Abstract
Objectives
Infants in neonatal units benefit from dependable peripheral intravenous access. However, peripheral intravenous access exposes infants to high rates of clinically minor and serious complications. Despite this, little is known about the interplay of risk factors. The aim of this study was to assess the incidence and evaluate the interactions of risk factors on the occurrence of peripheral intravenous complications in a neonatal population.
Design
This was a retrospective observational study.
Setting
The study was performed on the neonatal intensive care unit of the Women’s Wellness and Research Center, Hamad Medical Corporation, Qatar, as a single-site study.
Participants
This study included 12 978 neonates who required intravenous therapy.
Outcome measurements
The main outcome was the occurrence of any peripheral intravenous cannulation failure, leading to unplanned removal of the device before completion of the intended intravenous therapy.
Results
A mean dwell time of 36±28 hours was recorded in participants with no complications, whereas the mean dwell time was 31±23 hours in participants with an indication for premature removal of the peripheral intravenous catheter (PIVC) (p<0.001, t=11.35). Unplanned removal occurred in 59% of cases; the overall complication rate was 18 per 1000 catheter days. Unmodifiable factors affecting PIVC dwell time include lower birth (HR=0.23, 0.20 to 0.28, p<0.001) and current body weight (HR=1.06, 1.03 to 1.10, p=0.018). Cannulation site (HR=1.23, 1.16 to 1.30, p<0.001), the inserted device (HR=0.89, 0.84 to 0.94, p<0.001) and the indication for intravenous treatment (HR=0.76, 0.73 to 0.79, p<0.001) were modifiable factors.
Conclusion
Most infants experienced a vascular access-related complication. Given the high complication rate, PIVCs should be used judiciously and thought given prior to their use as to whether alternate means of intravenous access might be more appropriate.
Keywords: neonatology, anaesthetics, neonatal intensive & critical care, intensive & critical care
Strengths and limitations of this study.
This was an observational study including a large sample of 12 978 neonates.
This study provides information on the risk of complications regarding peripheral intravenous cannulation in neonates.
This study is based on retrospective analyses of collected data.
Introduction
Providing reliable vascular access in the neonatal intensive care unit (NICU) is essential to administer nutrition, fluids, medication and blood products.1 Critically ill and preterm infants benefit from early intravenous therapy.2 Currently, the main intravenous vascular access routes are via peripheral and central veins. Peripheral intravenous cannulation is the most frequently performed procedure in NICU.1 3 Preterm and ill infants are at an increased risk of peripheral intravenous catheter (PIVC)-related complications.1 3–6 In part, this is due to immature skin anatomy and physiology, immature immune system and smaller, fragile blood vessels.3–6 When making decisions about vascular access requirements, a ‘5Rs’ mnemonic (after Steere et al7) can be referred to as an aid to supporting patient safety and well-being.
PIVC-related complications are a major clinical concern in NICUs. Frequently encountered complications are infiltration and extravasation (peripheral intravenous intravasation and extravasation (PIVIE)), leakage, occlusion, thrombosis, phlebitis, infection, and dislodgement or accidental removal.1 4 8–12 According to Pettit,13 the incidence of complications has remained constant over recent decades irrespective of clinical innovations and changes in practice. Overall, the risk of a PIVC-related complication in this patient population is reported as up to 75%.1 5 6 9 12 14 Of particular concern is the risk of PIVIE, which, according to several sources, is high in the neonatal population, having an incidence of around 65%.1 4 9 13 Infection rates are highly variable, but have been documented as between 2 and 49 incidents per 1000 catheter days.15
Extrinsic modifiable factors influence PIVC dwell time, such as clinician training, exposure, experience, choice of the optimal PIVC for the right patient for the right therapy, site selection and preparation, insertion technology, maintenance care bundles, stabilisation materials and dressings.3–5 16 Recent evidence from large-scale studies in neonatal populations regarding factors influencing PIVC is lacking and absent for Middle Eastern settings and contexts. The current study aimed to identify and evaluate the relationships between unmodifiable and potentially modifiable factors with the presence of PIVC-related complications.
Methods
Design and setting
This retrospective observational study uses routinely collected anonymised data from January 2019 to July 2020. The outcome of the study was the occurrence of any complication in relation to PIVC use, leading to unplanned removal of the device before completion of the intended intravenous therapy. The study was carried out on the NICU (112 cots) of the Women’s Wellness and Research Center of Hamad Medical Corporation, Doha, Qatar.
Participants and sample size
Infants who were admitted to the NICU and who required intravenous therapy were included in this study. Participants were excluded from the sample if the data collection was incomplete or related to the use of other devices (centrally inserted central catheters or peripherally inserted central catheters).
Procedure
Peripheral intravenous cannulation was performed according to hospital policy based on international guidelines.17 In the study setting, peripheral intravenous cannulation is routinely performed by nurses from the NICU vascular access team (VAT). Proactive choices to prevent patients from running out of veins and being labelled as difficult vascular access patients are key in the selection of cannulation site and intravenous catheter.7 For that reason, saphenous and elbow veins generally are avoided for cannulation.17 The selection of suitable veins was done using the VeinViewer (Christie Medical Holdings, Lake Mary, Florida, USA). Vein length, valves and potential for the vein to fill and empty itself were previously assessed using a standardised approach to appraisal of the potential site. Short PIVCs were used if therapy was predicted for up to 2 days, including a Neoflon Pro (Becton Dickinson Infusion Therapy, Sandy, Utah, USA) of 26 or 24 gauge or a SuperCath Safety (ICU Medical, San Clemente, California, USA) of 26 gauge. Extended 22-gauge PIVCs were inserted when duration of therapy was expected to last for 5 days (LeaderFlex, Vygon, Lansdale, Pennsylvania, USA). In situations where intravenous therapy was expected to last more than 5 days, central venous access is preferred. According to hospital protocols and based on international guidelines, there is no evidence for routine rotation of vascular access devices (VADs) in the neonatal population.17
Measurements and data collection
The main outcome was the occurrence of any peripheral intravenous cannulation failure, leading to unplanned removal of the device before completion of the intended intravenous therapy. Patient demographics and baseline data included sex, gestational age at birth in weeks and days, birth weight and current body weight in gram. Data regarding the procedure of peripheral intravenous cannulation were the date and time of cannulation, as well as the number of attempts needed to successful cannulation; cannulation side (left or right); extremity of cannulation and the site on the extremity (dorsum of the hand, wrist and lower arm, elbow crease and upper arm, foot, ankle and lower leg, or knee and upper leg); size of device (22, 24 or 26 gauge); the indication for intravenous treatment (intravenous fluids, medications, total parenteral nutrition, blood and blood products, blood extraction or procedural); the date and time of removal of the PIVC; total dwell time of the PIVC in hours (calculated as the removal date and time minus the insertion date and time); and the reason for removal of the PIVC (therapy completed and elective removal, PIVIE, phlebitis, occlusion, dislodgement and accidental removal, discolouration, and patient transferred or expired). Furthermore, additional data points included the use of catheter securement glue, application of ivWatch (ivWatch LLC, Newport News, Virginia, USA), if the touch–look–compare (TLC) observation tool was used and calculation of the PIVIE Severity Score in percentages.18 19 The ivWatch was introduced into use in January 2020 and applied since then with infants weighing more than 1000 g.
Statistical analyses
Descriptive statistics were used to summarise the outcomes with the mean and its SD or median, and its range for continuous variables regarding its normal distribution, and absolute numbers with percentage for discrete variables. The assumption of normal distribution was proved with Kolmogorov-Smirnov testing. Differences regarding outcomes and measurements were demonstrated by using the χ2 test, Mann-Whitney U test or unpaired samples t-test, as appropriate. Stepwise Cox hazard regression analyses were used to provide correlations between variables regarding the outcome of this study and to obtain its OR with 95% CI. Items with a significant relationship (p<0.01) to the outcome of this study from a univariate analysis were entered in these analyses. The stepwise method was used to remove independent variables that did not make a significant contribution to the primary outcome variable using a backward elimination process based on the Wald statistic and level of significance, with the removal criteria set at p=0.01, to obtain a model with a minimal set of variables. Correlation between variables was measured by determining Pearson’s or Spearman’s r, as appropriate. Survival analyses of PIVC in terms of its dwell time were performed by plotting a Kaplan-Meier curve. Differences between survival time of the PIVC according to its reason for premature removal were represented with log-rank (Mantel-Cox) χ2. In addition, log-rank (Mantel-Cox) χ2 analyses were used for all comparisons regarding the different outcome measures on device dwell time. A p value of <0.05 was denoted to be statistically significant throughout this study. SPSS V.25.0 was used for statistical analyses.
Patient and public involvement
Study outcome measurements were based on recent literature and after a brainstorm session with the researchers. The study did not involve any patient nor member of the public in the conception, design and development of the study protocol. They were not also involved in data acquisition, analyses, interpretation and development of this manuscript.
Results
In total, data on 15 087 cannulation events in neonates were collected during the study period, of which data of 2109 participants were removed due to incompleteness, including failure to insert. The final database included 12 978 participants, with 7695 (59%) being of male sex. Mean gestational age was 34+6 (23 to 43) weeks. Current age in days after birth was 9 (0 to 29) days at the time that peripheral intravenous cannulation was performed. Mean weight at birth was 2334±975 g, with a mean current weight of 2410±931 g at the time of cannulation.
Successful peripheral intravenous cannulation at the first attempt was obtained in 8481 participants (65%). Twenty-four per cent needed two attempts; 8% and 2% needed three attempts; and a small number, under senior clinician oversight, needed more attempts to successfully insert a PIVC. Throughout the study, 19 329 insertion attempts were performed to create peripheral intravenous access. Data regarding the procedure of peripheral intravenous cannulation are summarised in table 1.
Table 1.
Procedural peripheral intravenous cannulation data
| Factor | Description | Total cohort | Successful first attempt | Unsuccessful first attempt | P value |
| N=12 978 | n=8481 | n=4497 | |||
| Side of cannulation | Left | 7120 (55%) | 4794 (57%) | 2326 (52%) | <0.001 |
| Right | 5854 (45%) | 3684 (43%) | 2170 (48%) | ||
| Site of cannulation on the selected extremity | Hand | 10 512 (81%) | 7078 (83%) | 3434 (76%) | <0.001 |
| Wrist/lower arm | 459 (4%) | 240 (3%) | 219 (5%) | ||
| Elbow/upper arm | 61 (<1%) | 33 (1%) | 28 (1%) | ||
| Foot | 1774 (14%) | 1025 (12%) | 749 (17%) | ||
| Ankle/lower leg | 119 (1%) | 78 (1%) | 41 (1%) | ||
| Knee/upper leg | 50 (<1%) | 25 (<1%) | 25 (<1%) | ||
| Scalp | 2 (<1%) | 1 (<1%) | 1 (<1%) | ||
| Size of the inserted catheter | 26 gauge | 12 403 (96%) | 8090 (96%) | 4313 (96%) | <0.001 |
| 24 gauge | 141 (1%) | 97 (1%) | 44 (1%) | ||
| 22 gauge | 434 (3%) | 294 (3%) | 140 (3%) | ||
| Indication for intravenous treatment | Intravenous fluids/medications | 7283 (56%) | 4781 (56%) | 2502 (56%) | <0.001 |
| Intravenous fluids/TPN | 4330 (33%) | 2844 (34%) | 1486 (33%) | ||
| Blood and blood products | 482 (4%) | 285 (3%) | 197 (4%) | ||
| Blood extraction | 708 (5%) | 455 (5%) | 253 (6%) | ||
| Procedure | 175 (2%) | 116 (2%) | 59 (1%) |
Data are represented as absolute number and percentages, which were calculated as a proportion within in the cell.
TPN, total parenteral nutrition.
Failure of the PIVC, resulting in premature removal, occurred in 7627 participants (59%). In 5145 participants (40%), the PIVC was removed after completion of intravenous therapy. In 142 cases (1%), the participant was transferred or expired (administrative censoring). A mean dwell time of 36±28 hours was recorded in participants with no complications, whereas the mean dwell time was 31±23 hours in participants with an indication for premature removal of the PIVC (p<0.001, χ2=5850.77, df=1). Subsequently, there was a correlation between dwell times and the occurrence of a PIVC-related complication (p<0.001, ρ=−0.099). The overall PIVC complication rate was 18 per 1000 catheter days. PIVIE was the most frequently observed complication throughout the studied cohort, with a relative risk of device failure of 3.14 (3.04 to 3.25). Additional information according to the reason for removal of the PIVC is shown in table 2.
Table 2.
Data representing the reason for removal of the peripheral intravenous catheter
| Factor | Description | Device dwell time (hours) | Total cohort |
| N=12 914 | |||
| Reason for removal of the VAD | Therapy completed/elective | 36±28 | 5145 (40%) |
| PIVIE | 31±24 | 5159 (40%) | |
| Phlebitis | 29±19 | 1590 (12%) | |
| Occlusion | 41±29 | 527 (4%) | |
| Dislodgement/accidental removal | 23±25 | 286 (2%) | |
| Swelling or discolouration | 22±20 | 65 (1%) | |
| Administrative censoring | 17±26 | 142 (1%) |
Data are represented as mean and its SD or as absolute number and percentages, which were calculated as a proportion within the cell. Device dwell time is represented in hours.
Data of 64 participants are missing.
PIVIE, peripheral intravenous intravasation and extravasation; VAD, vascular access device.
Total dwell time of the device in each participant until its moment of removal is represented in figure 1. Fifty per cent of PIVCs were removed within the first 38 hours. Dwell times differed regarding the reason for removal or the kind of PIVC-related complication (p<0.001, χ2=76.83, df=4).
Figure 1.
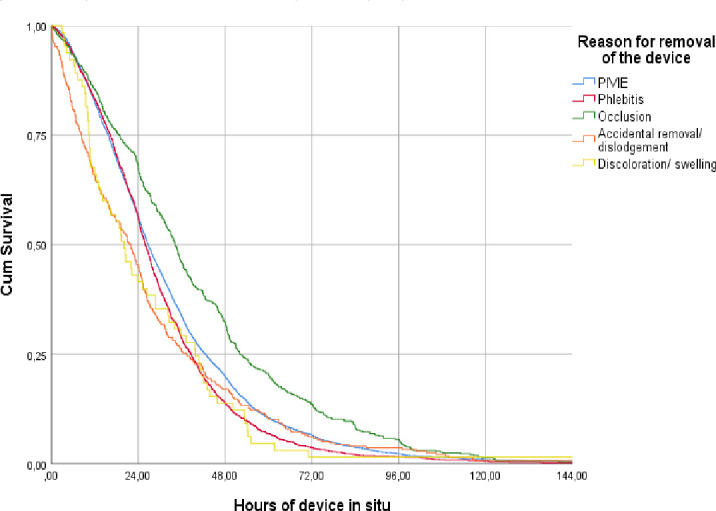
Kaplan-Meier survival analyses for peripheral intravenous catheters. Intravenous catheters were removed after the occurrence of a complication, of which dwell times were compared between the type of complications as measured in this study. PIVIE, peripheral intravenous infiltration and extravasation.
As shown in table 3, 12 variables had a significant relation with the outcome of interest in the univariate logistic analyses, resulting in premature removal of the device. These items were used for multivariate analyses, resulting in the smallest set of five variables correlating with the outcome of this study (table 4).
Table 3.
Univariate Cox hazard regression analyses with factors affecting the risk for failure of peripheral intravenous access devices
| Factor | β | HR | 95% CI | P value |
| Sex of the participant | 0.025 | 1.03 | 0.98 to 1.07 | 0.292 |
| Duration of gestation (weeks) | 0.014 | 0.98 | 0.98 to 0.99 | <0.001 |
| Current age since gestation (days) | 0.501 | 0.61 | 0.37 to 0.99 | 0.047 |
| Weight at birth (g) | 0.072 | 0.93 | 0.88 to 0.97 | 0.002 |
| Current weight (g) | 0.037 | 1.04 | 1.01 to 1.07 | 0.010 |
| Successful first attempt of cannulation | 0.006 | 1.01 | 0.95 to 1.06 | 0.810 |
| Number of attempts to successful cannulation | 0.005 | 0.99 | 0.96 to 1.03 | 0.758 |
| Side of cannulation | 0.161 | 1.18 | 1.12 to 1.23 | <0.001 |
| Site of cannulation on the extremity | 0.079 | 1.08 | 1.05 to 1.11 | <0.001 |
| Size of the inserted intravenous catheter | 0.080 | 0.92 | 0.89 to 0.96 | <0.001 |
| Indication for intravenous treatment | 0.292 | 0.75 | 0.72 to 0.78 | <0.001 |
| Time of the device in situ | 0.499 | 0.61 | 0.56 to 0.65 | <0.001 |
| Application of TLC observation | 0.265 | 1.30 | 1.13 to 1.50 | <0.001 |
| PIVIE Severity Score | 0.023 | 1.02 | 1.01 to 1.03 | <0.001 |
| Application of the ivWatch | 0.199 | 1.22 | 1.12 to 1.33 | <0.001 |
| Application of device fixation glue | 0.118 | 0.89 | 0.64 to 1.23 | 0.477 |
PIVIE, peripheral intravenous infiltration and extravasation; TLC, touch–look–compare.
Table 4.
Multivariate Cox hazard regression analyses with factors affecting the risk for failure of peripheral intravenous access devices
| Factor | β | HR | 95% CI | P value |
| Weight at birth (g) | 1.452 | 0.23 | 0.20 to 0.28 | <0.001 |
| Current weight (g) | 0.062 | 1.06 | 1.03 to 1.10 | 0.018 |
| Site of cannulation on the extremity | 0.207 | 1.23 | 1.16 to 1.30 | <0.001 |
| Size of the inserted intravenous catheter | 0.119 | 0.89 | 0.84 to 0.94 | <0.001 |
| Indication for intravenous treatment | 0.280 | 0.76 | 0.73 to 0.79 | <0.001 |
A lower weight at birth (HR=0.23, 0.20 to 0.28, p<0.001) and a lower current body weight (HR=1.06, 1.03 to 1.10, p=0.018) resulted in an increased risk of PIVC-related complications. Cannulation, on one hand, showed the lowest complication rate (57%), whereas most complications were reported after cannulation on the ankle or lower leg (72%) (p<0.001, χ2=112.65, df=6). Inserting a 22-gauge device resulted in 77% of cases in a complication; cannulation with a 26-gauge catheter led to complications in 49% of insertions (p=0.001, χ2=17.04, df=3). If TPN was the indication for starting up intravenous treatment, 64% resulted in premature removal of the device, whereas only 18% of insertion resulted in a complication if cannulation was performed per procedure and elective (p<0.001, χ2=288.33, df=4). Cannulation site (HR=1.23, 1.16 to 1.30, p<0.001), the inserted device (HR=0.89, 0.84 to 0.94, p<0.001) and the indication for intravenous treatment (HR=0.76, 0.73 to 0.79, p<0.001) were modifiable factors.
The PIVIE Severity Score was higher in participants with an indication for premature removal of the device (13.1±8.6) when compared with those without a VAD-related complication (0.8±4.1) (p<0.001, t=−25.409). PIVIE Severity Scores increased in participants suffering from PIVIE (13.8±8.0) and phlebitis (12.9±9.9). Furthermore, a correlation between the PIVIE Severity Score and device dwell time could be obtained (p<0.001, ρ=−0.122). The ivWatch was applied in 12% of participants, of which 63% suffered from premature removal. The added value of this device resulted in a sensitivity of 57% and a specificity of 56% (p<0.001, χ2=54.165, df=1). Catheter dwell times of 38±26 were seen after the application of ivWatch, which did not differ from dwell times of 31±25 in participants in whom the technique was not used (p<0.001, χ2=45.31, df=1). However, a correlation between the application of the ivWatch and device dwell times could not be obtained (p=0.705, ρ=−0.006). The TLC observation tool was applied in 67% of cases and detected complications in 61% of participants with an event (p=0.002, χ2=9.975, df=1). The use of the TLC observation tool resulted in a sensitivity of 97% and a specificity of 96% and correlated with device dwell time (p=0.001, ρ=−0.032). The use of glue for fixation of the PIVC increased the dwell time to 34±25 when compared with participants in which no glue was used (dwell time of 28±18), although the difference was not significant (p=0.623, χ2=0.24, df=1). A correlation could not be seen between the use of glue and PIVC dwell times (p=0.025, ρ=−0.106).
Discussion
The incidence of VAD failure is high in clinical practice, which negatively affects a neonate’s comfort and outcome.20 21 Failure of peripherally inserted PIVC, resulting in premature removal, occurred in 51% of participants, with a complication rate of 18 per 1000 device days. The most frequently reported complications were PIVIE and phlebitis. The risk of complications was increased in participants with a lower weight at birth and current body weight. Furthermore, the cannulation site, size and type of device, and the indication for intravenous treatment affected the risk of failure as well.
Although this study provides information on the risk of complications regarding peripheral intravenous cannulation in neonates, majority of it was reported in many articles. Nonetheless, to the best of our knowledge, a study including as many patients as the current study does was never published before on this topic.
PIVCs are often the primary and most commonly inserted devices used to obtain vascular access during hospitalisation.20 The incidence of device failure in the current study is slightly higher when compared with the 34% pooled incidence of failure in the recently published meta-analyses by Indarwati et al.22 It is difficult to give an unambiguous clarification for this, although the pattern of complications and their relative incidence does match.
PIVIE was the most common complication in infants admitted to the NICU, with an incidence of 34% in the current study. PIVIE is defined as an unintended infusion of fluids and/or medication in the surrounding tissue, in which infiltration is the infusion of non-vesicant fluids or medication and extravasation infusion of vesicants into surrounding tissues.5 The determination of PIVIE can be subjective, making it hard to compare the results of different studies. However, standardised training of a dedicated VAT and routine review of scores can improve consensus and reduce subjectivity. The incidence of infiltration reported elsewhere ranges from 6% to 87%, and the incidence of extravasation ranges between 2% and 77%.22 The use of the infiltration/extravasation staging instrument, as developed by Montgomery et al,23 could accomplish consensus on the definition of the condition and its severity.5 An explanation for the non-standard use of this instrument may be that it has not been externally validated.
Phlebitis (inflammation of the venous wall) can cause discomfort and tissue damage. The incidence was 10% in the current study, which is broadly in accord with other reports.22–27 According to Arias-Fernández et al,28 assessment of phlebitis is difficult because the consensus for the diagnosis is low. Furthermore, a lack of consensus on phlebitis measures has likely contributed to disparities in reported phlebitis incidence.29
Several tools are used in clinical practice to reduce the risk or severity for premature failure of PIVCs due to device-related complications. The TLC observation tool was developed at Cincinnati Children’s Hospital Medical Centre to reduce peripheral intravenous infiltration and extravasation injuries.18 This documented methodical hourly assessment of patients with a PIVC can help practitioners standardise their practice and reduce variations in quality of care.18 Our study showed highly discriminative effects of the TLC observation tool based on high sensitivity and specificity, which was denoted as the most decisive tool in detecting device-related complications earlier. Routine observations by combining the TLC observation tool and the PIVIE Severity Scoring instrument seem to result in the most optimal situation regarding the early detection of complications.
It is known that the preferred cannulation site is the dorsal hand, on which fewer attempts were required for successful cannulation, with fewer complications and extended dwell times.14 This is in accordance with the results of the current study. Moreover, phlebitis caused by mechanical irritation due to the device is thought to be an important factor for failure.21 Fixation of the device after insertion with glue increases the stability of the device. Despite the fact no significance could be obtained, dwell times were increased after using glue in this study. Highest incidence of premature removal of the device was seen with a 22-gauge device. Insertion of a 26-gauge catheter resulted in the lowest incidence of complications. Notwithstanding, most participants in this study received a 26-gauge device, possibly leading to a distorting result. To minimise the risk of phlebitis, the smallest gauge catheter possible should be inserted, and the use of extension tubes as an accessory to the device should be avoided.27
Preterm infants are extrasensitive to the development of PIVIE and phlebitis due to their immature immune systems.22 30 Beall et al30 concluded that the inadequate anti-inflammatory response may fail to release free radical scavengers, leading to endothelial apoptosis and injury of cell membranes and vessels. To add to this, it is thought that medications or fluids with a higher osmolality increase the risk of extravasation by irritating the endothelial lining of the vein.22 Early detection of signs and symptoms correlating positively with PIVC complications is crucial in limiting the risk of failure of the device. Assessing pain accurately in preverbal infants is challenging.31 Moreover, additional occlusive fixtures and bandages to secure the device add limits to identifying early stages of complications, and thus timely cessation of therapy and treatment to minimise harm.22 31 The incidence of complications could likely be reduced with consistent and quality insertion and maintenance practices. The Infusion Nurses Society provides specific recommendations for newborn infants offering further specific guidelines for insertion and management practice.17
Limitations
The current study was based on a retrospective collected dataset. In contrast to randomised studies, the method creates a risk of selection bias. In the present study, every infant with a PIVC was included in order to minimise the risk of selection bias. In addition, this current study was carried out according to the Strengthening the Reporting of Observational Studies in Epidemiology statement.32 Inter-rater variability might have affected the results; however, our use of standardised education and training and limiting vascular access to a small team (the VAT) will mitigate this variability in the data. Nonetheless, future research should focus on the development and validation of decisive tools and their integration with emerging technologies to identify complications early.
Conclusion and relevant implications
Most infants experienced a vascular access-related complication. Five variables were identified as factors affecting PIVC dwell time in patients admitted to the NICU. These factors include a lower weight at birth and current body weight, the cannulation site, size and type of device, and the indication for intravenous treatment affected the risk for failure as well. The PIVC complication rate was 18 per 1000 catheter days in the current study. The risk for the development of a PIVC-related complication, leading to premature removal of the device, increased with extended dwell times. It seems that when a PIVC is inserted, it is not the question of if the infant will have a complication, but only a matter of when. The most frequently observed complication in the neonatal population is a PIVIE, with a relative risk of 3.14 (95% CI 3.04 to 3.25). Consequently, we argue that PIVC should be used judiciously, and thought should be given prior to their use as to whether alternate means of intravenous access might be more appropriate.
Supplementary Material
Acknowledgments
The authors thank the neonatal intensive care unit (NICU)’s vascular access team and all our other colleagues in the NICU of Women’s Wellness and Research Center.
Footnotes
Contributors: MFPTvR was the main investigator, conceptualised and designed the study, coordinated and supervised the data collection, drafted the initial manuscript, and reviewed and revised the manuscript. KH drafted the initial manuscript and reviewed and revised the manuscript. MAM critically reviewed the manuscript for important intellectual content. MB reviewed and revised the manuscript. ALVF designed the data collection instruments, collected data, and reviewed and revised the manuscript. KLPG designed the data collection instruments, collected data, and reviewed and revised the manuscript. FHJvL conceptualised and designed the study, carried out the initial analyses, critically reviewed the manuscript for important intellectual content and revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
No data are available. Deidentified individual participant data will not be made available.
Ethics statements
Patient consent for publication
Obtained.
Ethics approval
The study protocol (MRC-01-20-594) was approved by the local institution review body (IRB). As the data source was anonymised, the local IRB deemed that participant consent was not feasible nor required as they determined the study a ‘chart review’. Participants and their parents were not involved in the design, conduct or reporting of this study.
References
- 1.Legemaat M, Carr PJ, van Rens RM, et al. Peripheral intravenous cannulation: complication rates in the neonatal population: a multicenter observational study. J Vasc Access 2016;17:360–5. 10.5301/jva.5000558 [DOI] [PubMed] [Google Scholar]
- 2.Ainsworth S, McGuire W. Percutaneous central venous catheters versus peripheral cannulae for delivery of parenteral nutrition in neonates. Cochrane Database Syst Rev 2015;10:CD004219. 10.1002/14651858.CD004219.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chenoweth KB, Guo J-W, Chan B. The extended dwell peripheral intravenous catheter is an alternative method of NICU intravenous access. Advances in Neonatal Care 2018;18:295–301. 10.1097/ANC.0000000000000515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Odom B, Lowe L, Yates C. Peripheral infiltration and extravasation injury methodology: a retrospective study. J Infus Nurs 2018;41:247–52. 10.1097/NAN.0000000000000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atay S, Sen S, Cukurlu D. Incidence of infiltration/extravasation in newborns using peripheral venous catheter and affecting factors. Rev Esc Enferm USP 2018;52:e03360. 10.1590/s1980-220x2017040103360 [DOI] [PubMed] [Google Scholar]
- 6.Unbeck M, Förberg U, Ygge B-M, et al. Peripheral venous catheter related complications are common among paediatric and neonatal patients. Acta Paediatr 2015;104:566–74. 10.1111/apa.12963 [DOI] [PubMed] [Google Scholar]
- 7.Steere L, Ficara C, Davis M, et al. Reaching one peripheral intravenous catheter (PIVC) per patient visit with lean multimodal strategy: the PIV5Rights™ bundle. J Assoc Vasc Access 2019;24:31–43. 10.2309/j.java.2019.003.004 [DOI] [Google Scholar]
- 8.Patregnani JT, Sochet AA, Klugman D. Short-Term peripheral vasoactive infusions in pediatrics: where is the harm? Pediatr Crit care Med a J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc 2017;18:e378–81. [DOI] [PubMed] [Google Scholar]
- 9.Danski MTR, Mingorance P, Johann DA, et al. [Incidence of local complications and risk factors associated with peripheral intravenous catheter in neonates]. Rev Esc Enferm USP 2016;50:22–8. 10.1590/S0080-623420160000100003 [DOI] [PubMed] [Google Scholar]
- 10.Boyar V, Galiczewski C, Kurepa D. Point-Of-Care ultrasound use in neonatal peripheral intravenous extravasation injuries: a case series. J Wound Ostomy Continence Nurs 2018;45:503–9. 10.1097/WON.0000000000000475 [DOI] [PubMed] [Google Scholar]
- 11.Desarno J, Sandate I, Green K, et al. When in doubt, pull the catheter out: implementation of an evidence-based protocol in the prevention and management of peripheral intravenous Infiltration/Extravasation in neonates. Neonatal Netw 2018;37:372–7. 10.1891/0730-0832.37.6.372 [DOI] [PubMed] [Google Scholar]
- 12.Ohki Y, Maruyama K, Harigaya A, et al. Complications of peripherally inserted central venous catheter in Japanese neonatal intensive care units. Pediatr Int 2013;55:185–9. 10.1111/ped.12033 [DOI] [PubMed] [Google Scholar]
- 13.Pettit J. Assessment of the infant with a peripheral intravenous device. Adv Neonatal Care 2003;3:230–40. [PubMed] [Google Scholar]
- 14.Monasor-Ortolá D, Cortés-Castell E, Martínez-Pascual C, et al. Factors influencing the success of peripheral venous access in neonates. J Pediatr Nurs 2019;47:e30–5. 10.1016/j.pedn.2019.04.017 [DOI] [PubMed] [Google Scholar]
- 15.Wu J, Mu D. Vascular catheter-related complications in newborns. J Paediatr Child Health 2012;48:E91–5. 10.1111/j.1440-1754.2010.01934.x [DOI] [PubMed] [Google Scholar]
- 16.Rickard CM, Marsh NM, Webster J, et al. Intravascular device administration sets: replacement after standard versus prolonged use in hospitalised patients-a study protocol for a randomised controlled trial (the RSVP trial). BMJ Open 2015;5:e007257. 10.1136/bmjopen-2014-007257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorski LA, Hadaway L, Hagle ME, et al. Infusion therapy standards of practice, 8th edition. J Infus Nurs 2021;44:S1–224. 10.1097/NAN.0000000000000396 [DOI] [PubMed] [Google Scholar]
- 18.Tofani BF, Rineair SA, Gosdin CH, et al. Quality improvement project to reduce infiltration and extravasation events in a pediatric hospital. J Pediatr Nurs 2012;27:682–9. 10.1016/j.pedn.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 19.Doellman D, Hadaway L, Bowe-Geddes LA, et al. Infiltration and extravasation: update on prevention and management. J Infus Nurs 2009;32:203–11. 10.1097/NAN.0b013e3181aac042 [DOI] [PubMed] [Google Scholar]
- 20.Alexandrou E, Ray-Barruel G, Carr PJ, et al. Use of short peripheral intravenous catheters: characteristics, management, and outcomes worldwide. J Hosp Med 2018;13. 10.12788/jhm.3039. [Epub ahead of print: 30 05 2018]. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Murayama R, Abe-Doi M, et al. Preventing peripheral intravenous catheter failure by reducing mechanical irritation. Sci Rep 2020;10:1550. 10.1038/s41598-019-56873-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Indarwati F, Mathew S, Munday J, et al. Incidence of peripheral intravenous catheter failure and complications in paediatric patients: systematic review and meta analysis. Int J Nurs Stud 2020;102:103488. 10.1016/j.ijnurstu.2019.103488 [DOI] [PubMed] [Google Scholar]
- 23.Montgomery LA, Hanrahan K, Kottman K, et al. Guideline for i.v. infiltrations in pediatric patients. Pediatr Nurs 1999;25:167-169,173-180. [PubMed] [Google Scholar]
- 24.Padilla-Sánchez C, Montejano-Lozoya R, Benavent-Taengua L, et al. Risk factors associated with adverse events in neonates with peripherally inserted central catheter. Enferm Intensiva 2019;30:170–80. 10.1016/j.enfie.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 25.Cho Y-H, Yen L-L, Yu K-L, et al. [Reducing the incidence of phlebitis related to intravenous injection in pediatric patients]. Hu Li Za Zhi 2015;62:49–57. 10.6224/JN.62.3S.49 [DOI] [PubMed] [Google Scholar]
- 26.Mihala G, Ray-Barruel G, Chopra V, et al. Phlebitis signs and symptoms with peripheral intravenous catheters: incidence and correlation study. J Infus Nurs 2018;41:260–3. 10.1097/NAN.0000000000000288 [DOI] [PubMed] [Google Scholar]
- 27.Sharp R, Cummings M, Childs J, et al. Measurement of vein diameter for peripherally inserted central catheter (PICC) insertion: an observational study. J Infus Nurs 2015;38:351–7. 10.1097/NAN.0000000000000125 [DOI] [PubMed] [Google Scholar]
- 28.Arias-Fernández L, Suérez-Mier B, Martínez-Ortega MDC, et al. Incidence and risk factors of phlebitis associated to peripheral intravenous catheters. Enferm Clin 2017;27:79–86. 10.1016/j.enfcle.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 29.Ray-Barruel G, Polit DF, Murfield JE, et al. Infusion phlebitis assessment measures: a systematic review. J Eval Clin Pract 2014;20:191–202. 10.1111/jep.12107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beall V, Hall B, Mulholland JT, et al. Neonatal extravasation: an overview and algorithm for evidence-based treatment. Newborn and Infant Nursing Reviews 2013;13:189–95. 10.1053/j.nainr.2013.09.001 [DOI] [Google Scholar]
- 31.Sangam SL. Quality improvement measures for early detection of severe intravenous infiltration in infants. BMJ Open Qual 2019;8:e000407. 10.1136/bmjoq-2018-000407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available. Deidentified individual participant data will not be made available.


