Abstract
For patients with acute lymphoblastic leukemia (ALL) who relapse following allogeneic hematopoietic stem cell transplantation (HSCT), treatment options are limited, and the clinical course and prognostic factors affecting outcome have not been well characterized. We retrospectively analyzed outcomes of 123 adult patients with ALL who relapsed after a first HSCT performed at our center between 1993– 2011. First line salvage included: Second HSCT (n=19), donor lymphocyte infusion (DLI) with or without prior chemotherapy (n=11), radiation therapy (n=6), cytoreductive chemotherapy (n=30), mild chemotherapy (n=27) or palliative care (n=23), with median post relapse overall survival (OS) of 10 months, 6.5 months, 3 months, 4 months, 4 months, and 1 month, respectively. Despite a complete remission (CR) rate of 38% following first line salvage in the treated patients, the OS remained limited with 1- and 2- year OS rates of 17% (13–29) and 10 % (95% CI 6–20), respectively. On univariate analysis, adverse factors for OS included active disease at the time of first HSCT and short time to progression from first HSCT (<6 months). There was no difference in the 6-month survival post-relapse in patients with isolated extra-medullary (EM) relapse (44%) compared with combined EM and bone marrow (BM) relapse (29%), or those with isolated BM relapse (34%) (P =0.8). Our data provides more insight into the disease behavior and treatment outcomes of ALL at relapse following HSCT against which future trials may be compared.
Keywords: Acute lymphoblastic leukemia, allogeneic transplantation, relapse, Category, Transplantation
INTRODUCTION
Treatment outcomes for adults with acute lymphoblastic leukemia (ALL) have improved during the last few decades. Measures such as optimized chemotherapy regimens, modeled after pediatric regimens 1, 2, risk stratification with minimal residual disease (MRD) monitoring3, 4, development of novel agents5, 6, and improvements in supportive care has led to improvement in survival rates. Results from a number of trials including the pivotal MRC/UKALL XII/ ECOG E2993 trial has led to more interest in the upfront use of allogeneic hematopoietic stem cell transplantation (HSCT) for both standard risk and high risk ALL patients7, 8
Patients with ALL who relapse following HSCT have a poor prognosis. Some patients do respond to subsequent treatment and prognostic factors for these patients are not well characterized. Few studies have evaluated management of ALL relapse after HSCT. Spyridonidis et al reviewed the registry data from the European Blood and Marrow Transplant group (EBMT), and demonstrated a median post-relapse survival of only 5.5 months, and an estimated 5 year post-relapse survival of 8 ± 1%9. Using a multivariate model, the authors reported a prognostic model at the time of relapse. The nature of a retrospective registry study however precludes more in-depth look at specific patient populations within the group, such as patients with extramedullary (EM) relapse. In addition, there has been limited data available on factors such as discontinuation of immunosuppressive therapy (IST), as well as more details on salvage therapy and its responses, and the impact of these factors on the outcomes of the patients. Examinations of these issues are warranted in order to guide post relapse management decisions, and to explore novel therapeutic interventions. We performed a single center retrospective analysis on adults with hematologic relapse after first HSCT for ALL in an attempt to better characterize these issues.
METHODS
Patient inclusion criteria and data collection
This retrospective analysis included all adult ALL patients, aged ≥ 18 years-old, who had undergone first HSCT at MD Anderson Cancer Center between 1993– 2011, and subsequently relapsed. Patients were treated on transplantation protocols that were available during the different time periods. Collected data included patient and disease characteristics at diagnosis, disease status at time of first HSCT, source of stem cells, allotype of donor, conditioning regimens, graft-versus-host disease (GVHD) prophylaxis, incidence of acute and chronic GVHD following transplantation, duration of post-transplantation remission, leukemia burden at relapse, details of salvage therapy and outcome variables including response, overall survival (OS) and cause of death. Institutional review board approval was obtained for this retrospective study.
Definitions
Cytogenetic abnormalities were classified based on previously published reports10_ENREF_10. Myeloablative and reduced intensity conditioning regimens were defined according to the CIBMTR criteria11. Criteria for complete response (CR) included normal cytogenetics, the absence of circulating blasts and less than 5% marrow blasts. The disease stage at transplantation was defined using established criteria. Response was documented as the best response occurring after day 30 following HSCT. Hematological relapse was defined by recurrence of blasts in the peripheral blood (PB) or infiltration of the bone marrow (BM) by > 5% blasts. Isolated EM relapse had to be proven with biopsy.
Immunosuppressive therapy was defined as being continued if not discontinued following relapse until time of last follow up or death, or if patients died within 2 weeks of stopping IST. Treatment regimens used for control of the leukemia after relapse was defined as intensive if a combination of cytotoxic agents were used. Mild therapy was defined as combinations of steroids and vincristine, use of a single cytotoxic drug such as clofarabine, nelarabine, vincristine, or hydroxyurea, use of targeted therapies such as NOTCH inhibitors or, tyrosine kinase inhibitors (TKI), or use of immunomodulatory agents such as antibodies or hypomethylating agents. Donor lymphocyte infusion (DLI) was defined as the infusion of unstimulated lymphocytes collected from the original donor. Acute GVHD was clinically graded as 0 to IV based on standard criteria12, 13 and chronic GVHD was classified as none, limited, or extensive as described previously 14.
Statistical Analysis
The primary outcome of interest was survival after relapse following HSCT. Actuarial OS was estimated by the method of Kaplan-Meier. Survival according to patients’ characteristics was compared using Cox’s proportional hazards regression analysis. Comparison was limited to univariate analysis because of sample size limitation and the heterogeneity of the salvage therapy following relapse. Continuous variables were compared using the Wilcoxon’s rank-sum test. Statistical significance was defined at the 0.05 level. Analysis was performed using STATA.11 (College Station, TX: StataCorp LP.)
RESULTS
Patient, disease and relapse characteristics
Between 1993–2011, 381 adults with ALL underwent a HSCT at M. D. Anderson Cancer center, and 123 (32%) subsequently relapsed. The patient characteristics are summarized by the salvage treatment they received at time of relapse in Table 1. For the whole group, the median age at diagnosis was 31 years (range 18–70 years, with 4 patients older than 60 years). A significant proportion of patients had high risk features including 37% (n=46) with high risk cytogenetics (including 23% who were Ph+). In addition, 78% of patients (n=95) were transplanted beyond CR1, with 33% (n=40) transplanted with active disease. The majority of patients received a myeloablative conditioning regimen (74%, n=91). Patients were transplanted from a sibling donor in 53% of cases (n=65), matched unrelated donor in 37% (n=45), mismatched related donor in 5% (n=6), and from a cord blood source in 6% (n=7). The source of stem cells was peripheral blood for the majority of patients (n=74). The median time from transplantation to relapse was 4 months (range 1–38 months). At the time of relapse, 80% (n=99) of patients had systemic relapse, while 20% (n=24) had isolated extramedullary relapse (Table 1).
Table 1.
Overall characteristics of relapsed patients by salvage treatment group
| No. of patients | Overall N=123 | HD chemo N=30 | HSCT±Chemo N=19 | DLI ± Chemo N=11 | Gentle chemo N=27 | Radiation or IT only N=6 | Palliative N=23 |
|---|---|---|---|---|---|---|---|
| Median Age, years (range) | 31 (18–70) | 38 (21–64) | 31 (19–51) | 35 (23–53) | 27 (18–70) | 34 (22–42) | 29 (18–68) |
|
| |||||||
| Response Pre-Transplant | |||||||
| Disease in remission | 83 (67%) | 25 (83%) | 15 (79%) | 5 (45%) | 19 (70%) | 3 (50%) | 11 (48%) |
| Active Disease | 40 (33%) | 5 (17%) | 4 (21%) | 6 (55%) | 8 (30%) | 3 (50%) | 12 (52%) |
|
| |||||||
| Histology | |||||||
| B-lineage | 95 (77%) | 23 (77%) | 17 (89%) | 8 (73%) | 22 (81%) | 3 (50%) | 16 (70%) |
| T-lineage | 28 (23%) | 7 (23%) | 2 (11%) | 3 (27%) | 5 (19%) | 3 (50%) | 7 (30%) |
|
| |||||||
| Cytogenetics Risk Category | |||||||
| High risk | 46 (37%) | 13 (43%) | 9 (47%) | 5 (45%) | 10 (37%) | 1 (17%) | 7 (30%) |
| Others | 53 (43%) | 12 (40%) | 9 (47%) | 3 (27%) | 12 (44%) | 1 (0%) | 12 (52%) |
| Unknown | 24 (20%) | 5 (17%) | 0 (0%) | 4 (36%) | 5 (19%) | 4 (67%) | 4 (17%) |
|
| |||||||
| Site of Relapse | |||||||
| Systemic Relapse | 99 (80%) | 23 (77%) | 17 (89%) | 10 (91%) | 23 (85%) | 0 (0%) | 19 (83%) |
| Isolated extramedullary** | 24 (20%) | 7 (23%) | 2 (11%) | 1 (9%) | 4 (15%) | 6 (100%) | 4 (17%) |
|
| |||||||
| Preparative Regimen | |||||||
| Myeloablative | 91 (74%) | 24 (80%) | 16 (84%) | 8 (73%) | 17 (63%) | 4 (67%) | 16 (70%) |
| RIC | 32 (26%) | 6 (20%) | 3 (16%) | 3 (27%) | 10 (37%) | 2 (33%) | 7 (30%) |
|
| |||||||
| Allo type | |||||||
| Matched unrelated donor | 45 (37%) | 9 (30%) | 4 (21%) | 7 (64%) | 9 (33%) | 1 (17%) | 12 (52%) |
| Matched related donor | 65 (53%) | 16 (53%) | 14 (74%) | 4 (36%) | 15 (56%) | 5 (83%) | 7 (30%) |
| Mismatched related | 6 (5%) | 4 (13%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (9%) |
| Umbilical Cord Blood | 7 (6%) | 1 (3%) | 1 (5%) | 0 (0%) | 3 (11%) | 0 (0%) | 2 (9%) |
|
| |||||||
| Time from HSCT to progression | |||||||
| ≤6 months | 81 (66%) | 15 (50%) | 7 (37%) | 7 (64%) | 22 (81%) | 4 (67%) | 21 (91%) |
| ≥6 months | 42 (34%) | 15 (50%) | 12 (63%) | 4 (36%) | 5 (19%) | 2 (33%) | 2 (9%) |
|
| |||||||
| PB blasts at relapse | |||||||
| ≤10% | 96 (78%) | 24 (80%) | 13 (68%) | 10 (91%) | 21 (78%) | 6 (100%) | 18 (78%) |
| ≥10% | 27 (22%) | 6 (20%) | 6 (32%) | 1 (9%) | 6 (22%) | 0 (0%) | 5 (22%) |
|
| |||||||
| EBMT score*** | (n=83) | (n=25) | (n=15) | (n=5) | (n=19) | (n=3) | (n=11) |
| Score 0 −1 | 43 (52%) | 18 (72%) | 10 (67%) | 4 (80%) | 7 (37%) | 1 (33%) | 2 (18%) |
| Score 2–3 | 40 (48%) | 7 (28%) | 5 (33%) | 1 (20%) | 12 (63%) | 2 (67%) | 9 (82%) |
HD indicates high dose; HSCT, hematopoietic stem cell transplantation; DLI, donor lymphocyte infusion; IT, intrathecal; RIC, reduced intensity conditioning; PB, peripheral blood; EBMT, the European group for blood and marrow transplantation; OS, overall survival.
Overall survival was calculated from the time of progression
Cytogenetic risk category based on National comprehensive cancer network (NCCN) guidelines 201210
Locations were Central Nervous System (n=16), lymph node (n=5), joints (n=2), testes (n=1), and others (n=5). Five patients had more than 1 site of extramedullary relapse
Based on study by Spyridonidis et al9. Applied to patients in study who underwent HSCT while in remission.
All patients died by 3 months.
Management of Relapse, Response, and Overall Survival
At the time of relapse, 23 patients received only supportive care while data on the treatment of 7 others were limited because they were treated at other hospitals following relapse. The remaining 93 patients received some form of anti-leukemic therapy as described in Table 2. The choice of initial salvage treatment was at the discretion of the attending physician and patient, and consisted of chemotherapy alone (mild 22% and intensive 24%), radiation therapy/intrathecal therapy alone (5%), DLI ± chemotherapy (9%), and HSCT± chemotherapy (15%). Of note, the majority of patients who received mild chemotherapeutic agents, including targeted or immunomodulatory agents were those transplanted after 2000. Among the Ph positive patients (n=28), the majority (n=20) received TKIs (+/−chemotherapy) as part of their salvage therapy. Imatinib, a first generation TKI was used in relapses between 2001–2005 (n=9), while in the majority of the relapses from 2006 onwards, second (dasatinib or nilotinib) or third (ponatinib) generation TKIs were usedfor first line or subsequent salvage chemotherapy (n=11).
Table 2:
Response and outcomes to salvage treatment
| Treatments | N = 93 (%) | CR rates (%) | Median OS (months) | Number, cause of death |
|---|---|---|---|---|
|
Mild therapy Single agent chemo (n=6): (Clofarabine, n= 2; nelarabine, n=2; azacitidine, n=1; hydrea, n=1) Novel therapeutic agents/ Trial Medications (n=13) Steroids / Gentle chemo (n=2) TKIs (n= 6) |
27 (29) | 41 | 4 | TRM (n=1) Disease relapse (n=25) |
|
Intensive chemotherapy MTX / Ara C (n= 4) HyperCVAD / Augmented HyperCVAD24 (n=20) MOAD25 ( n=4) Others (n=2) |
30 (32) | 27 | 4 | TRM (n=2) Disease relapse (n=24) Unknown (n=1) |
| Radiotherapy or IT alone | 6 (7) | 83 | 3 | TRM (n=1) Disease relapse( n=4) |
|
DLI + Intensive chemotherapy (n=8) + Mild chemotherapy (n= 3) |
11 (12) | 64 | 6.5 | TRM (n=2) Disease relapse (n=9) |
|
Second HSCT + Intensive chemotherapy (n=14) + Mild chemotherapy (n=5) |
19 (20) | 84 | 10 | TRM (n= 5) Disease relapse (n=8) |
OS indicates overall survival; TKIs, tyrosine kinase inhibitors; GCSF, granulocyte colony stimulating factor; MTX, methotrexate; Ara-C, cytarabine; IT, intrathecal; DLI, donor lymphocyte infusion; HSCT, hematopoietic stem cell transplantation; HyperCVAD: fractionated cyclophosphamide, vincristine, Adriamycin, and dexamethasone combined with methotrexate, cytarabine; MOAD: methotrexate, vincristine, L-asparaginase and dexamethasone
The decision to proceed to second transplant was at the discretion of the treating physician and involved patient. Patients who received second transplants were more likely to be in CR (n=14/19), and had a relatively prolonged duration of remission (>6 months) with their first transplant (n=12/19), Table 1. Donors for second HSCT were changed in 47% of cases (n=9/19). Outcomes and prognostic factors for second transplants have been reported previously15.
Overall, 38% of all patients achieved CR following their first line salvage. With a median follow-up among surviving patients of 11 months (range 1–107 months), the median OS in all patients was 4 months. The 1- and 2- year OS for all patients was 17% (95% CI 13–29) and 10 % (95% CI 6–20), respectively.
The median survival by treatment group was: 10 months in the second HSCT group, 6.5 months in the DLI group, 4 months in the chemotherapy only group with no difference in survival whether intensive or mild chemotherapy was administered, and 3 months in the radiation therapy or intrathecal therapy group (Table 2). For patients who received palliation only, the median survival was 1 month.
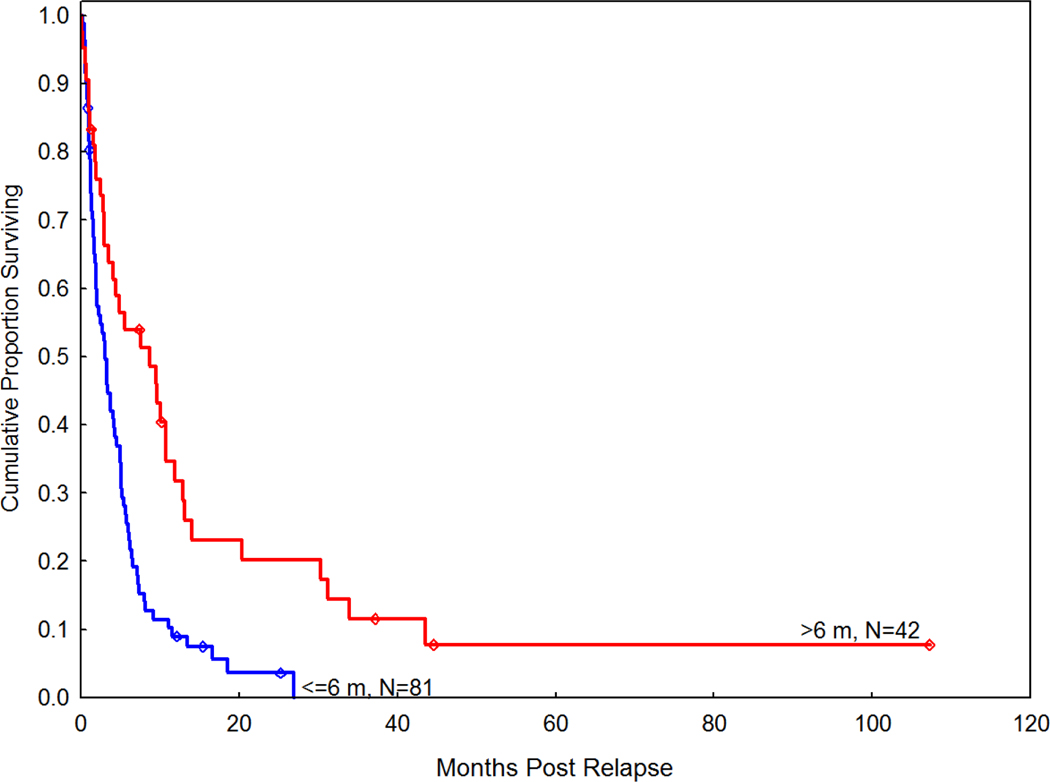
Disease status at time of first transplant and time to relapse following first transplant were found to be significant predictors for worse survival in univariate analysis (Table 3). The patients’ age, cytogenetic risk, and immunophenotype did not have a significant impact on overall survival. Figure 1 shows the OS among the patients who relapsed within 6 months after HSCT compared to those who relapsed later.
Table 3.
Univariate analysis of factors influencing 6-month OS after relapse
| Covariates | N (123) | HR | 95% CI | P value |
|---|---|---|---|---|
| Age at HSCT (years) | ||||
| <=30 | 55 | Ref. | ||
| >30 | 68 | 0.7 | 0.5–1.1 | 0.2 |
|
| ||||
| Sex | ||||
| Female | 39 | Ref. | ||
| Male | 84 | 1.7 | 1.01–2.8 | 0.05 |
| Lineage | ||||
| B-cell | 95 | Ref. | ||
| T-cell | 28 | 1 | 0.6–1.7 | 0.99 |
|
| ||||
| Cytogenetic | ||||
| Ph- | 71 | Ref. | ||
| Ph+ | 28 | 0.7 | 0.4–1.3 | 0.2 |
|
| ||||
| Status at HSCT | ||||
| CR1 | 28 | Ref. | ||
| CR2/CR3 | 55 | 2.02 | 0.9–4.3 | 0.07 |
| Active disease | 40 | 3.1 | 1.4–6.7 | 0.005 |
|
| ||||
| Preparative regimen | ||||
| RIC | 32 | Ref. | ||
| HD | 91 | 1.1 | 0.6–1.8 | 0.8 |
|
| ||||
| Allotype | ||||
| Matched unrelated | 45 | Ref. | ||
| Matched related | 65 | 0.7 | 0.5–1.2 | 0.2 |
| Cord blood | 7 | 0.8 | 0.3–2.2 | 0.6 |
| Mismatched related | 6 | 1.6 | 0.6–4.2 | 0.3 |
|
| ||||
| Time to relapse (months) | ||||
| >6 | 42 | Ref. | ||
| <=6 | 81 | 2.05 | 1.2–3.4 | 0.007 |
|
| ||||
| Site of relapse | ||||
| Systemic | 99 | Ref. | ||
| Isolated extramedullary | 24 | 0.8 | 0.4–1.4 | 0.4 |
Palliative group was excluded.
Palliative group and non-evaluable patients excluded.
CR indicates complete remission; PIF, primary induction failure; RIC, reduced intensity conditioning; HD, high dose conditioning; NR, no response
Figure 1:
Comparison of OS between patients who relapsed within 6 months of HSCT compared to those who relapsed after 6 months.
Treatment and outcomes of patients with extra-medullary relapse
At the time of relapse, 85 patients had isolated bone marrow relapse, while 38 had relapse in EM sites, either isolated (n=24) or with concurrent BM relapse (n=14) (Table 1). Among 38 patients with any EM relapse, 61% (n=23) had EM disease prior to HSCT. The central nervous system (CNS) was the most common site of EM relapse (n=25), followed by mediastinal masses/lymph node involvement (n=8) and testicular involvement (n=2). Five patients had more than one site of EM relapse. Among the 25 patients with CNS relapse, 52% (n=13) had CNS disease prior to their transplant. The median time from transplant to relapse was similar among the patients with isolated relapse (7 months, range 1–28) compared to the patients with systemic relapse (4 months, range 1–38), p =0.3.
Among the 24 patients with isolated EM relapse, 20 of these patients received treatment as described: radiation ± intrathecal (IT) therapy (n=6), DLI (n=1), chemotherapy ± radiotherapy/IT (n=11), and chemotherapy followed by HSCT (n=2). Complete response to salvage therapy was noted in 70% of patients (n=14), but 50% relapsed again, and ultimately died from disease related complications. Among the 7 patients who achieved a durable CR, only 3 patients with isolated CNS relapse remain alive and disease free, all having received systemic therapy (1 patient after chemotherapy and radiotherapy followed by a second transplant, and 2 patients following chemotherapy and radiotherapy only). Three patients have died of treatment related complications while in remission and one has been lost to follow-up. There was no significant difference in survival among patients with isolated EM relapse compared to those with combined BM/EM relapse or those with isolated BM relapse: 6 month OS: 44% (95% CI 24–63), 29% (9–52) and 34% (24–44), respectively.
Outcomes associated with IST withdrawal
Sixty-six patients had IST withdrawal at the time of relapse. Of these patients, 9 did not receive any further anti-leukemic therapy, did not show any response to IST withdrawal, and all have died of disease progression. Fifty-four patients received salvage therapy in addition to IST withdrawal, among which 44% (n=24) attained a CR with first line salvage. However, only 2 patients remain alive and disease-free (1 patient following a second transplant, and 1 patient still receiving intensive chemotherapy), while 45 have died of persistent disease, and 7 have died from various treatment related complications. Three patients were lost to follow up. Twenty-six percent of patients who had IST withdrawal at time of relapse (n=17) developed GVHD, with 17% (n=11) occurring within 3 months of stopping IST and 9% (n=6) developing after subsequent DLI. The incidence of acute GVHD, grades II-IV was 23% (n=15), and grades III-IV 3% (n=2). Two patients developed chronic GVHD.
Assessment of the EBMT scoring system
A recent report from the EBMT analyzing the prognostic factors that affected post relapse overall survival, indicated that by using a combination of 3 prognostic factors (disease status at the time of transplantation, interval from transplantation to relapse, and number of peripheral blasts at the time of relapse), 3 different prognostic groups for survival could be identified. When this EBMT prognostic score was applied to the patients in our study who were transplanted in remission, we found a similar trend: the median survival in patients with 0, 1, 2, and 3 risk factors were 10 months, 6 months, 3 months, and 2 months, respectively.
DISCUSSION
We present data on a large cohort of adult ALL patients who relapsed following HSCT from a single center, and were treated over an 18-year period. The relatively large sample size and long follow-up allowed us to investigate key issues in this patient population that has not been well studied. One important observation is the incidence, nature and prognosis of isolated EM relapses following HSCT. Among the 381 transplants performed for ALL during this study period, we report an EM relapse rate of 10%, with an isolated EM rate of 6%, which are very similar to that reported in the literature16–18. However, unlike other studies in the literature on EM relapse following allogeneic transplantation17, we found no differences in survival outcomes following isolated EM relapse compared to systemic relapse (Table 3). Importantly, our study was limited to only ALL. The inclusion of patients with other hematologic malignancies, in particular AML, may account for the conflicting results. A recent study by the Minnesota group looking solely at patients with AML and EM relapse following HSCT demonstrated a significantly better probability of 6-month survival in the patients with isolated EM relapse (69%) compared with those with combined BM and EM relapse (8%) or those with BM relapse alone (27%) (p < 0.01)19. In contrast, we report survival rates of 44% (95% CI 24–63), 29% (9–52) and 34% (24–44) in the isolated EM, combined BM/EM, and isolated BM relapse groups, respectively (p=0.8). The contrast in the findings between the studies suggests that the prognosis of isolated EM relapse following HSCT is dependent on the disease subtype, and may be poorer in patients with ALL compared to AML. Differences in disease biology, including the propensity of ALL to infiltrate immunological sanctuary sites such as the CNS and testis, as well as variation in treatment, including the upfront use of CNS prophylaxis in ALL, all likely contribute to the different outcomes for ALL and AML. The treatment options for isolated EM relapse have typically included radiotherapy and/or intrathecal therapy; the role of systemic therapy in this setting remains less clear. In our study, all three long term survivors with isolated EM disease received some form of systemic therapy.
We also attempted to draw some conclusions regarding the optimal management of IST at the time of relapse. Discontinuation of IST with the aim of inducing a graft versus leukemia (GVL) effect has been a common practice in patients who relapse after allogeneic transplantation. While there have been few case reports suggesting the possibility of inducing long term remission with IST withdrawal alone20, 21, larger studies looking at this have suggested minimal efficacy for this approach, especially in patients with acute leukemia22. One limitation of trying to evaluate the impact of IST withdrawal in a retrospective study is the fact that IST withdrawal is often used in combination with anti-leukemia therapy, rather than as a sole modality of treatment. In our study, 11 of the 17 patients who developed GVHD following IST withdrawal developed remission to their first line salvage therapy. The close proximity between the timing of IST withdrawal, the development of GVHD, and the use of anti-leukemia therapy in these patients however makes it difficult to ascertain the specific contribution of each event to GVL and leukemia response. Nevertheless, in our study, the findings that none of the 9 patients who had IST withdrawal alone attained remission, and the fact that there were only 2 patients who achieved long term remission amongst the 66 patients with IST withdrawal (both of whom had received other antileukemic therapy) suggest that there is likely minimal efficacy of such a strategy in this patient population. Furthermore, there is the concern of inducing GVHD following IST withdrawal, particularly in patients who relapse early following HSCT, or in patients who receive further chemotherapy that may induce a cytokine-abundant environment. We noted an acute grades II-IV GVHD rate of 17% following IST discontinuation. The subsequent salvage treatment, which commonly includes steroids in ALL, also likely impacts the GVHD rate. Thus, the complexity of the patient at time of transplant makes it difficult for us to make definitive conclusions. However, given the potential life-threatening toxicities associated with GVHD, and the minimal impact of IST withdrawal on disease control, it is reasonable to continue low-dose immune suppression at time of relapse.
The inclusion in our study of patients over an 18-year period provided a reflection of the paradigm shift in treatment strategies over time in this field. This included the increased use of non-cytotoxic therapeutic agents, such as monoclonal antibodies and TKI therapies during the last decade. The retrospective nature of our study, however, precluded the ability to determine the optimal salvage regimen or agents. The best survival was seen in patients who received a second transplant (Table 2), although these findings are likely biased by the fact that these patients had good response to salvage therapy, and needed to have survived long enough to receive their second transplant. More importantly, while a second transplant is likely the only curative options for these patients, the generally high TRM and poor outcomes of second transplants for relapsed ALL15 make it difficult to justify this procedure currently except for possibly a small selected subgroup of patients or in the context of clinical trials with novel therapeutic interventions. Whether the advent of promising novel agents including monoclonal antibody therapies will lead to an improvement in outcomes remains as yet unclear, and will be dependent on the results of prospective studies in this field.
In our study, the median overall survival following relapse was 4 months and the 2-year OS was 10%, which are very similar to the registry data reported by the EBMT group (median OS following relapse of 5.5 months and 2-year OS of 16±2%). In addition, in our univariate analysis for survival, our study findings were consistent with that of EBMT registry data with respect to the prognostic value of relapse-related characteristics, including active disease at the time of first HSCT (HR=1.8, p =0.01) and short duration of relapse (≤6months) from first HSCT (HR=2.05, p=0.007), as well as the lack of prognostic significance for disease related characteristics, such as immunophenotype and cytogenetic classification at diagnosis (Table 3). Finally, we were able to corroborate the EBMT scoring system to prognosticate outcome after transplant relapse. We noted a significant difference in median OS for the good-prognosis group (score 0/1) compared with the poor-prognosis group (score 2/3) (median OS of 7months vs. 3 months, p=0.009). However, given the constraints of a retrospective analysis, we cannot exclude the possibility that patients with shorter time to relapse, and more extensive prior therapy to transplant might have been more likely offered a palliative approach; hence biasing our findings.
In conclusion, survival of patients with ALL who relapse after transplant is extremely poor, with no difference in outcome between patients with isolated extra-medullary versus systemic relapse, suggesting that all relapse should be treated systemically. Furthermore, abrupt discontinuation of immune suppression at time of relapse does not result in clinical benefit, and may result in more GVHD, thus continued low-dose immune suppression may be the optimal approach. Finally, re-induction and second transplant in a highly selected patient group offers the best chance for prolonged survival. Ultimately, these data emphasize the need for continued research in preventing, rather than treating, relapse. Increased use of MRD monitoring, pre-emptive immunotherapeutic interventions, and post-transplant maintenance strategies should be considered23.
Significant message of manuscript:
Our study provides insight into the disease behavior and treatment outcomes of ALL at relapse following allogeneic transplantation. Our data confirm the poor outcomes of these patients regardless of treatment salvage strategies, emphasizing the need for continued research in preventing, rather than treating, relapse.
Acknowledgments
Funding sources declaration: This study had no funding sources to declare.
Financial disclosures: The authors have no relevant financial disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Haïat S, Marjanovic Z, Lapusan S, et al. Outcome of 40 adults aged from 18 to 55 years with acute lymphoblastic leukemia treated with double-delayed intensification pediatric protocol. Leukemia Research. 2011;35: 66–72. [DOI] [PubMed] [Google Scholar]
- 2.DeAngelo DJ, Dahlberg S, Silverman LB, et al. A Multicenter Phase II Study Using a Dose Intensified Pediatric Regimen in Adults with Untreated Acute Lymphoblastic Leukemia. ASH Annual Meeting Abstracts. 2007;110: 587-. [Google Scholar]
- 3.Bruggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107: 1116–1123. [DOI] [PubMed] [Google Scholar]
- 4.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113: 4153–4162. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22–calicheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. The Lancet Oncology. 2012;13: 403–411. [DOI] [PubMed] [Google Scholar]
- 6.Topp MS, Gökbuget N, Zugmaier G, et al. Long-term follow-up of hematological relapse-free survival in a phase 2 study of blinatumomab in patients with minimal residual disease (MRD) of Bprecursor acute lymphoblastic leukemia (ALL). Blood. 2012. [Google Scholar]
- 7.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111: 1827–1833. [DOI] [PubMed] [Google Scholar]
- 8.Cornelissen JJ, van der Holt B, Verhoef GE, et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no-donor comparison. Blood. 2009;113: 1375–1382. [DOI] [PubMed] [Google Scholar]
- 9.Spyridonidis A, Labopin M, Schmid C, et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012. [DOI] [PubMed] [Google Scholar]
- 10.National Clinical Practice Guidelines in Oncology (NCCN guidelines) for Acute Lymphoblastic Leukemia 2012. [Google Scholar]
- 11.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15: 1628–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15: 825–828. [PubMed] [Google Scholar]
- 13.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11: 945–956. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan KM, Shulman HM, Storb R, et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood. 1981;57: 267–276. [PubMed] [Google Scholar]
- 15.Poon LM, Bassett R Jr, Rondon G, et al. Outcomes of second allogeneic hematopoietic stem cell transplantation for patients with acute lymphoblastic leukemia. Bone Marrow Transplant. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee KH, Lee JH, Choi SJ, et al. Bone marrow vs extramedullary relapse of acute leukemia after allogeneic hematopoietic cell transplantation: risk factors and clinical course. Bone Marrow Transplant. 2003;32: 835–842. [DOI] [PubMed] [Google Scholar]
- 17.Shimoni A, Rand A, Hardan I, et al. Isolated Extra-Medullary Relapse of Acute Leukemia after Allogeneic Stem-Cell Transplantation; Different Kinetics and Better Prognosis Than Systemic Relapse. ASH Annual Meeting Abstracts. 2008;112: 2148-. [Google Scholar]
- 18.Stadler M, Diedrich H, Dammann E, et al. Extramedullary Acute Leukemias prior to and after Allogeneic Hematopoietic Stem Cell Transplantation: a Single Center Study. ASH Annual Meeting Abstracts. 2008;112: 3262-. [Google Scholar]
- 19.Solh M, DeFor TE, Weisdorf DJ, Kaufman DS. Extramedullary Relapse of Acute Myelogenous Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation: Better Prognosis Than Systemic Relapse. Biology of Blood and Marrow Transplantation. 2012;18: 106–112. [DOI] [PubMed] [Google Scholar]
- 20.Higano CS, Brixey M, Bryant EM, et al. Durable complete remission of acute nonlymphocytic leukemia associated with discontinuation of immunosuppression following relapse after allogeneic bone marrow transplantation. A case report of a probable graft-versus-leukemia effect. Transplantation. 1990;50: 175–177. [PubMed] [Google Scholar]
- 21.Odom LF, August CS, Githens JH, et al. Remission of relapsed leukaemia during a graft-versus-host reaction. A “graft-versus-leukaemia reaction” in man? Lancet. 1978;2: 537–540. [DOI] [PubMed] [Google Scholar]
- 22.Elmaagacli AH, Beelen DW, Trenn G, Schmidt O, Nahler M, Schaefer UW. Induction of a graft-versus-leukemia reaction by cyclosporin A withdrawal as immunotherapy for leukemia relapsing after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1999;23: 771–777. [DOI] [PubMed] [Google Scholar]
- 23.Alyea EP, DeAngelo DJ, Moldrem J, et al. NCI First International Workshop on The Biology, Prevention and Treatment of Relapse after Allogeneic Hematopoietic Cell Transplantation: report from the committee on prevention of relapse following allogeneic cell transplantation for hematologic malignancies. Biol Blood Marrow Transplant. 2010;16: 1037–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantarjian HM, O’Brien S, Smith TL, et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol. 2000;18: 547–561. [DOI] [PubMed] [Google Scholar]
- 25.Wiernik PH, Dutcher JP, Paietta E, et al. Long-term follow-up of treatment and potential cure of adult acute lymphocytic leukemia with MOAD: a non-anthracycline containing regimen. Leukemia. 1993;7: 1236–1241. [PubMed] [Google Scholar]