiama J, et al. Mortality and associated factors after initiation of pediatric antiretroviral treatment in the Democratic Republic of the Congo. Pediatr Infect Dis J 2009;28:35–40. 10.1097/INF.0b013e318184eeb9 [DOI] [PubMed] [Google Scholar]
- 8.Yin H, Ma Y, Yang X, et al. [Survival analysis on HIV-infected children aged 14 years old and younger in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020;41:850–5. 10.3760/cma.j.cn112338-20191129-00844 [DOI] [PubMed] [Google Scholar]
- 9.Oumer A, Kubsa ME, Mekonnen BA. Malnutrition as predictor of survival from anti-retroviral treatment among children living with HIV/AIDS in Southwest Ethiopia: survival analysis. BMC Pediatr 2019;19:474. 10.1186/s12887-019-1823-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wachholz NIR, Ferreira J. Adherence to antiretroviral therapy in children: a study of prevalence and associated factors. Cad Saude Publica 2007;23 Suppl 3:S424–34. 10.1590/S0102-311X2007001500010 [DOI] [PubMed] [Google Scholar]
- 11.Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep 2009;6:194–200. 10.1007/s11904-009-0026-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrasekaran P, Shet A, Srinivasan R, et al. Long-term virological outcome in children receiving first-line antiretroviral therapy. AIDS Res Ther 2018;15:23. 10.1186/s12981-018-0208-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh R, Mukherjee A, Singla M, et al. Immunological and virological responses to highly active antiretroviral therapy in HIV-1 infected children. Indian J Pediatr 2017;84:893–6. 10.1007/s12098-017-2441-y [DOI] [PubMed] [Google Scholar]
- 14.Paranjpe SM, Sarkate PP, Ingole NA, et al. Profiles of HIV-infected anti-retroviral therapy naïve children from Mumbai, India. World J Pediatr 2016;12:430–5. 10.1007/s12519-016-0035-9 [DOI] [PubMed] [Google Scholar]
- 15.Cleves M, Gould W, Gutierrez R, eds. An introduction to survival analysis using stata. College Station, Texas, USA: Stata Press, 2004. [Google Scholar]
- 16.Buchanan AL, Montepiedra G, Sirois PA, et al. Barriers to medication adherence in HIV-infected children and youth based on self- and caregiver report. Pediatrics 2012;129:e1244–51. 10.1542/peds.2011-1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin S, Elliott-DeSorbo DK, Wolters PL, et al. Patient, caregiver and regimen characteristics associated with adherence to highly active antiretroviral therapy among HIV-infected children and adolescents. Pediatr Infect Dis J 2007;26:61–7. 10.1097/01.inf.0000250625.80340.48 [DOI] [PubMed] [Google Scholar]
- 18.van Wyk BE, Davids L-AC. Challenges to HIV treatment adherence amongst adolescents in a low socio-economic setting in Cape Town. South Afr J HIV Med 2019;20:1002. 10.4102/sajhivmed.v20i1.1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marhefka SL, Farley JJ, Rodrigue JR, et al. Clinical assessment of medication adherence among HIV-infected children: examination of the Treatment Interview Protocol (TIP). AIDS Care 2004;16:323–37. 10.1080/09540120410001665330 [DOI] [PubMed] [Google Scholar]
- 20.Nasuuna E, Kigozi J, Muwanguzi PA, et al. Challenges faced by caregivers of virally non-suppressed children on the intensive adherence counselling program in Uganda: a qualitative study. BMC Health Serv Res 2019;19:150. 10.1186/s12913-019-3963-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biadgilign S, Deribew A, Amberbir A, et al. Barriers and facilitators to antiretroviral medication adherence among HIV-infected paediatric patients in Ethiopia: a qualitative study. Sahara J 2009;6:148–54. 10.1080/17290376.2009.9724943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikaako-Kajura W, Luyirika E, Purcell DW, et al. Disclosure of HIV status and adherence to daily drug regimens among HIV-infected children in Uganda. AIDS Behav 2006;10:85–93. 10.1007/s10461-006-9141-3 [DOI] [PubMed] [Google Scholar]
- 23.Meena R, Hemal A, Arora SK. Pediatric HIV disclosure in northern India: evaluation of its prevalence, perceptions amongst caregivers, and its impact on CLHIV. AIDS Res Treat 2018;2018:1–7. 10.1155/2018/2840467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reif LK, Abrams EJ, Arpadi S, et al. Interventions to improve antiretroviral therapy adherence among adolescents and youth in low- and middle-income countries: a systematic review 2015–2019. AIDS Behav 2020;24:2797–810. 10.1007/s10461-020-02822-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shearer WT, Easley KA, Goldfarb J, et al. Evaluation of immune survival factors in pediatric HIV-1 infection. Ann N Y Acad Sci 2000;918:298–312. 10.1111/j.1749-6632.2000.tb05499.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obimbo EM, Mbori-Ngacha DA, Ochieng JO, et al. Predictors of early mortality in a cohort of human immunodeficiency virus type 1-infected African children. Pediatr Infect Dis J 2004;23:536–43. 10.1097/01.inf.0000129692.42964.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu HW, Pelton S, Williamson JM, et al. Survival in children with perinatal HIV infection and very low CD4 lymphocyte counts. J Acquir Immune Defic Syndr 2000;25:269–75. 10.1097/00126334-200011010-00010 [DOI] [PubMed] [Google Scholar]
- 28.Walker AS, Mulenga V, Sinyinza F, et al. Determinants of survival without antiretroviral therapy after infancy in HIV-1-infected Zambian children in the CHAP trial. J Acquir Immune Defic Syndr 2006;42:637–45. 10.1097/01.qai.0000226334.34717.dc [DOI] [PubMed] [Google Scholar]
- 29.Chatterjee A, Bosch RJ, Hunter DJ, et al. Maternal disease stage and child undernutrition in relation to mortality among children born to HIV-infected women in Tanzania. J Acquir Immune Defic Syndr 2007;46:599–606. 10.1097/QAI.0b013e31815a5703 [DOI] [PubMed] [Google Scholar]
- 30.Venturini E, Turkova A, Chiappini E, et al. Tuberculosis and HIV co-infection in children. BMC Infect Dis 2014;14 Suppl 1:S5. 10.1186/1471-2334-14-S1-S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang SS, Swanson DS, Starke JR. New diagnostics for childhood tuberculosis. Infect Dis Clin North Am 2015;29:477–502. 10.1016/j.idc.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 32.Zar HJ, Connell TG, Nicol M. Diagnosis of pulmonary tuberculosis in children: new advances. Expert Rev Anti Infect Ther 2010;8:277–88. 10.1586/eri.10.9 [DOI] [PubMed] [Google Scholar]
- 33.Youngs J, Patil S, Jain Y. A prospective study evaluating the impact of cartridge-based nucleic acid amplification test (CBNAAT) on the management of tuberculosis in a low-resource high-burden Indian rural setting. J Family Med Prim Care 2018;7:982–92. 10.4103/jfmpc.jfmpc_104_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sachdeva K, Shrivastava T. CBNAAT: a boon for early diagnosis of tuberculosis-head and neck. Indian J Otolaryngol Head Neck Surg 2018;70:572–7. 10.1007/s12070-018-1364-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orikiriza P, Nansumba M, Nyehangane D, et al. Xpert MTB/RIF diagnosis of childhood tuberculosis from sputum and stool samples in a high TB-HIV-prevalent setting. Eur J Clin Microbiol Infect Dis 2018;37:1465–73. 10.1007/s10096-018-3272-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. Data access is limited due to ethical and legal considerations; however, request may be considered after data request submission and approval from relevant authorities and Ethics Committee.