Abstract
Robust surveillance testing is a key strategic plan to prevent COVID-19 outbreaks and slow the spread of the SARS-CoV-2 pandemic; however, limited resources, facilities and time often impair the implementation of a widespread surveillance effort. To mitigate these resource limitations, we employed a strategy of pooling samples, reducing reagent cost and processing time. Through utilizing academic faculty and labs, successful pooled surveillance testing was conducted throughout Fall 2020 semester to detect positive SARS-CoV-2 infections in a population of 4400 students. During the semester, over 25,000 individual COVID status evaluations were made by pooling eight individual samples into one quantitative reverse transcription polymerase chain reaction. This pooled surveillance strategy was highly effective at detecting infection and significantly reduced financial burden and cost by $3.6 million.
Keywords: : COVID-19, pandemic policy, pooled surveillance, qRT-PCR diagnostics
Diagnostic testing for SARS-CoV-2, the virus responsible for COVID-19, has been critical for early identification of infected individuals and implementation of subsequent isolation protocols in attempts to prevent further transmission and spread of the virus around the globe. Test kits and protocols were developed in early 2020 with the Center for Disease Control (CDC) releasing an emergency use authorization (EUA) primer/probe kit for quantitative reverse transcription polymerase chain reaction (qRT-PCR) to detect SARS-CoV-2 in early February 2020 [1,2]. With the expanding pandemic, research groups and companies raced to find better, more accurate and more sensitive diagnostics to detect SARS-CoV-2 as efficiently as possible [3]. qRT-PCR became the gold standard to accurately determine an individual’s SARS-CoV-2 infection status, but other methods have since been developed including isothermal nucleic acid amplification assays, CRISPR-based methods, antibody/serological tests and direct antigen tests with varying levels of sensitivity, accuracy, costs and time until results [4]. Studies show there is a direct correlation between the amount of testing and the additional years of life saved, with daily testing being optimal [5]. While daily testing is infeasible for most institutions, the bottom line is that the more testing that is done; the more people will stay healthy and not become infected with SARS-CoV-2.
Despite the obvious benefit of robust COVID-19 testing and surveillance, the costs, facilities and labor to collect, run and report the results are a limiting factor that has inhibited more widespread testing efforts. The US Air Force Academy (USAFA) is an institution of higher education granting Bachelors of Science degrees, but also has the commission from the Department of Defense to teach and train future officers for the US Air Force. The military training aspects of this institution required the students to be in-person for Fall 2020 courses. In order to facilitate in-person learning during a global pandemic, a robust testing plan was developed to ensure the safety of students and faculty. This plan included a dynamic mathematical equation to model the minimum amount of testing required each week to prevent COVID-19 outbreaks from occurring [6]. However, the levels of testing required severely strained the resources of our medical facilities. Our solution was to expand COVID-19 testing facilities to include academic scientists and labs already equipped to perform the diagnostics, working alongside medical professionals. If a similar strategy is employed across the US an additional 1.2–3.5 million tests could be processed each day [7].
Pooling samples for diagnostic testing has been proposed as an effective way to save resources and increase testing throughput, without significant loss to sensitivity and detection [8–10]. When disease prevalence is low, multiple individual samples can be combined into a single pool and tested simultaneously using one reaction [11–13]. Any positive pools can then be subjected to another round of testing to identify the infected individuals in the pool [14]. Despite the benefit of pools to conserve resources, there are concerns about pool size, sensitivity and accuracy of the test due to the inherent dilution that occurs from pooling samples together [15–18]. We report that after 6 months of pooled surveillance testing, our protocols successfully identified infected individuals while saving significant time, reagents and labor-hours compared with running individual tests.
Materials & methods
Sample collection
Medical professionals and staff used nasopharyngeal swabs to collect a sample from the student’s nasopharynx. Swabs were stored in viral transport media and stored at 4°C until further processing. Tubes were labeled with patient name and pool identifier. Samples were collected every morning from 6–7 am and processed the same day.
Pool formation
Cadets were assigned a pool number based on probable daily close contacts that may impact their exposure. To reduce the number of positive pools overall, likely close contacts, such as roommates, were placed in the same pool. Only a subset of students were pooled and tested daily; however, each day within a grouping of close contacts different individuals were selected for testing to increase the likelihood we would detect disease spread within that group. Once individual samples were collected, they were transported to the academic lab and 140 ul of each individual sample was taken and combined into one pool, with a maximum pool size of eight. Pool size was determined based on previous published studies that identified eight being optimal for saving resources without losing sensitivity [9,12,15,16]. Pooling of close contacts may not be feasible in other academic institutions, but the author’s institution has mandatory living arrangements that enabled highly efficient and intentional pool formation that saved time and resources.
RNA extraction
Pooled samples were subjected to RNA extraction using Qiagen QIAAMP Viral RNA Mini Kit (Cat # 52906) according to manufacturer’s protocol, with the exception of increasing the lysis buffer and ethanol to 1.2 ml volume. A vacuum manifold was used to increase efficiency.
qRT-PCR test
The INBIOS Smart Detect SARS-CoV-2-rRT-PCR Kit (COV-2-E) has US FDA EUA approval and was used for all qRT-PCR testing following the EUA guidelines and protocols.
Labor & cost calculations
Expenses and labor hours associated with COVID-19 testing in the academic labs were tracked for the entirety of Fall 2020 semester. Described cost comparisons of commercial individual tests are based on quotes acquired from companies bidding to do COVID-19 PCR testing for USAFA.
Data & statistical analysis
Data were collected and managed in microsoft excel spreadsheets. Changes in costs, labor burden and other metrics were represented in cost/time savings. Statistical analysis was not performed other than showing the total amount of money and time that would be saved with the described methods.
Ethics
Institutional Review Board (IRB) determination on the surveillance testing and mathematical modeling (FAC20200024N and FAC20200025N, respectively) were deemed Not Human Subjects Research in accordance with 32 CR 219, DoDI3216.02 and AFI 40-402.
Results
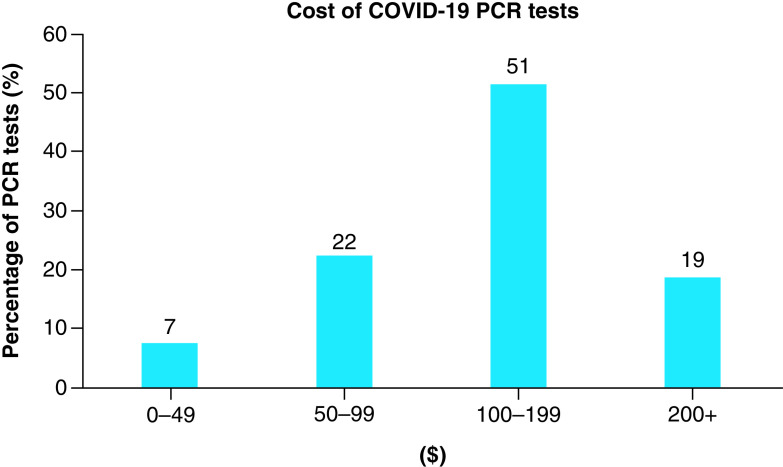
COVID-19 diagnostic tests can be very expensive [19]. From a recent survey of the largest hospitals across the US, COVID-19 diagnostics cost between $20–850 per test. Approximately 70% of COVID-19 PCR tests cost over $100, with the mean cost being around $150 (Figure 1) [20–22]. To implement an ongoing and widespread surveillance program that is robust enough to detect infection and prevent outbreaks, significant testing is required and its associated costs may be prohibitive. For example, testing a population of 5000 individuals at a rate of only 10% tested each week results in a monthly cost of $300,000 using the mean price of a test.
Figure 1. . Summary of costs of COVID-19 PCR diagnostics tests.
Data from 102 different hospitals across the US.
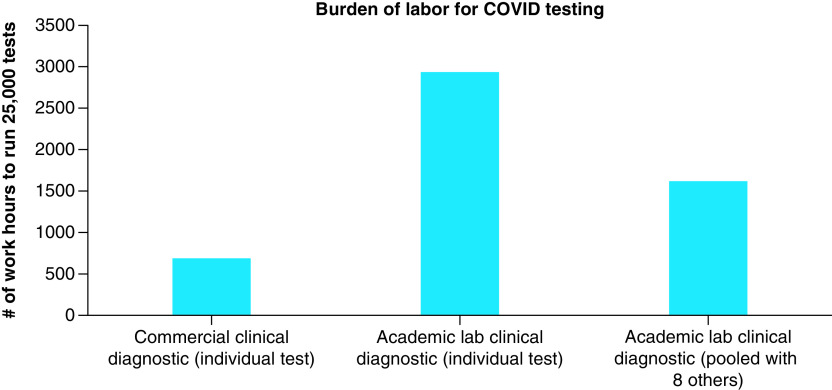
The USAFA began testing nearly 25% of the cadet population every week and over the course of the Fall 2020 semester, tested over 25,000 individuals for COVID surveillance. Using a typical commercial COVID-19 diagnostic test that costs $150 would have resulted in a $3,750,000 price tag. However, to implement a more cost effective approach, the USAFA utilized the expertise of its science faculty and medical professionals to do all testing internally. Using qRT-PCR testing protocols under an FDA EUA, and in-house academic labs and labor, COVID-19 diagnostics were run at $21.76 per sample. Using this approach saved USAFA $128.24 per sample compared with using an external commercial lab; however, processing that quantity of tests requires a significant amount of time to execute the clinical collection, labeling, processing, reaction setup, analyzing results and reporting. Based on logistics and planning, we estimated that running 25,000 individual tests would take nearly 700 h of labor to collect, process and send samples for commercial clinical testing. In contrast, to perform the same testing internally using academic labs, scientists would require nearly 3000 labor h for the same 25,000 samples (Figure 2).
Figure 2. . Labor requirement for different testing mechanisms.
We sought to further increase the efficiency to accommodate limited faculty and staff time. Collected nasal samples were pooled into groups of eight individuals as described in the methods section, then each pool was processed for RNA extraction and qRT-PCR. By pooling the samples, the number of tests to run was cut by eight and brought the total labor burden down to about 1600 h (reduced labor burden by 50%). It may seem that the burden would be reduced by a factor of eight, but despite the efficiency of pooling, every sample still needs to be labeled and processed into pools before the pooled testing can begin. More strikingly, with the shared resources of pooled testing, the cost of COVID-19 surveillance dropped to only $5.94 per sample (a 73% cost savings) and the time required was manageable. Table 1 summarizes the costs, labor and savings from doing pooled surveillance testing with academic labs for 25,000 diagnoses.
Table 1. . Labor and financial costs of COVID-19 testing.
| Costs |
||
|---|---|---|
| Type of test | Cost/test | Cost/25,000 tests |
| Commercial clinical diagnostic (individual test) | $150.00 | $3,750,000.00 |
| Academic lab clinical diagnostic (individual test) | $21.76 | $544,043.00 |
| Academic lab clinical diagnostic (pooled with eight others) | $5.94 | $148,551.50 |
| Academic lab savings | $3,205,957.00 | |
| Pooled savings: | $395,491.50 | |
| Total savings: | $3,601,448.50 |
|
Labor |
||
|---|---|---|
| Type of test | Labor h/25,000 tests | |
| Commercial clinical diagnostic (individual test) | 687.5 | |
| Academic lab clinical diagnostic (individual test) | 2937.5 | |
| Academic lab clinical diagnostic (pooled with eight others) | 1625.0 | |
Discussion
Pooled sample testing has been reported by many groups to be successful [23–25]. There are a variety of different pooling protocols including different number of samples to be included in each pool and various techniques to improve sensitivity in a pooled sample [26,27]. We report that pools of eight individual samples resulted in highly effective and efficient detection of SARS-CoV-2. Implementation of routine, random, pooled surveillance testing at the USAFA enabled in-person learning during Fall 2020 semester and prevented COVID-19 outbreaks on campus. Despite the ongoing pandemic and occasional infected student, individuals that were positive for SARS-CoV-2 were quickly identified and removed from the population through aggressive quarantine and isolation protocols. Very few false positives were detected through this pooled surveillance strategy, with nearly every positive pool correctly identifying an infected individual in that pool with follow-up individual clinical tests. This robust testing program was highly effective and became efficient through the use of academic labs and faculty helping with the testing burden. Additionally, pooling eight individual samples into one PCR reaction saved significant time, supplies and resources; resulting in $3.6 million in savings over the course of the whole semester and 25,000 individual tests.
Future perspective
Pooled sample testing has been shown to be effective and efficient at detecting pathogens in clinical samples. The authors believe that pooled testing may become the standard protocol for any medical emergency or infectious disease outbreak. Additionally, pooled testing may be a valid technique used before sporting events, large gatherings or departing on cruise or other travel arrangements in order to quickly and efficiently screen and check for certain pathogens in a given population.
Executive summary.
Background
Testing is the best way to detect and prevent the spread of COVID-19.
Limitation to testing is access to tests, time and resources.
Materials & methods
Samples collected from US Air Force Academy cadets.
Eight individual samples pooled together into one sample for further processing/analysis.
Testing performed by academic scientists in laboratory setting.
Results
Testing is expensive.
Academic testing requires significant amount of work, but pooling does increase efficiency.
Accurate detection of positive COVID cases while saving significant amounts of money and time.
Discussion
Pooled testing saved significant time and resources.
Testing detected positive cases and prevented serious outbreaks.
Acknowledgments
The 10th Medical Group collected the samples and managed the quarantine and isolation plans. Additionally, members of the Pandemic Math Team provided inputs and model refinements.
Footnotes
Disclaimer
The views expressed in this article are those of the author and do not reflect the official policy or position of the United States Air Force, Department of Defense or the US Government.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval. IRB determination on the surveillance testing and mathematical modeling (FAC20200024N and FAC20200025N, respectively) were deemed Not Human Subjects Research in accordance with 32 CR 219, DoDI3216.02 and AFI 40-402.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
- 1.CDC. Testing for COVID-19 (2020). https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html
- 2.CDC. CDC diagnostic tests for COVID-19 diagnostic testing 2019–2021 (2020). https://www.cdc.gov/coronavirus/2019-ncov/lab/testing.html
- 3.Vandenberg O, Martiny D, Rochas O, van Belkum A, Kozlakidis Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 19(3), 171–183 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter LJ, Garner LV, Smoot JWet al. Assay techniques and test development for COVID-19 diagnosis. ACS Cent. Sci. 6(5), 591–605 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Z, Pandey A, Bai Yet al. Comparative cost–effectiveness of SARS-CoV-2 testing strategies in the USA: a modelling study. Lancet Public Heal. 6(3), e184–e191 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cullenbine C, Rohrer J, Almand Eet al. Fizzle testing: an equation utilizing random surveillance to help reduce COVID-19 risks. Math. Comput. Appl. 26(1), 16 (2021). [Google Scholar]
- 7.Steel JJ, Sitko JC, Adkins MG, Hasstedt SC, Rohrer JW, Almand EA. Empowering academic labs and scientists to test for COVID-19. BioTechniques 69(4), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brault V, Mallein B, Rupprecht JF. Group testing as a strategy for the epidemiologic monitoring of COVID-19. arXiv (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yelin I, Aharony N, Tamar ESet al. Evaluation of COVID-19 RT-qPCR test in multi sample pools. Clin. Infect. Dis. 71(16), 2073–2078 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conger K. Testing pooled samples for COVID-19 helps Stanford researchers track early viral spread in Bay Area | News Center | Stanford Medicine. Stanford Med. (2020). http://med.stanford.edu/news/all-news/2020/04/testing-pooled-samples-to-track-early-spread-of-virus.html [Google Scholar]
- 11.Gupta E, Padhi A, Khodare Aet al. Pooled RNA sample reverse transcriptase real time PCR assay for SARS CoV-2 infection: a reliable, faster and economical method. PLoS ONE 15(7), e0236859 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohse S, Pfuhl T, Berkó-Göttel Bet al. Pooling of samples for testing for SARS-CoV-2 in asymptomatic people. Lancet Infect. 20(11), P1231–1232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perchetti GA, Sullivan KW, Pepper Get al. Pooling of SARS-CoV-2 samples to increase molecular testing throughput. J. Clin. Virol. 131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallapaty S. The mathematical strategy that could transform coronavirus testing. Nature 583(7817), 504–505 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Abid S, Ferjani S, El Moussi Aet al. Assessment of sample pooling for SARS-CoV-2 molecular testing for screening of asymptomatic persons in Tunisia. Diagn. Microbiol. Infect. Dis. 98(3), (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Ami R, Klochendler A, Seidel Met al. Large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin. Microbiol. Infect. 26(9), 1248 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khodare A, Padhi A, Gupta E, Agarwal R, Dubey S, Sarin S. Optimal size of sample pooling for RNA pool testing: an avant-garde for scaling up severe acute respiratory syndrome coronavirus-2 testing. Indian J. Med. Microbiol. 38(1), 18–23 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brynildsrud O. COVID-19 prevalence estimation by random sampling in population – optimal sample pooling under varying assumptions about true prevalence. BMC Med. Res. Methodol. 20(1), (2020). https://pubmed.ncbi.nlm.nih.gov/32703158/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neilan AM, Losina E, Bangs ACet al. Clinical impact, costs, and cost–effectiveness of expanded SARS-CoV-2 testing in Massachusetts medRxiv (2020). /pmc/articles/PMC7386528/ [DOI] [PMC free article] [PubMed]
- 20.Centers for Medicare and Medicaid Services. Medicare administrative contractor (MAC) COVID-19 test pricing May 19, 2020. https://www.cms.gov/files/document/mac-covid-19-test-pricing.pdf
- 21.Kliff S. Most coronavirus tests cost about $100. Why did one cost $2,315? – The New York Times. https://www.nytimes.com/2020/06/16/upshot/coronavirus-test-cost-varies-widely.html
- 22.Kurani N, Pollitz K, Cotliar D, Shanosky N, Cox C. COVID-19 test prices and payment policy. Peterson-KFF Heal. Syst. Tracker (2020). [Google Scholar]
- 23.Mahmoud SA, Ibrahim E, Thakre Bet al. Evaluation of pooling of samples for testing SARS-CoV-2 for mass screening of COVID-19. BMC Infect. Dis. 21(1), 1–9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Praharaj I, Jain A, Singh Met al. Pooled testing for COVID-19 diagnosis by real-time RT-PCR: a multi-site comparative evaluation of 5- & 10-sample pooling. Indian J. Med. Res. 152(1), 88–94 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garg A, Ghoshal U, Patel SSet al. Evaluation of seven commercial RT-PCR kits for COVID-19 testing in pooled clinical specimens. J. Med. Virol. 93(4), 2281–2286 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christoff AP, Cruz GNF, Sereia AFRet al. Swab pooling: a new method for large-scale RT-qPCR screening of SARS-CoV-2 avoiding sample dilution. PLoS ONE 16(2 February), e0246544 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am. J. Clin. Pathol. 153(6), 715–718 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]