Abstract
Background:
Triazole resistance is an emerging problem in the management of human aspergillosis globally and can arise in Aspergillus species which have been exposed to azole fungicides in the environment. We surveyed local government and council development areas in Lagos, Nigeria, to determine the distribution of Aspergillus species in the environment and their susceptibility to locally available triazole antifungal agents. We also reviewed the literature on the subject from the rest of Africa.
Methods:
A total of 168 soil samples from six locations in Lagos, Nigeria were processed and cultured on Saboraud dextrose agar impregnated with chloramphenicol to isolate Aspergillus species. Isolates were tested for susceptibility to itraconazole and voriconazole by microbroth dilution according to the European Committee on Antimicrobial Susceptibility Testing reference method. Relevant databases were searched to identify published work pertaining to triazole susceptibility of Aspergillus species in Africa.
Results:
A total of 117 Aspergillus species were isolated. Aspergillus niger was the most frequently isolated species (42.7%). Other species isolated were Aspergillus flavus, 37 (31.6%), Aspergillus terreus, 20 (17.1%), Aspergillus fumigatus, 5 (4.3%) and Aspergillus nidulans, 5 (4.3%). All isolates were susceptible to itraconazole and voriconazole. The literature review showed documented evidence of triazole-resistant Aspergillus species from East and West Africa.
Conclusions:
We found no triazole resistance in environmental isolates of Aspergillus in Lagos, Nigeria. Nevertheless, regular surveillance in clinical and environmental isolates is necessary in the light of findings from other African studies.
Keywords: Africa, aspergillosis, aspergillus, environmental, fungicide, Nigeria, resistance, triazole
Introduction
Aspergillosis affects millions of people worldwide. It is a spectrum which includes allergic bronchopulmonary aspergillosis, chronic pulmonary aspergillosis (CPA), aspergilloma and invasive aspergillosis (IA).1 This spectrum is frequently caused by species belonging to Aspergillus section Fumigati, although non-fumigatus species such as Aspergillus flavus, Aspergillus niger and Aspergillus terreus, are increasingly implicated.2 Human aspergillosis is poorly documented in most parts of Africa including Nigeria but certain factors suggest a significant burden. Pulmonary tuberculosis (TB), for which the country ranks high among 30 nations with the highest burden, is the commonest risk factor for CPA.3 Indeed, a prevalence of 8.7% for CPA was recently demonstrated in patients with smear-negative TB and TB treatment failure in Nigeria.4 The burden of IA, though undetermined, is likely to be significant because typical predispositions such as intensive care use, haematologic malignancies, solid organ and haematopoetic stem cell transplants are on the increase.5,6
Triazoles are the antifungal agents of choice for management of human aspergillosis. Resistance to these drugs among Aspergillus species is an emerging public health concern worldwide. As evidence of this, azole-resistant Aspergillus fumigatus was included as one of three pathogens on the watch list of the United States Centre for Disease Control 2019 antibiotics threat report: this report serves as a reference for information on antibiotic resistance, provides the latest antibiotic resistance burden estimates for human health and highlights emerging areas of concern and additional actions needed.7 With a global prevalence ranging from 0.6% to 27.8%, the growing trend of resistance to azoles in Aspergillus is worrisome because of the resultant possibility of treatment failure.2 Mortality rates as high as 88% have been reported in triazole-resistant cases of IA from The Netherlands and Germany.8 Resistance to these drugs would be particularly dire in a developing country like Nigeria where triazoles are the only readily available options for treatment of not just aspergillosis but all invasive fungal diseases.9
Acquired triazole resistance has been extensively studied and reported in A. fumigatus and occurs via two mechanisms: resistance developing gradually due to selection pressure during treatment with azoles (patient route) and de novo resistance caused by Aspergillus species which have gained resistance properties externally (environmental route).10 The environmental route has been linked to the use of azole fungicides in the agricultural sector.11,12 This phenomenon was first reported from the Netherlands in 2007.11 Subsequently, it was observed in other countries in Europe, Middle East and Asia.13–20
Triazole resistance may be associated with mutations affecting cyp51A, the gene coding for fungal sterol 14α demethylase enzyme which is the target of triazole antifungals.10 Depending on the type of mutation and its location in the genetic sequence, the resulting resistant strain can exhibit a range of phenotypes including a spectrum of triazole susceptibilities as well as single or pan-azole resistant qualities. The commonest cyp51A mutation associated with triazole resistance in Aspergillus species is TR34/L98H which consists of a tandem repeat (TR) sequence of 34 base pairs within the upstream promoter region of cyp51A and is characterized by substitution of leucine at the 98th amino acid position to histidine.10 Another TR mutation, TR46/Y121F/T289A is also commonly described. These TR-containing mutations are typically found in resistant isolates selected via the environmental route due to the use of azole fungicides. In addition, several point mutations such as G54, G138 or M220 can lead to resistant phenotypes due to disturbances in the docking of triazole antifungals to the cyp51A protein. Besides the cyp51A-related mutations, non-cyp51A mediated mutations have also been increasingly recognized in the development of triazole resistance, including mutations in other genes and efflux pumps.
There are no local statistics on the usage of pesticides in the agricultural sector, which contributes 29.25% to Nigeria’s gross domestic product.21 However, the azole fungicides hexaconazole and tricyclazole are approved by the National Agency for Food and Drug Administration and Control in Nigeria. Environmentally mediated triazole resistance is, therefore, a probable threat. This study aimed to determine the environmental distribution of Aspergillus species and their susceptibility to triazoles in Lagos, the most densely populated state in Nigeria. We also reviewed available data on triazole susceptibility among Aspergillus species from the rest of Africa.
Methods
Study design
This was a descriptive, cross-sectional study.
Study area
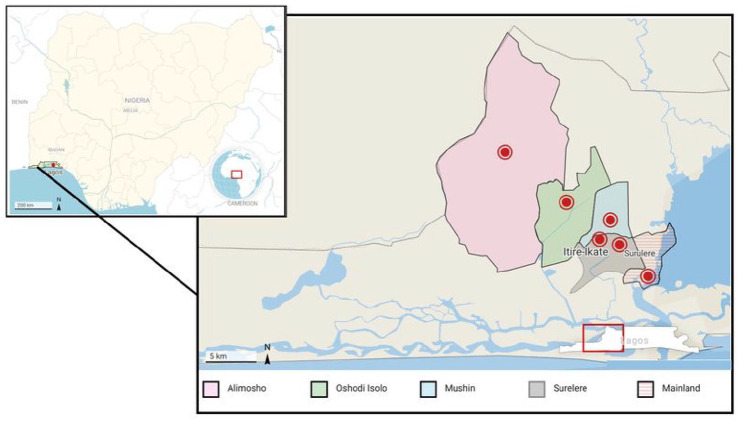
Lagos (Figure 1) is the most densely populated of the 36 states in Nigeria with a land mass of about 3577 square kilometres and a population of over 20 million people.22 The humid, tropical climate is characterized by two wet (April to July and October to November) and dry (August to September and December to March) seasons. Five of the 20 local government areas (LGAs) in the state and one local council development area (LCDA) were randomly selected for sampling: Yaba-Mainland, Oshodi Isolo, Itire-Ikate, Alimosho, Mushin and Surulere (Figure 1).
Figure 1.
Map of Lagos indicating sampling sites for Aspergillus species. The inset shows the location of Lagos state on the map of Nigeria.
Ethical approval
This study did not involve any human participants or animals and as such was exempt from ethical review.
Sample collection and isolation of fungi
Sampling of the selected areas was conducted from January to March, 2017. Seven different soil samples were collected from each of four sites in each LGA and LCDA, 168 samples in total. Sampling sites were in urban settlements and included soil from roadsides and markets. Samples were sieved through a mesh to remove stones and plant materials. Fungi were isolated following standard procedures as elaborated by Fred and Waksman.23 Briefly, 10 g of soil was added to a test tube containing 90 ml of sterile distilled water and shaken vigorously to obtain a stock solution. Exactly 1 ml of the stock solution was added to a test tube containing 9 ml of sterile distilled water from which a further series of dilutions was prepared up to the fifth dilution (1 in 105). Sterile swab sticks were dipped into the final dilution, streaked on Sabouraud Dextrose Agar (SDA) impreganted with chloramphenicol (0.5 g/l) and incubated at 37°C for 7 days.
Isolates were identified using cultural and microscopic characteristics as outlined by Larone.24
Antifungal susceptibility testing
Isolates were transported to the Mycology Reference Centre, Manchester, United Kingdom, on Sabouraud Dextrose agar slopes. In vitro susceptibility testing of the isolates to itraconazole (ITC) and voriconazole (VRZ) was performed by broth microdilution method, and results interpreted according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) breakpoints and epidemiological cut-off values for species where breakpoints have not been determined. The preparation of inocula, inoculation of microdilution plates, incubation, reading and interpretation of results were as described by Arendrup et al.25 in the EUCAST definitive document E.Def 9.2. A. flavus, A. fumigatus and Aspergillus nidulans isolates with ITC minimum inhibitory concentrations >1 mg/l were considered resistant, while A. niger and A. terreus isolates with minimum inhibitory concentrations greater than the epidemiological cut-off values of 4 mg/l and 0.5 mg/l, respectively were considered resistant. For A. fumigatus and A. nidulans, VRZ minimum inhibitory concentrations (MICs) >1mg/l were considered resistant, while A. flavus, A. niger and A. terreus isolates with epidemiological cut-off values >2 mg/l were considered resistant.
Data analysis
The percentage frequencies of occurrence of the various fungi were calculated for each sample area by the formula A/B × 100, where A = number of plates in which the species appeared, and B = total number of plates incubated for a given sample area. Results were presented as tables.
Literature search
We performed a literature search of published articles on triazole susceptibility in Aspergillus species from Africa between the period of 2007 to July 2021 using PubMed, Google Scholar and African Journals Online (AJOL) databases. The main search comprised individual searches using detailed medical subject heading (MeSH) terms for aspergillosis, aspergillus, and triazoles combined with relevant terms including broad terms such as ‘case report’, ‘environment’, and ‘survey’. The Boolean operators ‘AND’ and ‘OR’ were used to combine and narrow the searches. The references in all relevant papers were reviewed for additional publications that may not have been indexed.
Results
A total of 117 Aspergillus spp. were recovered from the 168 soil samples. The number of species of Aspergillus were: 50 A. niger (42.7%), 37 A. flavus (31.6%), 20 A. terreus (17.1%), five A. fumigatus (4.3%) and five A. nidulans (4.3%). Table 1 shows the diversity of aspergilli isolated in each local government area, as well as their corresponding percentage frequencies. Other saprophytic fungi isolated were Penicillium, Curvularia, Cunninghamella, Mucor and Fusarium species.
Table 1.
Distribution of Aspergillus spp. in Lagos state, Nigeria.
| Sampling area (number of isolates) | Aspergillus species | ||||
|---|---|---|---|---|---|
| Aspergillus niger, n (%) | Aspergillus flavus, n (%) | Aspergillus fumigatus, n (%) | Aspergillus terreus, n (%) | Aspergillus nidulans, n (%) | |
| Yaba-Mainland (24) | 12 (50.0) | 9 (37.5) | 3 (12.5) | 0 (0.0) | 0 (0.0) |
| Mushin (9) | 5 (55.6) | 4 (44.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Surulere (13) | 3 (23.1) | 6 (46.1) | 2 (15.4) | 2 (15.4) | 0 (0.0) |
| Itire-Ikate (32) | 13 (40.6) | 8 (25.0) | 0 (0.0) | 9 (28.1) | 2 (6.3) |
| Alimosho (17) | 7 (41.2) | 4 (23.5) | 0 (0.0) | 4 (23.5) | 2 (11.8) |
| Oshodi-Isolo (22) | 10 (45.4) | 6 (27.3) | 0 (0.0) | 5 (22.7) | 1 (4.5) |
Antifungal susceptibility
All isolates were susceptible to both ITC and VRZ (Table 2) according to EUCAST breakpoints and epidemiological cut-off values.25
Table 2.
In vitro antifungal susceptibility profile of Aspergillus species isolated (n = 117) against triazole antifungal agents.
| Species (number of isolates) | Drug | MIC (mg/l) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1.0 | 2.0 | ||
| Aspergillus niger (50) | ITC | 2 | – | – | – | – | – | – | – | 50 |
| VRZ | 1–2 | – | – | – | – | – | – | 12 | 38 | |
| Aspergillus flavus (37) | ITC | 0.06–0.50 | – | – | 10 | 5 | 10 | 12 | – | – |
| VRZ | 0.25–0.50 | – | – | – | – | 12 | 15 | – | – | |
| Aspergillus terreus (20) | ITC | 0.5 | – | – | – | – | – | 20 | – | – |
| VRZ | 0.5 | – | – | – | – | – | 20 | – | – | |
| Aspergillus fumigatus (5) | ITC | 0.06–0.25 | – | – | 2 | 2 | 1 | – | – | – |
| VRZ | 0.015–0.250 | 1 | 2 | – | 1 | 1 | – | – | – | |
| Aspergillus nidulans (5) | ITC | 0.125–0.500 | – | – | – | 3 | – | 2 | – | – |
| VRZ | 0.125–0.500 | – | – | – | 2 | 2 | 1 | – | – | |
ITC, itraconazole; MIC, Minimum Inhibitory Concentration; VRZ, voriconazole.
Literature review
Our search identified seven articles and a conference abstract pertaining to triazole susceptibility in Aspergillus isolates from Africa. The abstract alluded to a multinational survey including South Africa where no triazole resistance was documented; as details could not be obtained, it was excluded from the analysis. Table 3 shows the summary of the findings.
Table 3.
Studies reporting triazole-resistant Aspergillus fumigatus (TRAF) in Africa till date.
| Country | Source | Prevalence (TRAF/total number of Aspergillus fumigatus) | Resistance mechanisms detected | Reference |
|---|---|---|---|---|
| Tanzania | Environmental | 15/108 (13.9) | TR34/L98H | Chowdhary et al.26 |
| TR46/Y121F/T289A | ||||
| Tanzania | Clinical | 5/5 (100) | TR34/L98H | Mushi et al.27 |
| Kenya | Environmental | 26/97 (26.8) | NI | Kemoi et al.28 |
| Kenya | Environmental, Clinical | 13/48 (27.1) | TR34/L98H | Kemoi et al.29 |
| Tanzania | Environmental | 28/106 (26.4) | TR34/L98H | Sharma et al.30 |
| TR46 | ||||
| G54E | ||||
| Cameroon | Environmental | 0/51 (0) | NA | Ashu et al.31 |
| Burkina Fasso | Environmental | 1/51 (2) | F46Y/M172V/E427K | Yerbanga et al.32 |
| Nigeria | Environmental | 1/46 (2.2) | M172V | Resendiz-Sharpe et al.33 |
| Benin Republic | Environmental | 0/25 (0) | NA | Resendiz-Sharpe et al.33 |
NA, not applicable; NI, not indicated.
Discussion
Aspergillus species which may cause human aspergillosis are ubiquitous in the environment. The present study provides a snapshot of triazole susceptibility in environmental Aspergillus isolates from Nigeria. Resistance was not detected.
Aspergillus fumigatus, which accounted for 4.3% of the isolates, was absent from majority of soil samples. This contrasts sharply with most other series from Nigeria where A. fumigatus predominates, typically comprising slightly over half of isolates: 52.4% of isolates in a northern Nigerian survey; 51.9% in eastern Nigeria and 57.1% of clinical and environmental isolates in a study conducted in the southwest.34–36 Similarly, A. fumigatus accounted for 55.4% of soil isolates in an environmental survey conducted in Kenya.28 The absence of A. fumigatus from many of the soil samples in Lagos may be related to the time of year when the study was conducted (January to February) as this was the dry season. This finding warrants further investigation.
Intrinsic resistance to triazoles in Aspergillus is essentially restricted to non-fumigatus species. However, triazole resistance was not observed despite most isolates in the present study being non-fumigatus. Likewise, surveys in Kuwait and Iran did not report VRZ resistance in both clinical and environmental isolates of A. flavus.37,38 Secondary or acquired resistance is increasingly being observed in azole-naïve patients who acquire triazole-resistant A. fumigatus (TRAF) from the environment. Environmental TRAF has been linked to azole fungicide use for crop protection and material preservation. The hypothesis is strengthened by reports of clinically resistant strains demonstrating cross-resistance to common azole fungicides such as difeniconazole and tebuconazole.26,39 Acquired antifungal resistance is also possible in non-fumigatus species but few studies have researched triazole resistance in environmental isolates of Aspergillus species other than fumigatus and the characteristic mutations responsible have not been described in these species.2 Environmentally acquired TRAF has now been observed in many European countries.9,14–16 In Asia, prevalence rates of 7%, 7.5%, and 10.1% have been reported from India, Taiwan and Thailand respectively.18 Triazole susceptibility data from Latin America is limited, but a prevalence of 9.3% was found in Colombia, reportedly the fourth largest consumer of pesticides, of which 30% are azole fungicides.40
In Africa, Aspergillus triazole susceptibility data are generally lacking. Our review of the literature identified studies conducted in Tanzania, Kenya, Cameroon, Burkina Fasso, Benin Republic and Nigeria (Table 1) with the first evidence of TRAF originating from Tanzania.26 The prevalence of triazole resistance ranges from 0% to 27%, being absent in Cameroon and highest in Kenya.29,31 Clinical isolates were few, most studies reporting on environmental isolates. The relative scarcity of clinical data may be attributed to non-availability of mycological expertise and mycology reference laboratories, failure to routinely perform antifungal susceptibility, failure to store isolates and the generally low positivity rate of cultures.4,41 Molecular studies identified the tandem repeat, TR34/L98H as the predominant mutation but TR46/Y121F/T289A was also detected in Tanzania. In addition, G54E mutation was discovered in environmental isolates from a multi-national study which included Tanzania, Romania and India,30 while more recently, F46Y/M172V/E427K and M172V were found in Burkina Fasso and Nigeria, respectively.32,33 The isolation of TRAF bearing the TR34/L98H mutation from a handful of clinical specimens from Tanzania and Kenya, both in the eastern part of Africa, is ominous of ongoing acquisition from the environment in that region.27 A recent study conducted by Resendiz-Sharpe and colleagues which involved environmental samples from Nigeria revealed the presence of a single isolate of TRAF in Lagos Island which was not among the areas sampled in our study.
With respect to possible origin, Chowdhary et al.26 demonstrated a genetic relatedness of resistant genotypes from Tanzania to isolates from other parts of the world. Specifically, Tanzanian TR46/Y121F/T289A strains had a single genotype identical to Dutch isolates and the TR34/L98H isolates were identical to the Indian TR34/L98H genotype. These similarities in molecular epidemiology suggest the possibility of migration of isolates harbouring resistance traits. Furthermore, in Kenya, Kemoi et al.28 reported that although the prevalence of TRAF was higher in fungicide-experienced soil, TRAF was still present in the naïve soil. This may imply local spread of TRAF from areas of fungicide use to places where they are not used. Indeed, the capability of A. fumigatus to sporulate abundantly and survive in almost any environment facilitates dispersal across distances, long or short.
While there were no TRAF amongst A. fumigatus isolates from Cameroon, higher MICs were found in the sub-population from a particular location, Eloundem, where genetic studies provided evidence of frequent recombination arising from sexual reproduction.31 Increased genetic variation by means of sexual recombination has been demonstrated to promote adaptation to hostile environments in fungi and the authors alluded that the frequent sexual reproduction was a result of selective pressure, probably from azole fungicide use. We postulate that it is only a matter of time before TRAF emerges in that location. The foregoing provides possible hypotheses as to how TRAF may emerge in new locations: by migration from nearby countries; de novo emergence in areas of azole fungicide use as in Cameroon; or, possibly, a combination of both.
An obvious concern from reviewing the literature is that studies have been highly variable in the methods of sampling, selection of sampling sites and means of determining susceptibility. A standardised protocol will allow more meaningful inferences and comparisons to be made. This can be facilitated by collaborations with the Aspergillus Resistance Surveillance Working Group constituted by the International Society for Human and Animal Mycology and the European Confederation for Medical Mycology.10 A major limitation of our survey is that we did not target areas such as vegetable farms and horticultural gardens, where azole fungicides may have been used. Second, triazole resistance has been documented and studied more in A. fumigatus, and only five were isolated in this study. Thus, the data need cautious interpretation. Triazole resistance in environmental isolates is variable. Thus, another shortcoming is that the findings and deductions may not apply to other locations in Nigeria. Surveillance data from more regions in the country are needed to provide a holistic picture. Finally, due to lack of funding, genotypic identification of the isolates could not be done using the β-tubulin (bar-coding) gene. Classification based on colonial and microscopic morphology alone is prone to misidentification of cryptic species which tend to have higher minimum inhibitory concentrations. However, the susceptibility profiles detected do not suggest that any of such cryptic species were isolated.
The limitations notwithstanding, the environmental distribution of isolates established in this study is medically relevant because it may predict clinical isolates expected from patients with aspergillosis in the locale.42 The susceptibility data will be useful in drawing up local guidelines for treatment of IA based on the recommendations of an international expert panel which advised VRZ monotherapy as initial empiric treatment of IA in regions with minimal or no azole resistance.43 The report also raises awareness on a vital public health issue. Due to the pervading lack of mycological diagnostic facilities and dearth of research into fungal infections, mycology is a neglected discipline in many African countries. As such, antifungal resistance has not been addressed as part of the current global antimicrobial resistance crisis. The Nigerian Centre for Disease Control (NCDC), in 2017, undertook a situation analysis and developed a national action plan for antimicrobial resistance in Nigeria; no mention was made of environmentally mediated triazole resistance, which is becoming topical worldwide.44 As agriculture accounts for close to 30% of Nigeria’s gross domestic product, attention must be paid to fungicide use, the possible emergence of TRAF in the environment and subsequently in clinical isolates. The one health model which monitors antimicrobial use and resistance in farm animals should incorporate monitoring fungicide use and surveillance for resistance in environmental fungi.
In conclusion, A. niger and A. flavus are the predominant Aspergillus species in the studied locations of Lagos, Nigeria. There was no environmental triazole resistance in isolated species, implying that de novo resistance is unlikely to be an issue in managing naïve patients across the aspergillosis spectrum. However, the documented presence of TRAF in another study from Nigeria and other parts of Africa at large demands vigilance. This can be achieved by monitoring the volume and pattern of fungicide consumption in the country, followed by continuous surveillance to track azole resistance in both clinical and environmental isolates if and when it emerges. These measures are imperative to preserve the usefulness of triazoles, currently the only readily available drugs for the treatment of aspergillosis in Nigeria.
Acknowledgments
We acknowledge the assistance rendered by the staff of the Mycology Reference Centre, Manchester, in carrying out antifungal susceptibility testing.
We thank Ronald Olum for assisting with the maps of Nigeria and Lagos state.
Footnotes
Authors’ contributions: Cynthia Abosede Campbell: conception, design, data analysis, data interpretation, writing of original draft.
Iriagbonse Iyabo Osaigbovo: data analysis, writing of original draft, revision and editing for intellectual content.
Rita Okeoghene Oladele: conception, data analysis, supervision, revision and editing for intellectual content.
All authors have read and agreed to the submitted version of the manuscript.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Iriagbonse Iyabo Osaigbovo  https://orcid.org/0000-0001-8111-0743
https://orcid.org/0000-0001-8111-0743
Contributor Information
Cynthia Abosede Campbell, Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, Lagos, Nigeria.
Iriagbonse Iyabo Osaigbovo, Department of Medical Microbiology, School of Medicine, College of Medical Sciences, University of Benin, Benin City, Nigeria; Department of Medical Microbiology, University of Benin Teaching Hospital, Benin City, Nigeria.
Rita Okeoghene Oladele, Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, Lagos, Nigeria; Department of Medical Microbiology and Parasitology, Lagos University Teaching Hospital, Idi-Araba, Lagos, Nigeria.
References
- 1.European Centre for Disease Prevention and Control. Risk assessment on the impact of environmental usage of triazoles on the development and spread of resistance to medical triazoles in Aspergillus species. ECDC: Stockholm, 2013. [Google Scholar]
- 2.Hagiwara D, Watanabe A, Kamei K, et al. Epidemiological and genomic landscape of azole resistance mechanisms in Aspergillus fungi. Front Microbiol 2016; 7: 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwaghe AV, Umeokonkwo CD, Aworh MK.Evaluation of the national tuberculosis surveillance and response systems, 2018 to 2019: National Tuberculosis, Leprosy Buruli Ulcer Control Programme, Abuja, Nigeria. Pan Afr Med J 2020; 35: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oladele RO, Irurhe NK, Foden P, et al. Chronic pulmonary aspergillosis as a cause of smear-negative TB and/or TB treatment failure in Nigerians. Int J Tuberc Lung Dis 2017; 21: 1056–1061. [DOI] [PubMed] [Google Scholar]
- 5.Nwannadi IA, Alao OO, Bazuaye GN, et al. The epidemiology of haematological malignancies at the University of Benin Teaching Hospital: a ten-year retrospective study. Internet J Epidemiol 2010; 9: 1–6. [Google Scholar]
- 6.Bazuaye N, Nwogoh B, Ikponmwen D, et al. First successful allogeneic hematopoietic stem cell transplantation for a sickle cell disease patient in a low resource country (Nigeria): a case report. Ann Transplant 2014; 19: 210–213. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: U.S. Department of Health and Human Services, https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (2019, accessed 14 July 2021). [Google Scholar]
- 8.Van der Linden JWM, Snelders E, Kampinga GA, et al. Clinical implications of azole resistance in Aspergillus fumigatus, the Netherlands, 2007-2009. Emerg Infect Dis 2011; 17: 1846–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kneale M, Bartholomew JS, Davies E, et al. Global access to antifungal therapy and its variable cost. J Antimicrob Chemoter 2016; 71: 3599–3606. [DOI] [PubMed] [Google Scholar]
- 10.Sharpe AR, Lagrou K, Meis JF, et al. Triazole resistance surveillance in Aspergillus fumigatus. Med Mycol 2018; 56: S83–S92. [DOI] [PubMed] [Google Scholar]
- 11.Verweij PE, Snelders E, Kema GH, et al. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis 2009; 9: 789–795. [DOI] [PubMed] [Google Scholar]
- 12.Snelders E, Huis In’t Veld RA, Rijs AJ, et al. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 2009; 75: 4053–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chowdhary A, Kathuria S, Xu J, et al. Clonal expansion and emergence of environmental multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR34/L98H mutations in the cyp51A gene in India. PLoS One 2012; 7: e52871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mortensen KL, Mellado E, Lass-Florl C, et al. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark and Spain. Antimicrob Agents Chemother 2010; 54: 4545–4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prigitano A, Venier V, Cogliati M, et al. Azole-resistant Aspergillus fumigatus in the environment of Northern Italy, May 2011 to June 2012. Euro Surveill 2014; 19: 20747. [DOI] [PubMed] [Google Scholar]
- 16.Bader O, Tunnermann J, Dudakova A, et al. Environmental isolates of azole-resistant Aspergillus fumigatus in Germany. Antimicrob Agents Chemother 2015; 59: 4356–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chowdhary A, Sharma C, Kathuria S, et al. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J Antimicrob Chemother 2014; 69: 555–557. [DOI] [PubMed] [Google Scholar]
- 18.Tangwattanachuleeporn M, Minarin N, Saichan S, et al. Prevalence of azole-resistant Aspergillus fumigatus in the environment of Thailand. Med Mycol 2017; 55: 429–435. [DOI] [PubMed] [Google Scholar]
- 19.Badali H, Vaezi A, Haghani I, et al. Environmental study of azole-resistant Aspergillus fumigatus with TR34/L98H mutations in the cyp51A gene in Iran. Mycoses 2013; 56: 659–663. [DOI] [PubMed] [Google Scholar]
- 20.Ahmad S, Khan Z, Hagen F, et al. Occurrence of triazole-resistant Aspergillus fumigatus with TR34/L98H mutations in outdoor and hospital environment in Kuwait. Environ Res 2014; 133: 20–26. [DOI] [PubMed] [Google Scholar]
- 21.National Bureau of Statistics. Nigerian gross domestic product report. Quarter three. https://nigerianstat.gov.ng/elibrary (2019, accessed 10 May 2019).
- 22.About Lagos. https://lagosstate.gov.ng/about-lagos/ (2017, accessed 20 June 2019).
- 23.Fred EB, Waksman SA. Laboratory manual of general microbiology, with special reference to the micro-organisms of the soil. New York: McGraw-Hill Book Company, 1928, p.137. [Google Scholar]
- 24.Larone D.Medically important fungi: a guide to identification. 4th ed. Washington, DC: ASM Press, 2000. [Google Scholar]
- 25.Arendrup M, Hope W, Howard S. EUCAST definitive document E.Def 9.2 method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia forming moulds. EUCAST, http://eucast.org/astoffungi/previous_versions_of_documents/ (2014, accessed 5 May 2019).
- 26.Chowdhary A, Sharma C, Van den Boom M, et al. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother 2014; 69: 2979–2983. [DOI] [PubMed] [Google Scholar]
- 27.Mushi MF, Buname G, Bader O, et al. Aspergillus fumigates carrying TR34/L98H resistance allele causing complicated suppurative otitis media in Tanzania: call for improved diagnosis of fungi in sub-Saharan Africa. BMC Infect Dis 2016; 16: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kemoi EK, Nyerere A, Gross U, et al. Diversity of azole resistant Aspergillus species isolated from experienced and naïve soils in Nairobi county and Naivasha sub-county Kenya. Europ Sci J 2017; 13: 301–311. [Google Scholar]
- 29.Kemoi EK, Nyerere A, Bii CC.Triazole resistant Aspergillus fumigatus from fungicide-experienced soils in Naivasha subcounty and Nairobi county, Kenya. Int Journ Microbiol 2018; 2018: 7147938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma C, Hagen F, Moroti R, et al. Triazole resistant Aspergillus fumigatus harbouring G54 mutation: is it de novo or environmentally acquired? J Glob Antimicrob Resist 2015; 3: 69–74. [DOI] [PubMed] [Google Scholar]
- 31.Ashu EE, Korfanty GA, Jianping X.Evidence of unique genetic diversity in Aspergillus fumigatus isolates from Cameroon. Mycoses 2017; 60: 739–748. [DOI] [PubMed] [Google Scholar]
- 32.Yerbanga IW, Resendiz-Sharpe A, Bamba S, et al. First investigative study of azole-resistant Aspergillus fumigatus in the environment in Burkina Faso. Int J Environ Res Public Health 2021; 18: 2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Resendiz-Sharpe A, Dewaele K, Merckx R, et al. Triazole-resistance in environmental Aspergillus fumigatus in Latin American and African Countries. J Fungi (Basel) 2021; 7: 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwanashie CN, Kazeem HM, Abdu PA, et al. Distribution of Aspergillus species among apparently healthy birds in poultry farms in Kaduna state, Nigeria. Sci J Microbiol 2013; 2: 61–64. [Google Scholar]
- 35.Osoagbaka OU.Aspergillus and Candida species isolated from the sputa of patients with bronchopulmonary disorders in Nigeria. Mycoses 1981; 24: 547–551. [DOI] [PubMed] [Google Scholar]
- 36.Shittu OB, Adelaja OM, Obuotor TM, et al. PCR-internal transcribed spacer (ITS) gene sequencing and phyogenetic analysis of clinical and environmental Aspergillus species associated with HIV-TB coinfected patients in a hospital in Abeokuta, southwestern Nigeria. Afr Health Sci 2016; 16: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Wathiqi F, Ahmad S, Khan Z.Molecular identification and antifungal susceptibility profile of Aspergillus flavus isolates recovered from clinical specimens in Kuwait. BMC Infect Dis 2013; 13: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taghizadeh-Armaki M, Hedayati MT, Ansari S, et al. Genetic diversity and in vitro antifungal susceptibility of 200 clinical and environmental Aspergillus flavus isolates. Antimicrob Agents Chemother 2017; 61: e00004–e00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snelders E, Camps SMT, Karawajczyk A, et al. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 2012; e7: e31801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Pape P, Lavergne R, Morio F, et al. Multiple fungicide-driven alterations in azole-resistant Aspergillus fumigatus, Colombia, 2015. Emerg Infect Dis 2016; 22: 156–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oladele RO, Akase IE, Fahal A, et al. Bridging the knowledge gap on mycoses in Africa: setting up a pan-African Mycology Working Group. Mycoses 2020; 63: 244–249. [DOI] [PubMed] [Google Scholar]
- 42.Pasqualotto AC.Differences in pathogenicity and clinical syndromes due to Aspergillus fumigatus and Aspergillus flavus. Med Mycol 2009; 47(Suppl. 1): S261–S270. [DOI] [PubMed] [Google Scholar]
- 43.Verweij PE, Ananda-Rajah M, Andes D, et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist Updat 2015; 21: 30–40. [DOI] [PubMed] [Google Scholar]
- 44.Egwuenu A, Obasanya J, Okeke I, et al. Antimicrobial use and resistance in Nigeria: situation analysis and recommendations, 2017. Pan Afr Med J Conf Proc 2018; 8: 2. [Google Scholar]