Abstract
Background.
Preterm birth is an important risk factor for neurodevelopmental disabilities. The vast majority of these disabilities occur, however, among term births. The role of fetal growth restriction specifically among term babies has been incompletely described.
Methods.
We conducted a population-based study of term birth weight and its link to a range of neurodevelopmental outcomes using Norwegian health registries. To remove the influence of preterm birth, we restricted our analyses to 1.8 million singleton babies born during a narrow range of term gestational age (39–41 weeks). Babies with malformations were excluded. Analyses were adjusted simply for year of birth, as further adjustments for sex, parity, maternal age, smoking, marital status, immigrant status, and parental education had trivial influence. An additional sibling analysis controlled for unmeasured family-based confounding.
Results.
The risk of neurodevelopmental disabilities at term steadily increased at birth weights lower than 3.5 kg. Using the category of 3.5–3.9 kg as the reference, the odds reached 25-fold for cerebral palsy at the smallest weights (95% confidence interval 8.0–79), 16-fold for vision/hearing disability (4.0–65), 11-fold for intellectual impairment (6.9–17), 7-fold for schizophrenia (1.0–50), 5.4-fold for epilepsy (2.6–12), and 3.5-fold for autism spectrum (1.3–9.4) and behavioral disorders including attention deficit hyperactivity disorder (ADHD) (2.1–5.4). Associations remained robust with sibling controls.
Conclusions.
Reduced fetal growth is a powerful predictor of a wide variety of neurodevelopmental disabilities independent of preterm delivery.
Keywords: Birth weight, term birth, neurodevelopmental disability, neuropsychiatric disease, cohort study, developmental origin of disease
Introduction
Infants who are born preterm – and especially very preterm – are well known to be at risk of neurodevelopmental problems.1 However, preterm delivery accounts for a small fraction of all neurodevelopmental disabilities. In one population-based study, only 10% of all neurodevelopmental disabilities occurred in preterm births.1 Risk factors among term births are thus important to total risk.
Impaired fetal growth is a risk factor of particular interest. Prenatal conditions that increase the fetus’s susceptibility to neurodevelopmental disease often also prevent fetuses from reaching their full growth potential. The mechanisms of fetal damage may vary among neurodevelopmental disorders or may be shared among them. Chromosomal aneuploidy. infection, and placental dysfunction are all likely candidates for predisposing conditions, although the underlying causes of most cases remain unidentified. Small size at birth can thus serve as an early marker of exposure to such insults. Fetal growth restriction at term is well documented as a risk factor for cerebral palsy, with good evidence also for intellectual impairment. Data are more limited for other specific neurodevelopmental disabilities.
We investigated the relationship of fetal growth with seven neurodevelopmental conditions through adulthood, using data from 1.8 million Norwegian babies recorded in national health and administrative registries. To remove the influence of preterm birth, we restricted our analyses to babies born during a narrow range of term gestational age (39–41 weeks). We considered the following physician-diagnosed outcomes: cerebral palsy, intellectual impairment, disabilities of vision or hearing, epilepsy, autism spectrum disorder, attention-deficit hyperactivity disorder (ADHD), and schizophrenia.
Methods
Study design
We conducted a prospective population study of singleton babies recorded in the Medical Birth Registry of Norway. This registry comprises records of all deliveries in the country since 1967.2 By using the unique personal identifiers assigned at birth, we were able to link information from the birth registry on pregnancy, delivery, parents, and maternal and newborn health to other mandatory health and administrative registries, so as to follow each baby to adulthood.
To remove the influence of preterm delivery, we carefully restricted our sample to term births. The usual definition of term delivery is 37 or more completed weeks of gestation. We chose a more restricted definition of 39–41 completed weeks, which reduced the chance that even slightly misclassified preterm births might contribute to the observed associations. This definition also served to exclude post-term births (≥42 weeks). Gestational age was determined by fetal ultrasound if available, and otherwise by last menstrual period (LMP). Fetal ultrasounds have routinely been performed in Norway since 1986 and recorded in the birth registry since December 1998.3
This study was approved by the Regional Committee for Medical and Health Research Ethics in Norway and by the government agencies responsible for the registries. Names and personal identifiers were removed, and data were accessed through a secure server at the University of Bergen, Norway.
The outcomes
Neurodevelopmental diagnoses were retrieved from the National Insurance Scheme, which is the public social-security system in Norway.4 Relevant diagnoses included cerebral palsy (CP), intellectual impairment, autism spectrum disorder, behavioral disorders including ADHD, epilepsy, severe impairment of hearing or vision, and schizophrenia. Persons suffering any of these conditions are entitled to a basic financial benefit for illness-related expenses, plus an attendance benefit if the person requires special attention or nursing (both from early childhood), and a disability benefit from age 18 if the person’s working ability is permanently reduced by at least 50%.4 Financial compensation is without regard to wealth or income, so that virtually all persons with qualifying diagnoses are included in this recording system. The diagnoses are set by physicians according to the International Classification of Disease (ICD) versions 9 or 10, depending on the year, and independent of hospital stays (eTable 1). In a small validation study of 27 children with cerebral palsy according to hospital records, 19 were receiving insurance compensation for CP and 5 were compensated for a related disability (intellectual impairment or another neurologic condition). Three were not registered in the insurance program due to a mild level of disability.5
Covariates and exclusions
Most covariates were retrieved directly from the birth registry, including year of birth, infant sex, congenital malformations detected at birth (recorded according to ICD-8 and −10), and maternal parity, age, and presence of a partner. (Many couples in Norway are unmarried but in a stable partnership.) Information on parental education was obtained from the Norwegian National Education Database administered by Statistics Norway. Data on parental immigrant background was obtained from Statistics Norway. Children who died before age 5 (before full diagnosis of neurodevelopmental conditions was possible) were identified through linkage to the Cause of Death Registry and excluded from the main analysis.
Statistical analysis
We examined the association of birth weight with the various outcomes using logistic regression models. Results are reported as odds ratios (OR) and 95% confidence intervals (95%CI), which are interpretable as risk ratios due to the rare incidence of these outcomes in persons born at term (all less than 1%; eTable 2). We were able to follow all births through the end of 2014, with truncated follow-up of babies born late in this period. Birth weight was assessed in categories of 0.5 kilograms (kg) (<1.5, 1.5–1.9, etc., 5.0–5.4, ≥5.5kg). Babies weighing 3.5–3.9 kg had the lowest risk in most of our analyses and were the referent for all analyses.
Birth defects can appear as part of genetic syndromes and are associated with higher mortality and neurodevelopmental problems.6 In our data, malformations at birth (53,358 cases) were strongly related to birth weight (eFigure 1). All babies with registered malformations were excluded from analyses. We then investigated the association between fetal growth and the various neurodevelopmental conditions. As boys may be more affected by neurodevelopmental conditions (including autism, CP, ADHD, and schizophrenia 7,8), we assessed whether birth weight associations differed by sex.
We compared crude effect estimates to estimates adjusted for potential confounding factors. Since outcomes may vary in prevalence over time, we adjusted multivariable models for year of birth (5-year-groups in 1967–2015). We also adjusted for sex (male or female), maternal parity (0, 1, 2, 3, or ≥4), maternal age (≤19, 20–24, 25–29, 30–34, 35–39, or ≥40 years), maternal civil status (married/cohabitant or other), and immigrant background, defined as children born in Norway to immigrant parents (yes or no). We adjusted continuously for maternal and paternal highest level of education, using a scale from 0 (no formal education) to 8 (highest university degree, corresponding to a doctorate). Level 3 corresponds to 11–12 years and level 4 to 13 years of schooling.
We conducted several sensitivity analyses to assess the robustness of our findings. Most importantly, we carried out sibling-matched analyses for all outcomes using conditional logistic regression to compare full siblings born at 39–41 weeks in families with outcome-discordant children (i.e. families with at least one affected and one unaffected child). Within-family comparisons are able to control for familial confounders that persist across pregnancies, such as unmeasured shared genetic and stable lifestyle factors. These analyses also account for within-family correlations – unlike the main analyses, which treat all babies as independent observations irrespective of relatedness.
In additional sensitivity analyses, we restricted the sample to offspring with two independent measures of gestational age (LMP and fetal ultrasound) confirming 39–41 weeks of gestation. Although this reduced the sample size, it further limited the possibility of gestational-age misclassification. Moreover, as preeclampsia at term has been associated with neurodevelopmental outcomes,9 we reran the main analyses after excluding children born to mothers who suffered of preeclampsia or eclampsia during the pregnancy. Finally, we used information on maternal smoking (available only since 1999) in the subset of deliveries since 1999 to assess a possible confounding effect of smoking exposure (none or any during pregnancy). Statistical analyses were conducted using STATA 15 (StataCorp, College Station, Texas).
Results
A total of 2,880,401 pregnancies were registered in Norway between 1967 and 2015 (Figure 1). Birth weight data were missing for 0.3% and gestational-age data were missing for 4.2%. After excluding stillbirths, multiple births, and babies without recorded birth weight, there were 2,768,446 singleton babies (96%). After further restricting to those born at a gestational age of 39–41 weeks, 1,833,502 babies were available for analysis (75% of all term births). Among this group of narrowly defined term births, birth weight was normally distributed, with a mean of 3631g and a standard deviation of 468g (eFigure 2). Children with neurodevelopmental diagnoses were collectively less than 2% of these term births (eTable 2). Newborns were followed to an average age of 24 years (median 23 years, interquartile range 11–37 years), with maximum possible follow-up of 47 years.
Figure 1.
Flowchart of the study population selection
MBRN: Medical Birth Registry of Norway;
Factors associated with lighter birth weight included female sex, year of birth in earlier periods, younger mothers, nulliparous mothers, mothers without partners, and mothers with less education (Table 1). Adjustments by year of birth substantially changed some risk estimates, while adjustment for the additional potential confounders in Table 1 had little or no influence (eTable 2). We therefore are able to present the main analyses with adjustment only for year of birth.
Table 1.
Demographic characteristics of the Norwegian population born at term (39–41 weeks) in 1967–2015 according to their birth weighta
| Birth weight (in grams) | |||||||
|---|---|---|---|---|---|---|---|
| <2000 | 2000-<3000 | 3000-<3500 | 3500-<4000 | 4000-<5000 | ≥5000 | Any birth weight | |
|
|
|||||||
| N=1,010 (0.1%) | N=146,096 (8.0%) | N=582,523 (31.8%) | N=723,729 (39.5%) | N=372,734 (20.3%) | N=7,410 (0.4%) | N=1,833,502 (100%) | |
| Female | 57% | 61% | 55% | 47% | 39% | 29% | 49% |
| Weeks of gestation, mean | 39.7 | 39.7 | 39.9 | 40.0 | 40.2 | 40.4 | 40.0 |
| Birth year, mean | 1982 | 1989 | 1991 | 1992 | 1992 | 1993 | 1991 |
| Maternal age, years, mean | 26.7 | 26.9 | 27.4 | 28.0 | 28.7 | 29.8 | 27.9 |
| Maternal age ≥38 years | 6% | 4% | 4% | 4% | 5% | 8% | 4% |
| Nulliparous mothersb | 54% | 54% | 47% | 39% | 29% | 19% | 40% |
| Single mothers | 18% | 13% | 10% | 8% | 6% | 5% | 9% |
| Maternal educationc, mean | 3.5 | 3.9 | 4.1 | 4.3 | 4.4 | 4.3 | 4.2 |
| Paternal educationc, mean | 3.7 | 3.9 | 4.1 | 4.3 | 4.3 | 4.2 | 4.2 |
| Immigrant parents | 4% | 8% | 7% | 5% | 4% | 4% | 5% |
Abbreviations: N, number; SD, standard deviation;
Excluded are babies without registered birth weight, as well as stillbirths and multiple births.
Mothers that were nulliparous prior to birth of interest compared to parous.
Level of highest education on a national level scale of 0 (no formal education) to 8 (highest university degree, corresponding to a doctoral degree). Level 3 corresponds to 11–12 years and level 4 to 13 years of schooling.
Birth weight at term (39–41 weeks) was strongly associated with the risk of neurodevelopmental disabilities (Figure 2; eTable 2). There was a reverse J-shaped pattern of association for each of the neurodevelopmental outcomes. The pattern was strongest for cerebral palsy. Among babies weighing less than 3.5 kg, the risk of cerebral palsy increased steadily with smaller size, reaching 25-fold (95% CI: 8.0–79) for the smallest babies. The risk of hearing and vision disability was increased up to 16-fold (95% CI: 4.0–65), intellectual impairment up to 10.7-fold (95% CI: 6.9–17), schizophrenia nearly 7-fold (95% CI: 0.95–50), epilepsy 5.4-fold (95% CI: 2.6–12), and autism spectrum disorder and disorders of behavior including ADHD about 3.5-fold (95% CI: 1.3–9.4 and 2.1–5.4 respectively). Using babies weighing 3.5 to 3.9 kg as the referent, excess cases among smaller babies produced population attributable risks ranging from 19% for intellectual development and 18% for CP down to 5% for autism spectrum disorder. For most outcomes, there was also a slight upturn of risk at the heaviest weights, contributing negligible attributable risks.
Figure 2.
Odds ratios of various neurodevelopmental conditions among babies born at term (39–41 weeks) in the Norwegian population according to their birth weight
ORs are adjusted for year of birth (5-year-groups in 1967–2015). The neurodevelopmental conditions were retrieved from the National Insurance Scheme in Norway, where affected individuals are registered to receive social benefits (number with the condition and prevalence among term births of 39–41 weeks: CP, n=1,887, 1.1/1000; intellectual impairment, n=4,215, 2.4/1000; epilepsy, n=2,796, 1.6/1000; autism, n=3,516, 2.0/1000; hearing/vision disabilities, n=1,763, 1.0/1000; schizophrenia, n=2,138, 1.2/1000; ADHD, n=14,593, 8.2/1000). Individuals registered in the Norwegian Medical Birth Registry and not affected by these conditions served as comparison group. The last panel (All) shows that the association pattern is similar for all the conditions. Excluded are children without registered birth weight, multiple births, stillbirths, babies with registered congenital defects, and children who died before reaching age 5. Gestational age was estimated by ultrasound from 1999 and calculated from last menstrual period if ultrasound not available.
The associations of birth weight and neurodevelopmental outcomes were similar for boys and girls (eTable 3). Multivariable adjustment for measured confounding factors had little effect on these estimates (eTables 2 and 3). Exclusion of pregnancies with preeclampsia did not weaken our estimates (eTable 4). Adjustment for maternal smoking (available since 1999) also did not weaken the associations and in fact generally strengthened them (eFigure 3).
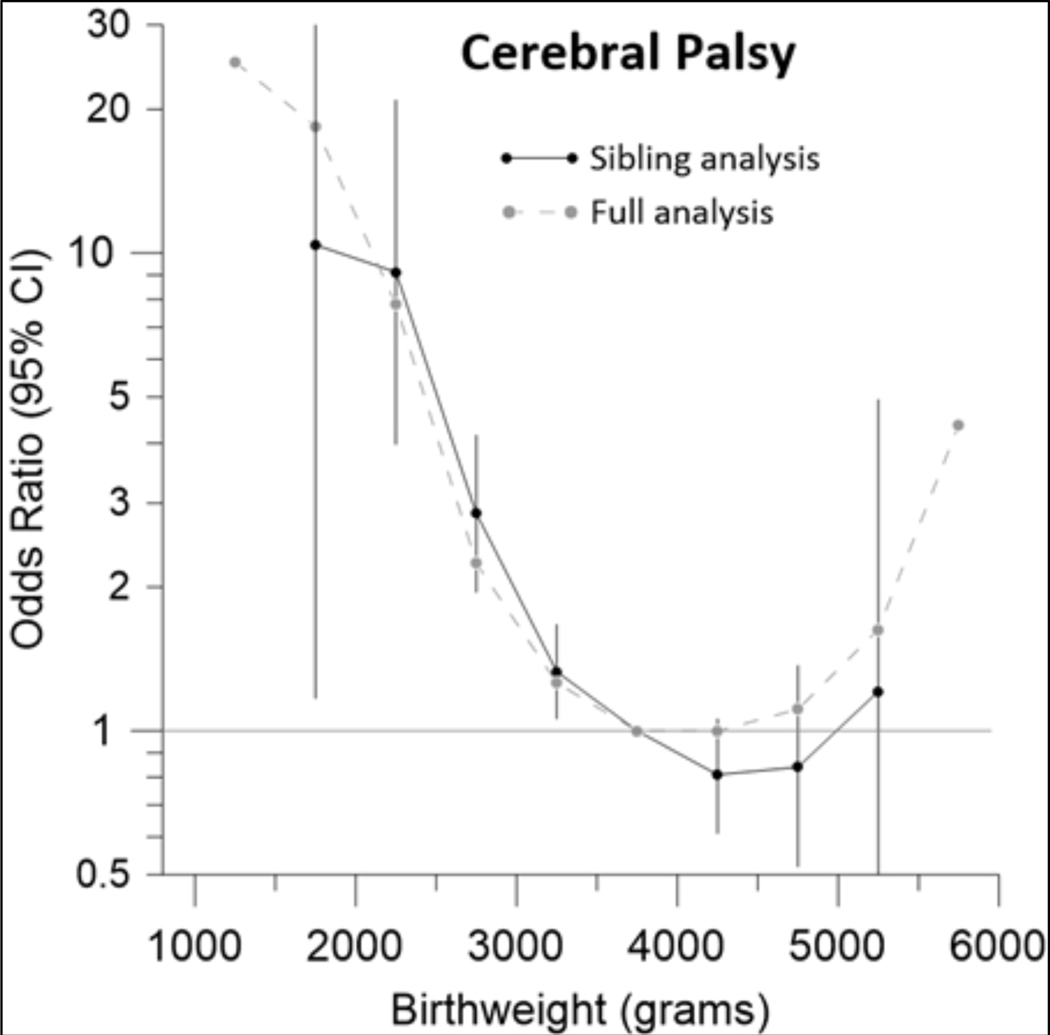
In order to better control for unmeasured confounders, we conducted a sibling analysis, comparing the weight-specific risk of our outcomes within families that had at least one affected and one unaffected child. Sibling analyses yielded patterns of birth weight-specific risk consistent with the main analyses, especially for low birth weights (Figure 3; eFigure 4).
Figure 3.
Odds ratios of cerebral palsy among siblings born at term (39–41 weeks) in the Norwegian population according to their birth weight
In the sibling analysis we matched and compared full siblings, of whom at least one developed CP. The full analysis shows results for all babies independent of relatedness (grey curve, compare Fig. 2). ORs of the sibling analysis are adjusted for year of birth (5-year-groups in 1967–2015), sex, maternal parity (0, 1, 2, 3, or ≥4), maternal age (≤19, 20–24, 25–29, 30–34, 35–39, ≥40 years), maternal civil status (married/cohabitant versus other) and, by design, for immigrant background, parental education, and shared genetic/environmental factors. CP diagnoses were retrieved from the National Insurance Scheme in Norway where affected individuals are registered to receive social benefits. Individuals registered in the Norwegian Medical Birth Registry and not affected by CP served as comparison group. Excluded are children without registered birth weight, multiple births, stillbirths, babies with registered congenital defects, and children who died before reaching age of 5. Gestational age was estimated by ultrasound from 1999 and calculated from last menstrual period if ultrasound not available.
Finally, we addressed the question of whether the smallest babies among our term births might include some misclassified preterm births. To more rigorously exclude this possibility, we conducted sensitivity analyses restricted to babies with gestational age of 39–41 weeks as determined by two independent assessments (maternal LMP and fetal ultrasound, available for 626,271 newborns). The patterns remained largely unchanged (Figure 4; eFigure 5).
Figure 4.
Odds ratios of cerebral palsy according to birth weight among babies in the Norwegian population born at term (39–41 weeks) as confirmed by two independent gestational age measures, maternal last menstrual period and fetal ultrasound (LMP & Ultrasound)
The grey curve (LMP) shows the results of the main analysis (compare Fig. 2), in which gestational age was estimated by ultrasound from 1999 and calculated from last menstrual period if ultrasound was not available. ORs are adjusted for year of birth (5-year-groups in 1967–2015). CP diagnoses were retrieved from the National Insurance Scheme in Norway, where affected individuals are registered to receive social benefits. Individuals registered in the Norwegian Medical Birth Registry and not affected by CP served as comparison group. Excluded are children without registered birth weight, multiple births, stillbirths, babies with registered birth defects, and children who died before reaching age of 5.
Discussion
There is a distinctive and pervasive pattern of neurodevelopmental morbidity related to birth weight at term. Every major category of disability showed increased risks among small babies reaching up to 25-fold, with slight increases also among large babies. These links between fetal growth and later neurologic function reinforce the importance of prenatal damage – independent of immaturity – in the etiology of neurodevelopmental problems.
Causal interpretation
It is unlikely that reduced fetal growth in itself causes neurodevelopmental damage. It is possible that, on rare occasions, small birth size could have direct effects, for example if small size were accompanied by limited reserve to overcome infection. However, such mechanisms are unlikely to play a significant role in developed countries. It is perhaps more plausible that large birth size could directly cause complications during labor that contribute to neurodevelopmental problems. Cerebral palsy in particular has been linked to difficult labor and delivery,10 although large babies are a minor contributor to CP.
The more likely interpretation is that birth size is a marker of prenatal insult.11,12 An example would be genetic syndromes, which can damage central nervous system development while impairing fetal growth. Other candidates are primary or secondary placental dysfunction, confined placental mosaicism, and intrauterine infection. There is growing evidence that subclinical in utero inflammation (infectious or non-infectious) can damage fetal growth, perhaps through effects on placental vasculature.13–15 Other pathologies of placentation or hypertensive disorders of pregnancy (e.g., preeclampsia) are known to reduce fetal growth,16 and preeclampsia has also been associated with lower cognitive abilities and an increased risk of CP, autism, and ADHD.9,17–19 Clinically unrecognized problems of placental development may contribute to our results. To the degree that such mechanisms are at work, strategies to improve outcomes simply by increasing size at birth are unlikely to be successful. The underlying causes of neurological damage and poor fetal growth would have to be addressed.
The link between large size and neurodevelopmental disability may also reflect the influence of prenatal factors, such as maternal BMI or maternal diabetes. Both obesity and diabetes (gestational, type 1, and type 2) are associated with heavier birth weights and also with impairments of neurologic development in the offspring.20–22
Previous literature
Exploratory clinical studies suggest a link between fetal growth and brain function. In one clinical report, brain metabolite differences by magnetic resonance spectroscopy were linked to subsequent reduced neurodevelopmental scores.23 A second clinical study found higher rates of immature EEG patterns associated with lower neurodevelopmental scores.24
Numerous epidemiologic studies have suggested broad associations between small size at term birth and risk of suboptimal or damaged neurodevelopment.25–44 Most are small or include only single outcomes, such as cognition, behavior, school performance, and general neurodevelopment at various ages. There are fewer studies of risk among term births for specific neurodevelopmental outcomes. Cerebral palsy has been the most thoroughly studied. Several projects based on population registries have described increased risk of cerebral palsy among small term births.45–47,48 The relation of intellectual impairment to term birth weight was well described in a recent population-based Swedish study, although the smallest 10% of babies were excluded from analysis.49 Less has been done on other neurodevelopmental disorders.
Swedish researchers have used national registries to assess fetal growth in relation to mental health conditions.50 Impaired fetal growth was related to autism and ADHD but not various mental-health difficulties (e.g. depression, alcoholism). Small size at term has also been linked to risk of schizophrenia,51,52 including a study based on Norwegian registries.53 While all these studies point to birth weight as a predictor of impaired brain function, even the best have grouped weights too coarsely to describe the high rates present among the smallest babies.48,54 For example, the comprehensive study of CP by Jarvis et al. reported relative risks in the range of 4 to 6 for the lowest ten percent of births,48 while we find a 25-fold risk in the very lowest weight category (95% CI 8–79).
Fetal growth restriction as a component of preterm risk?
There is a possibility that the well-known link between preterm delivery and neurodevelopmental problems is due not simply to immaturity but also to fetal growth restriction. It has been established that most preterm births are also growth restricted,55 suggesting a strong link between factors causing fetal growth restriction and factors causing preterm delivery. To the extent that small size at preterm delivery adds to the infant’s risk of subsequent disease, the neurodevelopmental risks observed with prematurity may be a combination of immaturity and impaired fetal growth. To our knowledge this possibility has not been discussed.
Strengths and limitations
Our study has the advantage of a prospective design with extended follow-up of an entire population. This minimizes the possibility of selection or ascertainment bias. There were few missing data for variables in the main analyses, further strengthening the validity of the findings. Fetal growth restriction was effectively separated from the correlated factor of preterm delivery by rigorously restricting births to a narrow group of full-term babies. Outcomes were based on physician diagnosis of disorders severe enough to warrant compensation, guaranteeing a high degree of specificity in the outcomes. A distinctive aspect of our study is the analysis of seven major neurodevelopmental outcomes across the full range of birth weights. In all cases, we find strong dose-response associations within the weights normally grouped as SGA (small-for-gestational-age) – a result that is not apparent with conventional analysis.
Unlike previous studies, we could systematically consider a range of neurodevelopmental conditions while controlling for some potential confounding factors. That said, the confounding factors for which we had data (other than year of birth) had virtually no impact on our results. This allowed us to provide results with a minimum of statistical modelling. Further support for the lack of important confounding emerged in our sibling analysis, which controlled for unmeasured confounding factors that are family-specific. The consistency of results in the sibship analysis provides further evidence that the underlying factors producing the observed associations are specific to the pregnancy, rather than to the mother and family.
Despite the large ORs among the smallest babies, excess risk among small birth weights amounted to only a modest proportion of term neurodevelopmental disabilities. In our analysis, the largest population attributable risk was 19% (for intellectual impairment). Small attributable risks are generally the case for adverse outcomes linked to small birth weights.
Our outcome definitions were limited by a lack of sensitivity, in that disabilities too mild to qualify for social benefits were not included. Still, the cases we do include are almost certainly true cases. We also miss very severe cases who died before reaching an age when their disability could be diagnosed – as is inevitably true for every study on this topic. Our exclusion of birth defects would not capture defects unrecorded at birth or emerging after birth.57 Although we followed births for up to several decades, the follow-up was too short for full identification of schizophrenia among those born in the most recent years. Finally, these data reflect occurrence in a relatively homogeneous Scandinavian population. It would be useful to confirm these findings in populations that differ in their culture, wealth, or ethnicity.
In sum, the risk of neurodevelopmental disabilities is increased many-fold among the smallest births at term. This signals the presence of prenatal insults that slow fetal growth and also lead to serious neurodevelopmental disability. These results provide further impetus for research on prenatal pathologies beyond immaturity that contribute to neuropsychiatric morbidity in later life.
Supplementary Material
References
- 1.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med 2008; 359(3): 262–73. [DOI] [PubMed] [Google Scholar]
- 2.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstet Gynecol Scand 2000; 79(6): 435–9. [PubMed] [Google Scholar]
- 3.Morken NH, Skjaerven R, Wilcox AJ. Ultrasound prediction of perinatal outcome: the unrecognised value of sibling data. BJOG 2015; 122(12): 1674–81. [DOI] [PubMed] [Google Scholar]
- 4.Norwegian Ministry of Labour and Social Affairs. The Norwegian Social Insurance Scheme. January2020. https://www.regjeringen.no/contentassets/03b0e088c8f44a8793ed0c0781556b11/a-0008-e-the-norwegian-social-insurance-scheme-2020.pdf (accessed August 11, 2020. [Google Scholar]
- 5.Moster D, Lie RT, Irgens LM, Bjerkedal T, Markestad T. The association of Apgar score with subsequent death and cerebral palsy: A population-based study in term infants. J Pediatr 2001; 138(6): 798–803. [DOI] [PubMed] [Google Scholar]
- 6.Agha MM, Williams JI, Marrett L, To T, Dodds L. Determinants of survival in children with congenital abnormalities: a long-term population-based cohort study. Birth Defects Res A Clin Mol Teratol 2006; 76(1): 46–54. [DOI] [PubMed] [Google Scholar]
- 7.Loomes R, Hull L, Mandy WPL. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry 2017; 56(6): 466–74. [DOI] [PubMed] [Google Scholar]
- 8.Romeo DM, Sini F, Brogna C, Albamonte E, Ricci D, Mercuri E. Sex differences in cerebral palsy on neuromotor outcome: a critical review. Dev Med Child Neurol 2016; 58(8): 809–13. [DOI] [PubMed] [Google Scholar]
- 9.Sun BZ, Moster D, Harmon QE, Wilcox AJ. Association of Preeclampsia in Term Births With Neurodevelopmental Disorders in Offspring. JAMA Psychiatry 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moster D, Wilcox AJ, Vollset SE, Markestad T, Lie RT. Cerebral palsy among term and postterm births. JAMA 2010; 304(9): 976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basso O, Wilcox AJ, Weinberg CR. Birth weight and mortality: causality or confounding? Am J Epidemiol 2006; 164(4): 303–11. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox AJ. On the importance--and the unimportance--of birthweight. Int J Epidemiol 2001; 30(6): 1233–41. [DOI] [PubMed] [Google Scholar]
- 13.Gomes J, Au F, Basak A, Cakmak S, Vincent R, Kumarathasan P. Maternal blood biomarkers and adverse pregnancy outcomes: a systematic review and meta-analysis. Crit Rev Toxicol 2019; 49(6): 461–78. [DOI] [PubMed] [Google Scholar]
- 14.Weckman AM, Ngai M, Wright J, McDonald CR, Kain KC. The Impact of Infection in Pregnancy on Placental Vascular Development and Adverse Birth Outcomes. Front Microbiol 2019; 10: 1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Welch BM, Keil AP, van ‘t Erve TJ, et al. Longitudinal profiles of plasma eicosanoids during pregnancy and size for gestational age at delivery: A nested case-control study. PLoS Med 2020; 17(8): e1003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Preeclampsia. Lancet 2016; 387(10022): 999–1011. [DOI] [PubMed] [Google Scholar]
- 17.Curran EA, O’Keeffe GW, Looney AM, et al. Exposure to Hypertensive Disorders of Pregnancy Increases the Risk of Autism Spectrum Disorder in Affected Offspring. Mol Neurobiol 2018; 55(7): 5557–64. [DOI] [PubMed] [Google Scholar]
- 18.Zhu T, Gan J, Huang J, Li Y, Qu Y, Mu D. Association Between Perinatal Hypoxic Ischemic Conditions and Attention-Deficit/Hyperactivity Disorder: A Meta-Analysis. J Child Neurol 2016; 31(10): 1235–44. [DOI] [PubMed] [Google Scholar]
- 19.Tuovinen S, Eriksson JG, Kajantie E, Raikkonen K. Maternal hypertensive pregnancy disorders and cognitive functioning of the offspring: a systematic review. J Am Soc Hypertens 2014; 8(11): 832–47 e1. [DOI] [PubMed] [Google Scholar]
- 20.Forthun I, Wilcox AJ, Strandberg-Larsen K, et al. Maternal Prepregnancy BMI and Risk of Cerebral Palsy in Offspring. Pediatrics 2016; 138(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nahum Sacks K, Friger M, Shoham-Vardi I, et al. Prenatal exposure to gestational diabetes mellitus as an independent risk factor for long-term neuropsychiatric morbidity of the offspring. Am J Obstet Gynecol 2016; 215(3): 380 e1–7. [DOI] [PubMed] [Google Scholar]
- 22.Godfrey KM, Reynolds RM, Prescott SL, et al. Influence of maternal obesity on the long-term health of offspring. Lancet Diabetes Endocrinol 2017; 5(1): 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simoes RV, Cruz-Lemini M, Bargallo N, Gratacos E, Sanz-Cortes M. Brain metabolite differences in one-year-old infants born small at term and association with neurodevelopmental outcome. Am J Obstet Gynecol 2015; 213(2): 210 e1- e11. [DOI] [PubMed] [Google Scholar]
- 24.Castro Conde JR, Gonzalez Campo C, Gonzalez Gonzalez NL, et al. Assessment of neonatal EEG background and neurodevelopment in full-term small for their gestational age infants. Pediatr Res 2020; 88(1): 91–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laerum AMW, Reitan SK, Evensen KAI, et al. Psychiatric symptoms and risk factors in adults born preterm with very low birthweight or born small for gestational age at term. BMC Psychiatry 2019; 19(1): 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larroque B, Bertrais S, Czernichow P, Leger J. School difficulties in 20-year-olds who were born small for gestational age at term in a regional cohort study. Pediatrics 2001; 108(1): 111–5. [DOI] [PubMed] [Google Scholar]
- 27.Matte TD, Bresnahan M, Begg MD, Susser E. Influence of variation in birth weight within normal range and within sibships on IQ at age 7 years: cohort study. BMJ 2001; 323(7308): 310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Keeffe MJ, O’Callaghan M, Williams GM, Najman JM, Bor W. Learning, cognitive, and attentional problems in adolescents born small for gestational age. Pediatrics 2003; 112(2): 301–7. [DOI] [PubMed] [Google Scholar]
- 29.Paz I, Gale R, Laor A, Danon YL, Stevenson DK, Seidman DS. The cognitive outcome of full-term small for gestational age infants at late adolescence. Obstet Gynecol 1995; 85(3): 452–6. [DOI] [PubMed] [Google Scholar]
- 30.Raznahan A, Greenstein D, Lee NR, Clasen LS, Giedd JN. Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci U S A 2012; 109(28): 11366–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth S, Chang TC, Robson S, Spencer JA, Wyatt JS, Stewart AL. The neurodevelopmental outcome of term infants with different intrauterine growth characteristics. Early Hum Dev 1999; 55(1): 39–50. [DOI] [PubMed] [Google Scholar]
- 32.Sommerfelt K, Andersson HW, Sonnander K, et al. Cognitive development of term small for gestational age children at five years of age. Arch Dis Child 2000; 83(1): 25–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strauss RS. Adult functional outcome of those born small for gestational age: twenty-six-year follow-up of the 1970 British Birth Cohort. JAMA 2000; 283(5): 625–32. [DOI] [PubMed] [Google Scholar]
- 34.Strauss RS, Dietz WH. Growth and development of term children born with low birth weight: effects of genetic and environmental factors. J Pediatr 1998; 133(1): 67–72. [DOI] [PubMed] [Google Scholar]
- 35.Takeuchi A, Yorifuji T, Takahashi K, et al. Behavioral outcomes of school-aged full-term small-for-gestational-age infants: A nationwide Japanese population-based study. Brain Dev 2017; 39(2): 101–6. [DOI] [PubMed] [Google Scholar]
- 36.Tosun A, Gurbuz-Ozgur B, Aksu H, Kaynak-Turkmen M. The long-term neurodevelopmental outcomes of infants born full-term with low birth weight. Turk J Pediatr 2017; 59(2): 169–76. [DOI] [PubMed] [Google Scholar]
- 37.Arcangeli T, Thilaganathan B, Hooper R, Khan KS, Bhide A. Neurodevelopmental delay in small babies at term: a systematic review. Ultrasound Obstet Gynecol 2012; 40(3): 267–75. [DOI] [PubMed] [Google Scholar]
- 38.Malin GL, Morris RK, Riley RD, Teune MJ, Khan KS. When is birthweight at term (>/=37 weeks’ gestation) abnormally low? A systematic review and meta-analysis of the prognostic and predictive ability of current birthweight standards for childhood and adult outcomes. BJOG 2015; 122(5): 634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eixarch E, Meler E, Iraola A, et al. Neurodevelopmental outcome in 2-year-old infants who were small-for-gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol 2008; 32(7): 894–9. [DOI] [PubMed] [Google Scholar]
- 40.Indredavik MS, Vik T, Heyerdahl S, Kulseng S, Brubakk AM. Psychiatric symptoms in low birth weight adolescents, assessed by screening questionnaires. Eur Child Adolesc Psychiatry 2005; 14(4): 226–36. [DOI] [PubMed] [Google Scholar]
- 41.Mello B, Gagliardo H, Goncalves V. Neurodevelopment of small-for-gestational age infants: behavioral aspects in first year. Arq Neuropsiquiatr 2014; 72(7): 517–23. [DOI] [PubMed] [Google Scholar]
- 42.Savchev S, Sanz-Cortes M, Cruz-Martinez R, et al. Neurodevelopmental outcome of full-term small-for-gestational-age infants with normal placental function. Ultrasound Obstet Gynecol 2013; 42(2): 201–6. [DOI] [PubMed] [Google Scholar]
- 43.Tamai K, Yorifuji T, Takeuchi A, et al. Associations of Birth Weight for Gestational Age with Child Health and Neurodevelopment among Term Infants: A Nationwide Japanese Population-Based Study. J Pediatr 2020. [DOI] [PubMed] [Google Scholar]
- 44.Yi KH, Yi YY, Hwang IT. Behavioral and intelligence outcome in 8- to 16-year-old born small for gestational age. Korean J Pediatr 2016; 59(10): 414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahlseng MO, Andersen GL, Irgens LM, Skranes J, Vik T. Risk of cerebral palsy in term-born singletons according to growth status at birth. Dev Med Child Neurol 2014; 56(1): 53–8. [DOI] [PubMed] [Google Scholar]
- 46.Jacobsson B, Ahlin K, Francis A, Hagberg G, Hagberg H, Gardosi J. Cerebral palsy and restricted growth status at birth: population-based case-control study. BJOG 2008; 115(10): 1250–5. [DOI] [PubMed] [Google Scholar]
- 47.Stoknes M, Andersen GL, Dahlseng MO, et al. Cerebral palsy and neonatal death in term singletons born small for gestational age. Pediatrics 2012; 130(6): e1629–35. [DOI] [PubMed] [Google Scholar]
- 48.Jarvis S, Glinianaia SV, Torrioli MG, et al. Cerebral palsy and intrauterine growth in single births: European collaborative study. Lancet 2003; 362(9390): 1106–11. [DOI] [PubMed] [Google Scholar]
- 49.Chen R, Tedroff K, Villamor E, Lu D, Cnattingius S. Risk of intellectual disability in children born appropriate-for-gestational-age at term or post-term: impact of birth weight for gestational age and gestational age. Eur J Epidemiol 2020; 35(3): 273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pettersson E, Larsson H, D’Onofrio B, Almqvist C, Lichtenstein P. Association of Fetal Growth With General and Specific Mental Health Conditions. JAMA Psychiatry 2019; 76(5): 536–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abel KM, Wicks S, Susser ES, et al. Birth weight, schizophrenia, and adult mental disorder: is risk confined to the smallest babies? Arch Gen Psychiatry 2010; 67(9): 923–30. [DOI] [PubMed] [Google Scholar]
- 52.Gunnell D, Rasmussen F, Fouskakis D, Tynelius P, Harrison G. Patterns of fetal and childhood growth and the development of psychosis in young males: a cohort study. Am J Epidemiol 2003; 158(4): 291–300. [DOI] [PubMed] [Google Scholar]
- 53.Eide MG, Moster D, Irgens LM, et al. Degree of fetal growth restriction associated with schizophrenia risk in a national cohort. Psychol Med 2013; 43(10): 2057–66. [DOI] [PubMed] [Google Scholar]
- 54.Leonard H, Nassar N, Bourke J, et al. Relation between intrauterine growth and subsequent intellectual disability in a ten-year population cohort of children in Western Australia. Am J Epidemiol 2008; 167(1): 103–11. [DOI] [PubMed] [Google Scholar]
- 55.Hutcheon JA, Platt RW. The missing data problem in birth weight percentiles and thresholds for “small-for-gestational-age”. Am J Epidemiol 2008; 167(7): 786–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.