Abstract
The present trial evaluated the effect of crossbred composition and Temperature and Humidity Index (THI) on vaginal temperature (VT) of Girolando dairy cows maintained under tropical pasture during warm seasons. The VT was monitored along 41 to 96 h in 615 Girolando cows with different Holstein (H) × Gir genetic composition (1/2 H = 284, 3/4 H = 248, and 7/8 H = 83) from six Brazilian farms in the summer of 2016 and 2017. VT of each cow at each hour of the day and the respective THI were averaged per hour across all monitoring days to generate an averaged value for VT and THI during 24 h. A linear mixed model with repeated measures using restricted maximum likelihood (REML) method for (co)variance components estimation procedure was employed. The final model adjusted the VT for the effects of cow, time, THI, farm, year, pregnancy status, body condition score (BCS), milk yield, genetic composition, and genetic composition*time interaction. Fixed effects were evaluated by ANOVA and tested with Tukey test in R software version 3.6.1 (R Core Team, 2019). Overall mean of VT, air temperature (AT), and THI were 39.06 ± 0.52 °C, 25.63 ± 0.40 °C, and 75.06 ± 3.96, respectively. VT had moderate positive correlation with THI (r² = 0.45, P < 0.001) and AT (r² = 0.46, P < 0.001). The VT had estimated linear increase of 0.05 °C for each THI unit increase (P < 0.001). Least square mean of VT varied among the farms (P < 0.001), pregnancy status (P < 0.001), and BCS (P < 0.05) but not for Milk yield (P > 0.05). The daily average VT was affected by genetic composition (P < 0.001) with highest temperature for 3/4 H (39.08 ± 0.06 °C a) and 7/8 H (39.09 ± 0.06 °C a) and lowest temperature for 1/2 H (38.95 ± 0.06 °C b). The difference of VT among the three crossbred groups varied in function of the time of the day, from 12:00 to 20:00 h (P < 0.001), with 3/4 Holstein and 7/8 Holstein cows reaching similar VT, above to the upper limit 39.1 °C and higher than 1/2 Holstein cows during all this period. In conclusion, Girolando cows are sensitive to heat stress in tropical condition during warm seasons. Moreover, Girolando cows with genetic composition higher than 3/4 Holstein display reduced thermoregulatory efficiency. Therefore, Girolando cows in tropical dairy farms require strategies to mitigate heat stress according to their genetic composition.
Keywords: core body temperature, crossbreed, dairy cow, grazing pasture, tropical environment
INTRODUCTION
Heat stress has been a problem to livestock production worldwide, in special on tropical and subtropical climate, known for hot and humid conditions (Renaudeau et al., 2012). Data of world milk production from 2008 to 2018 shows that six of the top ten milk produce countries in world are partially or completely under tropical or subtropical environment (USDA, 2019). In this period, around 48.5% of the world lactating cows were in India and Brazil, countries responsible for 33% of world milk production in 2018 (USDA, 2019).
Under stress condition, external body forces disrupt homeostasis, which stimulate the activation of physiological responses that can induce changes and adaptation of the organism to better fit to the environment (Scott, 1981). Cows under heat stress conditions activate thermolysis physiological mechanism, as the increasing of respiration rate and sweating, aiming to release body heat and keep body core temperature under physiological threshold. If thermal load exceeds thermolysis capability body temperature rises, which is associated with increasing of maintenance requirement, reduction of fertility, and milk yield (Berman, 2005; Baumgard et al., 2011). Intensity of heat stress can be measured by Temperature and Humidity Index (THI) and categorized in different levels of stress. One of the most cited considers cows to be in a stage of heat stress when THI is 72 or above (Armstrong, 1994).
Some factors are associated with bovine sensibility to heat stress, as metabolic status (e.g., lactating or pregnant) and breed (Vasconcelos et al., 2019). Holstein cows have been selected over the last decades to increase milk yield, however, the increase in productivity was followed by an increase of sensibility to the heat (Lucy et al., 2001). Indeed, Holstein cows can achieve high milk yield potential on temperate regions but can not express all genetic potential under tropical conditions (Guimarães et al., 2002; Mellado et al., 2011; Franzoni et al., 2018). On the other hand, zebu breeds are more resistant to environmental challenges, but have lower milk yield (Ruas et al., 2014). Farmers in tropical and subtropical regions use to raise Holstein × Zebu crossbred animals to take advantage of complementarity between desired traits from both strains, as the Holstein milk yield and the Zebu resistance to heat and ectoparasites (Porto-Neto et al., 2014; Franzoni et al., 2018). In Brazil, the most used dairy crossbred is the Holstein × Gir animal, which originated the Girolando breed. It is estimated that more than 70% of Brazilian dairy herds are composed by Girolando cows (Ruas et al., 2014).
Although crossbreeding could be beneficial for milk production traits in Bos taurus × Bos indicus animals in tropical regions (Bunning et al., 2019), Holstein proportion in crossbred dairy cows genetic composition (H) seems to impact crossbred cows thermoregulation. Under tropical heat stress condition Girolando cows have shown higher rectal temperature and respiratory rate than zebu animals, as Sindi breed and Nelore × Gyr crossbred cows (McManus et al., 2014; Vasconcelos et al., 2019). Rectal temperature of 3/4 H cows, in the afternoon of Brazilian semiarid conditions, tended to be higher than 1/2 H cows when exposed to moderate heat stress (average THI = 85; Costa et al., 2015a).
Detrimental effect of heat stress on milk yield and pregnancy rate in artificial insemination protocols still persists as reported for Girolando cows (Costa et al., 2015a, 2015b). To achieve successful conception 3/4 H cows needed more artificial insemination than 1/2 H cows, that had more confirmed pregnancy 30 days post insemination than 3/4 H cows (Costa et al., 2015a, 2015b). Indeed, rectal temperature and THI have a negative correlation with follicle size and oocyte viability at 90 days post-partum of Girolando cows (Alves et al., 2014) and heat stress performed in vitro reduces the development of both Holstein and Girolando embryos (Satrapa et al., 2011). Nevertheless, it is still unclear the effect of heat stress over Bos taurus × Bos indicus dairy crossbred cows. Studies are still required to better understanding how body temperature is influenced by breed composition, health status, and environmental conditions (Godyń et al., 2019).
Vaginal temperature (VT) monitoring is a precise technique to evaluate body core temperature continuously without effect of restraint stress observed on rectal temperature measurement, the classic way to evaluate body temperature in cattle (Vickers et al., 2010 and Burfeind et al., 2012). Studies have evaluated daily body core temperature variation by VT monitoring in Holstein cows (Kendall et al., 2009; Nabenishi et al., 2011) and crossbred beef cows (Dikmen et al., 2018; Davila et al., 2019) exposed to induced or natural heat stress. However, there are no reports of daily VT response of crossbred dairy cows to natural heat stress on tropical and subtropical conditions.
We hypothesized that Holstein proportion in crossbred dairy cows genetic composition, phenotypic traits, and THI would affect crossbred dairy cows body core thermoregulation. The present trial evaluated the effect of H, pregnancy status, body condition score (BCS), milk yield, THI, and time of day on VT of Girolando dairy cows maintained under tropical pasture during warm seasons.
MATERIAL AND METHODS
Animals and Farms
This study was approved by Embrapa’s Ethics Committee on Animal Use (CEUA – Embrapa) by the protocol no 07/2015. Data were collected during the summer season (January, February, and March) of 2016 and 2017 from four Brazilian properties with extensive dairy grazing systems: Campo Experimental José Henrique Bruschi (Farms 1 and 2, Coronel Pacheco, Minas Gerais; 21°33′25″S and 43°15′26″W; Köpper climate classification [Kcc]: Aw), Campo Experimental Santa Mônica (Farm 3, Valença, Rio de Janeiro; 22°21′44″S and 43°41′51″W, Kcc: Cwa), Fazenda Santa Luzia (Farms 4 and 5, Passos, Minas Gerais; 20°44′54”S and 46°26’01”W, Kcc: Cwa), and Fazendas do Basa (Farm 6, Leopoldina, Minas Gerais; 21°31′55″ S and 42°38′35″ W; Kcc: Aw). As Campo Experimental José Henrique Bruschi and Fazenda Santa Luzia properties had each one two independent milk parlors with distinguished grazing systems, and animals independent management, the data of each system was collected and analyzed as independent farms for these properties, summing in the final data analysis six farms (Table 1).
Table 1.
Summary information of vaginal temperature monitoring
| Farm | Monitoring period | Number of monitored cows1 | Tropical grasses | ||
|---|---|---|---|---|---|
| 1/2 H | 3/4 H | 7/8 H | |||
| 1 | February, 01st to 03rd of 2016 | 17 | 37 | 19 | Brachiaria sp. |
| March, 22nd to 24th of 2016 | 18 | 30 | 19 | Brachiaria sp. | |
| 2 | February, 17th to 19th of 2016 | 18 | 21 | 3 | Panicum sp. |
| 3 | March, 9th to 11th of 2016 | 33 | 11 | 5 | Cynodon sp. |
| March, 14th to 17th of 2016 | 31 | 11 | - | Brachiaria sp. | |
| March, 18th to 20th of 2016 | 16 | 14 | 1 | Brachiaria sp. | |
| 4 | January, 12th to 14th of 2016 | 30 | 25 | 10 | Panicum sp. |
| January, 27th to 30th of 2017 | 8 | 18 | 5 | Panicum sp. | |
| February, 03rd to 06th of 2017 | 20 | 23 | 10 | Panicum sp. | |
| 5 | January, 27th to 30th of 2017 | 5 | 33 | 6 | Cynodon sp. |
| February, 03rd to 06th of 2017 | 5 | 25 | 5 | Cynodon sp. | |
| 6 | February, 15th to 16th of 2017 | 83 | – | Brachiaria sp. | |
| Total | 284 | 248 | 83 |
1Genetic composition: proportion of Holstein (H) breed.
Farm: 1 and 2: Campo Experimental José Henrique Brushi; 3: Campo Experimental Santa Mônica; 4 and 5: Fazenda Santa Luzia; 6: Fazendas do Basa.
Cows were kept grazing on tropical grass all day long in all farms (Table 1). The paddocks had natural shades from trees and water ad libitum. Lactating cows were milked twice a day (around 5:00 and 15:00) and were supplemented with corn and soybean meal (1.5 to 4 kg/cow/day) after each milking accordingly to daily milk yield, except for farm 2. At this farm cows producing ≤10 L/day were milked once a day in the morning only; cows producing more than 10 L/day were milked also at afternoon. At farms 4 and 5, cows were cooled by water sprinklers and fans in the waiting area before milking and by fans while being milked. At farms 1, 2, and 3 the cows only were cooled by fans while being milked.
VT Monitoring
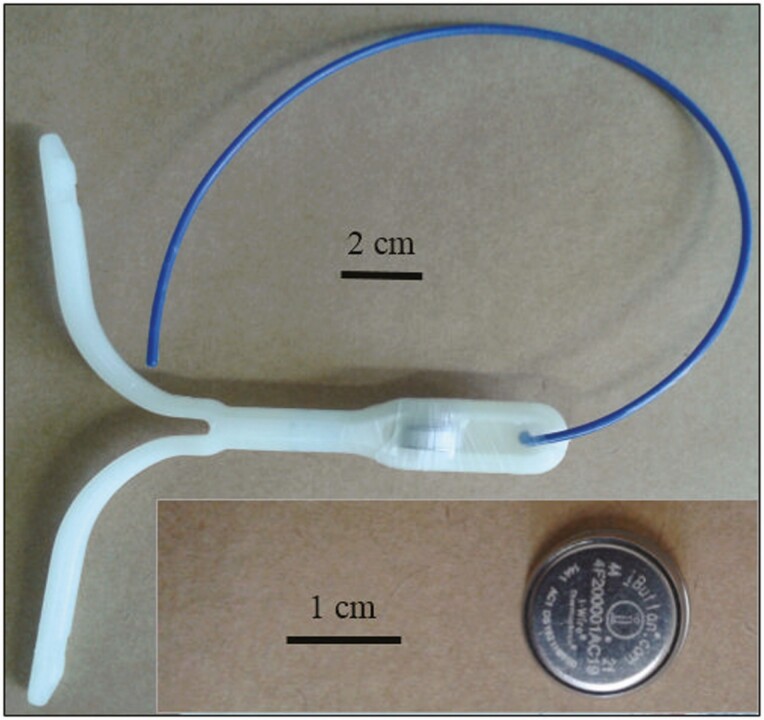
VT was recorded by iBotton data logger (DS1921H-F5# Thermochron High Res 15 to 46 °C, Maxim Integrated, San Jose, CA, USA) mounted with parafilm into a drilled hole in the stem of a controlled internal drug release device (Sincrogest, Ouro Fino, Cravinhos, SP, Brazil) without the progesterone-impregnated silicone film (Figure 1). Data of animals in estrus, within seven or less days post-partum or with health disorder (e.g., with mastitis, endometritis, or laminitis) during VT monitoring were excluded before data were compiled in the database.
Figure 1.
iBotton data logger fitted to a controlled internal drug release device (Sincrogest) without the progesterone-impregnated silicone film.
Detail: iBotton data logger.
Lactating and non-lactating Girolando cows (n = 775) from different genetic compositions were monitored at 30-min intervals for 41–96 h (average 53.0 ± 23.3 h), generating 82–192 measurements (average 106 ± 46.8) per cow. Due to the high variation on cows’ Holstein composition in the database, data were filtered to keep only animals 1/2 (n = 284), 3/4 (n = 248), and 7/8 (n = 83) of Holstein on their genetic composition, totalizing 615 monitored cows used for analysis (Table 1).
AT, °C and air relative humidity (RH, %) were recorded concurrently with VT with an environmental data logger AKSO AK 174 (AT ±0.5 °C accuracy and air humidity ±3% accuracy; Akso, Brazil) placed about 2 m from the ground in a shaded and rain-protected area close to farm’s milking parlor. The THI was calculated for each hour according to the following equation (Dikmen and Hansen, 2009): THI = (1.8 × AT + 32) − [(0.55 − 0.0055 × RH) × (1.8 × AT − 26)].
BCS 1 to 5 scale, 0.25 increments, and coat color (dark, white, or mottled) were taken at the time of introduction or removal of the progesterone-free intravaginal devices with data logger attached. Data of milk yield (correspondent to one daily observation of the closest register from VT first measurement day), days in milk, calving order, age, and pregnancy status were taken from each farm record system. Milk yield was grouped on seven milk production status: non-lactating (n = 255); 0.5–10 L/day (n= 53); 10.1–20 L/day (n = 177); 20.1–30 L/day (n = 79); 30.1–40 L/day (n = 21); 40.1–50 L/day (n = 21); 50.1–60 L/day (n = 9). Pregnancy status was categorized in four classes: nonpregnant (n = 242), 1° trimester (n = 105, <90 days of gestation), 2° trimester (n = 168, 90–180 days of gestation), and 3° trimester (n = 100, >180 days of gestation) of gestation.
Statistical Analysis
The VT of each cow at each hour of the day and the respective THI were averaged per hour across all monitoring days to generate an averaged value for VT and THI. A linear mixed model with repeated measures using REML method for (co)variance components estimation procedure was employed. The stepwise variable selection method was used considering in the initial model the inclusion of the main effects of all recorded variables, plus the interaction between time and genetic composition. The final model described below contained the effects of farm, year, proportion of Holstein genetic on crossbred composition (H), BCS, THI, time of the day, pregnancy status, milk yield, and the interaction between H and time:
where, a: farm 1, 2, 3, 4, 5, or 6; b: 2016 or 2017; c: 1/2 Holstein, 3/4 Holstein, or 7/8 Holstein; d: cow BCS; e: THI average of the same time of VT average; f: time (hour) of the day that VT and THI was registered; g: nonpregnant, 1° trimester, 2° trimester, or 3° trimester; h: the closest milk yield recorded of first VT monitoring day; δ i: random cow effect; and Ɛ abcdefghi,: error.
Fixed effects were evaluated by ANOVA and estimated marginal means were tested with Tukey test. Significance level was stipulated as 0.05. All analysis were performed using R 3.6.1 software (R Core Team, 2019).
RESULTS
Farm and Year
VT varied among farms (P < 0.001, Table 2). Farms 4 and 5 had higher VT than farms 1, 2, and 3. Mean VT for farm 6 had no difference from farms 1, 4, and 5. Farm 3 had the lowest VT among all evaluated farms. There was no difference between independent production systems of the properties Campo Experimental José Henrique Brushi (farms 1 and 2) and Fazenda Santa Luiza (farms 4 and 5). VT was higher in 2016 than 2017 (P < 0.01, Table 2).
Table 2.
Least square means (± standard error) of vaginal temperature in function of farm, year, genetic composition, pregnancy status, body condition score (BCS), and daily milk yield category
| Variable | n | Vaginal temperature (°C) ±SE | P-value | |
|---|---|---|---|---|
| Farm | 1 | 140 | 38.97 ± 0.07b,e | <0.001 |
| 2 | 42 | 38.86 ± 0.08c,e | ||
| 3 | 122 | 38.80 ± 0.08d | ||
| 4 | 149 | 39.24 ± 0.03a | ||
| 5 | 79 | 39.24 ± 0.04a | ||
| 6 | 83 | 39.15 ± 0.06a,b | ||
| Year | 2016 | 369 | 39.16 ± 0.03a | <0.01 |
| 2017 | 246 | 38.93 ± 0.07b | ||
| Genetic composition1 | 1/2 | 284 | 38.95 ± 0.06a | <0.001 |
| 3/4 | 248 | 39.08 ± 0.06b | ||
| 7/8 | 83 | 39.09 ± 0.06b | ||
| Pregancy status | Nonpregnant | 242 | 39.10 ± 0.04a | <0.001 |
| 1° trimester | 105 | 38.89 ± 0.04b | ||
| 2° trimester | 168 | 38.99 ± 0.04c | ||
| 3° trimester | 100 | 39.19 ± 0.05a | ||
| BCS (1–5; 0.25) | 2.5 | 52 | 39.18 ± 0.05a | <0.05 |
| 2.75 | 94 | 39.08 ± 0.04a,b | ||
| 3 | 200 | 39.03 ± 0.04b | ||
| 3.25 | 18 | 38.98 ± 0.07a,b | ||
| 3.5 | 214 | 39.00 ± 0.04b | ||
| 3.75 | 19 | 39.11 ± 0.08a,b | ||
| 4 | 18 | 38.92 ± 0.08b | ||
| Daily milk yield | Non-lactating | 255 | 39.00 ± 0.03 | >0.05 |
| 0.5–10L | 53 | 39.01 ± 0.04 | ||
| 10.1–20L | 176 | 39.11 ± 0.03 | ||
| 20.1–30L | 80 | 39.09 ± 0.04 | ||
| 30.1–40L | 21 | 38.99 ± 0.09 | ||
| 40.1–50L | 21 | 38.97 ± 0.09 | ||
| 50.1–60L | 9 | 39.14 ± 0.12 |
1Holstein proportion on crossbred composition.
a–cValues within a column with different superscripts differ significantly at P < 0.05 by Tukey test.
Phenotypic Factors
Pregnancy status affected VT (P < 0.001, Table 2). Nonpregnant cows and cows on third trimester of pregnancy had higher VT than cows on first and second trimester of pregnancy. Cows on first trimester of pregnancy had lower VT than cows on second trimester of pregnancy (Table 2). VT varied among different BCS (P < 0.05, Table 2). VT of cows with BCS equal to 2.5 was higher than cows with BCS equal to 3, 3.5, or 4. Milk yield did not interfere on VT of crossbred cows (P > 0.05, Table 2).
Daily VT and THI
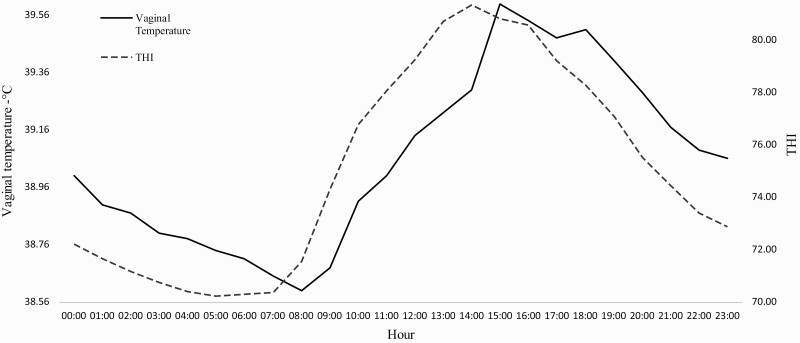
Overall mean of VT (± standard error), air temperature, and THI were, respectively, 39.06 ± 0.52 °C, 25.64 ± 4.09 °C, and 75.56 ± 4.43 °C. VT had moderate positive correlation with THI (r² = 0.45, P < 0.0001) and air temperature (r² = 0.46, P < 0.0001). Descriptive means of the variation body temperature rhythm for all animals and average THI considering all evaluated factors are shown in Figure 2. VT was aligned to THI daily variation, although there was a lag of approximately one hour between them (Figure 2). As a general pattern, when evaluating 00:00 to 05:00 h period THI reduced from 72.04 to 70.24. At this point THI entered in plateau until 07:00 h when started continuously increasing until 14:00 h, reaching 81.35, the highest THI throughout the day. Thereafter, THI decreased slowly until 16:00 h reaching 80.57, when started a vigorous decline until 23:00 h to 72.89 (Figure 2).
Figure 2.
Average daily variation of vaginal temperature of Girolando cows and temperature humid index (THI) during summer.
Mean VT vigorously decrease from 0:00 to 8:00 h, from 39.0 °C to the nadir VT 38.6 °C. At this point, VT started increasing and reached the highest value (39.6 °C) at 15:00 h, which was one hour later of the highest THI. Thenceforth, VT decreased just 0.11 °C until 18:00 h, the point that started a vigorous decrease to reaches 39.06 °C at 23:00 h (Figure 2). Cows reached 39.14 °C at 12:00 h when THI increased to 79.25 and decline below 39.1 °C at 22:00 h when THI decreased to 73.39.
Proposed model was able to explain 73% of VT variation (R2 = 0.73) (E-supplement Table S1). THI significantly affected VT (P < 0.001). An estimated linear increasing of 0.05 °C in VT for every THI unit increase was observed (E-supplement Table S1).
Genetic Composition
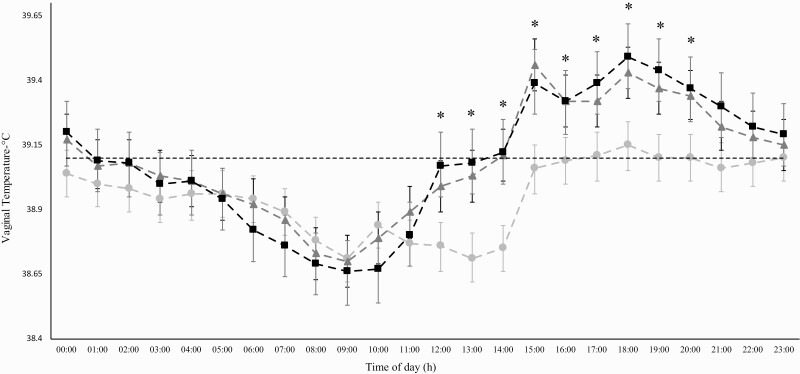
The daily average VT was affected (P < 0.001) by the proportion of Holstein (%H) on genetic composition (Table 2). 1/2 H cows had smaller VT than 3/4 H and 7/8 H cows on average. In addition, the difference of VT among the three crossbred groups varied in function of the time of the day. From 12:00 to 20:00 h it was observed difference in VT least squared means among genetic groups (P < 0.001, Figure 3). Throughout this period the average VT of 1/2 H cows remained about 0.5 to 1.0 °C below the average of TV of 3/4 H and 7/8 H cows. Interestingly, 3/4 and 7/8 H cows increased VT acutely from 11:00 to 12:00 whereas 1/2 H cows did not increase VT. At 12:00 h VT of 7/8H was equal to 39.7 °C whereas VT of 1/2 H was equal to 38.75 °C. VT of 1/2 H cows was kept close to a plateau between 11:00 and 14:00 h. An observed difference in mean VT around 1 °C between the 1/2 H group and others lasted until 15:00 h, when 1/2 H VT increased to 39.05 °C and 3/4 and 7/8 H VT increased around 39.5 °C. This VT difference of approximately 0.5 °C between 1/2 H and other groups remained from 15:00 to 20:00 h (Figure 3).
Figure 3.
Least square means and confidence interval of vaginal temperature of Girolando dairy cows from three genetic composition groups (1/2 Holstein [H], 3/4 H, and 7/8 H) in function of the hour of the day. Horizontal dotted line represents the upper limit vaginal temperature 39.1 °C. (*) 1/2 H vs. 3/4 H and 1/2 H vs. 7/8 H, P < 0.05.
DISCUSSION
Bos taurus × Bos indicus crossbred dairy cows has been used in tropic regions in the attempt to alleviate the effect of heat stress on production and reproduction (Porto-Neto et al., 2014). However, a harmful effect still remains in crossbred dairy cows like Girolando (Holstein × Gir) (Alves et al., 2014). In this study, we found that in warm seasons the pregnancy status and BCS have an effect on VT of Girolando cows in contrast to milk yield. We showed that diurnal rhythms of VT in Girolando cattle are influenced by genetic composition; cows 1/2 Holstein have lower fluctuation on VT along hot days than cows with 3/4 or 7/8 Holstein.
Body temperature had small variation of the means in this study as expected endothermic animals; however, fluctuations in body temperature were influenced by several factors as BCS, pregnancy stage, and genetic composition. Detection of differences was made possible by using VT monitoring, recording data every hour, without effect of animal restraint, which is a source of stress.
In this study, cows were supposedly under heat stress most part of the day. The average daily air temperature (25.63 ± 0.40 °C) was above the upper critical limit of 25 °C for lactating dairy cows (Berman et al., 1985; Hahn, 1999). The THI values were above heat stress threshold (72) between 8:00 and 23:00 h (Armstrong, 1994). It was shown recently that VT has a better correlation with THI and it seems to be more effective to determine thermal load than rectal temperature (Kaufman et al., 2018). Indeed, our study showed that THI affects Girolando’s VT.
Body temperature of 39.1 °C has been considered the upper limit for adult cattle; above this limit cows can undergo hyperthermia, affecting breath and heart rates, production, and fertility as well (Gwazdauskas, 1985; Wheelock et al., 2010; Polsky et al., 2017). VT average of 1/2 H cows kept all day long close to this upper limit, while the VT of 3/4 and 7/8 H cows had a significantly increase and kept 10 h above 39.1 °C in the afternoon, period that THI raised. Indeed, we found that the ability of Girolando cows to manage the VT is determined by the genetic composition, as those cows with higher proportion of Holstein breed than 3/4 are less able to thermoregulate.
Although Porto-Neto et al. (2014) find that genetic variation and correlation between retal temperature trait was not dependent of degree of bovine taurine or indicine content, Bos taurus content has been related to reduction on thermoregulatory ability in beef cattle. On pregnant Brahman-Angus cows on summer, animals with 4/8 or more of Brahman genetic composition, a zebu breed, had lower daily VT than the groups with lower Brahman genetic (Dikmen et al., 2018). On Brahman-Angus heifers, animals 1/4 Angus had no difference of VT than 1/2 Angus heifers, but had lower VT than Angus heifers (80% to 100% Angus) (Davila et al., 2019).
Zebu cattle are more adapted for body temperature regulation under heat stress, among other factors, due to the short coat length, size and number of sweat glands (Hansen, 2004). Coat length in Brahman-Angus cows decreases as zebu influence increases over than 1/2 and show high heritability (Davila et al., 2019). The reduced thermoregulation capacity showed by 3/4 and 7/8 Girolando cows in this trial may be due to reduced presence of zebu genes related to thermorregulation traits.
The highest THI did not occur simultaneously with the highest VT as well as the lowest THI with the lowest VT. There is 1 h lag between the THI and VT, indicating that cows body can withstand environmental heat load for a time and after that body core temperature increases. This 1 h lag is in agreement with a previous report in beef Brangus heifers (Hamblen et al., 2018).
Differences in meteorological conditions and other environmental trends influenced VT among farms and years. Animals from farms 4, 5, and 6 had the average of daily VT above 39.1 °C. In addition, animals from different productive systems in the same properties (farms 1 vs. 2 and 4 vs. 5) had similar vaginal temperature. Farms environmental conditions are described as the most important factors affecting animal’s thermoregulation (Godyń et al., 2019), otherwise, we found that some phenotypic characteristics can influence vaginal temperature.
The VT increased from early to late pregnancy cows, may be associated to the metabolic heat production by accelerate fetal growth at this stage of development. Indeed, more attention should be given to this category as heat stressed late pregnancy cows have reduced milk yield in subsequent lactation and their offspring have reduced development and production performance (Thompson et al., 2014 and Monteiro et al., 2016). Unexpectedly, non-pregnant cows had as higher VT as late pregnancy cows. Cows with calm behavior shown lower daily VT in relation to the agitated ones (Hamblen et al., 2018). In this trial, the nonpregnant group was composed by 52% of nulliparous and primiparous cows (data no shown), categories known to display agitated temperament in relation to multiparous dairy cows, what may have influenced this group VT.
Surprisingly, cows with high BCS had lower vaginal temperature. In Brangus heifer on summer, Hamblen et al. (2018) found that heavier heifers were able to maintain a lower VT relative to lighter weight heifers. The authors suggested that heifers that are better adapted to hot and humid environments tend to have higher average daily gain, what would explain the reduced VT of heavier cows (Hamblen et al., 2018).
Milk yield is associated with metabolic heat production, which contribute to increase cows’ body temperature (Berman, 2005). In this study, there was no significant effect of milk yield on VT. However, Dikmen and Hansen (2009) also did not find significant relationship between milk yield and rectal temperature in Holstein cows. The lack of effect of milk yield on VT would be because Girolando cows are not high milk producing, in this study only 14% of lactating cows were producing more than 30kg/day, which did not allow a robust comparison.
CONCLUSION
In conclusion, VT of Girolando cows increases as THI increases, with one-hour lag time in animal’s response and undergoes to variation among the hours of the day. Cows with 1/2 of Holstein on crossbred genetic composition have better thermoregulatory ability in the afternoon than cows with 3/4 and 7/8, that experience hyperthermia. Furthermore, phenotypic factors, as pregnancy status and BCS, and environmental factors, as farm and year can affect daily VT of Girolando cows.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank CAPES foundation for the graduate student LRC scholarship and Embrapa Dairy Cattle for the financial support on this research. The authors also thank Maurício Silveira Coelho (Fazenda Santa Luzia) and Evandro do Carmo Guimarães (Fazendas do BASA) for making their herds available for this study.
DECLARATION OF INTEREST
The authors declare that they have no conflict of interest.
ETHICS STATEMENT
This study was approved by Embrapa’s Ethics Committee on Animal Use (CEUA – Embrapa) by the protocol no 07/2015.
LITERATURE CITED
- Alves, B. G., Alves K. A., Lúcio A. C., Martins M. C., Silva T. H., Alves B. G., Braga L. S., Silva T. V., Viu M. A., Beletti M. E., . et al. 2014. Ovarian activity and oocyte quality associated with the biochemical profile of serum and follicular fluid from Girolando dairy cows postpartum. Anim. Reprod. Sci. 146:117–125. doi: 10.1016/j.anireprosci.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Armstrong, D. V. 1994. Heat stress interaction with shade and cooling. J. Dairy Sci. 77:2044–2050. doi: 10.3168/jds.S0022-0302(94)77149-6. [DOI] [PubMed] [Google Scholar]
- Baumgard, L. H., Wheelock J. B., Sanders S. R., Moore C. E., Green H. B., Waldron M. R., and Rhoads R. P.. . 2011. Postabsorptive carbohydrate adaptations to heat stress and monensin supplementation in lactating Holstein cows. J. Dairy Sci. 94:5620–5633. doi: 10.3168/jds.2011-4462. [DOI] [PubMed] [Google Scholar]
- Berman, A. 2005. Estimates of heat stress relief needs for Holstein dairy cows. J. Anim. Sci. 83:1377–1384. doi: 10.2527/2005.8361377x. [DOI] [PubMed] [Google Scholar]
- Berman, A., Folman Y., Kaim M., Mamen M., Herz Z., Wolfenson D., Arieli A., and Graber Y.. . 1985. Upper critical temperatures and forced ventilation effects for high-yielding dairy cows in a subtropical climate. J. Dairy Sci. 68:1488–1495. doi: 10.3168/jds.S0022-0302(85)80987-5. [DOI] [PubMed] [Google Scholar]
- Bunning, H., Wall E., Chagunda M. G. G., Banos G., and Simm G.. . 2019. Heterosis in cattle crossbreeding schemes in tropical regions: meta-analysis of effects of breed combination, trait type, and climate on level of heterosis. J. Anim. Sci. 97:29–34. doi: 10.1093/jas/sky406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burfeind, O., Suthar V. S., and Heuwieser W.. . 2012. Effect of heat stress on body temperature in healthy early postpartum dairy cows. Theriogenology. 78:2031–2038. doi: 10.1016/j.theriogenology.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Costa, A. N. L., Feitosa J. V., Júnior P. A., de Souza P. T., and de Araújo A. A.. . 2015a. Hormonal profiles, physiological parameters, and productive and reproductive performances of Girolando cows in the state of Ceará-Brazil. Int. J. Biometeorol. 59:231–236. doi: 10.1007/s00484-014-0838-0. [DOI] [PubMed] [Google Scholar]
- Costa, A. N. L., Feitosa J. V., P. A.Montezuma, Jr, de Souza P. T., and de Araújo A. A.. . 2015b. Rectal temperatures, respiratory rates, production, and reproduction performances of crossbred Girolando cows under heat stress in northeastern Brazil. Int. J. Biometeorol. 59:1647–1653. doi: 10.1007/s00484-015-0971-4. [DOI] [PubMed] [Google Scholar]
- Davila, K. M. S., Hamblen H., Hansen P. J., Dikmen S., Oltenacu P. A., and Mateescu R. G.. . 2019. Genetic parameters for hair characteristics and core body temperature in a multibreed Brahman-Angus herd1. J. Anim. Sci. 97:3246–3252. doi: 10.1093/jas/skz188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen, S., and Hansen P. J.. . 2009. Is the temperature-humidity index the best indicator of heat stress in lactating dairy cows in a subtropical environment? J. Dairy Sci. 92:109–116. doi: 10.3168/jds.2008-1370. [DOI] [PubMed] [Google Scholar]
- Dikmen, S., Mateescu R. G., Elzo M. A., and Hansen P. J.. . 2018. Determination of the optimum contribution of Brahman genetics in an Angus-Brahman multibreed herd for regulation of body temperature during hot weather. J. Anim. Sci. 96:2175–2183. doi: 10.1093/jas/sky133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoni, A. P. S., Glória J. R., Costa A. L. B. S. A., Martins R. A., Amaral T. F., Azevedo R. A., Campos E. F., and Coelho S. G.. . 2018. Metabolic and hormone profiles of Holstein × Gyr cows during pre- and postpartum. Pesqui. Agropecu. Bras. 53:371–377. doi: 10.1590/S0100-204X2018000300012. [DOI] [Google Scholar]
- Godyń, D., Herbut P., and Angrecka S.. . 2019. Measurements of peripheral and deep body temperature in cattle – a review. J. Therm. Biol. 79:42–49. doi: 10.1016/j.jtherbio.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Guimarães, J. D., Alves N. G., Costa E. P., Silva M. R., Costa F. M. J., and Zamperlini B.. . 2002. Reproductive and productive efficiencies in Holstein and Holstein × Zebu cows crossbreds. Rev. Bras. Zootec. 31:641–647. doi: 10.1590/S1516-35982002000300014. [DOI] [Google Scholar]
- Gwazdauskas, F. C. 1985. Effects of climate on reproduction in cattle. J. Dairy Sci. 68:1568–1578. doi: 10.3168/jds.S0022-0302(85)80995-4. [DOI] [PubMed] [Google Scholar]
- Hahn, G. L. 1999. Dynamic responses of cattle to thermal heat loads. J. Anim. Sci. 77(Suppl 2):10–20. doi: 10.2527/1997.77suppl_210x. [DOI] [PubMed] [Google Scholar]
- Hamblen, H., Hansen P. J., Zolini A. M., Oltenacu P. A., and Mateescu R. G.. . 2018. Thermoregulatory response of Brangus heifers to naturally occurring heat exposure on pasture. J. Anim. Sci. 96:3131–3137. doi: 10.1093/jas/sky224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, P. J. 2004. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 82–83:349–360. doi: 10.1016/j.anireprosci.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Kaufman, J. D., Kassube K. R., Almeida R. A., and Ríus A. G.. . 2018. Short communication: high incubation temperature in bovine mammary epithelial cells reduced the activity of the mTOR signaling pathway. J. Dairy Sci. 101:7480–7486. doi: 10.3168/jds.2017-13958. [DOI] [PubMed] [Google Scholar]
- Kendall, P. E., and Webster J. R.. . 2009. Season and physiological status affects the circadian body temperature rhythm of dairy cows. Livest. Sci. 125:155–160. doi: 10.1016/j.livsci.2009.04.004. [DOI] [Google Scholar]
- Lucy, M. C., Jiang H., and Kobayashi Y.. . 2001. Changes in the somatotrophic axis associated with the initiation of lactation. J. Dairy Sci. 84(E. Suppl.):E113–E119. doi: 10.3168/jds.S0022-0302(01)70205-6. [DOI] [Google Scholar]
- Mcmanus, C. M., Louvandini H., Paim T. P., e Silva F. C. P., and Bernal F. E. M.. . 2014. Factors affecting heat tolerance in crossbred cattle in central Brazil. Ciênc. Anim. Bras. 15:152–158. doi: 10.1590/1809-6891v15i2872. [DOI] [Google Scholar]
- Mellado, M., Coronel F., Estrada A., and Ríos F. G.. . 2011. Lactation performance of Holstein and Holstein × Gyr cattle under intensive condition in a subtropical environment. Trop. Subtrop. Agroecosyst. 14:927–931. [Google Scholar]
- Monteiro, A. P. A., Tao S., Thompson I. M. T., and Dahl G. E.. . 2016. In utero heat stress decreases calf survival and performance through the first lactation. J. Dairy Sci. 99:8443–8450. doi: 10.3168/jds.2016-11072. [DOI] [PubMed] [Google Scholar]
- Nabenishi, H., Ohta H., Nishimoto T., Morita T., Ashizawa K., and Tsuzuki Y.. . 2011. Effect of the temperature-humidity index on body temperature and conception rate of lactating dairy cows in southwestern Japan. J. Reprod. Dev. 57:450–456. doi: 10.1262/jrd.10-135t. [DOI] [PubMed] [Google Scholar]
- Polsky, L. B., Madureira A. M. L., Filho E. L. D., Soriano S., Sica A. F., Vasconcelos J. L. M., and Cerri R. L. A.. . 2017. Association between ambient temperature and humidity, vaginal temperature, and automatic activity monitoring on induced estrus in lactating cows. J. Dairy Sci. 100:8590–8601. doi: 10.3168/jds.2017-12656. [DOI] [PubMed] [Google Scholar]
- Porto-Neto, L. R., Reverter A., Prayaga K. C., Chan E. K., Johnston D. J., Hawken R. J., Fordyce G., Garcia J. F., Sonstegard T. S., Bolormaa S., . et al. 2014. The genetic architecture of climatic adaptation of tropical cattle. PLoS One 9:e113284. doi: 10.1371/journal.pone.0113284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team. 2019. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. https://www.R-project.org/. [Google Scholar]
- Renaudeau, D., Collin A., Yahav S., de Basilio V., Gourdine J. L., and Collier R. J.. . 2012. Adaptation to hot climate and strategies to alleviate heat stress in livestock production. Animal 6:707–728. doi: 10.1017/S1751731111002448. [DOI] [PubMed] [Google Scholar]
- Ruas, J. R. M., da Silva E. A., Queiroz D. S., Pereira M. E. G., Júnior J. A. G. S., Santos M. D., Júnior V. R. R., and da Costa M. D.. . 2014. Lactation productive characteristics of four genetic groups F1 Holstein x Zebu. Rev. Bras. Ciênc. Vet. 21:33–37. doi: 10.4322/rbcv.2014.014. [DOI] [Google Scholar]
- Satrapa, R. A., Nabhan T., Silva C. F., Simões R. A., Razza E. M., Puelker R. Z., Trinca L. A., and Barros C. M.. . 2011. Influence of sire breed (Bos indicus versus Bos taurus) and interval from slaughter to oocyte aspiration on heat stress tolerance of in vitro-produced bovine embryos. Theriogenology. 76:1162–1167. doi: 10.1016/j.theriogenology.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Scott, G. H. 1981What is animal stress and how is it measured? J. Anim. Sci. 52:150–153. doi: 10.2527/jas1981.521150x. [DOI] [PubMed] [Google Scholar]
- Thompson, I. M., Tao S., Monteiro A. P., Jeong K. C., and Dahl G. E.. . 2014. Effect of cooling during the dry period on immune response after Streptococcus uberis intramammary infection challenge of dairy cows. J. Dairy Sci. 97:7426–7436. doi: 10.3168/jds.2013-7621. [DOI] [PubMed] [Google Scholar]
- USDA. 2019. Market and trade data, PSD Online, Custom Query [accessed August 26, 2019]. https://apps.fas.usda.gov/psdonline/app/index.html#/app/advQuery.
- Vasconcelos, A. M., de Albuquerque C. C., de Carvalho J. F., Façanha D. A. E., Lima F. R. G., Silveira R. M. F., and Ferreira J.. . 2019. Adaptive profile of dairy cows in a tropical region. Int. J. Biometeorol. 64:105–113. doi: 10.1007/s00484-019-01797-9. [DOI] [PubMed] [Google Scholar]
- Vickers, L. A., Burfeind O., von Keyserlingk M. A., Veira D. M., Weary D. M., and Heuwieser W.. . 2010. Technical note: comparison of rectal and vaginal temperatures in lactating dairy cows. J. Dairy Sci. 93:5246–5251. doi: 10.3168/jds.2010-3388. [DOI] [PubMed] [Google Scholar]
- Wheelock, J. B., Rhoads R. P., Vanbaale M. J., Sanders S. R., and Baumgard L. H.. . 2010. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 93:644–655. doi: 10.3168/jds.2009-2295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.