Abstract
BACKGROUND
Dermatologic surgery is associated with low postoperative infection rates, averaging from approximately 1% to 4.25%. Often, postoperative infections are treated empirically based on clinical diagnosis of infection, given it can take 48 to 72 hours for a wound culture to identify a pathogen.
OBJECTIVE
We aimed to evaluate the efficacy of empiric antibiotics in dermatologic surgery postoperative infections and if wound cultures change postoperative antibiotic therapy.
METHODS
A 7-center, retrospective analysis of postoperative infections, with culture data, in dermatologic surgery patients was performed.
RESULTS
Of 91 cases of clinically diagnosed postoperative infection, 82.4% (n = 75) were successfully treated with empiric oral antibiotics (95% confidence interval [0.73–0.89], p < .0001). In 16 (17.6%) cases, initial empiric antibiotics were unsuccessful, and wound culture results altered antibiotic therapy in 9 cases (9.9%) with 6 (6.6%) of these cases requiring additional coverage for methicillin-resistant Staphylococcus aureus (MRSA).
CONCLUSION
Empiric antibiotic treatment is usually appropriate for patients with postoperative surgical-site infections with wound cultures altering antibiotic management in a minority of cases. When empiric antibiotics fail, lack of MRSA coverage is usually the cause; therefore, providers should be aware of local MRSA prevalence and susceptibilities.
Dermatologic surgery, including Mohs micrographic surgery, is associated with a low postoperative infection rate, averaging between 1% to 4.25% based on anatomic location and procedure type.1–6 This low infection rate is the result of often “clean” anatomical locations and is supported whether using sterile or nonsterile gloves during tumor extirpation and defect repair.7,8 A recent study has shown overall antibiotic use is increasing in dermatology procedures despite a relatively low incidence of infection.9 When postoperative infections do occur, empiric antibiotics are often given before the availability of culture results.1,3,4 It is unclear how often wound cultures alter patient management, and we aim to address this knowledge gap.
Postoperative infections are often clinically diagnosed by recognition of purulent drainage in combination with at least one of the following: warmth, pain, localized edema, tenderness, or erythema of the surgical site.2,3,10 A wound culture can be a tool used to assist in the diagnosis of a postoperative infection and to determine the pathogenic cause along with antibiotic sensitivities. Often a wound can show clinical signs of infection with a wound culture that does not isolate bacteria, and on the contrary, bacteria can be cultured from wounds that heal without clinical evidence of infection.4
The most common causative organism in dermatologic surgery postoperative infections is Staphylococcus aureus.1–3,5 Less frequent causes of postoperative infections include, Pseudomonas aeruginosa, S. epidermidis, Proteus mirabilis, Seratia marcescens, and Enterobacteriaceae.2,3 Knowledge of the most prevalent pathogens in postoperative infections allows providers to effectively initiate an empiric antibiotic, which is most often cephalexin.2
Many studies examining dermatologic surgery postoperative infections have relied on either clinical criteria and/or a wound culture to diagnose infection.1–4 Often, postoperative infections are treated empirically based on clinical suspicion of infection given that it can take 48 to 72 hours for a wound culture to identify a pathogen. In one study, only 2.7% of patients (n =2 of 75) with a postoperative infection did not respond to empiric antibiotic treatment.1 A rare potential complication in this already small population of non-responders is cellulitis, requiring intravenous (IV) antibiotics.1,3 Results from multiple studies in the dermatologic surgery literature have shown no mortalities in patients with postoperative infections or associated sequelae.1,2,4 We sought to determine the efficacy of empiric antibiotic therapy in dermatologic surgery postoperative infections and whether wound cultures direct therapy in the setting of a clinically diagnosed infection.
Methods
The authors conducted a multicenter, retrospective analysis of patient records among 7 academic institutions. The study was approved by the institutional review board at each participating university and was deemed exempt. The study involved consecutive patients managed by attending, fellowship-trained dermatologic surgeons at their respective institutions. The overall study duration was between August 2010 and August 2018, although duration of the retrospective review varied at each institution based on duration of faculty appointment.
Patients included were 18 years of age or older who were treated for skin cancer on the head, neck, trunk, or extremities with excision or Mohs micrographic surgery. Inclusion criteria required patients to present within 7 days postoperatively with a clinical diagnosis of postoperative infection and have undergone wound culture before initiation of antibiotic therapy. Clinical diagnosis of infection was defined as purulent drainage or the presence of 2 or more of the following documented clinical signs and symptoms: edema, erythema, or tenderness of the surgical site. Patients were excluded if they were on chronic antibiotics for other medical conditions, given empiric preoperative or postoperative antibiotics, presented to the clinic in the first 48 hours after surgery with a hematoma requiring evacuation and prophylactic antibiotics or if their wound closure was performed by an outside physician.
Data collected included the age and sex of the patient, tumor diagnosis, surgical site, surgical procedure (Mohs micrographic surgery, wide local excision, or staged excisional procedure), closure type, empiric antibiotic prescribed based on clinical diagnosis of infection, wound culture result, and further antibiotics prescribed based on culture result or clinical scenario. Data on the performance of incision and drainage (I&D) were not collected. Statistical analysis was performed using SAS Version 9.4, and a mixed-effect logistic regression was used to account for center variation.
Results
A total of 91 cases of postoperative infections after dermatologic surgery that met inclusion criteria were collected from 7 unique university sites. The 3 most common isolated wound culture pathogens were methicillin-sensitive S. aureus (MSSA) as the sole pathogen, n = 51 (56%), methicillin-resistant S. aureus (MRSA), n = 10 (11%), and MSSA mixed with other bacteria, n = 8 (8.8%) (Figure 1). Ten cases (11%) reported wound cultures that showed no growth or normal cutaneous flora, and 5 cases (5.5%) had wound cultures that grew coagulase-negative Staph., which is believed to be a nonpathogenic component of the normal skin flora.
Figure 1.
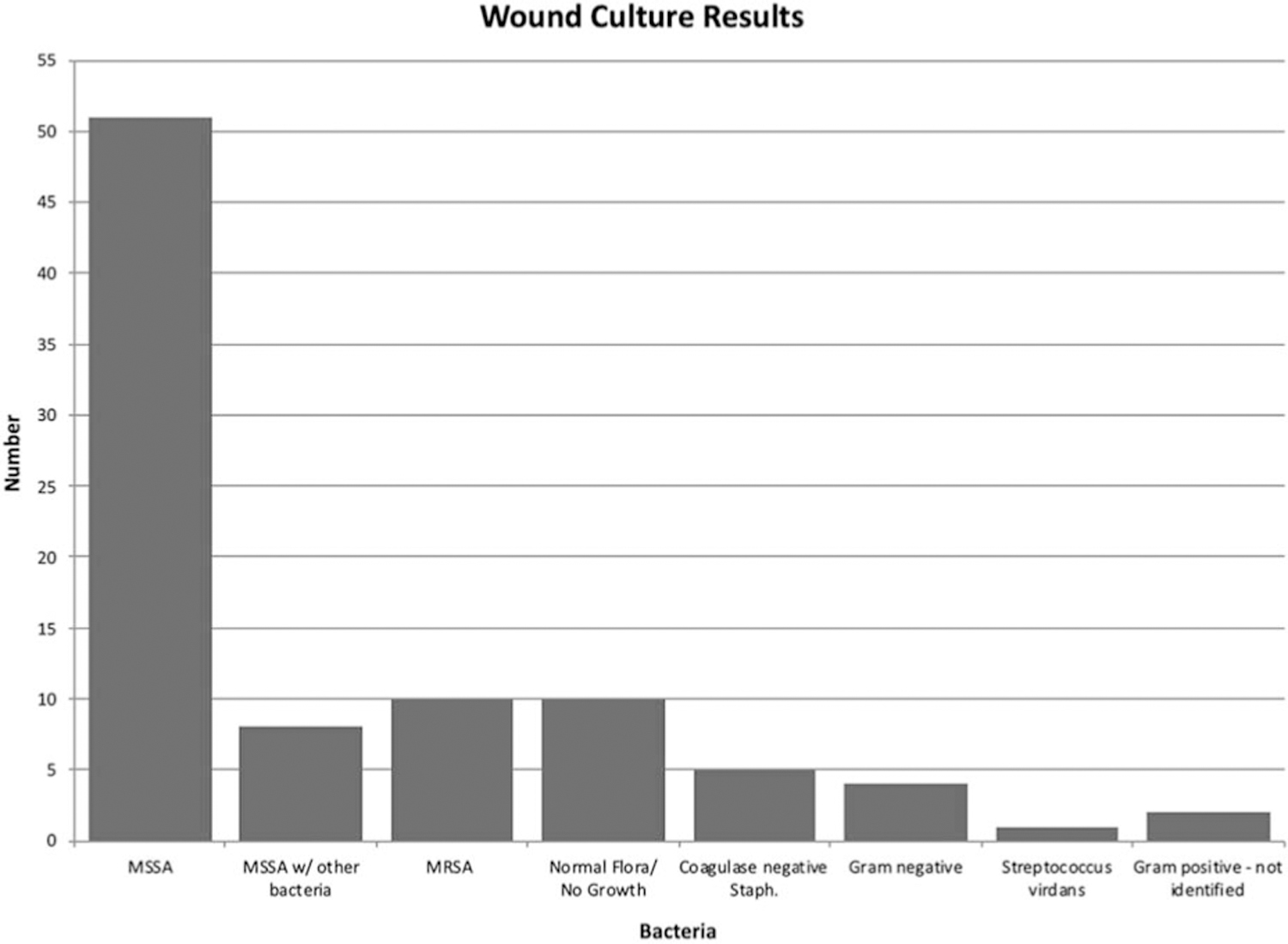
Microorganisms discovered on wound culture.
Of 91 cases, 82.4% (n = 75) were successfully treated with initial empiric oral antibiotics (95% confidence interval [0.73–0.89], p < .0001, α = 0.05) (Figure 2), and there was no significant difference among successful empiric antibiotic therapy and age (p = .89), gender (p = .4), surgical site (p = .97), type of surgery (p = .79), or closure type (p = .88) (Table 1). Infections treated with a tetracycline, trimethoprim/sulfamethoxazole double strength (TMP/SMX DS), or clindamycin as an empiric antibiotic were most often successful in 100%, 89.5%, and 83.3% of cases, respectively; however, this difference in efficacy was not significant when these 3 were grouped and compared with other empiric antibiotics (p = .7) (Table 2).
Figure 2.
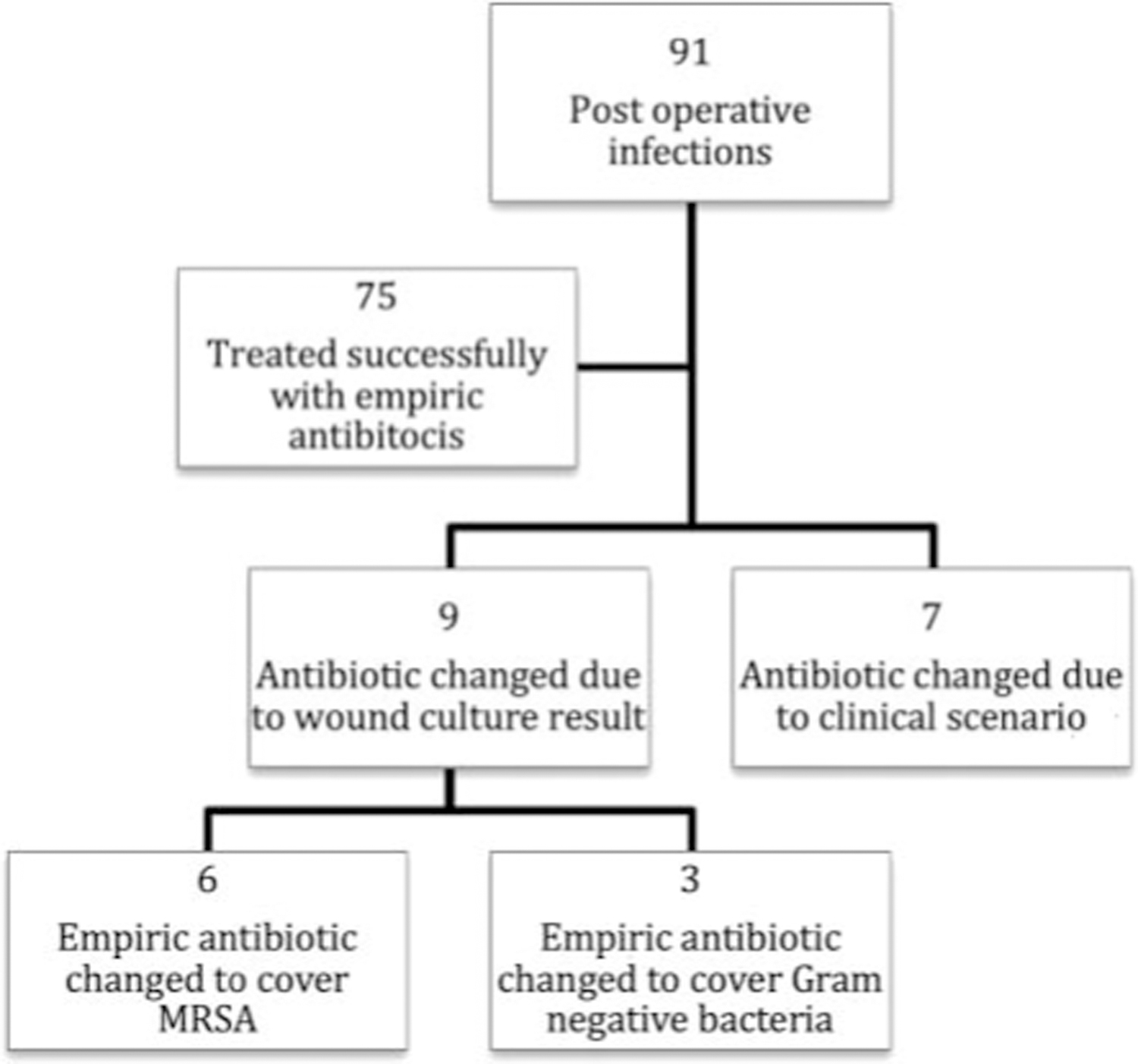
Study outline.
TABLE 1.
Empiric Antibiotic Treatment Success by Surgery Type, Location, and Closure Type
| Successful Empiric Antibiotic | Unsuccessful Empiric Antibiotic | Total # Cases | % Treated Successfully With Empiric Antibiotic | |
|---|---|---|---|---|
| Surgery type | ||||
| Mohs | 61 | 12 | 73 | 83.6% |
| Excision | 12 | 4 | 16 | 75.0% |
| Other | 2 | 0 | 2 | 100.0% |
| Location | ||||
| Scalp | 12 | 1 | 13 | 92.3% |
| Face | 31 | 3 | 34 | 91.2% |
| Trunk | 14 | 3 | 17 | 82.4% |
| Lower extremity | 9 | 4 | 13 | 69.2% |
| Ear | 4 | 2 | 6 | 66.7% |
| Upper extremity | 5 | 3 | 8 | 62.5% |
| Closure type | ||||
| Linear | 49 | 8 | 57 | 86.0% |
| Flap | 13 | 2 | 15 | 86.7% |
| Second intent | 11 | 5 | 16 | 68.8% |
| Graft | 2 | 1 | 3 | 66.7% |
| Total | 75 | 16 | 91 | 82.4% |
TABLE 2.
Empiric Antibiotic Treatment Success by Empiric Antibiotic
| Successful Empiric Antibiotic | Unsuccessful Empiric Antibiotic | Total # Cases | % Treated Successfully With Empiric Antibiotic | |
|---|---|---|---|---|
| Empiric antibiotic | ||||
| Tetracycline | 11 | 0 | 11 | 100.0% |
| TMP/SMX DS | 17 | 2 | 19 | 89.5% |
| Clindamycin | 10 | 2 | 12 | 83.3% |
| Cephalexin | 33 | 10 | 43 | 76.7% |
| Ciprofloxacin | 4 | 2 | 6 | 66.7% |
| Total | 75 | 16 | 91 | 82.4% |
Sixteen (17.6%) cases were not successfully treated with an initial empiric antibiotic, and of these cases, 9 (9.9%) required a change in antibiotic coverage due to wound culture results, whereas 7 (7.7%) had an antibiotic changed due to the clinical scenario (see Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A231). A wound culture result of MRSA directed a therapeutic change in 6 cases, which lacked MRSA coverage by the initial empiric antibiotic, whereas wound culture growth of gram-negative bacteria in 3 cases prompted an antimicrobial change.
Of the 7 cases that required an antibiotic change due to clinical scenario, 6 cases had a wound culture showing the initial empiric antibiotic was appropriate (Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A231). Poor clinical response to empiric antibiotic therapy and clinician preference were some of the clinical scenarios that prompted empiric antibiotic change. The final case had a wound culture that grew normal cutaneous flora, and empiric therapy with TMP/SMX DS was discontinued because of an allergic reaction. One case required hospital admission for intravenous antibiotics due to MRSA preseptal cellulitis. Some of the other reasons behind changing antibiotic therapy included persistent induration, lack of clinical response requiring I&D, and desire to increase the duration of antibiotic therapy, although with a different agent.
Discussion
Dermatologic surgery is associated with low postoperative infection rates that average from approximately 1% to 4.25%, and within this small population, our study found the majority, 82.4%, of infections were successfully treated with empiric antibiotics.1–6 Empiric antibiotics failed in 17.6% of our cases with 9.9% of cases having a wound culture drive antibiotic change and a unique 7.7% of cases having antibiotic therapy changed based on clinical scenario. In the 7.7% of cases where antibiotic was changed based on clinical scenario, it is unclear whether having the knowledge of the wound culture impacted clinical decision-making.
The Infectious Disease Society of America (IDSA) published updated practice guidelines in 2014 for the management of skin and soft tissue infections (SSTIs).11 Within these guidelines are algorithms for the management of purulent and nonpurulent SSTI and surgical-site infections (SSI). For SSTI, gram stain and culture is recommended for severe or moderate purulent infections, but treatment without these studies is reasonable in typical cases. For nonpurulent, mild or moderate SSTI, such as cellulitis, oral and intravenous antibiotics, respectively, are recommended, whereas wound swabs are not routinely recommended. For all SSIs, the only clear suggestion for microbial analysis is in cases less than 4 days postoperative where a gram stain can rule out streptococci and clostridia. The Infectious Disease Society of America also has the following management recommendations, listed as a strong recommendation with low-quality evidence, for SSI treatment: suture removal with I&D, a brief course of systemic antimicrobial therapy for SSI with systemic signs, and recommendation of a first-generation cephalosporin or antistaphylococcal penicillin for MSSA or an antibiotic that covers MRSA where risk factors for MRSA are high pending culture results, although they do not mention clear guidelines on when to culture. In our study, 44% of cases were given a first-generation cephalosporin as empiric antibiotic therapy and 51% cases were given an MRSA-covering empiric antibiotic; however, we did not asses for MRSA risk factors in our patient population. The IDSA recommends suture removal and I&D for SSI, not distinguishing between superficial incisional, deep incisional, and organ/space SSI, and in dermatologic surgery, this recommendation could lead to poor cosmetic outcomes.
From previous studies, selecting empiric antibiotic therapy to cover the most common causative organism in postoperative infections, S. aureus, can increase the success of empiric antibiotics.1–3,5 A study from Zabielinski and colleagues, examining S. aureus cultures in an outpatient dermatology facility, found the overall portion of MRSA was 35.7% and for MSSA was 64.3%, with the proportion of MRSA isolates increasing from the years 2008 to 2010, compared with 2005 to 2007.12 This study also found that MSSA is becoming more resistant to many antibiotics including ciprofloxacin, clindamycin, gentamicin, and TMP/SMX, although these data should be interpreted with caution given the likely broad clinical context of these cultures and, importantly, that antibiotic-resistance patterns vary by geographic locale.
With increasing antibiotic use associated with dermatology procedures, in the setting of an overall decrease in the use of antibiotics by dermatologists, appropriate selection of empiric antibiotic therapy is important.9,13 Covering for potential MRSA with initial empiric antibiotic therapy would have increased the efficacy of empiric antibiotic treatment from 82.4% to 89%; however, when empiric antibiotics that frequently cover for MRSA (tetracycline, TMP/SMX DS, and clindamycin) were compared with cephalexin and ciprofloxacin, there was no statistical difference in success of empiric antibiotic therapy. Given antibiotic resistance is a growing concern,14 it may not be reasonable to standardize empiric MRSA coverage for all patients although it is interesting that in our study cohort, tetracyclines, which are used very commonly for long periods of time in dermatology, also had a 100% efficacy rate. Assessing patient risk factors for MRSA, such as a prior history of MRSA infection or colonization,15 may allow judicious use of empiric antibiotics covering MRSA without increasing the threat of resistance.
A wound culture can identify a potential pathogen along with antibiotic sensitivity, which can help direct therapy, and cultures did directly impact therapy in 9.9% (n = 9/91) of our cases. At the University of Missouri, the approximate cost of a gram stain is $30, bacterial culture $60 to $75, and antibiotic sensitivity testing $75 to $80. These costs could be avoided by treating initially with an empiric antibiotic and only performing a wound culture if the patient does not clinically respond; however, patient preference on this matter is unclear. This approach should be undertaken with the caveat that it may delay treatment in the event of a serious infection as a wound culture may be needed to direct IV antibiotic therapy, infectious disease consultation, and/or inpatient admission.
Performing a wound culture necessitates the patient to be physically present in the clinic, requiring them to travel to the office and accrue expenses associated with travel and, sometimes, time off from work. In an age where not only telephone but also electronic communication between the patient and physician occurs via patient portals, those with postoperative infections could potentially be managed remotely and started on empiric antibiotic therapy without having a face-to-face clinical encounter, at the discretion of the physician. The American College of Mohs Surgery (ACMS) has identified SSIs as a measurable complication to include in their registry, with the goal to ultimately improve the quality of patient care.10 The ACMS registry recognizes that for postoperative infections, the gold standard for acceptable follow-up is in-person evaluation with the operative surgeon; however, the registry also states that telephone follow-up between the patient and the operating surgeon’s office is deemed as an “acceptable standard.” Further studies to validate the reliability of remote follow-up are needed.
In our study, there were only 2 notable complications (Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A231): preseptal cellulitis that required inpatient admission to the hospital for IV antibiotics and allergy to TMP/SMX DS requiring the antibiotic to be discontinued and treatment with diphenhydramine. In the case of preseptal cellulitis, the patient was presumed to have MRSA and empirically started on Bactrim; the wound culture confirmed suspicion so did not itself result in a therapeutic or management change.
Given the multisite nature of the study and broad geographical representation, we feel these results may be representative of dermatologic surgery practices nationwide, at least those at tertiary care centers. However, this study is not without its limitations, including a small sample size, which may be the result of strict inclusion and exclusion criteria including presentation within 7 days of surgery, as postoperative infections can occur after this time frame, and requiring awound culture be performed before initiation of antibiotic therapy. The lack of data on whether I&D was performed could have affected clinical outcomes, as complete drainage of a postoperative abscess may result in clinical improvement regardless of oral antibiotic therapy. The low overall rate of postoperative infections after dermatologic surgery was also a limitation for our sample size.
Conclusion
Empiric antibiotic treatment in the setting of postoperative dermatologic surgery infections is often appropriate and may obviate the need for wound cultures in select cases, including those without purulent drainage based on IDSA management recommendations. Reserving wound cultures for infections unresponsive to empiric antibiotics may expedite patient care and save cost. However, a lack of initial culture may delay further treatment if a patient does not respond to empiric antibiotic therapy as a wound culture did alter antibiotic management in approximately 10% of cases. When empiric antibiotics fail, lack of MRSA coverage is usually the cause. Covering for MSSA, the most common pathogen in dermatology surgery postoperative infections, is most appropriate, and consideration for empiric MRSA coverage is reasonable if incidence is common in a particular region or patient population.
Supplementary Material
Acknowledgments
Gary Dyer Dermatology Research Endowment Fund at the University of Missouri. The project described was supported by the National Institutes of Health through Grant Number UL1TR001857. The authors have indicated no significant interest with commercial supporters.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.dermatologicsurgery.org).
References
- 1.Dixon AJ, Dixon MP, Askew DA, Wilkinson D. Prospective study of wound infections in dermatologic surgery in the absence of prophylactic antibiotics. Dermatol Surg 2006;32:819–26. [DOI] [PubMed] [Google Scholar]
- 2.Nasseri E Prospective study of wound infections in Mohs micrographic surgery using a single set of instruments. Dermatol Surg 2015;41:1008–12. [DOI] [PubMed] [Google Scholar]
- 3.Futoryan T, Grande D. Postoperative wound infection rates in dermatologic surgery. Dermatol Surg 1995;21:509–14. [DOI] [PubMed] [Google Scholar]
- 4.Whitaker DC, Grande DJ, Johnson SS. Wound infection rate in dermatologic surgery. Dermatol Surg Oncol 1988;14:525–8. [DOI] [PubMed] [Google Scholar]
- 5.Rogers HD, Desciak EB, Marcus RP, Wang S, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol 2010;63:842–51. [DOI] [PubMed] [Google Scholar]
- 6.Liu A, Lawrence N. Incidence of infection after Mohs micrographic and dermatologic surgery before and after implementation of new sterilization guidelines. J Am Acad Dermatol 2014;70:1088–91. [DOI] [PubMed] [Google Scholar]
- 7.Rhinehart MB, Murphy MM, Farley MF, Albertini JG. Sterile versus nonsterile gloves during Mohs micrographic surgery: infection rate is not affected. Dermatol Surg 2006;32:170–6. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y, Cho S, Greenway HT, Zelac DE, et al. Infection rates of wound repairs during Mohs micrographic surgery using sterile versus nonsterile gloves: a prospective randomized pilot study. Dermatol Surg 2011;37:651–6. [DOI] [PubMed] [Google Scholar]
- 9.Barbieri JS, Etzkorn JR, Margolis DJ. Use of antibiotics for dermatologic procedures from 2008 to 2016. JAMA Dermatol 2019;155:465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Council ML, Alam M, Gloster HM Jr, Bordeaux JS, et al. Identifying and defining complications of dermatologic surgery to be tracked in the American College of Mohs Surgery (ACMS) Registry. J Am Acad Dermatol 2016;74:739–45. [DOI] [PubMed] [Google Scholar]
- 11.Stevens DL, Bisno AL, Chambers HF, Dellinger EP, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis 2014;59:e10–52. [DOI] [PubMed] [Google Scholar]
- 12.Zabielinski M, McLeod MP, Aber C, Izakovic J, et al. Trends and antibiotic susceptibility patterns of methicillin-resistant and methicillin-sensitive Staphylococcus aureus in an outpatient dermatology facility. JAMA Dermatol 2013;149:427–32. [DOI] [PubMed] [Google Scholar]
- 13.Barbieri JS, Bhate K, Hartnett KP, Fleming-Dutra K, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016. JAMA Dermatol 2019;155:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. AccessedJanuary 1, 2019.
- 15.Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med 2002;346:1871–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


