Abstract
Background and Aims:
Colonic stent placement in patients with large-bowel obstruction (LBO) secondary to extra colonic malignancy (ECM) has been evaluated in small series with heterogeneous results. Our aim is to better characterize the technical and clinical success of colonic stent placement and to identify factors that affect this success in ECM patients.
Methods:
All patients at a single high-volume center who presented for colonic stent placement for LBO due to ECM between 2001 and 2012 were retrospectively identified. The outcomes of interest were technical success, clinical success, stent occlusion rate and overall survival.
Results:
A total of 187 patients were identified. Mean age was 61.9 years (range 23–89) and 150 (80.2%) were female. The most common malignancy type and location was urogynecologic (n=104) and sigmoid colon (n=128), respectively. Overall, 142 patients (75.9%) achieved technical success and 102 patients (54.5%) achieved clinical success. Radiographic presence of peritoneal carcinomatosis (p<0.001) and multifocal disease (p<0.001) were associated with both decreased technical and clinical success. Procedure-related adverse events were seen in 12 patients (6.4%). In patients with clinical success, the incidence of stent occlusion at 3 months was 14.7% (95% CI, 7.8%–21.6%) and was higher in patients with prior radiation therapy (p=0.011). The median overall survival for all patients from time of attempted stent placement was 3.3 months (95% CI, 3.0–4.1 months).
Conclusion:
This study represents the largest retrospective series of colonic stent placement for LBO in ECM patients in the literature. Our technical success rate of 75.9%, clinical success rate of 54.5% and 3-month stent occlusion rate of 14.7% suggest that stent placement is a viable palliative option for patients with advanced disease due to ECM. Patients with peritoneal carcinomatosis and multifocal disease have reduced technical and clinical success. However, these factors should not dissuade an attempt at stent placement, if risk-benefit analysis is favorable.
Keywords: colon stent, extracolonic malignancy, obstruction, clinical outcome
Introduction
Acute malignant colonic obstruction remains a surgical emergency that has traditionally been treated with colostomy or resection. These interventions, however, may be associated with a high rate of perioperative adverse events and mortality. Colonic stent placement has been well described as an alternative palliative measure in patients with colonic obstruction secondary to colorectal cancer (CRC) 1–7. Colonic stent placement for large-bowel obstruction (LBO) secondary to extracolonic malignancy (ECM) has also been described, but has been less well characterized. Several studies have evaluated patients with ECM and colonic stent placement; these are generally small case series and case reports with variable technical success rates ranging from 42% to 100% and clinical success rates ranging from 20% to 90% 8–17. Due to the limited experience reported in the literature, we hope to better characterize the technical and clinical success rates of patients with colonic stent placement for ECM and to identify patients who may most benefit from this intervention. The primary aim of this study, therefore, is to identify clinical variables that may predict clinical outcomes of colon stent placement in ECM patients.
Methods
Study design and subjects
This is a retrospective study evaluating all patients who underwent attempted colon stent placement at our institution for palliation of LBO from ECM between March 2001 and September 2012. Patients were identified by retrospective review of the hospital-wide clinical database for diagnosis of non-colonic malignancy and for procedures including colonoscopy or flexible sigmoidoscopy with attempted or completed colonic stent placement.
A total of 187 patients were identified who had ECM and presented with colonic obstruction, leading to attempted stent placement. Medical records were reviewed for all of these patients. This study was approved by the institutional review board at Memorial Sloan Kettering Cancer Center (IRB WA0535-12).
Stent procedures
In all patients, colonic obstruction was diagnosed using a combination of clinical symptoms and radiographic or colonoscopic examination. All patients were treated in a multidisciplinary approach and were evaluated with surgical consultation before the stent placement procedure. All patients were considered for surgical management if clinical failure or an adverse event occurred from the stent placement. Colonic stents were placed by experienced physicians on the Gastroenterology service using standard techniques. All patients were sedated; initially, patients received intravenous conscious sedation, but our recent practice has been to use general anesthesia for all patients with colonic obstruction.
An Olympus flexible upper endoscope with a therapeutic channel or adult colonoscope is advanced to the site of obstruction. Initially, stent placement was only attempted if the obstruction could be traversed with an endoscope, either therapeutic channel scope or small caliber upper endoscope. Subsequently, technique was refined to use wire-guided fluoroscopic technique for colonic stent placement.
Under fluoroscopic guidance, a guidewire is advanced through the site of obstruction and positioned in the more proximal colon. Over the guidewire, a catheter is advanced and contrast is injected to define the length of the obstruction and to confirm positioning in the lumen of the colon. A controlled radial expansion (CRE) wire-guided balloon is advanced at the discretion of the endoscopist to aid in determination of the proximal edge of the obstruction; in this maneuver, the balloon is inflated in the proximal dilated colon and withdrawn until resistance is encountered. A radio-opaque marker is placed at this site to mark the proximal edge of the obstruction. The length of the obstruction is estimated using fluoroscopy. A self-expanding metal stent (Wallstent; Boston Scientific, Natick, Mass) is chosen according to the estimated length of obstruction plus approximately 1.5cm on either side of the obstruction to allow for stent expansion and introduced over the guidewire. The stent is positioned and deployed across the site of obstruction under fluoroscopic guidance. In cases which warranted longer stent placement or in which multifocal obstruction was noted, a second stent was deployed to ensure that the entire length of obstruction(s) was treated.
Variables
Patient information, including demographic data, disease information, and procedural information, was collected. The demographic and clinical variables compiled included age, gender, primary tumor site, history of chemotherapy or prior radiation therapy, radiographic evidence of peritoneal carcinomatosis, and prior abdominal or pelvic surgery. In addition, information regarding prior bevacizumab use was collected; bevacizumab use has been reported to be associated with increased rates of colonic perforations or bleeding. Procedural information gathered included site and focality of obstruction.
The primary outcomes assessed were technical success and clinical success of stent placement. Additional outcomes evaluated included incidence of stent occlusion and overall survival since stent placement. Technical success was defined as the ability to pass a wire guide through the area of obstruction and successfully deploy a stent. Clinical success was defined as technically successful stent insertion as well as the passage of stool and/or flatus with no procedure-related adverse events and no subsequent interventions for LBO (repeat colonic stent placement, colostomy, or drainage percutaneous endoscopic gastrostomy [dPEG]) within 7 days of stent placement. In patients who achieved clinical success, stent occlusion was defined as the time point at which the patient received a second stent placement, colostomy, or dPEG insertion, whichever event occurred first, with analysis including death as a competing end point.
Statistical analysis
The Fisher exact test was used to compare clinical characteristics between patients who had technical or clinical success of colon stent placement and patients who did not. Overall survival from the date of stent placement procedure was evaluated using the Kaplan-Meier method and compared between groups using the log-rank test. In patients with clinically successful stent placement, stent occlusion was analyzed using the Grey test, treating death as a competing risk. Three-month cumulative incidence of stent occlusion was estimated along with the 95% confidence interval. A test with p-value < 0.05 was considered statistically significant. All statistical analyses were performed in software packages SAS 9.4 (SAS Institute Inc, Cary, NC, USA), R version 3.1 (The R Foundation for Statistical Computing).
Results
A total of 187 patients were identified with ECM and colonic obstruction who underwent attempted stent placement. The mean age of all patients was 61.9 years (range 22–89). Of all patients, 150 (80.2%) were female and 37 (19.8%) were male. The most common malignancy was ovarian cancer with 73 patients (39.0%). Urogynecological malignancies combined (defined as ovarian, cervical, endometrial, fallopian tube and bladder cancers) were represented by the most patients with 104 individuals (55.6%). Non-colonic gastrointestinal malignancies (gastric, esophageal, appendix, and small bowel cancers) contained 41 (21.9%) individuals.
The most common site of obstruction was the sigmoid colon in 128 (68.4%) patients. In this study, 21 (11.2%) had multifocal sites of tumor, 33 (17.6%) had a prior history of radiation therapy, 154 (82.4%) had a history of prior abdominal or pelvic surgery, 42 (22.5%) had prior bevacizumab use, and 150 (80.2%) showed radiographic signs of peritoneal carcinomatosis at the time of procedure. Table 1 summarizes the demographic and clinical information of these patients.
Table 1:
Patient demographics
| All patients (N=187) | |
|---|---|
| N (%) | |
| Age, Mean ± SD (Range), years | 61.9 ± 13.2, (22–89) |
| Gender | |
| Female | 150 (80%) |
| Male | 37 (20%) |
| Tumor type | |
| Urogynecological | 104 (56%) |
| Gastrointestinal | 41 (22%) |
| Pancreaticobiliary | 15 (8%) |
| Breast | 10 (5%) |
| Other | 17 (9%) |
| Stent location | |
| Rectum | 12 (7%) |
| Sigmoid | 128 (68%) |
| Descending | 8 (4%) |
| Transverse | 30 (16%) |
| Ascending | 9 (5%) |
| Focality | |
| Unifocal | 166 (89%) |
| Multifocal | 21 (11%) |
| Radiation therapy | |
| No | 154 (82%) |
| Yes | 33 (18%) |
| Prior abdominal or pelvic surgery | |
| No | 33 (18%) |
| Yes | 154 (82%) |
| Bevacizumab use* | |
| No | 145 (78%) |
| Yes | 41 (22%) |
| Peritoneal carcinomatosis | |
| Absent | 37 (20%) |
| Present | 150 (80%) |
Of the 187 patients with attempted stent placement, 142 patients (76%) achieved technically successful stent placement. Several clinical factors were assessed for association with technical success (Table 2). The presence of multifocal disease (p<0.001) and peritoneal carcinomatosis (p<0.001) were associated with decreased technical success. Of all patients with radiographic evidence of carcinomatosis, 70.7% had technical success, whereas 97.3% of individuals without carcinomatosis achieved technical success. Similarly, only 42.9% with multifocal disease achieved technical success, whereas 80.1% of patients with unifocal disease achieved technical success.
Table 2:
Technical success
| Technical failure (N=45) | Technical success (N=142) | P Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Age, Mean ± SD (Range), years | 65 ± 11.9, (34–87) | 61 ± 13.5, (22–89) | 0.076 |
| Gender | 0.051 | ||
| Female | 41 (27%) | 109 (73%) | |
| Male | 4 (11%) | 33 (89%) | |
| Tumor type | 0.319 | ||
| Urogynecological | 28 (27%) | 76 (73%) | |
| Gastrointestinal | 6 (15%) | 35 (85%) | |
| Pancreaticobiliary | 4 (27%) | 11 (73%) | |
| Breast | 1 (10%) | 9 (90%) | |
| Other | 6 (35%) | 11 (65%) | |
| Stent location | 0.677 | ||
| Rectum | 1 (8%) | 11 (92%) | |
| Sigmoid | 33 (26%) | 95 (74%) | |
| Descending | 2 (25%) | 6 (75%) | |
| Transverse | 8 (27%) | 22 (73%) | |
| Ascending | 1 (11%) | 8 (89%) | |
| Focality | <0.001 | ||
| Unifocal | 33 (20%) | 133 (80%) | |
| Multifocal | 12 (57%) | 9 (43%) | |
| Radiation therapy | 0.026 | ||
| No | 42 (27%) | 112 (73%) | |
| Yes | 3 (9%) | 30 (91%) | |
| Prior abdominal or pelvic surgery | 0.114 | ||
| No | 4 (12%) | 29 (88%) | |
| Yes | 41 (27%) | 113 (73%) | |
| Peritoneal carcinomatosis | <0.001 | ||
| Absent | 1 (3%) | 36 (97%) | |
| Present | 44 (29%) | 106 (71%) |
Of all patients, 102 (54.5%) achieved clinical success after stent placement. Several clinical factors were assessed for association with clinical success (Table 3). The presence of multifocal disease (p<0.001) and peritoneal carcinomatosis (p<0.001) were associated with decreased clinical success. Of all patients with radiographic evidence of carcinomatosis, 66.7% had clinical success after stent placement, whereas 91.4% of individuals without carcinomatosis achieved clinical success. Location of obstruction did not affect clinical success. Similarly, only 19.0% with multifocal disease achieved clinical success, whereas 59.0% with unifocal disease achieved clinical success.
Table 3:
Clinical success
| Clinical failure (N=85) | Clinical success (N=102) | P Value | |
|---|---|---|---|
| N (%) | N (%) | ||
| Age, Mean ± SD (Range), years | 62.4 ± 13.1, (22–89) | 61.6 ± 13.3, (29–88) | 0.694 |
| Gender | 0.042 | ||
| Female | 74 (49%) | 76 (51%) | |
| Male | 11 (30%) | 26 (70%) | |
| Tumor type | 0.512 | ||
| Urogynecological | 51 (49%) | 53 (51%) | |
| Gastrointestinal | 14 (34%) | 27 (66%) | |
| Pancreaticobiliary | 8 (53%) | 7 (47%) | |
| Breast | 5 (50%) | 5 (50%) | |
| Other | 7 (41%) | 10 (59%) | |
| Stent location | 0.665 | ||
| Rectum | 4 (33%) | 8 (67%) | |
| Sigmoid | 63 (49%) | 65 (51%) | |
| Descending | 3 (37%) | 5 (63%) | |
| Transverse | 12 (40%) | 18 (60%) | |
| Ascending | 3 (33%) | 6 (67%) | |
| Focality | <0.001 | ||
| Unifocal | 68 (41%) | 98 (59%) | |
| Multifocal | 17 (81%) | 4 (19%) | |
| Radiation therapy | 0.564 | ||
| No | 72 (47%) | 82 (53%) | |
| Yes | 13 (39%) | 20 (61%) | |
| Prior abdominal or pelvic surgery | 0.336 | ||
| No | 12 (36%) | 21 (64%) | |
| Yes | 73 (47%) | 81 (53%) | |
| Peritoneal carcinomatosis | <0.001 | ||
| Absent | 5 (14%) | 32 (86%) | |
| Present | 80 (53%) | 70 (47%) |
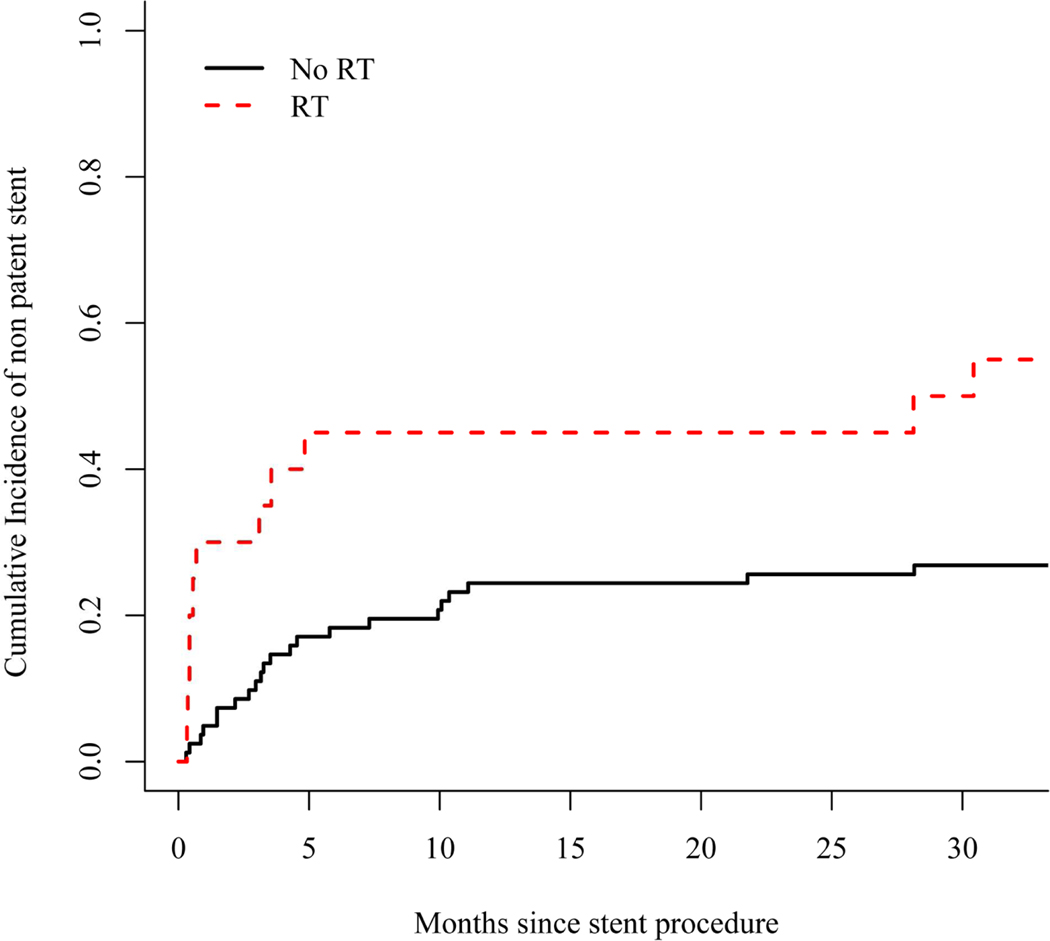
The 3-month incidence of stent occlusion observed in patients with clinically successful stent placement in this study was 14.7% (95% CI, 7.8–21.6%). Additionally, those with prior radiation therapy had significantly higher rates of stent occlusion than those without prior exposure (p=0.010) (Table 4). Figure 1 presents the incidence of stent occlusion over time in patients with and without prior radiation exposure.
Table 4:
Stent Occlusion
| 3-Month Stent Occlusion % (95%CI) | P Value | |
|---|---|---|
| Tumor type | 0.480 | |
| Urogynecological | 17 (7 – 27) | |
| Gastrointestinal | 15 (1 – 29) | |
| Pancreaticobiliary | 0 (0 – 0) | |
| Breast | 20 (0 – 63) | |
| Other | 10 (0 – 30) | |
| Stent location | 0.515 | |
| Rectum | 25 (0 – 58) | |
| Sigmoid | 15 (7 – 24) | |
| Descending | 20 (0 – 59) | |
| Transverse | 6 (0 – 16) | |
| Ascending | 17 (0 – 49) | |
| Focality | 0.108 | |
| Unifocal | 14 (7 – 21) | |
| Multifocal | 25 (0 – 82) | |
| Radiation therapy | 0.010 | |
| No | 11 (4 – 18) | |
| Yes | 30 (9 – 51) | |
| Prior abdominal or pelvic surgery | 0.170 | |
| No | 0 (0 – 0) | |
| Yes | 19 (10 – 27) | |
| Peritoneal carcinomatosis | 0.203 | |
| Absent | 16 (3 – 29) | |
| Present | 14 (6 – 23) |
Stent occlusion was analyzed using Grey’s test, treating death as a competing risk.
Figure 1:
Cumulative Incidence of Stent Occlusion
The median overall survival after stent placement was 3.3 months (95% CI, 3.0–4.1 months). At the time of analysis, 185 (98.9%) patients were deceased, one was lost to follow-up after 6 months and one was lost to follow-up after 10 months. Tumor type was significantly associated with 3 month overall survival (p=0.005) (Table 5). Figure 2 shows the survival curve of all patients in our study.
Table 5:
Overall Survival
| All Patients (N=187) | Patients with clinical success (N=102) | |||
|---|---|---|---|---|
| 3-Month OS % (95%CI) | P Value | 3-Month OS % (95%CI) | P Value | |
| Gender | 0.196 | 0.439 | ||
| Female | 61 (52 – 71) | 68 (59 – 80) | ||
| Male | 42 (29 – 63) | 50 (34 – 73) | ||
| Tumor type | 0.005 | 0.348 | ||
| Urogynecological | 62 (53 – 72) | 72 (61 – 85) | ||
| Gastrointestinal | 63 (50 – 80) | 63 (47 – 84) | ||
| Pancreaticobiliary | 40 (22 – 74) | 43 (18 – 100) | ||
| Breast | 30 (12 – 77) | 60 (29 – 100) | ||
| Other | 29 (14 – 61) | 40 (19 – 86) | ||
| Stent location | 0.821 | 0.433 | ||
| Rectum | 42 (21 – 81) | 50 (25 – 100) | ||
| Sigmoid | 58 (59 – 67) | 66 (56 – 79) | ||
| Descending | 50 (25 – 100) | 80 (52 – 100) | ||
| Transverse | 53 (38 – 75) | 56 (37 – 84) | ||
| Ascending | 56 (31 – 100) | 67 (40 – 100) | ||
| Focality | 0.120 | 0.023 | ||
| Unifocal | 55 (48 – 64) | 64 (56 – 75) | ||
| Multifocal | 57 (40 – 83) | 50 (19 – 100) | ||
| Radiation therapy | 0.311 | 0.142 | ||
| No | 56 (49 – 64) | 65.9 (56 – 77) | ||
| Yes | 55 (40 – 75) | 55 (37 – 82) | ||
| Prior abdominal or pelvic surgery | 0.178 | 0.350 | ||
| No | 46 (31 – 66) | 57 (40 – 83) | ||
| Yes | 58 (51 – 66) | 65 (56 – 77) | ||
| Peritoneal carcinomatosis | 0.398 | 0.723 | ||
| Absent | 60 (46 – 78) | 63 (48 – 8) | ||
| Present | 55% (47 – 63) | 64 (54 – 77) | ||
Figure 2:
Overall Survival Curve
In patients with technically successful stent insertion, procedure-related adverse events were noted in 12 patients (6.4%), including perforation in 7 patients (3.7%), cardiopulmonary events in 3 patients, significant pain necessitating same-day stent removal in 1 patient, and mucosal reaction requiring stent removal in 1 patient. There were 1 case of stent migration. In patients who received bevacizumab, no increased risk of adverse events including perforation or bleeding was noted.
Discussion
Malignant LBO remains a surgical emergency because of the risk of bowel perforation. Surgery in patients with late stage extracolonic malignant disease remains high risk with regard to morbidity and mortality. Studies show that in patients with bowel obstruction secondary to recurrent ovarian cancer, palliative surgery has a morbidity of 5% to 49% and mortality of 5–15%.18,19,20,21 ECM has been previously evaluated with variable rates of technical and clinical success. Additionally, few studies have previously investigated correlations between clinical variables and stent outcomes in ECM patients 9,11,15,17. Suh et al,15 in a series of 42 patients with CRC and 13 patients with ECM, demonstrated a statistically significant association between degree of luminal expansion at 48 hours after procedure and stent occlusion during follow-up. In 2009, Keswani et al5 compared 34 CRC patients and 15 ECM patients at a single institution who underwent successful colonic stent placement for LBO and reported clinical success rates of colonic stent placement in 94.1 % of CRC patients and in 20.0% of ECM patients. There were no clinical variables identified that correlated with clinical success; however, they did note that ECM patients were more likely to have peritoneal carcinomatosis at the time of stent placement as compared with CRC patients 5. The authors hypothesized that a combination of factors including peritoneal carcinomatosis and prior radiation therapy might contribute to the disparity in clinical success rates, though statistical significance was not demonstrated in their series5.
To our knowledge, these results describe the largest series of ECM patients with LBO treated with colonic stent placement. Additionally, this study investigates clinical variables such as presence of peritoneal carcinomatosis and tumor focality that have rarely been analyzed in this patient population. Similar to prior series, we did not find any association between tumor/stent location or prior abdominal/pelvic surgery and clinical outcomes
Our technical success rate of 75.9% and clinical success rate of 54.5% suggests that colonic stent placement for LBO in ECM patients is an effective palliative alternative to surgical intervention, and our low adverse event rate further attests to its safety. Our technical and clinical success rates are similar to other selected series. However, it must be noted that clinical success rates in ECM patients vary considerably in the existing literature, possibly because researchers use different definitions of clinical success or because of smaller numbers of cases in these series. Standardization of this definition may aid with future research.
Our results show that the presence of peritoneal carcinomatosis decreases both the technical and clinical success rates of colonic stent placement in this patient population. In patients with ECM and peritoneal carcinomatosis, individuals should be counseled about the possible need for alternative interventions. In our cohort, 70.7% and 46.7% of patients with peritoneal carcinomatosis still achieved technical and clinical success, respectively, suggesting that stent placement may still be considered. Conversely, the absence of peritoneal disease suggests a significant advantage, as 97.3% and 86.5% of such patients achieved technical and clinical success, respectively. In our study, multifocal disease was also associated with decreased technical and clinical success, with only 42.9% of patients achieving technical success and 19.0% of patients achieving clinical success.
Our study also shows that the incidence of stent occlusion may be affected by clinical factors, specifically prior radiation therapy. This may stem from the multifaceted effect of radiation therapy on gastrointestinal mucosa and the soft tissues surrounding the bowel. Previous studies have shown the link between radiation exposure and acute inflammation in the colon, submucosal edema, development of submucosal fibrosis and formation of multiple strictures.22,23,24 Interestingly, prior radiation therapy was actually associated with an increased technical success rate in our study. One can hypothesize that pre-procedure radiation therapy may reduce the size of tumor burden and physical compression, allowing for easier maneuvering and improved technical success. The effect of radiation therapy on stent occlusion may be mediated by other long term effects of radiation. The complex effect of radiation therapy on outcomes needs to be further evaluated.
The median overall survival in our study of 3.3 months after colonic stent placement is consistent with previously reported findings that bowel obstruction is a common presentation of disease progression in many ECM patients during the late stages of disease.16, 17, 25 Previous studies have shown that up to 31% of patients with ovarian cancer are admitted to the hospital with LBO during the last 6 months of life.26
The main limitation of our study is its retrospective design and single institution experience. Additionally, a limitation of not only our study, but of the related literature, is the variable use of the definition of clinical success. We defined clinical success as the passage of stool and/or flatus without any procedure-related adverse events or further interventions for LBO within 1 week of stent placement. Although we believe this is a reasonable definition for clinical success, changing the time period in the definition would inevitably change the rate of clinical success.
Conversely, the strengths of the study include the large number of patients, wide array of clinical variables evaluated, and the follow-up period. Although we believe that this study enhances our understanding of the use of colonic stents in ECM patients, further studies are needed to address the disparity in success rates between ECM and CRC patients.
Acronyms
- LBO
large bowel obstruction
- ECM
extra colonic malignancy
- CRC
colorectal cancer
- CRE
controlled radial expansion
- dPEG
drainage percutaneous endoscopic gastrostomy
Footnotes
Conflict of Interest Disclosure: None of the authors have any conflicts of interest to disclose regarding any of the topics or materials discussed in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1).van Hooft JE, van Halsema EE, Vanbiervliet G et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2014;46:990–1053. [DOI] [PubMed] [Google Scholar]
- 2).Sebastian S, Johnston S, Geoghegan T et al. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. The American Journal of Gastroenterology 2004;99:2051–57. [DOI] [PubMed] [Google Scholar]
- 3).Khot UP, Lang AW, Murali K et al. Systematic review of the efficacy and safety of colorectal stents. British Journal of Surgery 2002;89:1096–1102. [DOI] [PubMed] [Google Scholar]
- 4).Larkin JO, Moriarity AR, Cooke F et al. Self-expanding metal stent insertion by colorectal surgeons in the management of obstructing colorectal cancers: a 6-year experience. Techniques in Coloproctology 2014;18:453–8. [DOI] [PubMed] [Google Scholar]
- 5).Keswani RN, Azar RR, Edmundowicz SA et al. Stenting for malignant colonic obstruction: a comparison of efficacy and complications in colonic versus extracolonic malignancy. Gastrointestinal Endoscopy 2009;69:675–80. [DOI] [PubMed] [Google Scholar]
- 6).Yoon JY, Jung YS, Hong SP et al. Clinical outcomes and risk factors for technical and clinical failures of self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointestinal Endoscopy 2011;74:858–68. [DOI] [PubMed] [Google Scholar]
- 7).Manes G, de Bellis M, Fuccio L et al. Endoscopic palliation in patients with incurable malignant colorectal obstruction by means of self-expanding metal stent: analysis of results and predictors of outcomes in a large multicenter series. Archives of Surgery 2011;146:1157–62. [DOI] [PubMed] [Google Scholar]
- 8).Keränen I, Lepisto A, Udd M et al. Stenting for malignant colorectal obstruction: a single-center experience with 101 patients. Surgical Endoscopy 2012;26:423–30. [DOI] [PubMed] [Google Scholar]
- 9).Kim JY, Kim SG, Im JP et al. Comparison of treatment outcomes of endoscopic stenting for colonic and extracolonic malignant obstruction. Surgical Endoscopy 2013;27:272–77. [DOI] [PubMed] [Google Scholar]
- 10).Moon SJ, Kim SW, Lee BI et al. Palliative stent for malignant colonic obstruction by extracolonic malignancy: a comparison with colorectal cancer. Dig Dis Sci 2013;29:1891–7. [DOI] [PubMed] [Google Scholar]
- 11).Kim BK, Hong SP, Heo HM et al. Endoscopic stenting is not as effective for palliation of colorectal obstruction in patients with advanced gastric cancer as emergency surgery. Gastrointestinal Endoscopy 2012;75:294–301. [DOI] [PubMed] [Google Scholar]
- 12).Kim JH, Song HY, Park JH et al. Metallic stent placement in the palliative treatment of malignant colonic obstructions: primary colonic versus extracolonic malignancies. J Vasc Interv Radiol 2011;22:1727–32. [DOI] [PubMed] [Google Scholar]
- 13).Trompetas V, Saunders M, Gossage J et al. Shortcomings in colonic stenting to palliate large bowel obstruction from extracolonic malignancies. Int J Colorectal Dis 2010;25:851–54. [DOI] [PubMed] [Google Scholar]
- 14).Shin SJ, Kim TI, Kim BC et al. Clinical application of self-expandable metallic stent for treatment of colorectal obstruction caused by extrinsic invasive tumors. Dis Colon Rectum 2008;5:578–83. [DOI] [PubMed] [Google Scholar]
- 15).Suh JP, Kim SW, Cho YK et al. Effectiveness of stent placement for palliative treatment in malignant colorectal obstruction and predictive factors for stent occlusion. Surgical Endoscopy 2010;24:400–06. [DOI] [PubMed] [Google Scholar]
- 16).Caceres A, Zhou Q, Iasonos A et al. Colorectal stents for palliation of large-bowel obstructions in recurrent gynecologic cancer: an updated series. Gynecologic Oncology 2008;108:482–85. [DOI] [PubMed] [Google Scholar]
- 17).Jutzi L, Russell D, Ho S et al. The role of palliative colorectal stents in gynaecologic malignancy. Gynecologic Oncology 2014;134:566–69. [DOI] [PubMed] [Google Scholar]
- 18).Clarke-Pearson DL, Chin NO, DeLong ER et al. Surgical management of intestinal obstruction in ovarian cancer: I. Clinical features, postoperative complications, and survival. Gynecologic oncology 1987;26:11–18. [DOI] [PubMed] [Google Scholar]
- 19).Solomon HJ, Atkinson KH, Coppleson JV et al. Bowel complications in the management of ovarian cancer. Australian and New Zealand Journal of Obstetrics and Gynaecology 1983;23:65–68. [DOI] [PubMed] [Google Scholar]
- 20).Redman CW, Shafi MI, Ambrose S et al. Survival following intestinal obstruction in ovarian cancer. European journal of surgical oncology 1988;14:383–386. [PubMed] [Google Scholar]
- 21).Pothuri B, Vaidya A, Aghajanian C et al. Palliative surgery for bowel obstruction in recurrent ovarian cancer: an updated series. Gynecologic oncolog 2003;89:306–313. [DOI] [PubMed] [Google Scholar]
- 22).François A, Fabien M, Olivier G et al. Inflammation and immunity in radiation damage to the gut mucosa. BioMed Research International 2013;2013:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23).Coia LR, Lawrence R, Myerson RJ et al. Late effects of radiation therapy on the gastrointestinal tract. International Journal of Radiation Oncology* Biology* Physics 1995;31:1213–36. [DOI] [PubMed] [Google Scholar]
- 24).Stone HB, Coleman CN, Anscher MS et al. Effects of radiation on normal tissue: consequences and mechanisms. The Lancet Oncology 2003;4:529–36. [DOI] [PubMed] [Google Scholar]
- 25).Adler DG. Management of malignant colonic obstruction. Curr Treat Options Gastroenterol 2005;8:231–37. [DOI] [PubMed] [Google Scholar]
- 26).von Gruenigen VE, Frasure HE, Reldy AM et al. Clinical disease course during the last year in ovarian cancer. Gynecologic Oncology 2003;90:619–24. [DOI] [PubMed] [Google Scholar]