Abstract
Point-of-care ultrasound has become firmly established in acute and critical care settings, and is now increasingly being used as an important tool in the assessment of the lungs. In this article, we briefly describe the technique of lung ultrasound and the various lines and signs commonly encountered during sonography of the lung, namely the normally visualised A- and T-lines and the bat sign, sliding sign (power slide sign on colour Doppler), sea-shore sign, curtain sign, and the lung pulse. We have also described signs seen in various pathological conditions like B-lines seen in cases of increased lung density; the quad sign, sinusoid sign, thoracic spine sign, plankton sign and the jelly fish sign seen in pleural effusion; the stratosphere sign and the lung point sign seen in pneumothorax; the shred/fractal sign and tissue-like sign in consolidation, and the double lung point sign seen in transient tachypnoea of the newborn. With adequate and appropriate training, lung ultrasound can be effectively utilised as a point-of-care investigation.
Keywords: sonography, emergency ultrasound, POCUS, BLUE protocol
Introduction
It is highly desirable to have accurate and easily reproducible imaging techniques to diagnose pathologies, and implement and monitor treatment – especially in critically ill patients. Point-of-care ultrasound (POCUS) has become firmly established in acute and critical care settings (FAST, vascular access, echocardiography), and is now increasingly being used as an important tool in the assessment of the lungs(1,2).
There are many advantages of using Lung Ultrasound (LUS), namely it is a fast, easily repeatable, widely available, cheap, and portable method. Most importantly, it is safe and does not use harmful ionising radiation, and avoids potentially dangerous transfers within the hospital. The diagnostic accuracy of LUS is superior to that of physical examination and chest radiography combined(3,4).
When the ultrasound beam is directed to image the lungs, most (99%) of the waves are reflected back on striking the soft tissue and air interface(4,5). This implies that structures beneath the pleura (soft tissue) in an air-filled lung will only be sparsely visualised, and mostly artefacts will be seen. The appearance of these artefacts will change depending on the relative amount of air and fluid in the lungs/pleural cavity(4–6) (normal lung – 98% air; interstitial syndromes – 90% air; alveolar syndromes – 10% air, and atelectasis – 5% air(4); all with different sonographic appearances ranging from specific artefacts to the visualisation of true structures). LUS relies on the direct visualisation of structures and artefact interpretation. In this article, we briefly describe the technique of lung ultrasound; the various lines and signs commonly encountered during sonography of the lungs are discussed, and their interpretation in various pulmonary conditions, both physiological and pathological, is presented.
Technique
Sonography of the lungs is best performed by using a low-frequency curvilinear transducer (3–6 MHz)(7). Microconvex transducers (if available) exhibit the additional benefit of having a smaller footprint for better intercostal placement and application in paediatric patients. High-frequency linear transducers (7–12 MHz) are helpful7 in better visualisation of certain signs including the sliding sign and A-lines (see below); they are also preferred in paediatric patients. It should be noted that the imaging of the lungs relies mostly on the interpretation of artefacts, so various filters like Tissue Harmonic Imaging; Compound Imaging etc., which are designed to reduce these, should be turned off for better visualisation of the artefacts(6–8) (unless mentioned otherwise, all the sonographic images presented in this article were obtained by using a low-frequency curvilinear transducer; 3–6 MHz).
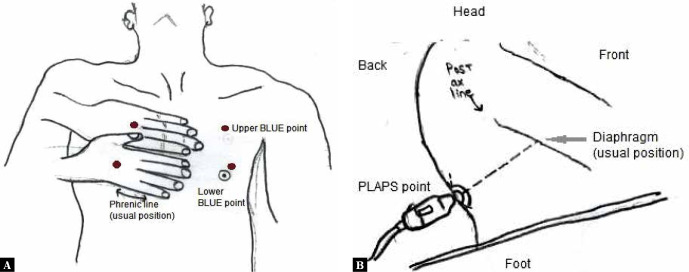
LUS is performed with the patient in the supine and/or the sitting (from back) position. Many different methods have been described in the literature regarding the points/areas to be examined during lung sonography. In our opinion, lung imaging as described in the BLUE (bedside lung ultrasound in emergency) protocol is an easy and effective way to complete the lung examination quickly and adequately(3,4,9–11). In this protocol, the patient lies supine on the examination couch and each lung is examined at three sites (Fig. 1); the upper anterior point, the lower anterior point, and the PLAPS (posterior lateral alveolar or pleural) point. Imaging should be carried out by placing the probe at each point in the longitudinal orientation. The probe should be nearly perpendicular to skin surface because excessive angulation will misplace the beam and make the ultrasound images difficult to interpret.
Fig. 1.
Lung ultrasound examination sites. Both hands are placed, side by side, on the anterior chest wall (as shown); the little finger of the upper hand rests along the lower border of clavicle.(position of the thumbs are not considered). The area covered by the hands, corresponds to the location of the underlying lung. When placed this way, the little finger of the lower hand aligns with the phrenic line or the lower border of the lung. This method defines three standardised points: (i) The upper BLUE-point is at the middle of the upper hand; (ii) The lower-BLUE-point is at the middle of the lower hand and the (iii) The PLAPS-point (posterolateral alveolar or pleural syndromes), which is indicated by the intersection of a horizontal line at the level of the lower BLUE-point and a vertical line at the posterior axillary line. However, the probe should be moved as posteriorly as possible, along this line to get more information in supine patients. The PLAPS point is located slightly above the level of the diaphragm
Bat sign
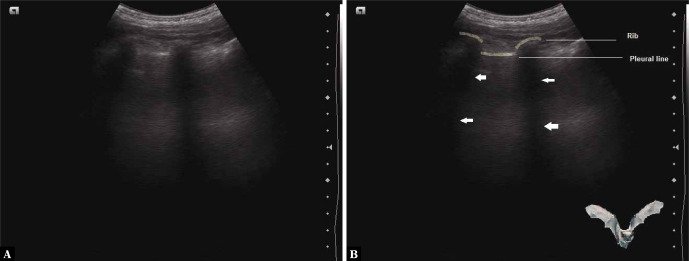
The bat sign refers to the characteristic appearance of the pleural line (which represents the parietal pleura) along with the adjacent ribs(4,5). The ribs resemble the wings of the bat, while the pleural line which lies about half a centimetre below the ribs mimics the body of the bat (Fig. 2). It is seen when the probe is placed longitudinally on the chest wall. If placed obliquely along the intercostal space (avoiding the ribs), only the pleural line is seen, which is not interrupted by the ribs (Fig. S1). The bat sign is seen in all conditions except for subcutaneous emphysema, as the air in the subcutaneous tissues prevents adequate imaging of the structures underneath.
Fig. 2.
A. Grey scale ultrasound image showing the longitudinal scan of the intercostal space depicting the bat sign; B. Labelled view; Ribs and pleural line are outlined in white, arrows point to rib shadows
A-lines
A-lines (Fig. 3) are echogenic, gradually fading horizontal lines arranged at equal intervals below the pleural line, and represent the repetition artefact of the parietal pleura. Their presence indicates that air/gas is present below the pleura, which reflects the ultrasound waves back to the probe, causing the movement of waves to and fro between the transducer and the air beneath the pleura, resulting in this artefact(4,7,12). The distance between A-lines is almost the same as the distance between the skin surface and the pleural line(7). As they originate due to air/gas being present beneath the pleura, A-lines are seen both in the normal lung and in pneumothorax. A-lines are better appreciated with the linear transducer.
Fig. 3.
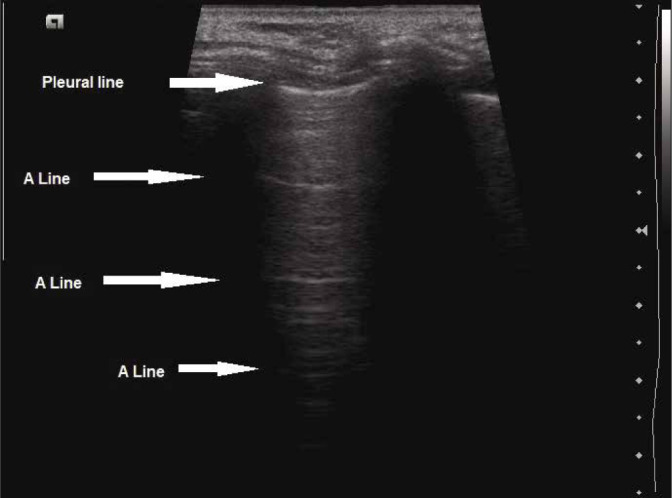
Grey scale longitudinal ultrasound scan of the intercostal space using high-frequency linear transducer (7–12 MHz) depicting A-lines
Curtain sign
The curtain sign is the sonographic characteristic of the inferior-most part of the lateral lung which occupies the costophrenic recess. The depiction of this sign is an indicator of normality in this region. Normally, the aerated tissue of the lung in the costophrenic recess overlaps part of the upper abdomen (liver/spleen) and the diaphragm, creating a demarcated leading edge of the lung air artefact, giving the appearance of a curtain (of air) over them(13,14) (Fig. 4).
Fig. 4.
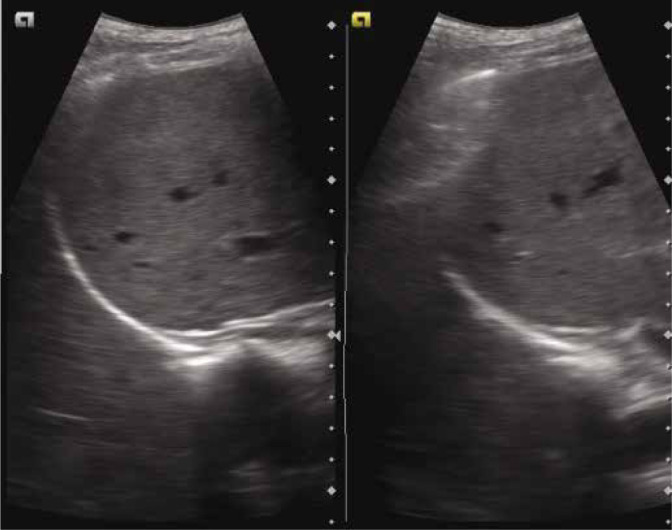
Grey scale ultrasound image depicting the curtain sign – the aerated lung in the costophrenic recess overlaps part of liver and diaphragm creating a demarcated leading edge of the lung air artefact, giving the appearance of curtain (of air). Both images are in different part of the respiratory cycle. CS – curtain sign; L – liver; D – diaphragm
Normal curtain sign: the curtain sign in a normal lung will demonstrate two features, (i) dynamic movement of the lung curtain which is due to the expansion and retraction of the lung (the lung curtain moves down and up covering varying proportions of the upper abdomen and diaphragm, Fig. 4 and Fig. S2), and (ii) the lateral aspect of the diaphragm and the upper abdomen is always covered/obscured by the lung curtain, irrespectively of the phase of the respiratory cycle (Video 1A).
The absence of either or both of these features results in the abnormal curtain sign which is commonly seen in pleural effusion.
In cases of small pleural effusion, the fluid occupies the costophrenic angle/recess, causing minimal compressive atelectasis of the surrounding lung tissue. In this scenario, the dynamic motion of the lung curtain can still be seen, however the curtain does not overlap the diaphragm as much as in the absence of effusion, and uncovers it at peak expiration (Video 1B).
As the amount of effusion increases, the curtain progressively covers less and less of the diaphragm, uncovering it even at inspiration. With massive effusion, the dynamic component is also lost.
The identification of this sign is crucial during lung ultrasound, as its presence implies that the basal and peripheral parts of the lung (in the costophrenic recesses) have been adequately scanned and are normal(13,15). Any changes to the normal curtain sign point towards early or subtle pulmonary abnormality involving the peripheral lung bases.
In emergency and critical care ultrasound use, the recognition of changes to the CS is very useful for detecting early pulmonary pathological processes occurring at the lateral lung bases and costophrenic recesses.
Lung sliding sign and power slide sign
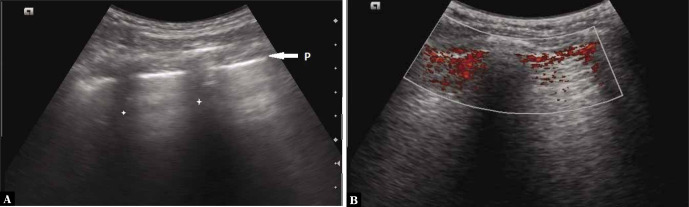
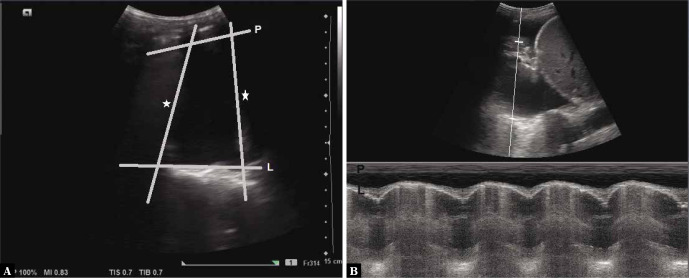
The lung sliding sign refers to the movement between the two pleural layers (at the pleural line, Fig. 5A) that occurs during respiration. Its presence indicates that the two pleural layers are in apposition to each other, and sliding with respiration(4). Similarly to A-lines, this sign is better visualised when a higher frequency linear transducer is used (Video 2A and Video 2B).
Fig. 5.
A. Grey scale longitudinal ultrasound scan of the intercostal space depicting the Pleural line-P (arrow; stars indicate the rib shadows); B. Power Doppler image of normal sliding lung. The presence of colour indicates movement at the pleural line
This sign is not seen in conditions in which the two pleural layers are not opposed to each other as in pneumothorax (air) or in pleural effusion (fluid). Similarly, it is not seen when the pleura are firmly adherent to each other like in pneumonia complicated by adhesions, in pleurodesis, or in cases where no respiration is present (pneumonectomy, one lung intubation)(5–7). It needs to be noted that even though the sign is not seen in pneumothorax, its absence does not always mean that pneumothorax is present(16).
It has been reported that the positive predictive value of absent lung sliding for the detection of pneumothorax is only 87% in the general population, falls to 56% in the critically ill, and to 27% in patients with respiratory failure(3,5,17,18).
The power slide sign is seen when power Doppler is used to help identify the lung sliding sign. If lung sliding is present, the colour will overlap the pleural line, making its visualisation easier (Fig. 5B; Fig. S3). The technique is especially helpful to observe subtle movements of the pleural line in cases of overinflation of the lungs when minimal sliding is seen(19,20). Similarly, this technique is useful when the pleural line may not be adequately visualised in subcutaneous emphysema. It is to be noted that while elucidating this sign, the movement of the patient or probe may also give rise to false colour flow over the pleural line(19–21).
Sea-shore sign and stratosphere sign (also termed barcode sign)
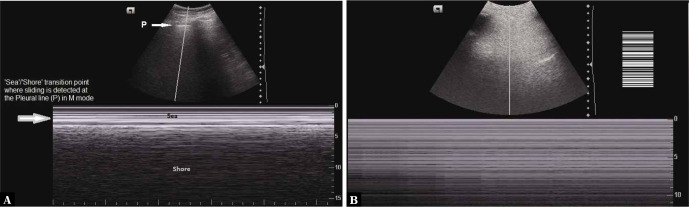
Lung sliding seen on the M (motion) mode images appears as the sea-shore sign in which the pleura and the overlying structures appear as horizontal echogenic lines, while the underlying lung gives a grainy/sandy appearance.
The stratified appearance above the pleural line is due to the motionless chest wall (sea waves); whereas below the pleural line, the movement of the lung shows a sandy pattern (the shore)(4–7) (Fig. 6A, Video 3).
Fig. 6.
A. M-mode ultrasound image depicting the sea-shore sign; P indicates the pleural line; B. The stratosphere/barcode sign in which no movement is seen below the pleural line
If lung sliding is absent, it will appear on the M-mode images as uniform horizontal straight lines known as the stratosphere sign or the barcode sign(4,6,8) (Fig. 6B), where the grainy/sandy appearance is not seen due to the absence of motion. The stratosphere sign suggests pneumothorax as a probable cause.
B-lines
B-lines (also known as ultrasound lung comets) are due to discreet vertical reverberation artefacts that originate from the pleural line, extend to the depth of the image without decreasing in intensity, and move synchronously with lung sliding(12) (Fig. 7A). Their characteristic feature is that they obscure A-lines. An increase in the density of the underlying lung caused by the replacement of air by exudate, transudate, collagen, blood, etc. lowers the acoustic mismatch between the pleura and the underlying structures. This causes the reflection of the ultrasound beam back to the transducer, and to and fro movement of the reflected beam, producing distinctive comet--tail artefacts(22,23).
Fig. 7.
A. Grey scale longitudinal ultrasound scan of the intercostal space depicting the vertically coursing B-lines (arrows); B. Confluent B-lines (arrows) are seen. The B-lines obscure the horizontal A-lines which are seen in the adjacent intercostal space (depicted by asterisk)
B-lines are not observed in pneumothorax, as they are seen only at pleura/tissue acoustic interface (for which the apposition of both pleural layers is required, whereas in pneumothorax both layers are separated by air in between them). Occasional B-lines (up to two) can be seen in normal lungs (commonly at the bases). They are considered significant if three or more B-lines are seen in a single image between two ribs.
Sonographically, interstitial syndrome appears as multiple B-lines. Their number increases as air content of the lungs decreases, and there is an increase in lung density. When closely spaced (≤3 mm) (Fig. 7B), they appear confluent and are due to sub-pleural fluid filled alveoli corresponding to ground-glass opacities on the CT scan. In case of interstitial oedema, which is a precursor of alveolar oedema, their presence or absence may guide the fluid administration(4). It has been proposed that their appearance suggest that further fluids should be administered with caution or restricted, as they may precipitate alveolar oedema. Similarly, B-lines disappear with the treatment of pulmonary oedema.
Quad sign and the sinusoid sign
In cases of pleural effusion, where the two pleural layers are separated by fluid in-between, the visceral pleura appears as a line (the lung line), which is regular and almost parallel to the parietal pleura (the pleural line). Together with the shadows from the adjacent ribs, these lines draw a four-sided figure which is known as the quad sign (Fig. 8A).
Fig. 8.
A. Grey scale longitudinal ultrasound scan of the intercostal space depicting the quad sign. The area of pleural effusion is framed within four regular borders: the pleural line-P, the shadow of the ribs (stars), and the almost regular deep border, which is the lung line-L; B. M--mode image of pleural effusion depicting the sinusoid sign
When examined on the M-mode ultrasound, a sinusoid pattern is seen which is due to the movement of the lung line towards the pleural line on inspiration, and back on expiration. This is referred to as the sinusoid sign (Fig. 8B and Fig. S4), the visualisation of which indicates the presence of low-viscosity fluid in between the two pleural layers(5,7,24). If indicated, the pleural fluid may be aspirated from this site using a small calibre needle. The sinusoid sign may also be of use in differentiating between pleural effusion and pleural thickening, as its presence indicates the movement of free fluid in an effusion(24,25).
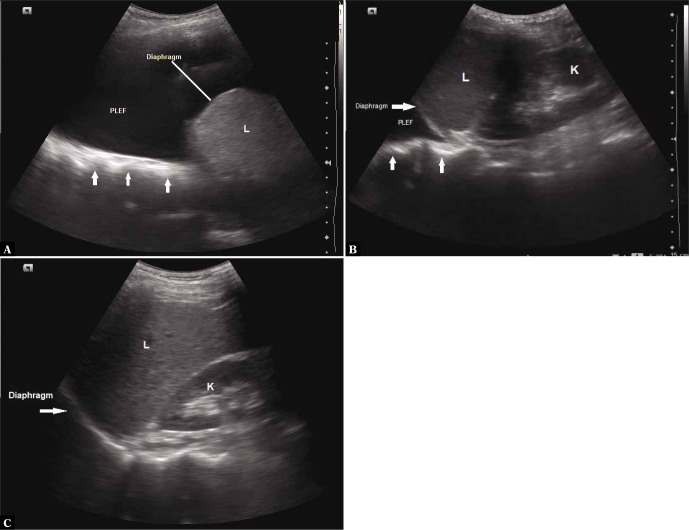
Jelly fish sign
In case of significant pleural effusion, the collapsed lung may be seen moving with respiration, and appears as a swimming or flapping jellyfish, which is referred to as the jellyfish sign(4–7) (Fig. 9 and Video 4A and Video 4B). Visualisation of this sign implies the absence of consolidation and pleural adhesions in the region(26). Free movement indicated by this sign means that low viscosity fluid is present, which suggests that the pleural effusion is of transudative type (as opposed to high viscosity associated with exudative effusion which may obstruct the free movement of the collapsed lung).
Fig. 9.
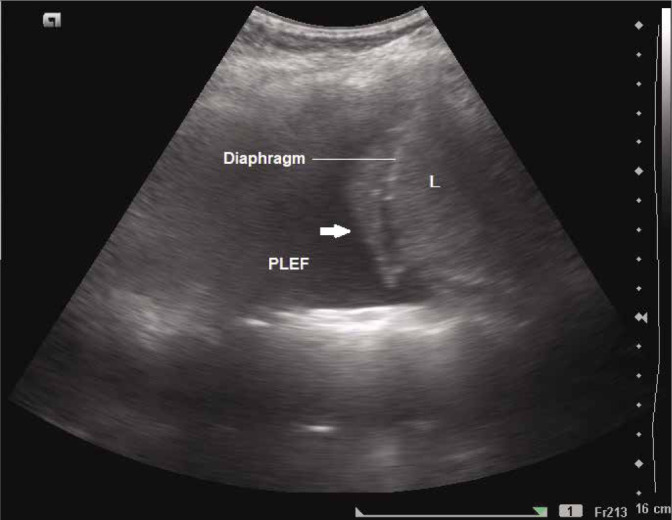
Grey scale longitudinal ultrasound scan of the intercostal space showing collapsed lung (arrow) which appears as moving jellyfish on real time sonography. L – liver; PLEF – pleural effusion
A similar appearance may also be noted in cases of rupture of the hydatid cyst of the lung into the pleural cavity (Video 5).
Plankton sign
The plankton sign (Fig. 10) refers to the floating debris in a pleural effusion which appears as punctiform internal echoes swirling with respiration or cardiac pulsations on dynamic lung ultrasound. When seen, this sign suggests that the effusion is likely to be exudative or haemorrhagic in nature(27) (the term plankton refers to small and microscopic organisms floating in large bodies of water).
Fig. 10.
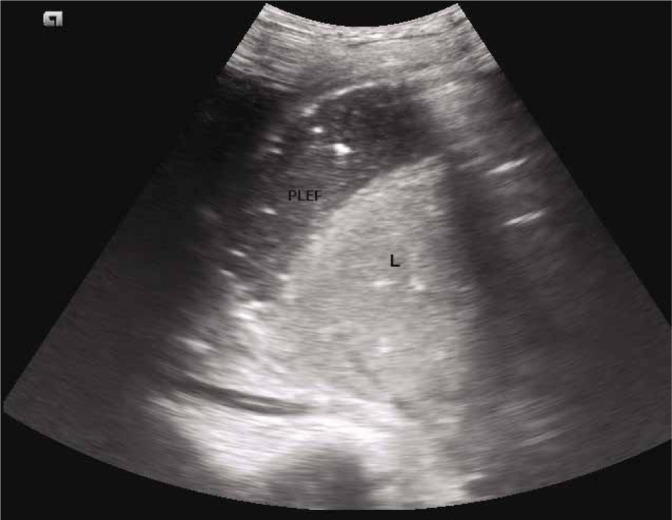
Grey scale longitudinal ultrasound scan of the intercostal space showing multiple punctuate foci representing floating debris – the plankton sign. L – liver; PLEF – pleural effusion
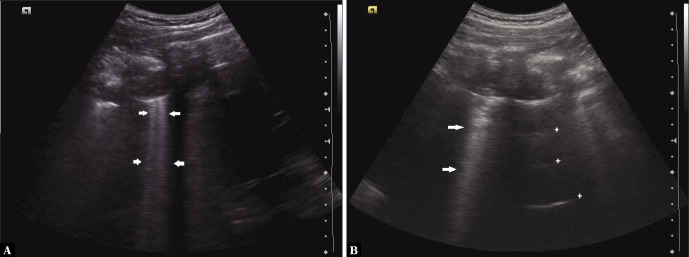
Thoracic spine sign or spine sign
This sign is seen in pleural effusion(4,28,29). The presence of fluid collection in between the two pleural layers creates an acoustic window which allows the visualisation of the vertebral bodies above the diaphragm (Fig. 11A and Fig. 11B). Normally, the vertebral bodies are not seen through the aerated lungs (Fig. 11C).
Fig. 11.
Grey scale longitudinal ultrasound scan of the intercostal space depicting the visualisation of vertebral bodies above the diaphragm in a case of pleural effusion (A and B). The vertebral bodies are not normally seen above the level of the diaphragm (C); L – liver; K – kidney; PLEF – pleural effusion
Shred sign or fractal sign and tissue-like sign
Seen when the margin between the consolidated and aerated lung is an irregular (shredded/fractal) line which is opposed to the lung line(4,7).
If the area of consolidation is large, the deeper border is not appreciated, and the consolidated lung appears as a tissue(4,9) i.e. as continuity of the liver on the right side, and as spleen on the left side (though separated from them by the diaphragm) (Fig. 12).
Fig. 12.
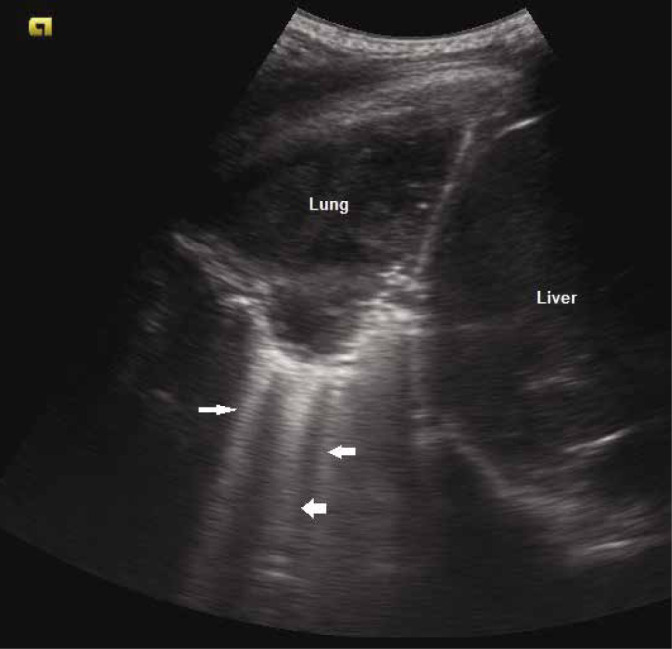
Grey scale ultrasound image depicting the tissue sign with consolidated lung visualised directly beneath the pleura. Multiple B-lines are also seen (arrows)
Lung point sign
This sign is seen in pneumothorax and is highly specific for this condition(4,9,13). The point where the healthy sliding lung meets the absent sliding in pneumothorax is referred to as the lung point. It gives an indication of the size of pneumothorax by its location(30).
If lung sliding is not seen anteriorly, the probe should be moved gradually to more lateral and posterior positions on the thoracic wall, searching for the location of the lung point. The more lateral or posterior is the position where it is identified, the larger is the pneumothorax.
It must be noted, however, that the lung point sign is not seen in every case of pneumothorax, especially in significant pneumothoraces, where the lung is collapsed and the lung sliding sign is absent globally(19,21,30). The lung point sign has an overall sensitivity of 66%, and a specificity of 100% in detecting pneumothorax.
Double lung point sign
In infants with TTN (transient tachypnoea of the newborn), there is a sonographic difference in lung echogenicity between the upper and lower lung fields. The double lung point sign refers to a sharp boundary found between relatively aerated (normal) superior lung fields and coalescent “B-lines” (representing interstitial oedema) in the lower lung fields. (Fig. 13). This sign is very specific for TTN (with reported specificity of up to 100%) and is not seen in normal lungs or in atelectasis, pneumothorax, pneumonia, or pulmonary haemorrhage(31).
Fig. 13.
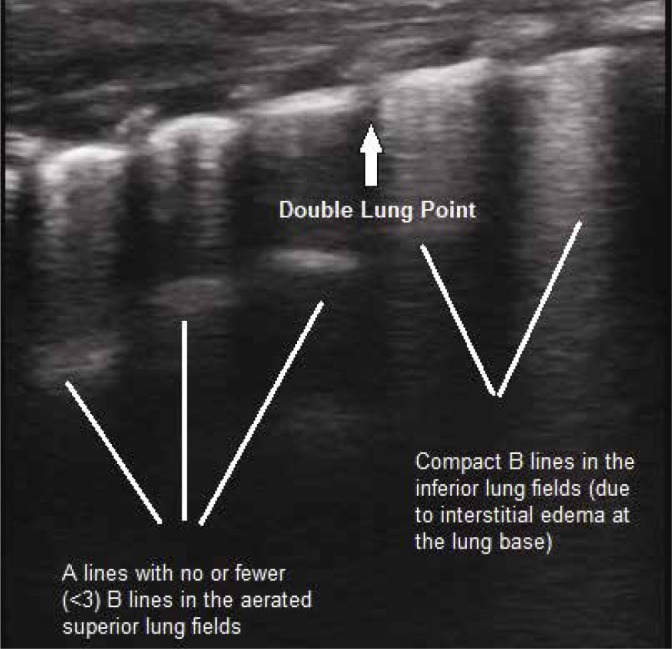
Grey scale longitudinal ultrasound scan of the lung using a high-frequency linear transducer (7–12 MHz), depicting the ‘double lung point’ sign in an infant with TTN. Compact B--lines due to interstitial oedema are seen in the lower lung field
Lung pulse and T-lines
Lung pulse refers to the subtle movement of the pleural layers that is synchronous with cardiac pulsations transmitted to the pleura through the lung(5,12,32). This results in a very small amount of periodic motion of the pleura. It is best viewed in the lung areas adjacent to the heart, especially in poorly aerated lungs(32). In normal well-aerated lungs, it is not appreciated, as lung sliding is dominant and masks cardiac pulsations.
It is easier to detect in the M-mode, where it causes T-lines (Fig. 14), i.e. vertical lines extending from the pleural line to the bottom of the image(12,32,33).
Fig. 14.
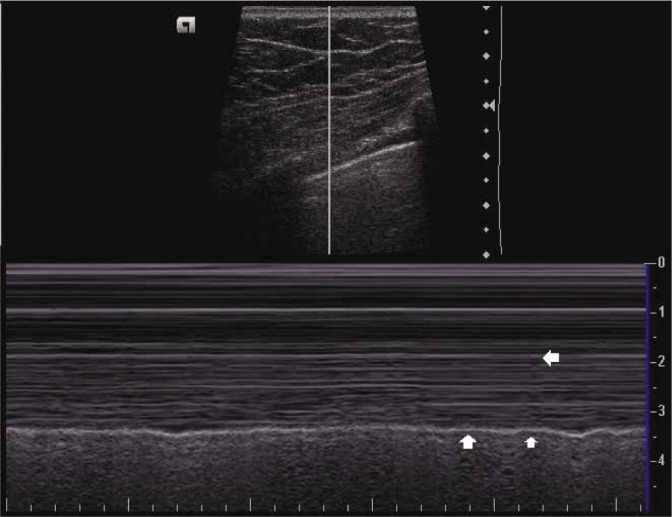
M-mode ultrasound image depicting the sea-shore sign. Arrows indicate the position of T-lines
Similarly to respiratory movements, cardiac pulsations will not be transmitted to the pleura if air is present in the pleural cavity. Therefore, lung pulse is not seen in pneumothorax.
Limitations
As structures beneath the pleura in an air-filled lung will only be sparsely visualised – mostly artefacts will be seen in LUS. LUS relies on direct visualisation of structures and artefact interpretation. It should be always kept in mind that LUS does not rule out lung abnormalities that do not reach up to the pleura. This limitation is of vital importance when looking for consolidations, as they may be medially located, which will prevent their visualisation by sonography because these will be surrounded by aerated lung parenchyma. Similarly, a focally located interstitial syndrome (as in perilesional oedema) may signify a more sinister medially located abnormality, warranting further investigation, i.e. a CT scan.
Adequate visualisation during lung sonography may also be prevented by emphysema or large thoracic dressings. LUS may also be difficult in obese patients due to increased subcutaneous tissue thickness.
Conclusion
Lung ultrasound provides for rapid, accurate, safe bedside assessment of the lungs. It is a relatively easy technique to perform, though it still requires proper understanding of the ultrasound physics, and relies on the correct clinical interpretation of the sonographic pulmonary patterns which can be mastered by practice. In this article, we have attempted to demonstrate and explain the lines and signs commonly encountered during sonography of the lungs, and discuss their interpretation in various pulmonary conditions, both physiological and pathological. The knowledge of these characteristic signs which are typically seen on lung ultrasound will be of substantial help in differentiating between normal and abnormal lung conditions, and in the early diagnosis of subtle lung pathologies.
Footnotes
Conflict of interest
The Authors do not report any financial or personal connections with other persons or organisations which might negatively affect the contents of this publication and/or claim authorship rights to this publication. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. No animals were used during the study.
References
- 1.Alonso JV, Turpie J, Farhad I, Ruffino G: Protocols for point-of-care-ultrasound (POCUS) in a patient with sepsis; an algorithmic approach. Bull Emerg Trauma 2019; 7: 67–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnittke N, Damewood S: Identifying and overcoming barriers to resident use of point-of-care ultrasound. West J Emerg Med 2019; 20: 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lichtenstein DA, Mezière GA: Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller A.: Practical approach to lung ultrasound. BJA Education 2016; 16: 39–45. [Google Scholar]
- 5.Lichtenstein DA: Lung ultrasound in the critically ill. Ann Intensive Care 2014; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouhemad B, Zhang M, Lu Q, Rouby JJ: Clinical review : bedside lung ultrasound in critical care practice. Crit Care 2007; 11: 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saraogi A: Lung ultrasound: present and future. Lung India 2015; 32: 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gargani L, Volpicelli G: How I do it: lung ultrasound. Cardiovasc Ultrasound 2014; 12: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parlamento S, Copetti R, Di Bartolomeo S: Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am J Emerg Med 2009; 27: 379–384. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein DA: BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015; 147: 1659–1670. [DOI] [PubMed] [Google Scholar]
- 11.Patel CJ, Bhatt HB, Parikh SN, Jhaveri BN, Puranik JH: Bedside lung ultrasound in emergency protocol as a diagnostic tool in patients of acute respiratory distress presenting to Emergency Department. J Emerg Trauma Shock 2018; 11: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lichtenstein DA, Mezière GA, Lagoueyte JF, Biderman P, Goldstein I, Gepner A: A-lines and B-lines: lung ultrasound as a bedside tool for predicting pulmonary artery occlusion pressure in the critically ill. Chest 2009; 136: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 13.Lee FCY: The curtain sign in lung ultrasound. J Med Ultrasound 2017; 25: 101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee FC: Lung ultrasound – a primary survey of the acutely dyspneic patient. J Intensive Care 2016; 4: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doerschug KC, Schmidt GA: Intensive care ultrasound: III. Lung and pleural ultrasound for the intensivist. Ann Am Thorac Soc 2013; 10: 708–712. [DOI] [PubMed] [Google Scholar]
- 16.Murphy M, Nagdev A, Sisson C: Lack of lung sliding on ultrasound does not always indicate a PTX. Resuscitation 2008; 77: 270. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein D, Menu Y: A bedside ultrasound sign ruling out pneumothorax in the critically ill: lung sliding. Chest 1995; 108: 1345–1348. [DOI] [PubMed] [Google Scholar]
- 18.Soldati G, Testa A, Sher S, Pignataro G, La Sala M, Silveri NG: Occult traumatic pneumothorax: diagnostic accuracy of lung ultrasonography in the emergency department. Chest 2008, 133: 204–2011. [DOI] [PubMed] [Google Scholar]
- 19.Parab SY, Solanki SL: Lung point and power slide signs help to improve the accuracy of lung ultrasound to diagnose pneumothorax. Saudi J Anaesth 2017; 11: 121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunningham J, Kirkpatrick AW, Nicolaou S, Liu D, Hamilton DR, Lawless B. et al. : Enhanced recognition of „lung sliding” with power color Doppler imaging in the diagnosis of PTX. J Trauma 2002; 52: 769–771. [DOI] [PubMed] [Google Scholar]
- 21.Husain LF, Hagopian L, Wayman D, Baker WE, Carmody KA: Sonographic diagnosis of pneumothorax. J Emerg Trauma Shock. 2012; 5:76– 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira JR, Castro MR, Guimarães TP, Pinheiro AJT, Figueiredo ACTC, Martins BJ. et al. : Evaluation of pulmonary B lines by different intensive care physicians using bedside ultrasonography: a reliability study. Rev Bras Ter Intensiva 2019; 31: 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichtenstein D, Mezière G, Biderman P, Gepner A: The comet-tail artifact, an ultrasound sign ruling out pneumothorax. Intensive Care Med 1999; 25: 383–388. [DOI] [PubMed] [Google Scholar]
- 24.Lichtenstein DA, Mauriat P: Lung Ultrasound in the Critically Ill Neonate. Curr Pediatr Rev 2012; 8: 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ünlüer EE, Karagöz A: Bedside lung ultrasound versus chest X-ray use in the emergency department. Interv Med Appl Sci 2014; 6: 175–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han J, Xiang H, Ridley WE, Ridley LJ: Jellyfish sign: pleural effusion. J Med Imaging Radiat Oncol 2018; 62Suppl 1: 33. [DOI] [PubMed] [Google Scholar]
- 27.Han J, Xiang H, Ridley WE, Ridley LJ: Plankton sign: pleural effusion. J Med Imaging Radiat Oncol 2018; 62Suppl 1: 35. [DOI] [PubMed] [Google Scholar]
- 28.Ahmed AA, Martin JA, Saul T, Lewiss RE: The thoracic spine sign in bedside ultrasound. Three cases report. Med Ultrason 2014; 16: 179–181. [DOI] [PubMed] [Google Scholar]
- 29.Koeze J, Nijsten MW, Lansink AO, Droogh JM, Ismael F: Bedside lung ultrasound in the critically ill patient with pulmonary pathology: differential diagnoses with comparable chest X-Ray opacification. Crit Ultrasound J 2012; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lichtenstein D, Mezière G, Biderman P, Gepner A: The „lung point”: an ultrasound sign specific to pneumothorax. Intensive Care Med 2000; 26: 1434–1440. [DOI] [PubMed] [Google Scholar]
- 31.Copetti R, Cattarossi L: The ‘double lung point’: an ultrasound sign diagnostic of transient tachypnea of the newborn. Neonatology 2007; 91: 203–209. [DOI] [PubMed] [Google Scholar]
- 32.Lichtenstein D, Lascols N, Prin S, Mezière G: The lung pulse: an early ultrasound sign of complete atelectasis. Intensive Care Med 2003; 29: 2187–2192. [DOI] [PubMed] [Google Scholar]
- 33.Kirkpatrick AW, Sirois M, Laupland KB, Liu D, Rowan K, Ball CG. et al. : Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: The extended focused assessment with sonography for trauma (EFAST). J Trauma 2004; 57: 288–295. [DOI] [PubMed] [Google Scholar]