Abstract
Malnutrition is associated with treatment-related toxicities (TRT) in adults with solid tumors and in children with leukemia. Few studies have assessed whether malnutrition in pediatric patients treated for solid tumors impacts risk for TRT, relapse, and/or survival. To address this knowledge gap, this retrospective study evaluated the association between body mass index (BMI) at diagnosis, and imputed BMI during therapy, on the prevalence of TRT, specific toxicities, relapse, and survival in pediatric patients with solid tumors treated with cisplatin-containing regimens. Kaplan-Meier curves and regression models evaluated the association between patient-specific characteristics (including BMI) and TRT, relapse, and survival. The cohort included 221 patients, of whom 22% were malnourished at diagnosis (10% were underweight and 12% were obese). Most patients (60%) experienced at least one severe TRT, and 30% developed more than one severe TRT. Most patients with obesity at diagnosis remained obese during therapy (62%). In multivariable analysis, obesity at diagnosis was significantly associated with a more than threefold greater risk for developing severe TRT (p=0.037), specifically for acute or chronic kidney injury (p=0.014). Obesity at diagnosis and adolescent and young adult age (≥15 years at diagnosis) were associated with worse event-free survival (hazard ratio [HR] 2.32, p=0.024 and HR 2.28, p=0.010, respectively) and overall survival (HR 3.69, p=0.006 and HR 2.6, p=0.012, respectively). Obese and older patients therefore constitute populations at-risk for poorer outcomes. Prospective studies are warranted to gain further insight into the mechanism and role of obesity and adolescence in developing TRT and/or treatment failure.
Keywords: cisplatin, childhood cancer, obesity, adolescent and young adult, body mass index
Introduction
Treatment-related toxicities (TRT) pose significant challenges to the management of children with solid tumors. In addition to necessitating chemotherapy dose adjustments and plan modifications which may affect risk of treatment failure, comorbidity from TRT profoundly impacts quality of life both during and long after therapy. Emerging evidence in adults with solid tumors suggests nutritional status is associated with TRT and outcomes. Worse survival has been demonstrated in adults who develop excessive (≥10%) weight loss or gain during therapy.1–4 Similarly, in those treated specifically with cisplatin-containing regimens, extremes of weight are associated with greater toxicity and poorer survival.5,6 Identifying the role of body composition in moderating risk for TRT could potentially enable strategies to reduce toxicity and thus improve therapy delivery and quality of life.
In pediatric malignancies, corresponding data for the impact of malnutrition and body composition (as assessed by body mass index [BMI]) are sparse. Children with acute lymphoblastic leukemia and extreme weight (obese or underweight) have inferior event-free survival (EFS) and greater TRT.11 Similarly, pediatric patients treated for Ewing sarcoma and certain forms of rhabdomyosarcoma who develop excessive weight loss and/or abnormal BMI experience more hospital days, poorer response to treatment, worse overall survival (OS) 2–4, and, in some reports, greater TRT overall.3,12 Prior studies for BMI in pediatric solid tumors have been disease-specific with inconsistent findings for risk of TRT. While preclinical and clinical data indicate BMI may impact cisplatin clearance7–9, very few studies have focused on the association of BMI with TRT from cisplatin-based therapy.10 As cisplatin-based regimens constitute nearly 40% of all childhood malignancies, the aim of this study was therefore to evaluate the association between BMI, TRT, and treatment outcome in this population.
Methods
Patient Population
We established a retrospective cohort of patients treated between January 2009 and December 2017 at Children’s Hospital Los Angeles with cisplatin-based chemotherapy regimens for a previously untreated non-hematologic malignancy. Additional inclusion criteria included availability of height and weight measurements at diagnosis, age 2–20 years for calculation of BMI in a United States population,13 and treatment and toxicity data for at least one cycle of chemotherapy. Demographic (age, sex, ethnicity, and BMI), diagnosis, and treatment (chemotherapy, radiation therapy, and autologous bone marrow transplant [ABMT]) information were abstracted from the electronic medical record.
Toxicities
Thresholds were established for clinically significant TRT resulting in organ damage and/or affecting therapy-delivery to formulate a list of specific toxicities of interest. Toxicity categories of interest included neurologic, gastrointestinal (GI), renal/genitourinary (GU), and infectious. Individual toxicities of interest included mucositis, acute kidney injury/chronic kidney disease (AKI/CKD), neuropathy/neuralgia, and documented blood stream infection (BSI). Laboratory results and clinical documentation were reviewed for each potential toxicity. A complete list of all screened toxicities is included in Table S1. Toxicities were graded using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 except for ototoxicity, which was graded per the consensus International Society of Pediatric Oncology (SIOP) Boston Ototoxicity Scale14, and electrolyte disturbances, which were included as “severe” if requiring intervention. The grading threshold for “severe” toxicity was specified for each adverse event for purposes of this study to reflect clinical relevance. If a patient received ABMT consolidation as part of their first therapy attempt, toxicities were captured from start of therapy until the time of transplantation.
BMI and AYA classification
BMI percentile was calculated using the Centers for Disease Control and Prevention age/sex-adjusted population norms for individuals between 2 and 20 years of age.15 All patients <20 years of age were then classified at diagnosis as underweight (UW, BMI <5th percentile), obese (OB, BMI≥95th percentile), or not underweight or obese (not UW/OB, 5–94.9 percentile). To enable interpolation of BMI to the time of TRT, BMI during therapy was evaluated by classifying BMI as OB at the start and end of therapy (Always OB), underweight at the start and end of therapy (Always UW), or not underweight or OB at the start and end of therapy (Never UW/OB). Adolescent and young adult (AYA) patients were defined as age ≥15 years at diagnosis.
Study Endpoints and Statistical Approach
The primary endpoint consisted of the presence of at least one severe TRT. Other clinical endpoints of interest were the presence of multiple severe TRT, association with toxicity categories, specific targeted toxicities, treatment-related mortality (TRM), pediatric intensive care unit (PICU) admissions, and chemotherapy dose reductions. Prevalence of each endpoint was compared across BMI categories at diagnosis and BMI during therapy. All comparisons used the Pearson’s chi-square or Fisher’s exact test as appropriate. Lowess regression was used to evaluate the association between BMI at diagnosis as a continuous variable and probability of severe TRT. Multivariable logistic regression for the primary endpoint included BMI category at diagnosis or during therapy and cancer diagnosis, sex, and AYA. Ethnicity was then tested against each model and retained if alpha <0.15. EFS was defined as time from start of therapy to relapse, disease progression, second cancer, or death. OS was defined as time from start of therapy to death or last follow-up. Kaplan-Meier curves were constructed for EFS and OS and compared by log-rank test (LRT). A Cox multiple regression model was constructed to explore the effect of BMI category on EFS and OS using backwards stepwise selection with variables retained at an alpha <0.15. BMT was included as a time-dependent covariate. All tests were two-sided unless otherwise stated and p<0.05 was considered significant. All statistical analyses were conducted using STATA/SE 15.16
Results
Patient characteristics and BMI classification
A total of 221 patients were included in this cohort. Patient characteristics are summarized in Table 1. Most patients were under 15 years of age (76%, 167/221), male (58%, 129/221), and of Hispanic ethnicity (52%, 114/221). The majority of patients were not UW/OB at diagnosis (79%, 174/221) and remained a healthy BMI during therapy (83%, 144). Most of those who were OB at diagnosis (12%, 26/221) similarly remained OB at end of therapy (62%, 16/26). There were no significant differences for BMI category in AYA compared to younger patients (p=0.922). More Hispanic than non-Hispanic patients were OB at diagnosis (63% vs 37%, p=0.034). TRT was common as 60% (132/221) of the cohort experienced at least one severe TRT and approximately one-third developed more than one severe TRT (median of 3 TRT) (Table 2). A minority of patients required dose-reductions (20%, 44/221) or PICU admission (8%, 17/221) due to TRT. Of note, treatment-related mortality was rare (0.5%, 1/221).
TABLE 1:
Patient Characteristics
| Number (%) | |
|---|---|
| Total | 221 (100) |
| AYA at diagnosis | |
| <15 years | 167 (76) |
| ≥15 years | 54 (24) |
| Sex | |
| Female | 92 (42) |
| Male | 129 (58) |
| Ethnicity | |
| Not Hispanic | 93 (42) |
| Hispanic | 114 (52) |
| Unknown | 14 (6) |
| BMI at diagnosis | |
| Not UW/OB | 174 (79) |
| UW | 21 (10) |
| OB | 26 (12) |
| Diagnosis | |
| CNS tumor | 59 (26) |
| Germ cell tumor | 48 (22) |
| Hepatic tumor | 20 (9) |
| Neuroblastoma | 31 (14) |
| Osteosarcoma | 48 (22) |
| Other BSTT | 15 (7) |
| Radiation therapy | |
| None | 120 (54) |
| Prior to EOT | 53 (24) |
| After EOT** | 48 (22) |
| Radiation site | |
| None | 120 (69) |
| Cranium±Spine | 41 (24) |
| Extra-cranial1 | 9 (5) |
| Skeletal only | 3 (2) |
extracranial sites include head and neck, chest, & abdomen
AYA = adolescent and young adult; BMI = body mass index; UW = underweight (BMI <5%); OB = obese (BMI ≥95%); CNS = central nervous system; BSTT = bone and soft tissue tumor; EOT = end of therapy.
TABLE 2:
Prevalence of severe toxicity
| No TRT* | TRT* | p-value1 | |||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Entire cohort | 89 | (40) | 132 | (60) | |
| AYA at diagnosis | 0.021 | ||||
| <15 years | 60 | (36) | 107 | (64) | |
| ≥15 years | 29 | (54) | 25 | (46) | |
| Sex | 0.092 | ||||
| Female | 31 | (34) | 61 | (66) | |
| Male | 58 | (45) | 71 | (55) | |
| Ethnicity | 0.001 | ||||
| Not Hispanic | 27 | (29) | 66 | (71) | |
| Hispanic | 51 | (45) | 63 | (55) | |
| Unknown | 11 | (79) | 3 | (21) | |
| BMI at diagnosis | 0.077 | ||||
| Not UW/OB | 76 | (44) | 98 | (56) | |
| UW | 4 | (19) | 17 | (81) | |
| OB | 9 | (35) | 17 | (65) | |
| Diagnosis | <0.001 | ||||
| CNS tumor | 14 | (24) | 45 | (76) | |
| Germ cell tumor | 40 | (83) | 8 | (17) | |
| Hepatic tumor | 9 | (45) | 11 | (55) | |
| Neuroblastoma | 5 | (16) | 26 | (84) | |
| Osteosarcoma | 15 | (31) | 33 | (69) | |
| Other BSTT | 6 | (40) | 9 | (60) | |
| Radiation therapy | 0.003 | ||||
| None | 61 | (51) | 59 | (49) | |
| Prior to EOT | 14 | (26) | 39 | (74) | |
| After EOT** | N/A | N/A | N/A | N/A | |
| Radiation site | 0.016 | ||||
| None | 61 | (51) | 59 | (49) | |
| Cranium±Spine | 12 | (29) | 29 | (71) | |
| Extra-cranial2 | 2 | (22) | 7 | (78) | |
| Skeletal only | 0 | (0) | 3 | (100) | |
Frequency TRT reported per co-variable (row);
Included in survival analyses.
chi-square or Fisher exact test for TRT vs no TRT.
extracranial sites include head and neck, chest, & abdomen.
TRT= treatment-related toxicity; AYA = adolescent and young adult; BMI = body mass index; UW = underweight (BMI <5%); OB = obese (BMI ≥95%); CNS = central nervous system; BSTT = bone and soft tissue tumor; N/A = not applicable; EOT = end of therapy.
BMI and TRT
In multivariable analysis inclusive of cancer diagnosis, there was a significant association between OB at diagnosis and the presence of at least one severe TRT during therapy (odds ratio [OR] 3.36, 95% confidence interval [95%CI] 1.07–10.51, p=0.037) (Table 3). The association of interpolated obesity during therapy (i.e., those OB at diagnosis and EOT) and TRT did not reach the threshold for significance (OR 2.04, 95%CI 0.55–7.63, p=0.289) (Table 3). Of note, there was increased prevalence of severe TRT at both extremes of BMI at diagnosis (Figure S1) and increased risk for TRT at the extremes in both multivariable models (Table 3). Prevalence of multiple severe TRT, PICU admissions, and dose-reduction were not associated with BMI category at diagnosis (p=0.293, p=0.429, p=0.543, respectively) or BMI during therapy (p=0.260, p=0.817, and p=0.153 respectively) (Table 4). There was an association between BMI at diagnosis and renal/GU toxicities (p=0.031), but not among BMI at diagnosis and any other toxicity category or targeted toxicity (Table 4). On multivariable analysis of AKI/CKD inclusive of age and diagnosis, obesity was associated with significantly greater risk for AKI/CKD (OR 3.89, 95%CI 1.31–11.54, p=0.014) (Table S2). Only one patient (0.5%) developed cardiomyopathy during therapy. Analysis of the subset of patients who did not change BMI category between diagnosis and EOT found no association between BMI category during therapy and prevalence of any individual toxicity category or targeted toxicity (Table 4). Ethnicity was not a significant risk factor for TRT.
TABLE 3:
Multivariable analysis of severe treatment-related toxicity
| At Diagnosis | During Therapy* | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% Confidence Interval | p-value | Odds Ratio | 95% Confidence Interval | p-value | |||
| BMI Classification | ||||||||
| Not UW/OB | 1 | -- | -- | 1 | -- | -- | ||
| UW | 3.71 | 0.98–14.01 | 0.053 | 6.39 | 0.97–42.26 | 0.054 | ||
| OB | 3.36 | 1.07–10.51 | 0.037 | 2.04 | 0.55–7.63 | 0.289 | ||
| Sex-Female | 1.62 | 0.84–3.12 | 0.149 | 1.55 | 0.76–3.17 | 0.232 | ||
| AYA- ≥15 years | 1.45 | 0.63–3.30 | 0.381 | 1.63 | 0.65–4.08 | 0.295 | ||
| Diagnosis | ||||||||
| Germ Cell Tumor | 1 | -- | -- | 1 | -- | -- | ||
| Hepatoblastoma | 11.04 | 2.98–40.91 | <0.001 | 10.69 | 2.50–45.76 | 0.001 | ||
| CNS | 23.97 | 7.70–74.58 | <0.001 | 19.66 | 5.56–69.55 | <0.001 | ||
| Neuroblastoma | 39.14 | 9.94–154.14 | <0.001 | 28.37 | 6.18–130.19 | <0.001 | ||
| Osteosarcoma | 15.48 | 5.18–46.25 | <0.001 | 13.08 | 4.06–42.11 | <0.001 | ||
| Other | 9.98 | 2.49–40.09 | 0.001 | 7.80 | 1.72–35.46 | 0.008 | ||
Includes patients in same BMI category at diagnosis and end of therapy (“Always Obese,” “Always Underweight”).
UW= underweight; OB= obese; AYA= adolescent and young adult; CNS= central nervous system
TABLE 4:
Prevalence of toxicities by weight classification at diagnosis and during therapy
| BMI Classification at Diagnosis | BMI Classification During Therapy | |||||||
|---|---|---|---|---|---|---|---|---|
| UW (n=21) | Not UW/ OB (n=174) | OB (n=26) | p-value** | Always UW (n=10) | Never UW/OB (n=144) | Always OB (n=16) | p-value** | |
| n (%)* | n (%)* | n (%)* | n (%)* | n (%)* | n (%)* | |||
| Toxicity Categories | ||||||||
| Neuro | 9 (43) | 35 (20) | 4 (15) | 0.057 | 3 (30) | 29 (20) | 1 (6) | 0.299 |
| GI | 10 (48) | 65 (37) | 9 (35) | 0.582 | 4 (40) | 52 (36) | 2 (13) | 0.144 |
| Renal/GU | 3 (14) | 23 (13) | 9 (35) | 0.031 | 1 (10) | 16 (11) | 3 (19) | 0.611 |
| Infection | 6 (29) | 35 (20) | 6 (23) | 0.582 | 4 (40) | 25 (17) | 2 (13) | 0.203 |
| Individual Targeted Toxicities | ||||||||
| Mucositis | 5 (24) | 42 (24) | 4 (15) | 0.683 | 2 (20) | 39 (27) | 1 (6) | 0.229 |
| Acute/Chronic Kidney Injury | 2 (10) | 22 (13) | 7 (27) | 0.162 | 0 (0) | 16 (11) | 2 (13) | 0.658 |
| Neuropathy/Neuralgia | 2 (10) | 14 (8) | 2 (8) | 0.906 | 0 (0) | 11 (8) | 0 (0) | 0.801 |
| Ototoxicity | 5 (24) | 20 (11) | 3 (12) | 0.303 | 2 (20) | 19 (13) | 1 (6) | 0.558 |
| BSI | 6 (29) | 35 (20) | 6 (23) | 0.582 | 4 (40) | 25 (17) | 2 (13) | 0.203 |
| Multiple Severe TRT | 9 (43) | 49 (28) | 7 (27) | 0.293 | 4 (40) | 35 (24) | 2 (13) | 0.260 |
| PICU Admission | 3 (14) | 13 (7) | 1 (4) | 0.429 | 1 (10) | 10 (7) | 1 (6) | 0.817 |
| Dose Reduction | 5 (24) | 36 (21) | 3 (12) | 0.543 | 0 (0) | 30 (21) | 1 (6) | 0.153 |
number of patients and % of BMI group with toxicity endpoint.
p-value comparing among BMI and each toxicity category and targeted toxicity.
BMI= body mass index; UW= underweight; OB= obese; GI = gastro-intestinal toxicity; GU= genitourinary; BSI= documented blood stream infection; TRT= treatment-related toxicity PICU= pediatric intensive care unit
BMI and Survival
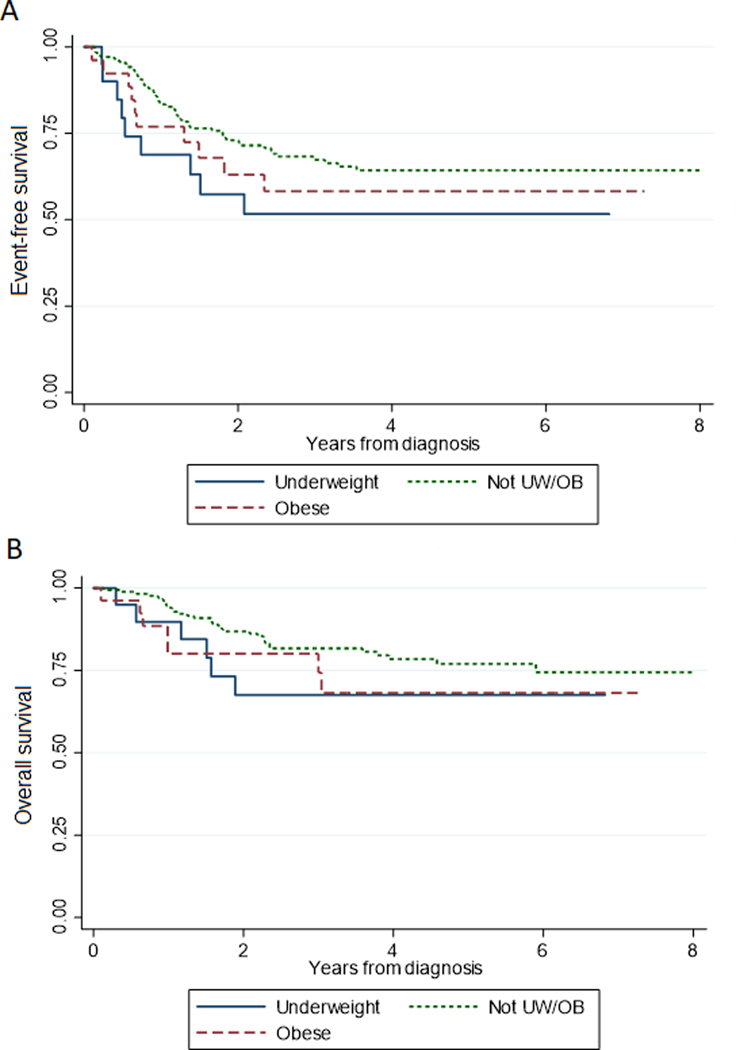
The median duration of follow-up was 3.1 years from diagnosis. While in univariable analysis no association was found between BMI category at diagnosis and EFS (LRT p=0.194) or OS (LRT p=0.316) (Figures 1A and B), in multivariable analysis (Table 5), obesity at diagnosis was associated with poorer EFS (hazard ratio [HR] 2.32, 95%CI 1.12–4.83, p=0.024) and OS (HR 3.69, 95%CI 1.47–9.37, p=0.006). AYA age was significantly associated with poorer EFS (HR 2.28, 95%CI 1.22–4.26, p=0.010), and OS (HR 2.60, 95%CI 1.23–5.47, p=0.012) even after accounting for impact of diagnosis.
Figure 1: Event-free and overall survival stratified by body mass index at diagnosis.
Kaplan-Meier curves stratified by body mass index (BMI) category at diagnosis for event–free survival (A), and overall survival (B). BMI category defined using CDC sex/age-adjusted norms for underweight (UW) defined as BMI <5%, obese (OB) ≥95%, and Not UW/OB as BMI 5–94.9%.
TABLE 5:
Multivariable analysis of BMI at diagnosis and EFS and OS
| Covariable* | EFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-value | HR | 95%CI | p-value | |
| BMI at Diagnosis | ||||||
| Not UW/OB | 1 | -- | -- | 1 | -- | -- |
| UW | 1.59 | 0.77–3.29 | 0.208 | 2.21 | 0.88–5.55 | 0.092 |
| OB | 2.32 | 1.12–4.83 | 0.024 | 3.69 | 1.47–9.26 | 0.006 |
| AYA, ≥15 years | 2.28 | 1.22–4.26 | 0.010 | 2.60 | 1.23–5.47 | 0.012 |
| Diagnosis | ||||||
| Germ Cell Tumor | 1 | -- | -- | 1 | -- | -- |
| Hepatoblastoma | 3.14 | 0.91–10.83 | 0.070 | 13.15 | 4.50–69.19 | 0.002 |
| CNS | 1.02 | 0.40–2.60 | 0.974 | 8.01 | 1.99–32.22 | 0.003 |
| Neuroblastoma | 0.65 | 0.23–1.83 | 0.415 | 4.87 | 1.03–23.15 | 0.046 |
| Osteosarcoma | 4.04 | 1.64–9.95 | 0.002 | 8.88 | 2.15–36.65 | 0.003 |
| Other | 3.15 | 0.91–11.00 | 0.071 | 16.76 | 3.22–87.17 | 0.001 |
| Dose Reduction | 1.74 | 0.94–3.24 | 0.080 | NS | ||
| Radiation Therapy | NS | 0.71 | 0.32–1.59 | 0.405 | ||
| Treatment-Related Toxicity | NS | 0.56 | 0.28–1.11 | 0.098 | ||
| ABMT** | 15.41 | 6.67–35.58 | <0.001 | 4.55 | 1.76–11.76 | 0.002 |
Individual multivariable models constructed for each endpoint using stepwise selection, see Methods.
Included as time-varying covariable.
EFS= event-free survival; OS= overall survival; HR= hazard ratio; CI= confidence interval; BMI= body mass index; UW= underweight; OB=obese; AYA= adolescent and young adult; ABMT= autologous bone marrow transplant; NS= not significant
Discussion
In our cohort of children and adolescents with solid tumors receiving cisplatin-containing chemotherapy regimens, we found evidence that body composition potentially influences risk for treatment toxicity and survival. Obesity was associated with increased risk for developing severe TRT, with a trend suggesting increased risk in the underweight group as well. Interestingly, of the various TRT examined, the risk for renal injury in the obese was significant, a concerning finding inasmuch as this is a dose-limiting toxicity. Independent of TRT and dose-reductions, obesity at diagnosis was also associated with poorer EFS and OS. Thus, malnutrition during cisplatin-therapy potentially impacts both risk for acute TRT and survival.
Known toxicities of cisplatin include ototoxicity, neurotoxicity, nephrotoxicity, myelosuppression, and cardiovascular disease.17,18 Yet, few studies have reported the association between BMI classification, TRT, and survival in patients with solid tumors. In adult patients, BMI at diagnosis impacted toxicity and survival for primary advanced endometrial cancer and other solid tumors.5,6 In pediatric patients, two studies of solid tumors explored the impact of BMI in non-cisplatin containing regimens and found conflicting results. In one study limited to patients with rhabdomyosarcoma, >10% weight loss at 24 weeks was associated with more toxicities and hospital days, and patients with borderline underweight status had inferior survival. However, there was no difference in survival between average weight and overweight or obese patients.3 Conversely, in patients with Ewing sarcoma, patients of Hispanic ethnicity and younger patients were more likely to develop grade 3 or 4 toxicities, but BMI was not independently associated with toxicity.12 In the lone study of pediatric patients receiving cisplatin, those being treated for osteosarcoma with high BMI developed more renal dysfunction and potentially myelosuppression as well as significantly poorer OS.2 In our cohort, obesity was also associated with risk for renal injury. By approaching this question from the perspective of exposure to chemotherapy agent, and not diagnosis, our findings validate the concern for elevated nephrotoxic risk in the obese across the range of cisplatin-treated tumors. Moreover, after accounting for cancer diagnosis, BMI was associated with risk for developing at least one severe TRT, thus underscoring the impact of malnutrition on chemotherapy toxicity. That these associations were strongest only when evaluating BMI at diagnosis signifies the importance of early nutritional intervention and optimization.
Differences in toxicity and outcome are likely due to variations in cisplatin pharmacokinetics in the obese and underweight. Body composition impacts all phases of drug metabolism including absorption, protein binding, blood-to-tissue delivery, hepatic conjugation, and renal clearance.19 Prior studies, including animal models, have proposed mechanisms for the association of BMI and TRT from cisplatin. Compared to non-obese mice, obese mice have a significant reduction in expression of glomerular filtration proteins, and this expression is further reduced by cisplatin.9 Similarly, adults with increased adipose tissue mass have reduced cisplatin clearance.7 Similarly, children undergoing treatment for all types of cancer have been found to have lower body cell mass and higher percentage of body fat and fat mass than age-matched healthy controls.20 In comparing obese and non-obese mice who received cisplatin, obese mice have increased renal toxicity evidenced by a significantly decrease in creatinine clearance,8,9 similar to the renal injury found in our pediatric cohort. Thus, current literature supports biological plausibility for an association between BMI and TRT following cisplatin administration, and specifically for potential renal toxicity in the obese. As nephrotoxicity is one of the most common toxicities seen with cisplatin, and as cisplatin is eliminated by and directly toxic to the kidney, it is perhaps not surprising that the present study found the strongest association between BMI and renal toxicity but no clear association with other individual TRT. Other confounding host factors such as older age21,22 contribute to differences in TRT and survival, potentially indicating similar pharmacokinetic differences based on age. Future characterization of patient demographic, socio-behavioural, and genetic predictors of toxicity and survival endpoints are necessary first steps toward incorporating pharmacogenomics to individualize treatment plans to maximize cure and minimize harm in the treatment of pediatric solid tumors.
The limitations of our study are primarily related to its retrospective nature. Toxicity data was captured from what was routinely reported in the medical record; while we acknowledge this may not capture every TRT, this likely captured clinically significant, severe TRT as was the focus of this study. Interestingly, the rate of obesity was unexpectedly low as compared to the general pediatric population.23 Weight loss surrounding tumor presentation may have led to underrepresentation of patients who were otherwise obese at diagnosis. Similarly, interpolation of BMI across therapy from diagnosis and EOT measurements instead of serial BMI data cannot account for potential fluctuations in BMI during treatment; however, the impact of these potential fluctuations in the analysis was limited somewhat by focusing only on patients who were at the extremes of BMI at both time-points (<5% and ≥95%). Our study also did not include individual doses of multi-agent chemotherapy, which may also be nephrotoxic, or treatment intensity, instead incorporating diagnosis/regimen as surrogate measures along with dose reductions. Finally, as pediatric solid tumors are rare, we must acknowledge the limited power to detect differences within the BMI categories for underweight or obese and individual clinical endpoints. Nonetheless, even in this context, the rarity of PICU admissions and other severe endpoints is in and of itself notable. With the scarcity of BMI and toxicity data for pediatric solid tumors, this study represents a large, unselected cohort of children treated with cisplatin. These hypothesis-generating findings highlight the need for larger, prospective studies to evaluate the association between malnutrition, TRT, and survival in pediatric patients with non-hematologic malignancies.
Supplementary Material
Acknowledgments
Funding sources: This work was supported by the Biostatistics Core at Children’s Hospital Los Angeles, jointly supported by The Saban Research Institute and the Southern California Clinical and Translational Science Institute (NIH/NCATS UL1TR000130). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosure statement: E.O. served on an advisory board for Servier Pharmaceuticals outside the scope of this research. Winston Huh is a consultant for Lilly, Inc. No other authors report potential conflicts to disclose.
Data Sharing Statement: Authors agree to make their data available upon reasonable request.
References
- 1.O’Donoghue N, Shrotriya S, Aktas A, et al. Clinical significance of weight changes at diagnosis in solid tumours. Support Care Cancer 2019;27:2725–33. [DOI] [PubMed] [Google Scholar]
- 2.Altaf S, Enders F, Jeavons E, et al. High-BMI at diagnosis is associated with inferior survival in patients with osteosarcoma: a report from the Children’s Oncology Group. Pediatr Blood Cancer 2013;60:2042–6. [DOI] [PubMed] [Google Scholar]
- 3.Burke ME, Lyden ER, Meza JL, et al. Does body mass index at diagnosis or weight change during therapy predict toxicity or survival in intermediate risk rhabdomyosarcoma? A report from the Children’s Oncology Group Soft Tissue Sarcoma Committee. Pediatr Blood Cancer 2013;60:748–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein G, Shemesh E, Frenkel T, Jacobson JM, Toren A. Abnormal body mass index at diagnosis in patients with Ewing sarcoma is associated with inferior tumor necrosis. Pediatr Blood Cancer 2015;62:1892–6. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee D, Roy S, Hazra A, Dasgupta P, Ganguly S, Das AK. Variation of adverse drug reaction profile of platinum-based chemotherapy with body mass index in patients with solid tumors: an observational study. Indian J Pharmacol 2014;46:222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modesitt SC, Tian C, Kryscio R, et al. Impact of body mass index on treatment outcomes in endometrial cancer patients receiving doxorubicin and cisplatin: a Gynecologic Oncology Group study. Gynecol Oncol 2007;105:59–65. [DOI] [PubMed] [Google Scholar]
- 7.Gerina-Berzina A, Hasnere S, Kolesovs A, Umbrashko S, Muceniece R, Nakurte I. Determination of cisplatin in human blood plasma and urine using liquid chromatography-mass spectrometry for oncological patients with a variety of fatty tissue mass for prediction of toxicity. Exp Oncol 2017;39:124–30. [PubMed] [Google Scholar]
- 8.Kaveripakam S, Adikay S. Development of an Experimental Model of Nephrotoxicity Co-existing With Obesity in Rats. Indian Journal of Pharmaceutical Sciences 2018;80:844–51. [Google Scholar]
- 9.Ribeiro RS, Passos CS, Novaes AS, et al. Precocious obesity predisposes the development of more severe cisplatin-induced acute kidney injury in young adult mice. PLoS One 2017;12:e0174721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joffe L, Dwyer S, Glade Bender JL, Frazier AL, Ladas EJ. Nutritional status and clinical outcomes in pediatric patients with solid tumors : A systematic review of the literature. Seminars in oncology 2019;46:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orgel E, Sposto R, Malvar J, et al. Impact on survival and toxicity by duration of weight extremes during treatment for pediatric acute lymphoblastic leukemia: A report from the Children’s Oncology Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014;32:1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharib JM, Cyrus J, Horvai A, et al. Predictors of acute chemotherapy-associated toxicity in patients with Ewing sarcoma. Pediatr Blood Cancer 2012;59:611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuczmarski R, Ogden C, Grummer-Strawn L, et al. CDC growth charts: United States. Advance data from vital and health statistics. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- 14.Brock PR, Knight KR, Freyer DR, et al. Platinum-induced ototoxicity in children: a consensus review on mechanisms, predisposition, and protection, including a new International Society of Pediatric Oncology Boston ototoxicity scale. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012;30:2408–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BMI Percentile Calculator for Child and Teen. 2019. at https://www.cdc.gov/healthyweight/bmi/calculator.html.)
- 16.StataCorp. Stata Statistical Software. College Station, TX: StataCorp LP; 2017. [Google Scholar]
- 17.Travis LB, Beard C, Allan JM, et al. Testicular cancer survivorship: research strategies and recommendations. J Natl Cancer Inst 2010;102:1114–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.In: Pizzo PA, Poplack DG, Adamson PC, Blaney SM, Helman LJ, eds. Principles and Practice of Pediatric Oncology. 7th ed2015. [Google Scholar]
- 19.Hanley MJ, Abernethy DR, Greenblatt DJ. Effect of obesity on the pharmacokinetics of drugs in humans. Clin Pharmacokinet 2010;49:71–87. [DOI] [PubMed] [Google Scholar]
- 20.Murphy AJ, White M, Davies PS. Body composition of children with cancer. Am J Clin Nutr 2010;92:55–60. [DOI] [PubMed] [Google Scholar]
- 21.Moke DJ, Tsai K, Hamilton AS, et al. Emerging Cancer Survival Trends, Disparities, and Priorities in Adolescents and Young Adults: A California Cancer Registry-Based Study. JNCI Cancer Spectr 2019;3:pkz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tricoli JV, Bleyer A. Adolescent and Young Adult Cancer Biology. Cancer J 2018;24:267–74. [DOI] [PubMed] [Google Scholar]
- 23.Skinner AC, Skelton JA. Prevalence and Trends in Obesity and Severe Obesity Among Children in the United States, 1999–2012. JAMA pediatrics 2014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.