Abstract
Context
Icariin has attracted increasing attention because of its wide variety of pharmacological effects.
Objective
This study investigates whether icariin could promote fracture healing in young and old rats and its mechanisms.
Materials and methods
A Wistar rat model for the tibia fracture in relatively young and old rats, respectively, was established. The rats were divided into four groups: model group, L-icariin (50 mg/kg icariin), M-icariin (100 mg/kg icariin) and H-icariin (200 mg/kg icariin), and intragastric administration of icariin was performed for 10 days or 20 days. In addition, isolated and cultured rat bone mesenchymal stem cells (rBMSCs) from young and old rats were cultured with 5% and 20% of icariin-containing serum, respectively, then cell viability and alkaline phosphatase (ALP) activity were measured.
Results
Icariin administration induced the expression of Runx2, Osterix, BMP-2, p-Smad5 and osteocalcin secretion (young rats: model: 2.50 ± 0.71; L-icariin: 10.10 ± 1.55; M-icariin: 24.95 ± 2.19; H-icariin: 36.80 ± 2.26; old rats: model: 1.55 ± 0.49; L-icariin:6.55 ± 0.50; M-icariin: 15.00 ± 0.85; H-icariin:20.50 ± 2.27) at the fracture site, and increased the levels of bone formation markers (OC, BAP, NTX-1 and CTX-1) in a dose-dependent manner. In vitro, icariin treatment promoted rBMSC viability, increased ALP activity and the expression of BMP-2/Smad5/Runx2 pathway proteins.
Discussion and conclusions
Icariin may accelerate fracture healing by activating the BMP-2/Smad5/Runx2 pathway in relatively young and old rats. The research on the mechanism of icariin to promote fracture healing can provide a theoretical basis for the clinical application and promotion of icariin.
Keywords: Epimedium, rat bone mesenchymal stem cells, alkaline phosphatase, BMP-2/Smad5/Runx2 pathway
Introduction
Currently, fractures are very common all over the world. Healing in the vast majority of patients with fractures occurs by natural healing rather than by accelerated healing, and natural healing involves a long and slow physiological process (Giannoudis, Tzioupis, et al. 2007; Dimitriou et al. 2011). Therefore, it is necessary to find and study drugs or interventions that can accelerate fracture healing, and recent studies have identified certain substances that can indeed accelerate this process (Giannoudis, Psarakis, et al. 2007). Xu et al. (2015) pointed out that Sry-related high-mobility group box 11 can accelerate fracture healing and reduce delayed unions or nonunions by regulating mesenchymal stem cell differentiation and migration. Additionally, traditional Chinese medicine has been widely used in the treatment of fractures, osteoporosis and other bone-related diseases for thousands of years, and they can stimulate bone regeneration and accelerate fracture healing (Zhou et al. 2004). The roles of traditional Chinese medicine, such as Epimedium, in treating fractures have attracted researchers’ attention (Huang and You 1997; Zhang, Niu, et al. 2017).
Epimedium brevicornu Maxim. (Berberidaceae) (Epimedium) has been used in Chinese ethnopharmacology for the treatment of metabolic bone diseases. Icariin is a flavonoid and the active compound in Epimedium, which has been used to cure different diseases, including bone fractures, osteoporosis and tumours (Iqbal et al. 2018; Wang et al. 2018). In osteoporosis and osteoporotic fractures with different causes, oral medication of icariin can promote bone formation by inhibiting bone resorption and improve peak bone mineral density and bone quality (Cheng et al. 2014). Wu et al. (2018) found that icariin promoted bone formation, inhibited bone loss and effectively restored bone structure and strength in chronic high-dose alcohol-induced osteopenic rats. In vitro, icariin promoted the proliferation of rat BMSCs by activating the ERK and p38 MAPK signalling pathways (Qin et al. 2015). Despite the increase in the clinical usage of the Epimedium plant genus, the mechanisms underlying its therapeutic effects of the pharmacologically active constituent (icariin) on fracture healing are still not clearly understood.
The BMP-2/Smad5/Runx2 pathway is one of the most important pathways in osteoblast proliferation and differentiation (Dai et al. 2013). Based on this information, we suspect that the therapeutic effects of icariin on fracture healing are related to the BMP-2/Smad5/Runx2 pathway. Therefore, we established a rat model of fracture in relatively young and old rats, and isolated rat bone mesenchymal stem cells (rBMSCs) from the model rats and then intragastrically administered icariin or cocultured the cells with it. We detected indicators that indicate fracture healing to ultimately reveal the underlying mechanisms of the therapeutic effects of icariin on fracture healing in relatively young and old rats.
Materials and methods
Animals and regents
Twenty-four 6-month-old Wistar rats (male/female 1:1; 280–330 g) and twenty-four 22-month-old Wistar rats (male/female 1:1; 440–500 g) were purchased from the Medical Laboratory Animal Center of Guangdong Province. The experimental studies were approved by the Institutional Animal Care and Use Committee of Guangxi University of Chinese Medicine (approval no. DW20180328-31). The rats were housed in a temperature-controlled environment (25 °C ± 2 °C) with a 12 h light/dark cycle and free access to food and water. The icariin standard (HPLC > 94%) was purchased from Sigma-Aldrich (#I1286, Shanghai, China).
Modelling of fractures caused by trauma in young and old rats, and treatment
Forty-eight Wistar rats (6 months and 22 months) were anaesthetized using 2% isoflurane, respectively. After animals were successfully anaesthetized, 1–3 pounds of weight were dropped from a height (10–15 cm) on the middle of the tibia, causing a tibia fracture. Postoperative intraperitoneal injection of painkillers and antibiotics were provided to prevent postoperative pain and infection after modelling.
After generating the fracture model, the rats were randomly divided into four groups: model group (gavaged 1 mL saline), low-dose group (L-icariin, gavaged 50 mg/kg icariin in 1 mL saline), medium-dose group (M-icariin, gavaged 100 mg/kg icariin in 1 mL saline) and high-dose group (H-icariin, gavaged 200 mg/kg icariin in 1 mL saline), with six rats in each group. The above intragastric administration was prepared once a day for a total of 10 days or 20 days. All rats were free to drink water. The doses of icariin (50, 100 and 200 mg/kg) were obtained from clinical experience and preliminary experiment in rats.
Sampling methods
On the 1st day, 10th day and 20th day, the rats were subjected to digital radiography (DR) examination (Goodsee, #GDA32-02, China) of their fracture healing, and scored the degree of fracture healing using the Lane–Sandhu scoring standard (Wang et al. 2007). On the 10th day, three rats of each group were anaesthetized using 2% isoflurane; the coeliac arterial blood was immediately isolated and centrifuged at 5000 rpm for 10 min, and the serum was used for ELISA assay. Then, the rats were euthanized by exsanguination under 2% isoflurane anaesthesia. The entire right tibia was taken for histological examination, RT-PCR and Western blot.
Haematoxylin and eosin (HE) staining
The entire right tibia was removed and fixed in 4% paraformaldehyde-phosphate-buffered saline (PBS) solution for three days. After decalcification of the entire right tibia, it was embedded in paraffin and then cut into 5 μm sections. The sections were stained as follows: 70% ethyl alcohol for 10 s, diethylpyrocarbonate-treated water for 5 s, haematoxylin with RNase inhibitor for 20 s, 70% ethyl alcohol for 30 s and eosin Y in 100% ethyl alcohol for 20 s, followed by dehydration in a series of alcohol for 30 s each and in xylenes for 2 min. HE staining images were captured at ×400 magnification (CX71, Olympus Corporation, Tokyo, Japan).
RNA preparation and RT-PCR
The bone tissue was located at the fracture site, placed in liquid nitrogen, and ground; then, the total RNA was extracted by using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized using a cDNA Synthesis Kit (Takara, Dalian, China). Quantitative RT-PCR (qRT-PCR) was performed to detect the expression levels of mRNA using SYBR Green and a LightCycler 480 detection system (Roche Diagnostics, Indianapolis, IN). GAPDH mRNA levels were used for normalization. The primer sequences were as follows: GAPDH: 5′-TGACAACTTTGGCATCGTGG-3A and 5′-GGGCCATCCACAGTCTTCTG-3GC Runx2: 5′-GCCTTCAAGGTTGTAGCCCT-3C and 5′-TGAACCTGGCCACTTGGTTT-3AA BMP-2: 5′-GGACGTCCTCAGCGAGTTT-3A and 5′-CAGGTCGAGCATATAGGGGG-3GGTOsterix: 5′-GGCTGAGGAAGAAGCCCATT-3C and 5′-AAGTGGGCTTTCAGATGCGA-3GT Smad5: 5′-TGTTGGGCTGGAAACAAGGT-3T and 5′-GTGACACACTTGCTTGGCTG-3GAC. qRT-PCR results were analysed and expressed as relative mRNA levels based on the CT value, which was then converted to fold change.
Western blot assay
The bone tissue was located at the fracture site, placed in liquid nitrogen, and ground; then, it was lysed using lysis buffer, and the total protein concentration of lysates was measured using a micro BCA protein assay kit (Pierce, Rockford, IL). The samples were separated on 12% SDS-polyacrylamide gels and then electrophoretically transferred to polyvinylidene difluoride membranes. After blocking in 5% non-fat milk solution for 1 h, the membranes were incubated with anti-Osterix (Abcam, ab209484, Cambridge, MA, dilution 1:1000), anti-BMP-2 (Abcam, ab14933, Cambridge, MA, dilution 1:500), anti-phosphorylated-Smad5 (Abcam, ab92698, Cambridge, MA, dilution 1:200), anti-Smad5 (Abcam, ab40771, Cambridge, MA, dilution 1:1000), anti-Runx2 (Abcam, ab76956, Cambridge, MA, dilution 1:200) and GAPDH antibodies for 2 h at 37 °C. Then, the membranes were washed three times and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. Next, the membranes were treated with ECL solution (Millipore, Darmstadt, Germany) and then imaged using ImageJ (Bethesda, MD). The grey values of each protein band were measured by Photoshop CS5 software (Adobe, San Jose, CA). The relative band intensity was assessed as the ratio of the grey value of each protein to that of the corresponding GAPDH.
Immunohistochemistry assay
After decalcifying the entire right tibia, it was embedded in paraffin and then cut into 5 μm sections. Following hydration and blocking, the slides were treated with peroxide. An anti-osteocalcin antibody (Abcam, ab13420, Cambridge, MA, dilution 1:500) was incubated with the slides at 37 °C for 2 h. After incubating with a secondary antibody at 37 °C for 1 h, the slides were treated with 3,3′-diaminobenzidine (DAB) solution. Finally, the slides were lightly counterstained with haematoxylin and dehydrated. Visual analysis was performed with an Olympus fluorescence microscope (Olympus, CX71, Tokyo, Japan) at ×400 magnification. The medium optical density (MOD) of osteocalcin expression in each group was analysed by Image-Pro Plus software (Media Cybernetics, Rockville, MD).
ELISA
A Rat OC ELISA kit (Cloud-Clone Corp., #SEA471Ra, Wuhan, China), Rat BAP ELISA kit (Jiancheng, #A059-1, Nanjing, China), Rat NTX-1 ELISA kit (Cloud-Clone Corp., #CEA639Ra, Wuhan, China) and Rat CTX-1 ELISA kit (Cloud-Clone Corp., Wuhan, #CEA665Ra, China) were used for OC, BAP, NTX-1 and CTX-1 measurements in this study. All assays were performed exactly following the manufacturer’s instructions.
Isolation, culture and identification of rBMSCs
Six-month-old/22-month-old rats were sacrificed by euthanized by exsanguination under 2% isoflurane anaesthesia. Under sterile conditions, 1 mL of bone marrow was extracted from the femur and tibia using a syringe prefilled with 2 mL of heparin for anticoagulation. Bone marrow mononuclear cells were separated by gradient density centrifugation and cultured in osteogenic differentiation medium of Wistar rat BMSCs (Cyagen Biosciences, RAWMX-90021, Santa Clara, CA) supplemented with 20% foetal bovine serum (HyClone, Logan, UT), 100 U/mL penicillin and 100 U/mL streptomycin at 37 °C in a 5% CO2 atmosphere (Thermo, Waltham, MA). Forty-eight hours later, nonadherent cells were removed, and the adherent cells were cultured and expanded in another flask. After 2–3 weeks of continuous culture, homogenous rBMSCs from young and old animals were obtained and identified by immunofluorescence.
The identification of rBMSCs was performed by immunofluorescence using a fluorescently labelled antibody. rBMSCs were fixed in 4% (w/v) paraformaldehyde in PBS for 20 min and then incubated with anti-CD29-FITC (eBioscience, #11-0291-82, Shanghai, China), anti-CD44-FITC (eBioscience, #MA5-17522, Shanghai, China) and anti-CD45-FITC (eBioscience, #11-0461-82, Shanghai, China). Visual analysis was performed with an Olympus fluorescence microscope (Olympus, CX71, Tokyo, Japan) at ×400 magnification.
Preparation of icariin-containing serum
Six adult Wistar rats (male/female 1:1; weight, 280–330 g, 6 months old) were orally gavaged with 200 mg/kg icariin once a day for seven days (Zhang et al. 2020). After seven days, the rats were euthanized by exsanguination under 2% isoflurane anaesthesia, and blood samples were collected. The icariin-containing serum was prepared by centrifugation at 2000×g at 4 °C for 15 min. rBMSCs were cultured with 5% and 20% icariin-containing serum or 5% and 20% icariin-free serum, and then RT-PCR, Western blot, CCK-8 and alkaline phosphatase (ALP) activity assays were performed.
CCK-8 assay
Homogenous rBMSCs from young and old animals were seeded in triplicate in 96-well plates (5 × 103 cells/well), and cultured in osteogenic differentiation medium supplemented with 5% and 20% icariin-containing serum, or 5% and 20% icariin-free serum for 72, 96 and 120 h, respectively. After treatment, the CCK-8 working solution was added to the wells for 1 h of incubation, and then cell viability was assessed daily by absorbance at 490 nm using a microplate reader (model 680 Microplate Reader, Bio-Rad, Hercules, CA).
ALP activity assay
rBMSCs from young and old animals were seeded in triplicate in 24-well plates (5 × 104 cells/well), treated with 5% and 20% icariin-containing serum and 5% and 20% icariin-free serum for 120 h, and then the ALP activity of the different rBMSC was detected. ALP activity was determined using an ALP activity kit (Jiancheng, #A059-1, Nanjing, China).
Statistical methods
Data were statistically analysed using SPSS 17.0 (SPSS Inc., Chicago, IL). All results are presented as the mean values ± standard deviations. *p< 0.05, **p< 0.01 and ***p< 0.001 were considered statistically significant. Statistically significant differences between groups were determined by one-way analysis of variance (ANOVA) with the Bonferroni post hoc test when equal variances were assumed, or with Dunnett's T3 post hoc test when equal variances were not assumed. Figures were graphed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA).
Results
Icariin accelerated fracture healing
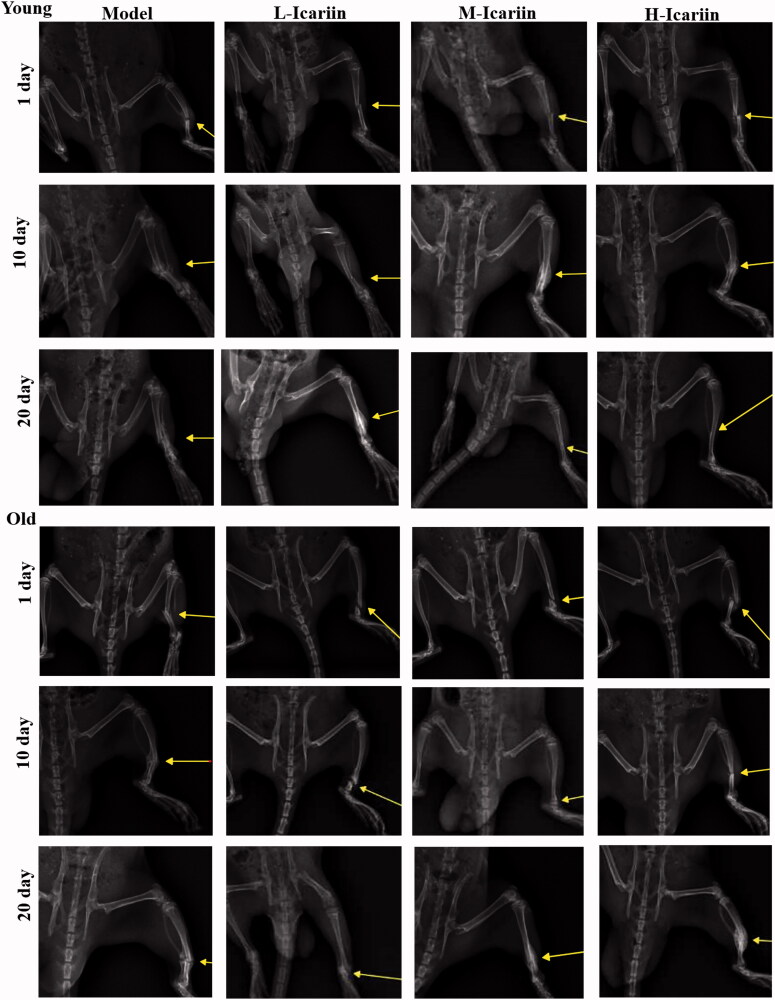
DR of rat tibias showed incomplete healing in rats subjected to natural healing on the 1st day, 10th day and 20th day in both young and old rats. Compared with the model rats with natural healing, at the 10th day and 20th day, the fracture model rats treated with icariin exhibited better fracture healing and Lane–Sandhu’s scores (Table 1). Additionally, the fracture healing speed and Lane–Sandhu’s scores of young rats were higher and faster than that of old rats (Table 1). DR images (Figure 1) indicated that icariin can accelerate fracture healing in both young and old rat models of fractures in a dose-dependent manner.
Table 1.
Lane–Sandhu’s scores of DR in each group.
| Groups | Time (days) | n | Model | L-Icariin | M-Icariin | H-Icariin |
|---|---|---|---|---|---|---|
| Young | 1 | 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 10 | 6 | 2.33 ± 0.52 | 3.33 ± 0.52# | 5.16 ± 0.41*,# | 5.50 ± 0.55*,# | |
| 20 | 3 | 4.67 ± 0.58 | 5.33 ± 0.58# | 8.33 ± 0.58*,# | 9.33 ± 0.58*,# | |
| Old | 1 | 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 10 | 6 | 2.17 ± 0.41 | 2.33 ± 0.52 | 4.17 ± 0.41* | 4.83 ± 0.41* | |
| 20 | 3 | 4.00 ± 1.00 | 4.33 ± 0.58 | 5.00 ± 0.00* | 5.67 ± 0.58* |
p< 0.05 vs. corresponding time’s model group.
p< 0.05 vs. corresponding time’s old group.
Figure 1.
Icariin accelerated fracture healing in the rat model of fractures. Digital radiography (DR) was used to observe fracture healing on the 1st day, 10th day and 20th day.
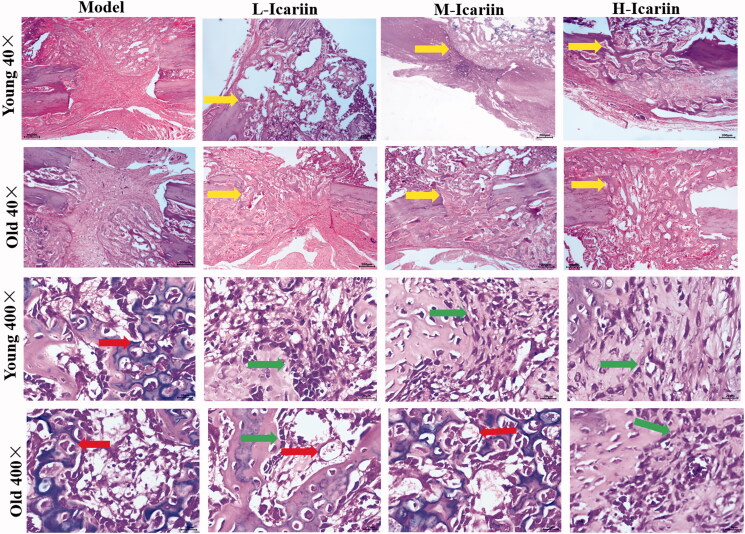
After 10 days of icariin administration, compared with the model group with natural healing and the L-icariin group, low magnification (×40) images showed faster transformation of trabecular bone into compact bone in the H-icariin group, and high magnification (×400) images showed that the H-icariin group had more osteoblasts and faster compact bone substance formation. Additionally, compared with the same treatment, the number of osteoblasts and bone marrow cells in young rats was higher than it was in old rats. DR (Figure 1) and HE staining (Figure 2) indicated that icariin can induce bone formation and accelerate fracture healing in young and old rat models of fracture in a dose-dependent manner.
Figure 2.
HE staining images of the fracture site in the rat model of fractures. Magnification ×40 and ×400. Yellow arrow: trabecular bone. Red arrow: chondrocytes. Green arrow: osteoblasts.
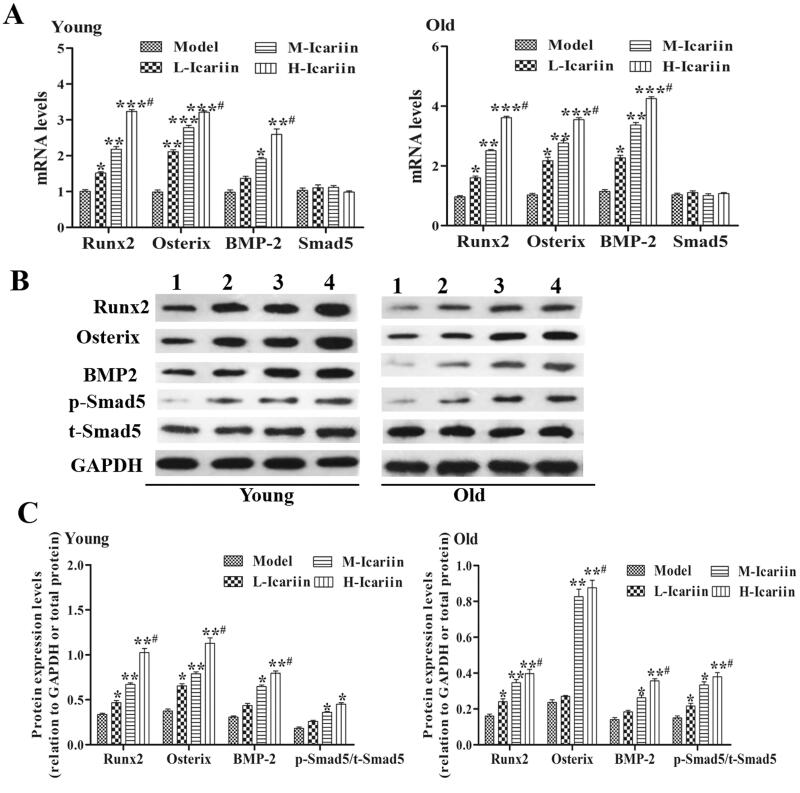
Icariin activated the BMP-2/Smad5/Runx2 pathway in the rat fracture model
RT-PCR and Western blot detected the expression levels of Runx2, Osterix, BMP-2 and Smad5 mRNAs and proteins in rat fracture models (Figure 3). In the young rat fracture models, after 10 days of icariin administration, the levels of Runx2, Osterix, BMP-2 and p-Smad5 in the fracture section were significantly higher than those of the model group (p < 0.05). Compared with the L-Icariin group, the levels of Runx2, Osterix, BMP-2 and p-Smad5 in the H-Icariin group were significantly increased. Additionally, icariin presented the same effects in the old rat fracture models. These data showed that icariin can activate the BMP-2/Smad5/Runx2 pathway in a dose-dependent manner.
Figure 3.
Icariin activates the BMP-2/Smad5/Runx2 pathway in the rat model of fractures. (A) RT-qPCR was performed to detect the mRNA expression levels of Runx2, Osterix, BMP-2 and Smad5. GAPDH was used as the control. (B) The expression levels of Runx2, Osterix, BMP-2, p-Smad5 and t-Smad5 proteins were detected by Western blotting. (1) Model group, (2) L-icariin group, (3) M-icariin group and (4) H-icariin group. (C) The relative expression levels of Runx2, Osterix, BMP-2, p-Smad5 and t-Smad5 were calculated by normalizing to the levels of GAPDH. Data are represented as the means ± SD (n = 3). *p< 0.05, **p< 0.01 and ***p< 0.001 vs. the model group. #p< 0.05 vs. the corresponding L-icariin group.
Icariin regulated the levels of bone formation- and bone resorption-specific markers in rat fracture models
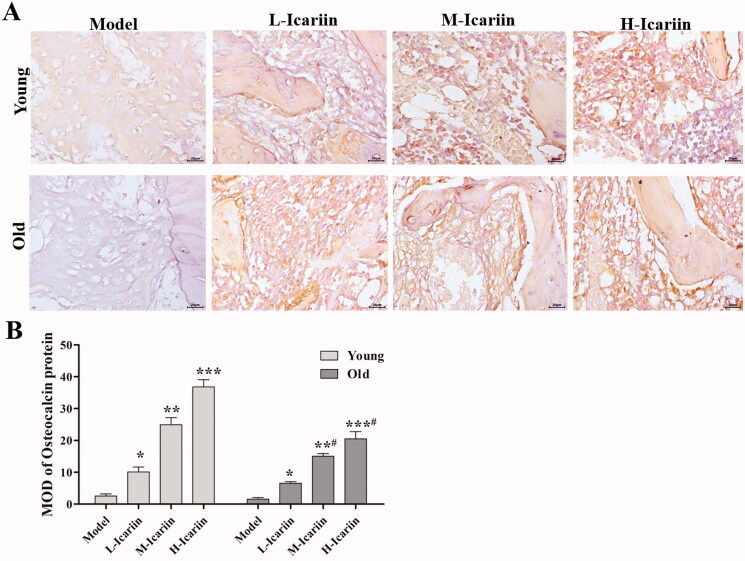
We detected the levels of bone formation- and bone resorption-specific markers. The results in Table 2 show that the levels of OC, BAP, NTX-1 and NCTX-1 in icariin-treated rats were critically higher than they were in model rats. Surprisingly, compared with the L-Icariin group, the levels of OC, BAP, NTX-1 and NCTX-1 in the H-Icariin group were significantly increased. Similar results were also obtained both rat fracture models. In addition, we measured the expression levels of OC at the fracture section using an immunohistochemistry assay on the 10th day and found that icariin treatment induced OC expression in a dose-dependent manner (Figure 4). These results revealed that icariin could accelerate bone formation and bone resorption in the young and old fracture rats.
Table 2.
The concentrations of OC, BAP, NTX-1 and CTX-1 in our rat model of fractures on the 10th day (n = 3).
| Groups | Markers | Model | L-Icariin | M-Icariin | H-Icariin |
|---|---|---|---|---|---|
| Young | OC (ng/mL) | 1.78 ± 0.065 | 1.79 ± 0.067 | 1.83 ± 0.057 | 2.27 ± 0.064*,# |
| BAP (U/mg prot) | 36.3 ± 1.57 | 41.05 ± 1.81 | 48.86 ± 1.10* | 60.85 ± 2.03*,# | |
| NTX-1 (ng/mL) | 1.05 ± 0.057 | 1.15 ± 0.062 | 1.21 ± 0.062* | 1.59 ± 0.071*,# | |
| CTX-1 (ng/mL) | 0.50 ± 0.041 | 0.55 ± 0.048 | 0.67 ± 0.052* | 1.18 ± 0.067*,# | |
| Old | OC (ng/mL) | 0.94 ± 0.058 | 1.42 ± 0.061* | 1.51 ± 0.063* | 1.86 ± 0.064*,# |
| BAP (U/mg prot) | 22.45 ± 1.64 | 25.34 ± 2.15 | 32.69 ± 1.50* | 49.65 ± 2.41*,# | |
| NTX-1 (ng/mL) | 0.71 ± 0.052 | 0.85 ± 0.049 | 0.95 ± 0.058* | 1.20 ± 0.063*,# | |
| CTX-1 (ng/mL) | 0.28 ± 0.058 | 0.41 ± 0.053* | 0.51 ± 0.055* | 0.96 ± 0.053*,# |
Data are represented as the means ± SD (n = 3).
p< 0.05 vs. model group.
p< 0.05 vs. L-Icariin group.
Figure 4.
Icariin induces the expression of osteocalcin in the rat model of fractures. (A) Immunohistochemistry to detect the expression of osteocalcin protein. (B) The MOD of osteocalcin expression was calculated using Image-Pro Plus 6.0 software. Data are represented as the means ± SD (n = 3). *p< 0.05, **p< 0.01 and ***p< 0.001 vs. the model group. #p< 0.05 vs. the corresponding young group.
Icariin increased cell viability and ALP activity in rBMSCs from young and old rats
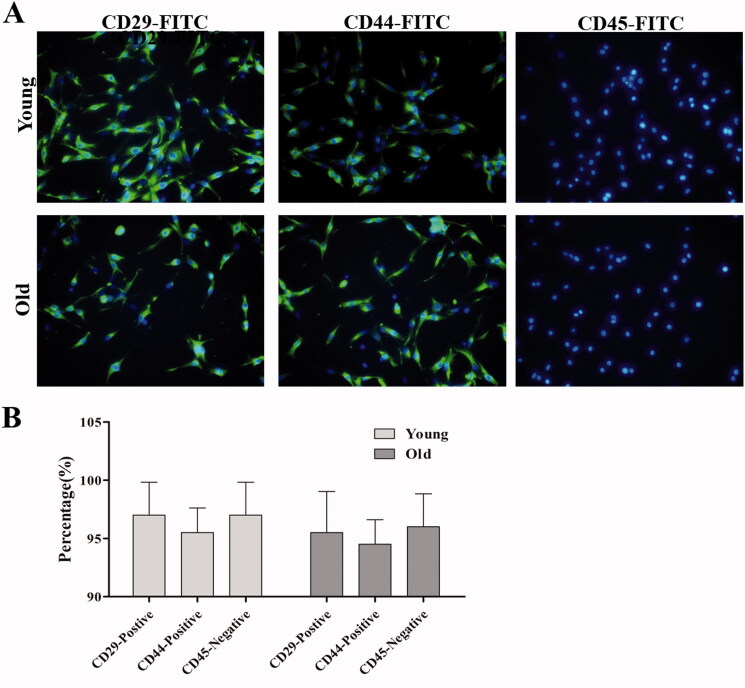
After isolation of the rBMSCs from young and old rats, immunofluorescence was used to identify the purity of the rBMSCs using a fluorescently labelled antibody. As shown in Figure 5(A), the percentage of CD29-positive and CD44-positive rBMSCs exceeded 95%, and the percentage of CD45-negative rBMSCs exceeded 95% (Figure 5(B)). Similar results were also obtained in rBMSCs from both young and old rats. Immuophenotypic studies have shown that cultured rBMSCs are CD29- and CD44-positive and CD45-negative (Qin et al. 2015; Wu et al. 2018). Therefore, these data show that the purity of rBMSCs (95%) was high enough and could be used for follow-up experiments.
Figure 5.
Immunofluorescence identification of the rBMSCs isolated from young and old rats. (A) Immunofluorescence was used to identify the purity of rBMSCs using a fluorescently labelled antibody. (B) The percentage of CD29-positive, CD44-positive and CD45-negative rBMSCs from young and old rats. Magnification ×400. Data are represented as the means ± SD (n = 5).
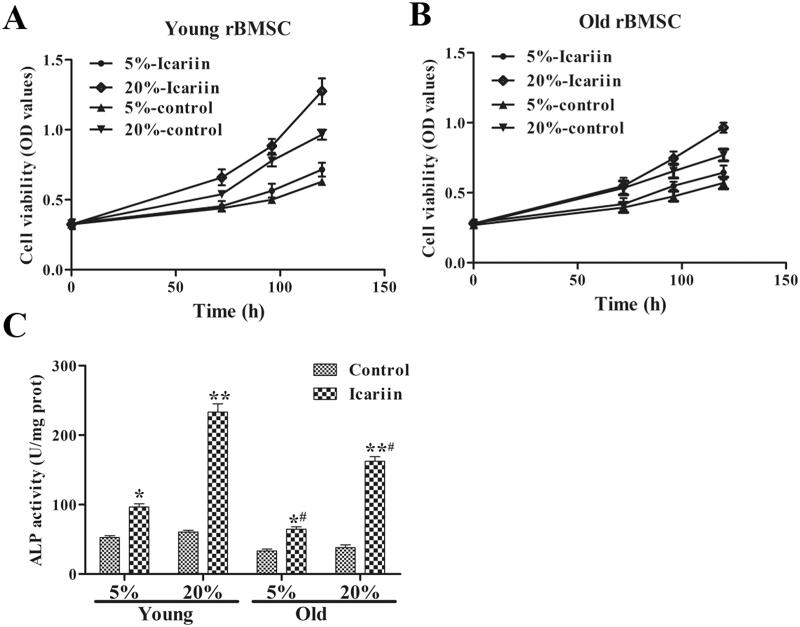
Then, we performed CCK-8 and ALP activity assays to show the effects of icariin on rBMSC viability and ALP activity. As shown in Figure 5, at the same serum concentration (5% and 20%), the rBMSC viability and ALP activity of the icariin-containing serum group were significantly higher than those of the icariin-free serum group. For the various concentrations of icariin-containing serum (5% and 20%), rBMSC viability and ALP activity were upregulated (Figure 6). Surprisingly, compared with young rBMSCs, rBMSC viability and ALP activity in older rBMSCs were significantly decreased. These results revealed that icariin increased rBMSC viability and ALP activity in rBMSCs from young and old rats.
Figure 6.
The rBMSC viability (A, B) and ALP activity (C) assay of rBMSCs from young and old rats after icariin treatment. Data are represented as the means ± SD (n = 6). *p< 0.05; **p< 0.01 and #p< 0.05 vs. its control group.
Icariin activated the BMP-2/Smad5/Runx2 pathway in rBMSCs from young and old rats
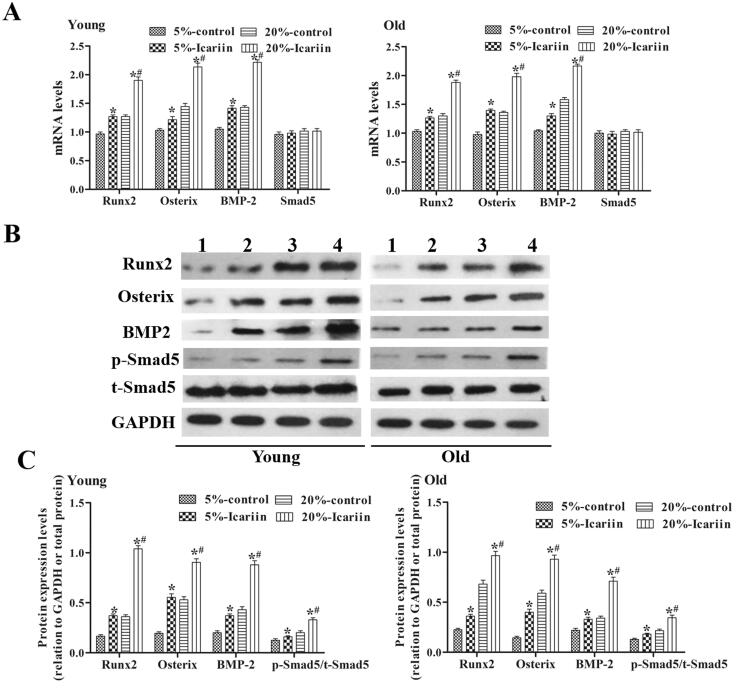
RT-PCR and Western blot experiments were used to detect the expression levels of Runx2, Osterix, BMP-2 and Smad5 mRNAs and proteins in rBMSCs from young and old rats (Figure 7). Compared with the rBMSCs cultured in icariin-free serum, the levels of Runx2, Osterix and BMP-2 in rBMSCs cultured with 5% and 20% icariin-containing serum were significantly higher (p < 0.05). Compared with the 5% icariin-containing serum group, the levels of Runx2, Osterix and BMP-2 in the 20% icariin-containing serum group were significantly increased. Additionally, icariin exhibited the same effects on rBMSCs from young and old rats, and icariin promoted Smad5 phosphorylation. These data showed that icariin can activate the BMP-2/Smad5/Runx2 pathway in rBMSCs from young and old rats.
Figure 7.
Icariin activated the BMP-2/Smad5/Runx2 pathway in rBMSCs from young and old rats. (A) RT-qPCR was performed to detect the mRNA expression levels of Runx2, Osterix, BMP-2 and Smad5. GAPDH was used as the control. (B) The expression levels of Runx2, Osterix, BMP-2, p-Smad5 and t-Smad5 proteins were detected by Western blotting. (1) 5% icariin-control group, (2) 5% icariin-containing group, (3) 20% icariin-control group and (4) 20% icariin-containing group. (C) The relative expression levels of Runx2, Osterix, BMP-2, p-Smad5 and t-Smad5 were calculated by normalizing to the levels of GAPDH. Data are represented as the means ± SD (n = 4). *p< 0.05 vs. its icariin-control group. #p< 0.05 vs. the 5% icariin-containing group.
Discussion
Epimedium has been used as a medicine in China for thousands of years, and its pharmacological effects and underlying mechanism are currently being studied in depth (Dai et al. 2013; Ti et al. 2017; Xi et al. 2018). Various studies have shown that icariin, as the major active component in Epimedium, has important pharmacological effects on metabolic bone diseases, including bone fractures and osteoporosis (Cheng et al. 2014; Qin et al. 2015; Wang et al. 2018; Wu et al. 2018). Our team studied the effects of icariin on fracture healing in relatively young and old rats, respectively, in vitro and in vivo. After intragastric administration of icariin for 10 days, we found that icariin could increase the levels of OC, BAP, NTX-1 and CTX-1 in serum, induce the expression of Runx2, Osterix, BMP-2 and p-Smad5, and promote viability and increase ALP activity in rBMSCs from young and old rats. In summary, our study showed that the therapeutic mechanism of icariin on fracture healing in relatively young and old rats.
Under normal circumstances, bone formation markers (e.g., OC and BAP) and bone resorption markers (e.g., NTX-1, CTX-1 and TRACP-5) are at relatively low levels in blood. When fractures occur, their levels increase significantly, and when the fractures heal, their levels return to normal levels (Froberg et al. 1999). Among them, OC, a secreted osteoblast-specific protein, is a unique noncollagenous protein of the extracellular matrix of bone that circulates in blood, and serum markers such as OC and ALP reflect osteoblastic activity in part during bone formation (Taniguchi et al. 2003; Bailey et al. 2017). Osteocalcin levels increased both in the bone and blood in femoral fracture model rats, and the higher the levels were, the faster the fracture healed (Aydin et al. 2014). In this study, we found that icariin could upregulate the levels of OC, BAP NTX-1 and CTX-1 in serum and increase OC expression in bone. These results indicate that icariin could regulate bone formation and bone resorption, resulting in the regulation of fracture healing.
The BMP-2/Smad5/Runx2 pathway is one of the most important pathways in bone metabolism. Bone morphogenetic proteins (BMPs) trigger intracellular signalling and activate Smad complexes that regulate the transcription of BMP-responsive genes, including Runx2 (Leboy et al. 2001). Runx2 (Cbfαl) is a transcription factor required for bone formation, and it functions synergistically with Smad1 and Smad5 to regulate bone-specific genes when BMPs induce osteogenesis (Ito and Miyazono 2003). A previous study indicated that the BMP-2/RUNX2 pathway is involved in osteogenic differentiation and mineralization of MC3T3-E1 osteoblastic cells in vitro and that it protects against ovariectomy-induced bone loss in vivo (Li et al. 2016). In ovariectomized rats, Epimedium promoted cartilage ossification of the callus and enhanced bone strength and bone quality via the CKIP-1/Runx2 pathway in the process of fracture healing (Shi et al. 2017). In bone infection caused by Staphylococcus aureus, Zhang, Shen, et al. (2017) found that icariin promoted bone formation by upregulating the BMP2/Runx2 and OPG/RANKL pathways under high local concentrations of vancomycin treatment. This study found that icariin can induce the expression of Runx2, Osterix, BMP-2 and p-Smad5 in our rat fracture model in a dose-dependent manner. Based on the roles of the BMP2/Runx2 pathway in bone metabolism, our results implied that icariin regulates bone formation and fracture healing via the BMP-2/Smad5/Runx2 pathway.
With the exception of haematopoietic stem cells, bone mesenchymal stem cells (BMSCs) are the main cells in the bone matrix environment, and they can differentiate into multiple types of cells, such as those in the chondrocytic and osteocytic lineages (Deans and Moseley 2002). It is becoming increasingly well accepted that BMSCs are involved in fracture repair and the bone regeneration process. When a fracture occurs, it is accompanied by the systemic mobilization of osteoblastic precursors to the fracture site, and increasing the number of BMSCs can accelerate fracture healing (Huang et al. 2015). ALP is mainly a biochemical marker of bone turnover, and it is usually used to monitor metabolic bone disease; higher serum ALP levels are associated with the incidence of fracture (Maruyama et al. 2014). A previous study pointed out that icariin enhanced human BMSC proliferation, motility and osteogenic differentiation through the STAT-3/CXCR4 pathway, and icariin may be a potential therapeutic for improving bone health (Lim et al. 2018). Our study found that icariin coculture could promote rBMSC viability, increase ALP activity and activate the BMP-2/Smad5/Runx2 pathway in rBMSCs from young and old animals, which indicates that icariin may induce osteogenic proliferation and differentiation of BMSCs by increasing ALP activity and activating the BMP-2/Smad5/Runx2 pathway.
Conclusions
This study found that icariin may accelerate fracture healing in relatively young and old rats, respectively, by activating the BMP-2/Smad5/Runx2 pathway. Therefore, Epimedium and its active component icariin can be developed as a fracture healing treatment drug. In the future, our team will study the regulatory relationships between icariin and the BMP-2/Smad5/Runx2 pathway and will explore in depth other relevant molecular mechanisms.
Funding Statement
This work was supported by the National Natural Science Foundation of China (No. 81760796), the Guangxi Young Teachers' Basic Ability Improvement Project (Nos. 2019KY0352 and KY2016YB204) and Natural Science Foundation of Guangxi Province (No. 2020GXNSFBA159053).
Disclosure statement
The authors declare that they have no competing interests.
References
- Aydin A, Halici Z, Akoz A, Karaman A, Ferah I, Bayir Y, Aksakal AM, Akpinar E, Selli J, Kovaci H.. 2014. Treatment with α-lipoic acid enhances the bone healing after femoral fracture model of rats. Naunyn-Schmiedeberg's Arch Pharmacol. 387(11):1025–1036. [DOI] [PubMed] [Google Scholar]
- Bailey S, Karsenty G, Gundberg C, Vashishth D.. 2017. Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann NY Acad Sci. 1409(1):79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K, Ge B, Chen K, Zhen P, Zhou J, Ma X, Song P, Ma H.. 2014. Oral medication of icariin enhances peak bone mineral density and bone quality in rats. Chin J Osteoporos. 20:120–124. [Google Scholar]
- Dai J, Li Y, Zhou H, Chen J, Chen M, Xiao Z.. 2013. Genistein promotion of osteogenic differentiation through BMP2/SMAD5/RUNX2 signaling. Int J Biol Sci. 9(10):1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans RJ, Moseley AB.. 2002. Mesenchymal stem cells. Bull Exp Biol Med. 133:103–109. [DOI] [PubMed] [Google Scholar]
- Dimitriou R, Jones E, McGonagle D, Giannoudis PV.. 2011. Bone regeneration: current concepts and future directions. BMC Med. 9:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froberg MK, Garg UC, Stroncek DF, Geis M, McCullough J, Brown DM.. 1999. Changes in serum osteocalcin and bone-specific alkaline phosphatase are associated with bone pain in donors receiving granulocyte-colony-stimulating factor for peripheral blood stem and progenitor cell collection. Transfusion. 39(4):410–414. [DOI] [PubMed] [Google Scholar]
- Giannoudis P, Psarakis S, Kontakis G.. 2007. Can we accelerate fracture healing? A critical analysis of the literature. Injury. 38(Suppl. 1):S81–S89. [DOI] [PubMed] [Google Scholar]
- Giannoudis P, Tzioupis C, Almalki T, Buckley R.. 2007. Fracture healing in osteoporotic fractures: is it really different? A basic science perspective. Injury. 38(Suppl. 1):S90–S99. [DOI] [PubMed] [Google Scholar]
- Huang S, Xu L, Zhang Y, Sun Y, Li G.. 2015. Systemic and local administration of allogeneic bone marrow-derived mesenchymal stem cells promotes fracture healing in rats. Cell Transplant. 24(12):2643–2655. [DOI] [PubMed] [Google Scholar]
- Huang HF, You JS.. 1997. The use of Chinese herbal medicine on experimental fracture healing. Am J Chin Med. 25(3–4):351–356. [DOI] [PubMed] [Google Scholar]
- Iqbal M, Zhang H, Mehmood K, Li A, Jiang X, Wang Y, Zhang J, Iqbal MK, Rehman MU, Yao W, et al. 2018. Icariin: a potential compound for the recovery of tibial dyschondroplasia affected chicken via up-regulating BMP-2 expression. Biol Proced Online. 20:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Miyazono K.. 2003. RUNX transcription factors as key targets of TGF-β superfamily signaling. Cur Opin Genet Dev. 13(1):43–47. [DOI] [PubMed] [Google Scholar]
- Leboy P, Grasso-Knight G, D'Angelo M, Volk SW, Lian JV, Drissi H, Stein GS, Adams SL.. 2001. Smad–Runx interactions during chondrocyte maturation. J Bone Joint Surg Am. 83-A(Suppl. 1, Pt 1):S15–S22. [PubMed] [Google Scholar]
- Li J, Hao L, Wu J, Zhang J, Su J.. 2016. Linarin promotes osteogenic differentiation by activating the BMP-2/RUNX2 pathway via protein kinase A signaling. Int J Mol Med. 37(4):901–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RZL, Li L, Yong EL, Chew N.. 2018. STAT-3 regulation of CXCR4 is necessary for the prenylflavonoid icaritin to enhance mesenchymal stem cell proliferation, migration and osteogenic differentiation. Biochim Biophys Acta Gen Subj. 1862(7):1680–1692. [DOI] [PubMed] [Google Scholar]
- Maruyama Y, Taniguchi M, Kazama JJ, Yokoyama K, Hosoya T, Yokoo T, Shigematsu T, Iseki K, Tsubakihara Y.. 2014. A higher serum alkaline phosphatase is associated with the incidence of hip fracture and mortality among patients receiving hemodialysis in Japan. Nephrol Dial Transplant. 29(8):1532–1538. [DOI] [PubMed] [Google Scholar]
- Qin S, Zhou W, Liu S, Chen P, Wu H.. 2015. Icariin stimulates the proliferation of rat bone mesenchymal stem cells via ERK and p38 MAPK signaling. Int J Clin Exp Med. 8(5):7125–7133. [PMC free article] [PubMed] [Google Scholar]
- Shi XL, Liang BC, Wu P, Wang B, Shi ZY, Kong LC, Yao JL, Huang JJ, Li CW.. 2017. Effect and mechanism of total flavone of Epimedium on primary callus formation in ovariectomized rats with fractures. Zhongguo Gu Shang. 30:743–750. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Matsumoto T, Shindo H.. 2003. Changes of serum levels of osteocalcin, alkaline phosphatase, IGF-I and IGF-binding protein-3 during fracture healing. Injury. 34(7):477–479. [DOI] [PubMed] [Google Scholar]
- Ti H, Wu P, Xu L, Wei X.. 2017. Anti-inflammatory neolignans from Epimedium pseudowushanese. Nat Prod Res. 31(22):2621–2628. [DOI] [PubMed] [Google Scholar]
- Wang CJ, Liu HC, Fu TH.. 2007. The effects of extracorporeal shockwave on acute high-energy long bone fractures of the lower extremity. Arch Orthop Trauma Surg. 127(2):137–142. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wang D, Yang D, Zhen W, Zhang J, Peng S.. 2018. The effect of icariin on bone metabolism and its potential clinical application. Osteoporos Int. 29(3):535–544. [DOI] [PubMed] [Google Scholar]
- Wu JZ, Liu PC, Liu R, Cai M.. 2018. Icariin restores bone structure and strength in a rat model of chronic high-dose alcohol-induced osteopenia. Cell Physiol Biochem. 46(4):1727–1736. [DOI] [PubMed] [Google Scholar]
- Xi HR, Ma HP, Yang FF, Gao YH, Zhou J, Wang YY, Li WY, Xian CJ, Chen KM.. 2018. Total flavonoid extract of Epimedium herb increases the peak bone mass of young rats involving enhanced activation of the AC10/cAMP/PKA/CREB pathway. J Ethnopharmacol. 223:76–87. [DOI] [PubMed] [Google Scholar]
- Xu L, Huang S, Hou Y, Liu Y, Ni M, Meng F, Wang K, Rui Y, Jiang X, Li G.. 2015. Sox11-modified mesenchymal stem cells (MSCs) accelerate bone fracture healing: Sox11 regulates differentiation and migration of MSCs. FASEB J. 29(4):1143–1152. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Chen YP, Zhang C, Zhang X, Xia T, Han J, Yang N, Song SL, Xu CH.. 2020. Icariin accelerates fracture healing via activation of the WNT1/β-catenin osteogenic signaling pathway. Curr Pharm Biotechnol. 21(15):1645–1653. [DOI] [PubMed] [Google Scholar]
- Zhang K, Niu LC, Yuan FJ, Liu SP.. 2017. Research on promotory effect of traditional Chinese medicine on fracture healing in cell and molecular level. Zhongguo Gu Shang. 30(8):777–782. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Shen L, Mao Z, Wang N, Wang X, Huang X, Hu Y, Shou D, Wen C.. 2017. Icariin enhances bone repair in rabbits with bone infection during post-infection treatment and prevents inhibition of osteoblasts by vancomycin. Front Pharmacol. 8:784–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FH, Zhao MJ, Zhao HY.. 2004. Iontophoresis with traditional Chinese herbal medicine accelerates the healing of bone fracture. J First Military Med Univ. 24:708–710. [PubMed] [Google Scholar]