Abstract
Backgrounds
Alzheimer’s disease is the most common cause of dementia, and currently, there is no disease-modifying treatment. Favorable functional outcomes and reduction of amyloid levels were observed following transplantation of mesenchymal stem cells (MSCs) in animal studies.
Objectives
We conducted a phase I clinical trial in nine patients with mild-to-moderate Alzheimer’s disease dementia to evaluate the safety and dose-limiting toxicity of three repeated intracerebroventricular injections of human umbilical cord blood–derived MSCs (hUCB-MSCs).
Methods
We recruited nine mild-to-moderate Alzheimer’s disease dementia patients from Samsung Medical Center, Seoul, Republic of Korea. Four weeks prior to MSC administration, the Ommaya reservoir was implanted into the right lateral ventricle of the patients. Three patients received a low dose (1.0 × 107 cells/2 mL), and six patients received a high dose (3.0 × 107 cells/2 mL) of hUCB-MSCs. Three repeated injections of MSCs were performed (4-week intervals) in all nine patients. These patients were followed up to 12 weeks after the first hUCB-MSC injection and an additional 36 months in the extended observation study.
Results
After hUCB-MSC injection, the most common adverse event was fever (n = 9) followed by headache (n = 7), nausea (n = 5), and vomiting (n = 4), which all subsided within 36 h. There were three serious adverse events in two participants that were considered to have arisen from the investigational product. Fever in a low dose participant and nausea with vomiting in another low dose participant each required extended hospitalization by a day. There were no dose-limiting toxicities. Five participants completed the 36-month extended observation study, and no further serious adverse events were observed.
Conclusions
Three repeated administrations of hUCB-MSCs into the lateral ventricle via an Ommaya reservoir were feasible, relatively and sufficiently safe, and well-tolerated. Currently, we are undergoing an extended follow-up study for those who participated in a phase IIa trial where upon completion, we hope to gain a deeper understanding of the clinical efficacy of MSC AD therapy.
Trial registration
ClinicalTrials.gov NCT02054208. Registered on 4 February 2014. ClinicalTrials.gov NCT03172117. Registered on 1 June 2017
Supplementary Information
The online version contains supplementary material available at 10.1186/s13195-021-00897-2.
Keywords: Alzheimer’s disease, Mesenchymal stem cell, Intracerebroventricular injection, Phase I/IIa
Introduction
Alzheimer’s disease (AD), an age-related neurodegenerative disorder, is the most common cause of dementia. Currently, disease-modifying therapies that can change the course of the disease are not yet available. Numerous clinical trials that targeted single patho-mechanisms such as amyloid-beta (Aβ) or tau, which are core pathological features of AD, fell short of robust data to demonstrate the clinical efficacy of single-target therapy. Since AD has multiple patho-mechanisms, multi- instead of single-target therapies may be more effective in shifting the clinical course of the disease.
Mesenchymal stem cells (MSCs) are a promising candidate to treat neurodegenerative disorders including AD [1–5]. When MSCs are injected into the brains of AD mouse models, rather than differentiating into neurons and glial cells, MSCs secrete various cytotrophic factors that can tackle the multiple patho-mechanisms of AD [6]. More specifically, previous preclinical studies showed that soluble intracellular adhesion molecule-1 (sICAM-1) and AgRP secreted by human umbilical cord blood–derived MSCs (hUCB-MSCs) reduced Aβ level [7–9], hUCB-MSC–derived galectin-3 protected against Aβ neurotoxicity [10], and hUCB-MSCs enhanced endogenous adult neurogenesis and synaptic activity via secretion of GDF-15 and Activin A [11]. Based upon these animal studies, we previously completed a phase I clinical trial where we conducted stereotactic injections of hUCB-MSCs into the hippocampus and precuneus of nine AD dementia patients [12]. Through this trial, we first confirmed the safety of MSC therapy. Considering the progressive nature of AD; however, we considered that it would be both crucial and imperative to perform repeated deliveries of MSCs for future studies so as to preserve the therapeutic efficacy of hUCB-MSCs for a prolonged period of time. According to reports that we recently published [13, 14], there was a low persistence of naive human MSCs administered into the parenchyma or lateral ventricle of wild-type mice at 1 week post-transplant. In a mouse model, only 5.7% of human Wharton’s jelly–derived MSCs injected into the intracerebroventricular space persisted a week following transplantation [14]. Many groups have also noted the poor survival of MSCs following administration as a limitation of MSC-based therapy [15, 16]. Such results further reinforced the need to perform repeated injections in order to substitute for the lost MSCs destined for short survival, as reported in a mouse model study [17]. Although we previously reported the safety of parenchymal injection of MSCs, it is too invasive to perform multiple parenchymal injections by applying the stereotactic approach. Thus, to account for the importance of performing repeated injections, an Ommaya reservoir was utilized to deliver the MSCs. The Ommaya reservoir was implanted into the lateral ventricle. Among the various administration routes to deliver MSCs to the brain, the intracerebroventricular route may be advantageous in that a wide distribution of MSCs in the parenchyma can be achieved [18, 19]. It has been reported that MSCs can migrate into the brain parenchyma by initially adhering to the walls of the lateral ventricle [17, 20]. Indeed, we previously demonstrated that hUCB-MSCs transplanted into the intracerebroventricular space of a canine model migrated from the lateral ventricle towards the cortex [21].
Here, we conducted a phase I clinical trial where we evaluated the safety and dose-limiting toxicity of repeated intracerebroventricular injections of hUCB-MSCs in nine patients with mild-to-moderate AD dementia.
Methods
Study design
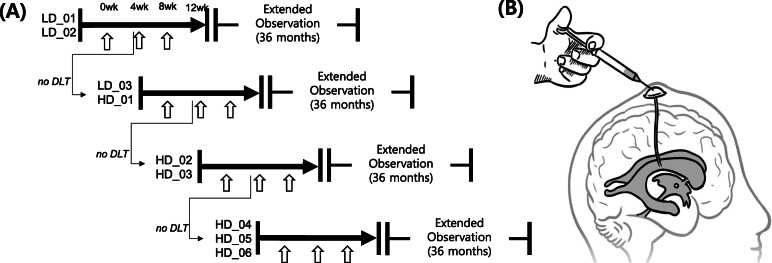
This was an open-label, single-center, phase I clinical trial performed at Samsung Medical Center, Seoul, Republic of Korea. First, participants underwent Ommaya reservoir implantation into the right lateral ventricle under local anesthesia, and then 4 weeks later, the participants received their first injection of MSCs followed by an additional two repeated hUCB-MSC injections at 4-week intervals (Fig. 1A, B). The participants received sequential hUCB-MSC (NEUROSTEM®) injections in a dose-escalating manner (Fig. 1). The rationale for the dose of injected hUCB-MSCs is provided in supplementary data. The first two participants (LD_01 and LD_02) received injections of a low dose (1.0 × 107 cells/2 mL) of hUCB-MSCs into the right lateral ventricular space via an Ommaya reservoir. Four weeks after the first injection, the dose-limiting toxicity (DLT) was evaluated. DLT was assessed according to the National Cancer Institute - Common Toxicity Criteria for Adverse Event (NCI-CTCAE) classification which encompassed grade 3 or higher toxicities [22]. After confirming the absence of DLT, the first two participants (LD_01 and LD_02) received their second hUCB-MSC injections, and the next two participants (LD_03 and HD_01) were subsequently enrolled to receive injections of a low (1.0 × 107 cells/2 mL) and high (3.0 × 107 cells/2 mL) dose of MSCs, respectively. During the entire study period, DLT was evaluated 4 weeks after each injection for each participant. We decided to continue repeated injections or enroll the next participants only when the absence of DLT was confirmed.
Fig. 1.
Study flow diagram for a phase I protocol in participants with Alzheimer’s disease. A Participants underwent three repeated hUCB-MSC injections in a dose-escalating, open-label fashion with two sequential doses (1.0 × 107 cells/2 mL in the low dose [LD] group and 3.0 × 107 cells/2 mL in the high dose [HD] group). Before continuing the study with subsequent participants, we confirmed that there was no DLT. Empty arrows indicate hUCB-MSC injection. B Schematic drawing of right intracerebroventricular injection of hUCB-MSCs via the Ommaya reservoir
This trial was registered at ClinicalTrial.gov for 12 weeks of follow-up (Identifier: NCT02054208) and for 36 months of extended observation (Identifier: NCT03172117). We obtained written informed consent from every patient and legally authorized representatives in cases of impaired capacity. This study was approved by the Institutional Review Board of Samsung Medical Center and Ministry of Food and Drug Safety (MFDS).
Participants
We enrolled nine AD dementia patients at Samsung Medical Center from March 2014 to June 2017. Eligible patients were 50 to 85 years of age, fulfilled the criteria for probable AD dementia according to the National Institute on Aging-Alzheimer’s Association workgroup (NIA-AA) [23], and had a Mini-Mental State Examination (MMSE) score of 18–26 at screening. Eligible patients were further screened with amyloid positron emission tomography (PET), [18F] fluoro-2-deoxy-d-glucose (FDG) PET, and magnetic resonance imaging (MRI) and were enrolled when they satisfied the following criteria: (1) had significant amyloid burden assessed by 11C-Pittsburgh compound B (PiB) PET or 18F-Florbetaben PET and (2) had neuronal degeneration assessed by atrophy on structural brain MRI or hypometabolism on FDG PET.
Patients with one or more of the following conditions were excluded: (1) neurodegenerative diseases other than AD dementia; (2) severe white matter hyperintensities on fluid-attenuated inversion recovery (FLAIR) MR images at baseline, which were defined as a cap or band (periventricular white matter hyperintensities) ≥ 10 mm, and deep white matter lesions (deep white matter hyperintensities) ≥ 25 mm as modified from the Fazekas ischemia criteria [24]; (3) major psychiatric disorders; (4) history of stroke within 3 months of enrollment; (4) hepatic, renal, hematologic, or active pulmonary disorders; (5) current or previous history of malignancy; and (6) difficult to perform Ommaya reservoir insertion. Patients were required to have sustained a stable dose of acetylcholinesterase inhibitors or memantine for at least 60 days prior to enrollment and the same dose of medication was continued throughout the study.
Preparation of hUCB-MSCs
To be used for clinical purposes, hUCB-MSC manufacture, cell quality control, and quality assurance were performed in compliance with the Korea Good Manufacturing Practices requirements by the MFDS. hUCB tissue was obtained after receiving written informed consent from normal women in their full-term pregnancy. hUCB-MSCs were grown in α-Minimum Essential Medium (α-MEM, Gibco/Life Technologies, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco/Life Technologies). Cells were cryopreserved at − 150 °C or lower by using 10% dimethyl sulfoxide.
To prepare for intracerebroventricular injection, frozen hUCB-MSCs were first thawed, seeded, and cultured. The cells were harvested 5 days after seeding, repeatedly washed to remove impurities such as fetal bovine serum and trypsin, and re-suspended in an appropriate amount of phenol red-free α-MEM. Therefore, fetal bovine serum was not present in the final drug product, NEUROSTEM®. hUCB-MSCs were tested for viability, phenotype, and presence of endotoxins, bacteria, and mycoplasma. After testing, 50 million cells were re-suspended in 1 mL of phenol red-free α-MEM, and a total volume of 2 mL was used to inject MSCs into a single patient of the low dose group. For the high dose group, from the adjusted final concentration of 150 million cells per 1 mL of phenol red-free MEM-α, a total volume of 2 mL was prepared for administration into each individual patient. hUCB-MSCs for the low dose group were stored at 4–20 °C and had a shelf life of about 60 h from the time of manufacture, and hUCB-MSCs for the high dose group were stored at 4–12 °C and had a shelf life of about 48 h. Based on flow cytometric analyses, the expressions of surface antigens of the cells were consistently positive for CD73, CD90, CD105, and CD166 but negative for CD45, CD14, and HLA-DR. The final products were delivered to the patient’s physician on the day of injection.
Ommaya insertion and intracerebroventricular injection of hUCB-MSCs
Thin-sliced brain CT scans of each patient were acquired for intraoperative navigation before surgery. Under local anesthesia, burr holes (approximately 15 mm) were made in the patient’s right Kocher’s point. An electromagnetic navigation system was used to guide the pathway to the lateral ventricle to avoid possible misplacement of the catheter and intra-parenchymal hemorrhage. The catheter was inserted about 6 cm from the dura opening and was connected to the reservoir chamber (Integra, NJ, USA). To assure that the catheter was securely inserted inside the lateral ventricle, cerebrospinal fluid (CSF) filling following pumping of the reservoir was checked, and intraoperative CT scans were also taken.
The average time interval between screening and Ommaya insertion was 13.7.0 ± 7.3 days. Four weeks following Ommaya reservoir implantation, the participants were admitted to the hospital and received baseline evaluation in the morning, and in the afternoon, a neurologist injected hUCB-MSCs into the intracerebroventricular space via the Ommaya reservoir (low dose 1.0 × 107 cells/2 mL or high dose 3.0 × 107 cells/2 mL). The participants were hospitalized for 2 days and were observed for signs of acute adverse events.
Safety assessments
Our primary goal was to assess the safety and DLT of two ascending doses of hUCB-MSCs in a total of three repeated injections. Safety was evaluated at each visit by examining the vital signs and weight, physical and neurologic examination, electrocardiogram, and clinical laboratory testing (hematologic and serum chemical testing). DLT was evaluated 4 weeks after each hUCB-MSC injection. Adverse events were assessed at each visit according to NCI-CTCAE [22]. In the extended observation study, adverse events were assessed at 18, 24, and 36 months after the end of the phase I trial. C&R Research Inc. served as external monitors of the study.
To detect immunologic reactions between the implanted hUCB-MSCs and the recipient, a mixed lymphocyte reaction was assessed at baseline and at 12 weeks after the first hUCB-MSC injection.
In order to monitor the complications (e.g., parenchymal or intraventricular hemorrhage) related to the Ommaya reservoir implantation, patients underwent brain CT scans at 24 h and 4 weeks after insertion of the Ommaya reservoir.
Clinical and laboratory assessments
To measure the clinical changes, patients underwent 11-item AD Assessment Scale-Cognitive Subscale (ADAS-Cog 11) testing (score range 0–70, lower score indicates better performance) [25], Seoul Instrumental Activities of Daily Living (S-IADL) (score range 0–45, lower score indicates better performance) [26], MMSE (score range 0–30, higher score indicates better performance), Clinician’s Interview-Based Impression of Change Plus Caregiver Input (CIBIC-Plus) [27], and Caregiver-Administered Neuropsychiatric Inventory (CGA-NPI) (sum of frequency × severity of 12 behavioral domains, score range 0–144, higher score indicates worse behavior) [28] at baseline, 4 weeks, 8 weeks, and 12 weeks after the first hUCB-MSC injection. In the extended observation study, the same assessment was done at 12 and 24 months after the end of the phase I trial.
We evaluated the changes in CSF white blood cell (WBC) count, AD biomarkers (CSF Aβ, total-tau, and phosphorylated-tau), and hUCB-MSC–related markers (Galactine-3, sICAM-1, progranulin, GDF-15, and Decorin) at baseline, 1 day, and 4 weeks after the 1st hUCB-MSC injection, 1 day and 4 weeks after the 2nd hUCB-MSC injection, and 1 day and 4 weeks after the 3rd hUCB-MSC injection. CSF was collected via the Ommaya reservoir.
To evaluate the structural changes, patients underwent brain MRI at baseline and 12 weeks after the first hUCB-MSC injection and at 12 and 24 months after the end of the phase I trial. To measure the changes in parenchymal Aβ deposition, patients underwent PiB-PET or florbetaben PET at baseline and 12 weeks after the first hUCB-MSC injection. Changes in standardized uptake value ratio (SUVR) were assessed.
Statistical analysis
Due to the small number of participants, absence of sham-surgery-control, and the fact that it was an open-labeled study design, we did not perform statistical analysis to compare the outcome measures of both the low- and high-dose hUCB-MSC recipients.
Results
Compliance and safety
The baseline characteristics of each individual subject are listed in Table 1. The mean age was 62.3 years in the low dose group and 63.0 years in the high dose group. All of the enrolled patients completed the phase I 12-week follow-up trial. For the extended follow-up study, three patients (LD_01, LD_03, and HD_04) declined to participate, and thus, only six out of nine patients were enrolled. During the extended follow-up study, one patient (LD_02) also dropped out of the study due to withdrawal of consent, and another patient (HD_06) was not included in the final clinical assessment at 24 months because the patient refused.
Table 1.
Baseline characteristics of participants
| Subject | Age | Gender | Education, years | Medication | MMSE | ADAS-Cog | APOE genotype | Amyloid PET |
|---|---|---|---|---|---|---|---|---|
| LD_01 | 59 | M | 9 | Donepezil 5 mg | 21 | 19 | e3/e4 | Positive |
| LD_02 | 65 | F | 12 | Donepezil 10 mg | 26 | 19 | e3/e3 | Positive |
| LD_03 | 63 | F | 12 | Donepezil 10 mg | 25 | 16 | e4/e4 | Positive |
| HD_01 | 60 | M | 18 | Donepezil 10 mg | 18 | 20 | e3/e4 | Positive |
| HD_02 | 76 | F | 12 | Donepezil 7.5 mg | 17 | 27 | e3/e3 | Positive |
| HD_03 | 50 | F | 14 | Rivastigmine 9.5 mg/24 h | 14 | 29 | e3/e3 | Positive |
| HD_04 | 66 | F | 6 | Memantine 10 mg, donepezil 10 mg | 24 | 19 | e3/e4 | Positive |
| HD_05 | 53 | F | 16 | Donepezil 10 mg | 24 | 13 | e3/e3 | Positive |
| HD_06 | 73 | M | 12 | Rivastigmine 9.5 mg/24 h | 21 | 29 | e4/e4 | Positive |
LD, low dose; HD, high dose; AChE-I, acetylcholine esterase inhibitor; MMSE, Mini-Mental State Examination; ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive Subscale
The Ommaya reservoir insertion procedure was well tolerated in all nine participants. However, Ommaya site pain or procedural pain occurred in four participants, and headache occurred in one participant. Brain CT scans taken within 24 h after Ommaya reservoir insertion confirmed that there was no cerebral hemorrhage in any of the participants.
After each hUCB-MSC injection, the participants were hospitalized for 2 days to be monitored for acute adverse events. After hUCB-MSC injection, all nine (100%) participants experienced fever. Out of a total of nine low-dose injections (three repeated injections for three participants), fever occurred after seven injections. Out of a total of 18 high-dose injections (three repeated injections for six participants), fever occurred after 13 injections. Fever subsided within 1–2 days with oral or IV antipyretics, and no antibiotics or antiviral agents were necessary. Other acute adverse events were headaches 7/9 (77.8%), followed by nausea 5/9 (55.6%), vomiting 4/9 (44.4%), myalgia or chills 2/9 (22.2%), and paresthesia on both arms and legs 2/9 (22.2%). Other events were abdominal pain 1/9 (11.1%), dyspepsia 1/9 (11.1%), pain other than the Ommaya site 1/9 (11.1%), musculoskeletal stiffness 1/9 (11.1%), arthralgia 1/9 (11.1%), periarthritis 1/9 (11.1%), and impetigo 1/9 (11.1%) (Table 2).
Table 2.
Adverse events that were observed in at least 1 subject during the 12-week follow-up period (all causalities)
| Low dose (n = 3) | High dose (n = 6) | |
|---|---|---|
| No. of subjects (%) [no. of events] | No. of subjects (%) [no. of events] | |
| Before hUCB-MSC injection | ||
| Total | 2 (66.7) [4] | 5 (83.3) [5] |
| Ommaya site pain | 0 [0] | 2 (33.3) [2] |
| Procedural pain | 1 (33.3) [1] | 1 (16.7) [1] |
| Headache | 1 (33.3) [2] | 0 [0] |
| Device malfunction | 0 [0] | 1 (16.7) [1] |
| Enteritis | 1 (33.3) [1] | 0 [0] |
| Otolithiasis | 0 [0] | 1 (16.7) [1] |
| After hUCB-MSC injection | ||
| Total | 3 (100.0) [21] | 6 (100.0) [48] |
| Nervous system disorders | ||
| Headache | 2 (66.7) [5] | 5 (83.3) [12] |
| Paraesthesia | 0 [0] | 2 (33.3) [4] |
| Gastrointestinal disorders | ||
| Nausea | 2 (66.7) [E2] | 3 (50.0) [7] |
| Vomiting | 1 (33.3) [2] | 3 (50.0) [6] |
| Abdominal pain | 0 [0]E | 1 (16.7) [1] |
| Dyspepsia | 1 (33.3) [1] | 0 [0] |
| General disorders and administration site conditions | ||
| Fever | 3 (100) [7] | 6 (100) [13] |
| Chills | 0 [0] | 1 (16.7) [1] |
| Ommaya site pain | 1 (33.3) [1] | 0 [0] |
| Pain other than the Ommaya site | 0 [0] | 1 (16.7) [1] |
| Musculoskeletal and connective tissue disorders | ||
| Musculoskeletal stiffness | 0 [0] | 1 (16.7) [1] |
| Myalgia | 1 (33.3) [2] | 0 [0] |
| Arthralgia | 0 [0] | 1 (16.7) [1] |
| Periarthritis | 0 [0] | 1 (16.7) [1] |
| Infections and infestations | ||
| Impetigo | 1 (33.3) [1] | 0 [0] |
| Psychiatric disorders | ||
| Delusion | 0 [0] | 0 [0] |
Among the aforementioned adverse events, three serious adverse drug reactions occurred in two participants. A 59-year-old male in the low dose group (LD_01) experienced a 38.0 °C fever 1 day after the second hUCB-MSC injection, which extended hospitalization by a day for close observation. One day after the third hUCB-MSC injection, the patient experienced a 38.6 °C high fever, which again extended hospitalization by a day. In both events, the fever subsided the next day without the use of antibiotics or anti-viral medication. According to CTCAE, the degree of adverse event was grade 1 and deemed by investigators as probably related to the investigational product. The second patient was a 65-year-old female who experienced nausea and vomiting 1 day after the second low-dose (LD_02) hUCB-MSC injection, which extended hospitalization by a day for close observation. The symptom subsided the next day without specific treatment. According to CTCAE, the degree of adverse event was grade 1 and deemed by investigators as probably related to the investigational product.
Brain MR images that were taken 12 weeks after the first hUCB-MSC injection, and at 12 and 24 months during extended observation, confirmed that there were no structural abnormalities including tumor, hydrocephalus, or subdural hemorrhages. Furthermore, dose-limiting toxicities were not evident for either the low- or high-dose hUCB-MSC groups. The results of the mixed lymphocyte reaction performed at 12 weeks after the first hUCB-MSC injection showed that all nine subjects were immunologically stable.
Clinical and laboratory outcome measures
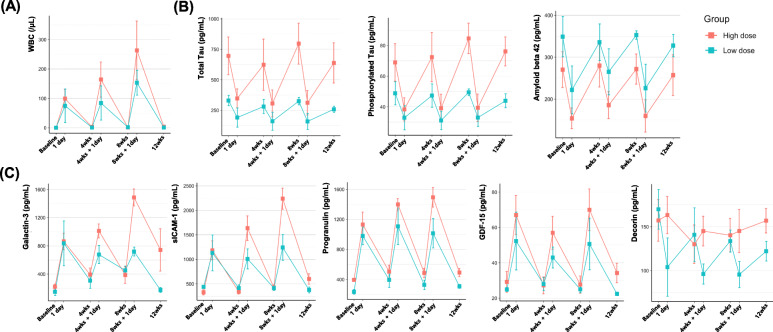
Scores of ADAS-Cog, MMSE, SIADL, and NPI at each time point are shown in Table 3. The number of patients in each category of CIBIC-Plus is shown in Table 4. CSF WBC count increased 1 day after each hUCB-MSC injection which then normalized after 4 weeks. The WBC increment amplified on repeated injections and a higher WBC increment were observed from the high dose group (Fig. 2A).
Table 3.
Scores of ADAS-Cog, S-IADL, MMSE, and NPI at each time point
| ` | ADAS-Cog | S-IADL | MMSE | NPI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low dose | High dose | Total | Low dose | High dose | Total | Low dose | High dose | Total | Low dose | High dose | Total | ||
| n = 3 | n = 6 | n = 9 | n = 3 | n = 6 | n = 9 | n = 3 | n = 6 | n = 9 | n = 3 | n = 6 | n = 9 | ||
| Screening | Mean ± SD | 15.0 ± 2.0 | 22.5 ± 6.2 | 20.0 ± 6.3 | 9.3 ± 4.0 | 13.0 ± 5.8 | 11.8 ± 5.3 | 24.0 ± 1.7 | 21.3 ± 2.7 | 22.2 ± 2.6 | 10.0 ± 7.8 | 1.5 ± 1.5 | 4.3 ± 5.9 |
| Median | 15 | 23 | 19.7 | 10 | 14.7 | 13 | 24 | 20.7 | 22 | 6 | 1.3 | 2.7 | |
| Min–Max | 13–17 | 12–30 | 12–30 | 5–13 | 5–20 | 5–20 | 22–25 | 18–25 | 18–25 | 5–19 | 0–4 | 0–19 | |
| Baseline | Mean ± SD | 18.0 ± 1.7 | 22.8 ± 6.5 | 21.2 ± 5.8 | 8.3 ± 4.6 | 15.3 ± 5.9 | 13.0 ± 6.3 | 24.0 ± 2.6 | 19.7 ± 4.0 | 21.1 ± 4.1 | 1.0 ± 1.7 | 5.3 ± 8.9 | 3.9 ± 7.4 |
| Median | 18 | 23.5 | 19.5 | 8.3 | 17.5 | 13 | 25 | 19.5 | 21.8 | 1 | 1.5 | 1 | |
| Min–Max | 16–19 | 13–29 | 13–29 | 3–11 | 7–21 | 3–21 | 21–26 | 14–24 | 14–26 | 0–3 | 0–23 | 0–23 | |
| Week 4 | Mean ± SD | 17.3 ± 3.8 | 22.2 ± 5.5 | 20.6 ± 5.3 | 12.3 ± 5.9 | 16.5 ± 9.4 | 15.1 ± 8.2 | 24.3 ± 1.5 | 21.5 ± 3.3 | 22.4 ± 3.1 | 5.7 ± 2.3 | 3.5 ± 3.6 | 4.2 ± 3.2 |
| Median | 19 | 21.3 | 19.3 | 10 | 15.5 | 12 | 24 | 21.5 | 23.3 | 5.7 | 3 | 4 | |
| Min–Max | 13–20 | 16–29 | 13–29 | 8–19 | 5–28 | 5–28 | 23–26 | 17–25 | 17–26 | 3–7 | 0–9 | 0–9 | |
| Week 8 | Mean ± SD | 15.7 ± 6.1 | 23.2 ± 6.7 | 20.7 ± 7.2 | 10.3 ± 2.1 | 17.8 ± 6.3 | 15.3 ± 6.3 | 26.0 ± 2.0 | 21.5 ± 3.6 | 23.0 ± 3.7 | 5.0 ± 5.6 | 5.3 ± 6.7 | 5.2 ± 6.0 |
| Median | 17 | 24 | 20.3 | 11 | 17.5 | 13 | 26 | 22 | 23.5 | 4 | 4 | 4 | |
| Min–Max | 9–21 | 13–31 | 9–31 | 8–12 | 10–28 | 8–28 | 24–28 | 16–26 | 16–28 | 0–11 | 0–18 | 0–18 | |
| Week 12 | Mean ± SD | 18.7 ± 2.5 | 25.2 ± 8.2 | 23.0 ± 7.4 | 10.3 ± 5.0 | 16.8 ± 6.5 | 14.7 ± 6.6 | 24.0 ± 4.4 | 20.3 ± 3.6 | 21.6 ± 4.0 | 2.3 ± 2.1 | 3.7 ± 6.0 | 3.2 ± 4.9 |
| Median | 19 | 23.5 | 21 | 11 | 17 | 14 | 26 | 20 | 21 | 3 | 3.2 | 2 | |
| Min–Max | 16–21 | 17–36 | 16–36 | 5–15 | 8–26 | 5–26 | 19–27 | 16–25 | 16–27 | 0–4 | 0–14 | 0–14 | |
| Mean change from baseline to week 12 | Mean ± SD | 0.7 ± 4.0 | 2.3 ± 5.0 | 1.8 ± 4.5 | 2.0 ± 2.0 | 1.5 ± 3.0 | 1.7 ± 2.6 | 0.0 ± 2.0 | 0.7 ± 1.6 | 0.4 ± 1.7 | 1.3 ± 2.3 | − 1.7 ± 5.8 | − 0.7 ± 5.0 |
| Median | 0 | 3.5 | 3 | 2 | 1.5 | 1.7 | 0 | 0.5 | 0.3 | 1.3 | − 1.5 | − 0.5 | |
| Min–Max | − 3–5 | − 4–9 | − 4–9 | 0–4 | − 3–6 | − 3–6 | − 2–2 | − 1–3 | − 2–3 | 0–4 | − 9–8 | − 9–8 | |
| Week 52 | n = 1 | n = 5 | n = 6 | n = 1 | n = 5 | n = 6 | n = 1 | n = 5 | n = 6 | n = 1 | n = 5 | n = 6 | |
| Mean ± SD | 19 | 30.0 ± 9.8 | 28.2 ± 9.8 | 17 | 23.4 ± 9.0 | 22.3 ± 8.4 | 23 | 16.0 ± 6.0 | 17.2 ± 6.0 | 41 | 2.6 ± 2.1 | 9.0 ± 15.8 | |
| Median | 19 | 31.0 | 28.5 | 17 | 21 | 19.5 | 23 | 17.0 | 18.5 | 41 | 3.0 | 3.5 | |
| Min–Max | 16–42 | 16–42 | 14–37 | 14–37 | 6–21 | 6–23 | 0–5 | 0–41 | |||||
| Week 104 | n = 0 | n = 4 | n = 4 | n = 0 | n = 4 | n = 4 | n = 0 | n = 4 | n = 4 | n = 0 | n = 4 | n = 4 | |
| Mean ± SD | 35.3 ± 13.6 | 35.3 ± 13.6 | 30.0 ± 9.2 | 30.0 ± 9.2 | 13.3 ± 8.0 | 13.3 ± 8.0 | 0.8 ± 1.0 | 0.8 ± 1.0 | |||||
| Median | 32 | 32 | 31 | 31 | 14 | 14 | 0.7 | 0.7 | |||||
| Min–Max | 23–54 | 23–54 | 18–40 | 18–40 | 3–22 | 3–22 | 0–2 | 0–2 | |||||
ADAS-Cog, Alzheimer’s Disease Assessment Scale-Cognitive Subscale; S-IADL, Seoul Instrumental Activities of Daily Living; MMSE, Mini-Mental State Examination; NPI, neuropsychiatric inventory; hUCB-MSC, human umbilical cord blood–derived mesenchymal stem cell; SD, standard deviation
Table 4.
Number of patients in each category of CIBIC-Plus
| Baseline | Week 4 | Week 8 | Week 12 | |||||
|---|---|---|---|---|---|---|---|---|
| Low dose | High dose | Low dose | High dose | Low dose | High dose | Low dose | High dose | |
| Marked worsening | ||||||||
| Moderate worsening | 1 | 1 | 3 | |||||
| Minimal worsening | 2 | 2 | 1 | |||||
| No change | 3 | 3 | 2 | 2 | 2 | 1 | ||
| Minimal improvement | 1 | 1 | 1 | 2 | 2 | 1 | 1 | |
| Moderate improvement | 1 | 1 | 1 | |||||
| Marked improvement | 1 | |||||||
CIBIC-Plus, Clinician’s Interview-Based Impression of Change Plus Caregiver Input
Fig. 2.
White blood cell count (A), Alzheimer’s disease biomarkers (B), and MSC-related markers (C) in the cerebrospinal fluid. Bars indicate standard error. WBC, white blood cell; sICAM-1, soluble intercellular adhesion molecule-1; GDS-15, growth differentiation factor 15
AD biomarkers (total tau, phosphorylated tau, and Aβ42) decreased 1 day after each hUCB-MSC injection which then increased to the baseline level after 4 weeks (Fig. 2B).
hUCB-MSC–related markers (Galactin-3, sICAM-1, progranulin, and GDF-15) increased 1 day after each hUCB-MSC injection which then decreased to the baseline level after 4 weeks. However, such patterns were not observed from the CSF decorin levels (Fig. 2C).
Aβ burden measured by amyloid-PET showed that in two participants of the low dose group, PiB SUVR changed from 2.57 ± 0.40 at baseline to 1.92 ± 0.03 at 12-week follow-up; in one participant of the low dose group, florbetaben SUVR changed from 1.70 at baseline to 1.60 at 12-week follow-up; and in six participants in the high dose group, florbetaben SUVR changed from 1.79 ± 0.07 at baseline to 1.71 ± 0.06 at 12-week follow-up.
Discussion
In this phase I clinical trial, we repeatedly injected hUCB-MSCs into the right lateral ventricle of nine mild-to-moderate AD dementia patients via an Ommaya reservoir. Three patients received a low dose (1.0 × 107 cells/2 mL) and six patients received a high dose (3.0 × 107 cells/2 mL) of hUCB-MSCs 3 times at 4-week intervals. We report that this was feasible, safe, and well-tolerated. However, within 24 h after each MSC administration, we consistently observed transient fever and increased WBC count in the CSF samples of the patients. The fever subsided after 1–2 days, and WBC was normalized by the next CSF examination that took place 4 weeks later.
First, in regard to safety, side effects associated with the Ommaya reservoir implantation were not remarkable. Implantation was performed under local anesthesia and was well tolerated in all patients. Following surgery, minor adverse events such as pain at the site of Ommaya reservoir implantation, procedural pain, and headaches were noted, but major complications such as hemorrhages or infections were not observed. Second, with respect to the complications related to the intracerebroventricular injection of hUCB-MSC into the lateral ventricle, the most common adverse events were fever followed by headache, nausea, vomiting, myalgia, or chills. Three events in two participants were assessed as serious adverse drug reactions as the event extended hospitalization by 1 day. However, the symptom subsided the next day without complications. In the extended observation study, serious adverse drug reactions were not observed. No structural abnormalities such as tumor development, hydrocephalus, or hemorrhage were observed from the five participants who completed the extended observation. These results are consistent with our previous study where we reported the safety of performing stereotactic brain injections of hUCB-MSC in AD patients [12].
Unexpectedly, however, all of the participants developed fever within 6–24 h after receiving hUCB-MSC injections which subsided within the next 36 h. This was surprising because in our previous study, fever was not observed following stereotactic injections of hUCB-MSCs into the hippocampus and precuneus [12]. We presumed that infection, composition of the suspension media, or the MSCs themselves could have caused the fever. However, out of the three possible explanations, infection seemed unlikely since microorganisms were not identified from the CSF culture and the fever subsided spontaneously without the use of antibiotics or antiviral agents. The second hypothesis was that MSCs per se caused the fever. Increased WBC count in the CSF was accompanied by fever which normalized by the next CSF test performed 4 weeks later, right before performing another round of MSC injections. The WBC count was higher for the high dose group. Since we carried out repeated hUCB-MSC injections, the increment of WBC count also amplified. This suggests that immune responses against hUCB-MSCs were sensitized in the second and third injections. We also noted that an increase in the dose of MSC injection led to an increase in WBC count. Although the inflammatory response was transient, we do acknowledge that repeated injections of MSCs could possibly lead towards widespread encephalopathy. Further studies are warranted to find the optimal number of repeated MSC injections considering the repeated inflammatory response and potential therapeutic effects. In addition, we need further studies to search for ways to prolong the survival of MSCs and to thus sustain their therapeutic effects. Mitigating immune responses generated from the transplanted MSCs must also be addressed to enhance the efficacy of stem cell therapy. Evaluating such factors will contribute towards the development of AD stem cell therapy.
This immune reaction was not expected since MSCs are known to express low levels of the major histocompatibility complex (MHC) class I and negative or no levels of MHC class II [13, 29]. However, based on the following observations, we suspect that immune reactions occurred following the administration of hUCB-MSCs. We recently reported that a high infiltration of immune cells was discovered at the injection site a week after performing the administration of human MSCs into the parenchyma or caudate putamen of wild-type mice [13]. While the most prominent and highest expression of immune cells was identified from the group that received transplantation of xenogeneic MSCs, compared to the syngeneic group, mice from the allogeneic group also demonstrated a pronounced expression of immune cells at the injection site [13]. Another group also demonstrated that allo-reactivity was discovered following the administration of bone marrow–derived allogeneic MSCs into the caudate nucleus of immuno-competent rhesus macaques [30]. Interestingly, the extent of host allo-immune response varied depending on the degree of MHC class I and II mismatch [30]. These findings along with the results acquired from this current study challenge the presumption that MSCs are intrinsically immune-privileged. The third possibility was that certain components of the MSC culture media (α-MEM) might have elicited chemical or foreign body reactions. Although further study is required to rule out this possibility, it seems unlikely that α-MEM is the culprit since the amount of α-MEM was identical for both the low and high dose groups. Furthermore, in our mouse experiment, when α-MEM was injected into the parenchyma of wild-type mice, a scarcity of immune cell infiltration was exhibited at the injection site [13].
As this was an open-label phase I clinical trial, we could not prove the clinical efficacy of hUCB-MSC injection. Nevertheless, we noted reduced levels of total tau, phosphorylated tau, and Aβ42 in the CSF samples collected 1 day after performing the hUCB-MSC injections. The increase in total tau, phosphorylated tau, and Aβ42 levels observed 4 weeks following hUCB-MSC transplantation suggest that the therapeutic effects exerted by the hUCB-MSCs may not have lasted for a prolonged period due to the short life span of the hUCB-MSCs. It is interesting to point out that while a striking increase in the expression levels of Galactin-3, sICAM-1, progranulin, and GDF-15 was noted a day following MSC transplantation, the levels dropped dramatically within 4 weeks. Such results support that performing repeated administrations (possibly shortening the interval between the injections) may be necessary to preserve the therapeutic efficacies exerted by the hUCB-MSCs. We also cannot rule out potential dilution effects considering that normal saline was administered right after each injection of hUCB-MSCs. Therefore, CSF biomarker changes observed from the hUCB-MSC injection group should be compared to those of the placebo group in future studies. Placebo-controlled, randomized phase IIa clinical trial of 36 patients is currently under extended follow-up.
Limitations
A limitation of this study is that the hUCB-MSC injection dose used in this trial was based on mouse studies. The injection dose in this trial was calculated by referring to the mouse to human CSF volume ratio. However, detailed studies to evaluate the optimal cell dose in AD patients are further warranted.
Conclusions
In this phase I clinical trial, we repeatedly injected hUCB-MSCs into the right lateral ventricle of nine mild-to-moderate AD dementia patients via an Ommaya reservoir. We report that this was feasible, safe, and well-tolerated. However, we consistently observed transient fever following each MSC administration, which subsided after 1–2 days. To date, we are currently conducting an extended follow-up study for those who participated in a placebo-controlled, randomized phase IIa clinical trial. Upon completion, ensuing analyses will be published, and through the analyses, we hope to gain a deeper understanding of the clinical therapeutic efficacy of MSC AD therapy.
Supplementary Information
Additional file 1. The optimal injection dose of hUCB-MSCs.
Acknowledgements
The authors thank Yoon Sun Yang and Wonil Oh from MEDIPOST Co, Ltd. for providing human umbilical cord blood–derived mesenchymal stem cells.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
Amyloid-beta
- MSCs
Mesenchymal stem cells
- hUCB-MSCs
Human umbilical cord blood–derived MSCs
- DLT
Dose-limiting toxicity
- NCI-CTCAE
National Cancer Institute - Common Toxicity Criteria for Adverse Event
- MFDS
Ministry of Food and Drug Safety
- NIA-AA
National Institute on Aging-Alzheimer’s Association workgroup
- MMSE
Mini-Mental State Examination
- PET
Positron emission tomography
- FDG
Fluoro-2-deoxy-d-glucose
- MRI
Magnetic resonance imaging
- PIB
11C-Pittsburgh compound B
- FLAIR
Fluid-attenuated inversion recovery
- α-MEM
α-Minimum Essential Medium
- CSF
Cerebrospinal fluid
- ADAS-Cog
Alzheimer’s Disease Assessment Scale-Cognitive Subscale
- S-IADL
Seoul Instrumental Activities of Daily Living
- CIBIC-Plus
Clinician’s Interview-Based Impression of Change Plus Caregiver Input
- CGA-NPI
Caregiver-Administered Neuropsychiatric Inventory
- WBC
White blood cell
- SUVR
Standardized uptake value ratio
- MHC
Major histocompatibility complex
Authors’ contributions
Hee Jin Kim and Duk L. Na contributed to the study concept and design, acquisition of the data, analysis and interpretation of the data, and draft and revision of the manuscript for content. Kyung Rae Cho and Jung Il Lee performed the Ommaya insertion surgery. Na Kyung Lee contributed to the interpretation of the data and drafting of the manuscript. Young Hee Jung, Hyemin Jang, Jun Pyo Kim, and Sang Won Seo collected the clinical data. Seongbeom Park, Sung Tae Kim, and Seung Whan Moon collected the imaging data and performed the imaging analysis. Jong Wook Chang, Soo Jin Choi, and Sung Tae Kim contributed to the study concept and design. All authors read and approved the final manuscript.
Funding
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI12C1821).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The institutional review board of Samsung Medical Center approved this study, and informed consent was obtained from the patients and caregivers.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Human umbilical cord blood–derived mesenchymal stem cells were supplied by MEDIPOST Co, Ltd.; the sponsor was not involved in the interpretation of the data, writing of the report, or the decision to submit the manuscript for publication.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lewis CM, Suzuki M. Therapeutic applications of mesenchymal stem cells for amyotrophic lateral sclerosis. Stem Cell Res Ther. 2014;5(2):32. doi: 10.1186/scrt421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosser A, Svendsen CN. Stem cells for cell replacement therapy: a therapeutic strategy for HD? Mov Disord. 2014;29(11):1446–1454. doi: 10.1002/mds.26026. [DOI] [PubMed] [Google Scholar]
- 3.Lee PH, Lee JE, Kim HS, Song SK, Lee HS, Nam HS, Cheong JW, Jeong Y, Park HJ, Kim DJ, Nam CM, Lee JD, Kim HO, Sohn YH. A randomized trial of mesenchymal stem cells in multiple system atrophy. Ann Neurol. 2012;72(1):32–40. doi: 10.1002/ana.23612. [DOI] [PubMed] [Google Scholar]
- 4.Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017;8(1):111. doi: 10.1186/s13287-017-0567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gugliandolo A, Bramanti P, Mazzon E. Mesenchymal stem cells: a potential therapeutic approach for amyotrophic lateral sclerosis? Stem Cells Int. 2019;2019:3675627–3675616. doi: 10.1155/2019/3675627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee NK, Na DL, Chang JW. Killing two birds with one stone: the multifunctional roles of mesenchymal stem cells in the treatment of neurodegenerative and muscle diseases. Histol Histopathol. 2018;33(7):629–638. doi: 10.14670/HH-11-951. [DOI] [PubMed] [Google Scholar]
- 7.Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ, Seo SW, Lee JI, Na DL, Yang YS, Oh W, Chang JW. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-beta plaques. Cell Death Differ. 2012;19(4):680–691. doi: 10.1038/cdd.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee NK, Park SE, Kwon SJ, Shim S, Byeon Y, Kim JH, Na DL, Chang JW. Agouti related peptide secreted via human mesenchymal stem cells upregulates proteasome activity in an Alzheimer’s disease model. Sci Rep. 2017;7(1):39340. doi: 10.1038/srep39340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer’s disease mouse model. Neurosci Lett. 2009;450(2):136–141. doi: 10.1016/j.neulet.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Kim DH, Kim DS, Kim JH, Jeong SY, Jeon HB, Lee EH, Yang YS, Oh W, Chang JW. Galectin-3 secreted by human umbilical cord blood-derived mesenchymal stem cells reduces amyloid-beta42 neurotoxicity in vitro. FEBS Lett. 2010;584(16):3601–3608. doi: 10.1016/j.febslet.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Park SE, Lee J, Chang EH, Kim JH, Sung JH, Na DL, Chang JW. Activin A secreted by human mesenchymal stem cells induces neuronal development and neurite outgrowth in an in vitro model of Alzheimer’s disease: neurogenesis induced by MSCs via activin A. Arch Pharm Res. 2016;39(8):1171–1179. doi: 10.1007/s12272-016-0799-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Seo SW, Chang JW, Lee JI, Kim CH, Chin J, Choi SJ, Kwon H, Yun HJ, Lee JM, Kim ST, Choe YS, Lee KH, Na DL. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer’s disease dementia: a phase 1 clinical trial. Alzheimers Dement (N Y). 2015;1(2):95–102. doi: 10.1016/j.trci.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang JW, Lee NK, Yang JH, Son HJ, Bang SI, Chang JW, et al. A comparison of immune responses exerted following syngeneic, allogeneic, and xenogeneic transplantation of mesenchymal stem cells into the mouse brain. Int J Mol Sci. 2020;21(9). 10.3390/ijms21093052. [DOI] [PMC free article] [PubMed]
- 14.Lee NH, Myeong SH, Son HJ, Hwang JW, Lee NK, Chang JW, et al. Ethionamide preconditioning enhances the proliferation and migration of human Wharton’s jelly-derived mesenchymal stem cells. Int J Mol Sci. 2020;21(19). 10.3390/ijms21197013. [DOI] [PMC free article] [PubMed]
- 15.Lee S, Choi E, Cha MJ, Hwang KC. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid Med Cell Longev. 2015;2015:632902–632909. doi: 10.1155/2015/632902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li L, Chen X, Wang WE, Zeng C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2016;2016:9682757–9682714. doi: 10.1155/2016/9682757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim DH, Lee D, Chang EH, Kim JH, Hwang JW, Kim JY, Kyung JW, Kim SH, Oh JS, Shim SM, Na DL, Oh W, Chang JW. GDF-15 secreted from human umbilical cord blood mesenchymal stem cells delivered through the cerebrospinal fluid promotes hippocampal neurogenesis and synaptic activity in an Alzheimer’s disease model. Stem Cells Dev. 2015;24(20):2378–2390. doi: 10.1089/scd.2014.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Lee NK, Yoo D, Lee J, Choi SJ, Oh W, Chang JW, Na DL. Lowering the concentration affects the migration and viability of intracerebroventricular-delivered human mesenchymal stem cells. Biochem Biophys Res Commun. 2017;493(1):751–757. doi: 10.1016/j.bbrc.2017.08.115. [DOI] [PubMed] [Google Scholar]
- 19.Park SE, Lee NK, Na DL, Chang JW. Optimal mesenchymal stem cell delivery routes to enhance neurogenesis for the treatment of Alzheimer’s disease: optimal MSCs delivery routes for the treatment of AD. Histol Histopathol. 2018;33(6):533–541. doi: 10.14670/HH-11-950. [DOI] [PubMed] [Google Scholar]
- 20.Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75(5):321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Park SE, Jung NY, Lee NK, Lee J, Hyung B, Myeong SH, Kim HS, Suh YL, Lee JI, Cho KR, Kim DH, Choi SJ, Chang JW, Na DL. Distribution of human umbilical cord blood-derived mesenchymal stem cells (hUCB-MSCs) in canines after intracerebroventricular injection. Neurobiol Aging. 2016;47:192–200. doi: 10.1016/j.neurobiolaging.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Postel-Vinay S, Collette L, Paoletti X, Rizzo E, Massard C, Olmos D, Fowst C, Levy B, Mancini P, Lacombe D, Ivy P, Seymour L, le Tourneau C, Siu LL, Kaye SB, Verweij J, Soria JC. Towards new methods for the determination of dose limiting toxicities and the assessment of the recommended dose for further studies of molecularly targeted agents - Dose-Limiting Toxicity and Toxicity Assessment Recommendation Group for Early Trials of Targeted therapies, an European Organisation for Research and Treatment of Cancer-led study. Eur J Cancer. 2014;50(12):2040–2049. doi: 10.1016/j.ejca.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 23.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dementia. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fazekas F, Kleinert R, Offenbacher H, Schmidt R, Kleinert G, Payer F, Radner H, Lechner H. Pathologic correlates of incidental MRI white matter signal hyperintensities. Neurology. 1993;43(9):1683–1689. doi: 10.1212/WNL.43.9.1683. [DOI] [PubMed] [Google Scholar]
- 25.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141(11):1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 26.Ku HMKJ, Kwon EJ. A study on the reliability and validity of Seoul-Instrumental Activities of Daily Living(S-IADL) J Korean Neuropsychiatr Assoc. 2004;43:189–199. [Google Scholar]
- 27.Boothby H, Mann AH, Barker A. Factors determining interrater agreement with rating global change in dementia: the CIBIC-Plus. Int J Geriatr Psychiatr. 1995;10(12):1037–1045. doi: 10.1002/gps.930101208. [DOI] [Google Scholar]
- 28.Choi SH, Na DL, Kwon HM, Yoon SJ, Jeong JH, Ha CK. The Korean version of the neuropsychiatric inventory: a scoring tool for neuropsychiatric disturbance in dementia patients. J Korean Med Sci. 2000;15(6):609–615. doi: 10.3346/jkms.2000.15.6.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs SA, Roobrouck VD, Verfaillie CM, Van Gool SW. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol Cell Biol. 2013;91(1):32–39. doi: 10.1038/icb.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isakova IA, Lanclos C, Bruhn J, Kuroda MJ, Baker KC, Krishnappa V, Phinney DG. Allo-reactivity of mesenchymal stem cells in rhesus macaques is dose and haplotype dependent and limits durable cell engraftment in vivo. PLoS One. 2014;9(1):e87238. doi: 10.1371/journal.pone.0087238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The optimal injection dose of hUCB-MSCs.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.