Abstract
Background:
Limited reports have documented the effect cardiac implantable electronic devices (CIEDs) have on arteriovenous (AV) access patency. Current recommendations suggest placing the access on the contralateral side of the CIEDs, as there is concern for increased central venous stenosis and access failure. The goal of this study is to review our single-center AV access patency rates for dialysis patients with an ipsilateral or contralateral side CIED.
Methods:
A retrospective review was performed from 2008 to 2016 at a single institution identifying all patients who have received a CIED and the diagnosis of end-stage renal disease (ESRD). Medical records were queried to identify each patient’s dialysis access and whether it was ipsilateral or contralateral to the CIED. Primary outcomes of study were primary and secondary patency rates.
Results:
A total of 44 patients were identified to have ESRD and CIED. Of these patients, 28 patients with fistulas or grafts (13 ipsilateral and 15 contralateral) had follow-up with regards to their AV access. There were 3 primary failures in both groups. For patients who had the CIED placed after already starting the dialysis, patency was based on when the cardiac device was implanted. Primary patency for ipsilateral and contralateral access was 20.2 and 22.2 months, respectively. With secondary interventions, ipsilateral and contralateral mean patency was 39 and 48.8 months, respectively. Six-month and 1-year primary patency for arteriovenous fistula or arteriovenous graft on patients with ipsilateral access was 69.2% and 53.8%, respectively. Ipsilateral 1-year cumulative patency was 39 months.
Conclusions:
CIED may lead to stenosis or occlusion to one’s AV access; however, primary assisted and secondary patency rates are still acceptable at 6 months and 1 year compared to Kidney Disease Outcomes Quality Initiative guidelines. Despite a CIED, a surgeon’s algorithm should not lead to the abandonment of an ipsilateral access if the central venous system is patent.
INTRODUCTION
Nearly 500,000 patients in the United States are treated with dialysis for end-stage renal disease (ESRD). Additionally, ESRD is frequently associated with cardiac disease. This includes coronary artery disease, cardiomyopathy, congestive heart failure, and valvular disease.1 Pacemakers and implantable cardioverter defibrillators (ICDs), collectively known as cardiac implantable electronic devices (CIEDs), are frequently utilized for the treatment of cardiac dysfunction in patients with ESRD.2 In the United States, CIEDs are present in up to approximately 10.5% of the dialysis population. Studies have proven the survival benefit of CIEDs in renal failure patients3,4; however, this is not without the risk of complications and poor outcomes. If the decision is made to proceed with a CIED in the dialysis patient, the affect it may have on a patient’s dialysis access must also be addressed.
There have been multiple studies identifying the correlation between transvenous cardiac leads and central venous occlusion and stenosis, ranging from 3.6% to 33% for occlusion and 10% to 65% for stenosis.5–7 Clinical symptoms occur in only 1–5% of patients; however, this statistic includes all patients with a CIED, and not just those with renal failure. Current recommendations suggest placing the access on the contralateral side of the CIED, as there is concern for increased central venous stenosis and access failure, but limited reports have documented the true effect CIEDs have on arteriovenous (AV) access patency (Fig. 1). The goal of this study is to review our single-center AV access patency rates for dialysis patients with an ipsilateral or contralateral side CIED.
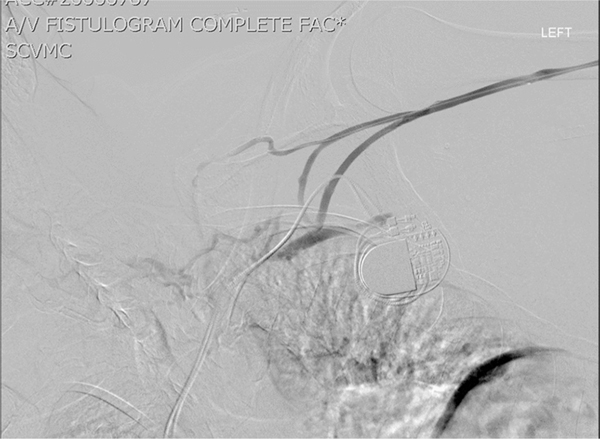
Fig. 1.
Demonstration of central venous stenosis from CIED.
METHODS
Patient Sample
A retrospective review of a single institutional database was performed from 2008 to 2016. The Institutional Review Board from Santa Clara Valley Medical Center approved the study prior to any gathering of patient information. All patient information was deidentified and handled on a secured network. The hemodialysis and cardiology databases were both reviewed and cross-referenced during this timeframe to confirm inclusion of all dialysis access patients (chronic kidney disease and ESRD) with a CIED. Demographic data were gathered on all patients. This included age, race, gender, as well as all cardiovascular comorbidities that lead to the need for both dialysis and a cardiac device.
Patients were included if they were using an AV fistula (AVF) or AV graft (AVG) in the upper extremity for dialysis. Based on operative reports and X-ray imaging, the access was determined to be either ipsilateral or contralateral. Patients using a tunneled dialysis catheter were excluded. All patients were included no matter which was placed first, the AV access or the CIED. However, the access must have been documented as functional for a minimum of 1 month to be included for patency rates. Location of the CIED, either trans-jugular or trans-subclavian, was also documented based on the operative report.
Outcomes
Outcomes were compared between those with ipsilateral or contralateral AV access in relation to the CIED. The failure rates, primary, primary assisted, and secondary patency rates were all evaluated comparing the 2 patient populations. The longevity of the fistula was determined by the amount of time the fistula was functional while the patient also had a CIED. If the patient had the access in place first, then the start date for the patency was based on the day the CIED was placed. If the CIED was placed prior to the access, then the patency start date was based on the first documentation of access use by the dialysis clinic. By definition, primary assisted patency was categorized as patients who had an intervention prior to their access occluding/failing. Secondary patency was defined as all patients who had an occluded access which was subsequently recanalized. Student’s t-test and Wilcoxon rank-sum test were used to compare the 2 groups, as well as Kaplan–Meier curves to look at overall longevity of the access patency.
RESULTS
After reviewing both the dialysis access and the cardiac database sets at a single institution, approximately 1,500 patients with either an AV access or a cardiac device were cross-referenced to identify those with both. A total of 44 patients were found within this retrospective review to have both upper extremity dialysis access and an implantable cardiac device. Thirty-four patients had acceptable follow-up (18 contralateral and 16 ipsilateral). Seven patients excluded had no follow-up and therefore were eliminated from analysis. Three others were excluded because failure of the access occurred prior to implantation of the cardiac device. Of the remaining patients, 28 patients with fistulas or grafts [13 (47%) ipsilateral and 15 (53%) contralateral] had adequate follow-up with regards to their AV access (Table I). Eight of 13 (62%) patients had AVFs, while 5 of 13 (38%) patients had some form of graft construction (loop grafts were included). Primary patency for ipsilateral and contralateral access was 20.23 and 22.21 months, respectively. Primary assisted patency was 21.2 and 38.01 months, respectively. With secondary interventions, ipsilateral and contralateral mean patency was 39 and 48.8 months, respectively. Six-month and 1-year primary patency for AVF or AVG on patients with ipsilateral access was 69.2% (9/13) and 53.8% (7/13), respectively. For contralateral access, the 6-month and 1-year primary patency rates were 86.6% (13/15) and 53.3% (8/15), respectively. There was no statistically significant difference comparing cumulative ipsilateral and contralateral 6-month and 1-year patency (P = 0.375 and 0.62, respectively) (Table II). Six of 13 ipsilateral access patients had intervention on their access prior to failure, giving a primary assisted patency rate. When this subset of patients was included in the primary patency population, an increase in the average patency to 40.7 months was seen.
Table I.
Demographics of those with ipsilateral and contralateral AV access
| Ipsilateral access (n = 13) | Contralateral access (n = 15) | P value | |
|---|---|---|---|
| Age | 65.23 | 66.75 | 0.7 |
| Gender (female) | 10/13 (77%) | 8/15 (53%) | 0.25 |
| Race (Caucasian) | 4/13 (31%) | 4/15 (26%) | 1.00 |
| Hypertension | 13/13 (100%) | 14/15 (93%) | 0.49 |
| Diabetes | 13/13 (100%) | 12/15 (80%) | 0.11 |
| CHF | 6/13 (46%) | 5/15 (33%) | 0.47 |
| CAD | 6/13 (46%) | 5/15 (33%) | 0.47 |
| Cardiac dysfunction | 7/13 (54%) | 7/15 (46%) | 0.71 |
| Type of access | |||
| Fistula | 8/13 (62%) | 12/15 (75%) | 0.69 |
| Graft | 5/13 (38%) | 4/15 (25%) | 0.69 |
| Location of CIED | |||
| Subclavian | 10/13 (77%) | 13/15 (81%) | 1.0 |
| Jugular | 2/13 (15%) | 3/15 (19%) | 1.0 |
| Epicardial | 1/13 (7%) | – | N/A |
CAD, coronary artery disease; CHF, congestive heart failure; N/A, not applicable.
Table II.
Primary and secondary patency rates for ipsilateral and contralateral access
| Patency rates | Ipsilateral (n = 13) | Contralateral (n = 15) | P value |
|---|---|---|---|
| Primary | 20.2 months | 22.2 months | |
| Primary assisted | 21.2 | 38.0 | |
| Secondary | 39 | 48.8 | |
| Six-month patency rate | 69.2% | 86.6% | 0.375 |
| One-year patency rate | 53.8% | 60% | 0.62 |
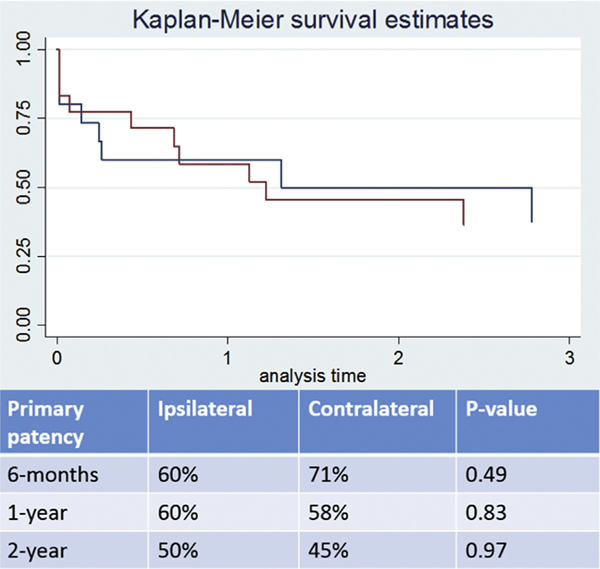
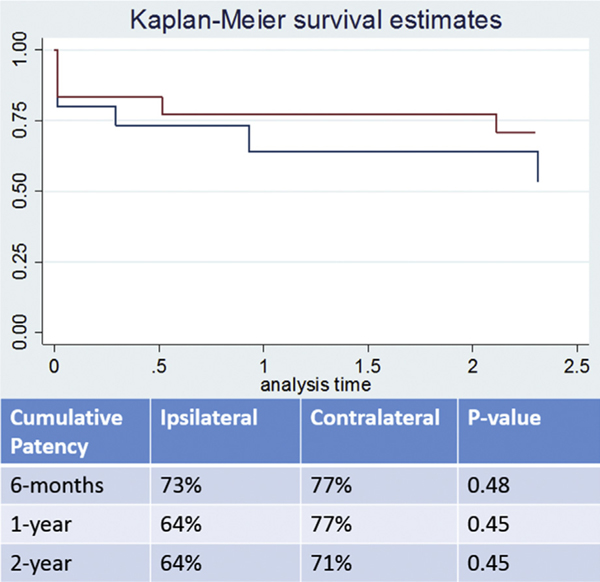
Multivariate analysis demonstrated no significant difference between the patency of ipsilateral contralateral access. After censoring the patient population over the timeframe eliminating those that did not have extended follow-up, Kaplan–Meier curves demonstrate no statistically significant difference over 6-month, 1-year, and 2-year follow-up (Figs. 2 and 3).
Fig. 2.
Kaplan–Meier curves demonstrating primary patency rates. There were no statistically significant differences associated between the 2 groups.
Fig. 3.
Kaplan–Meier curves demonstrating cumulative (primary assisted and secondary) patency rates.
DISCUSSION
Compared to the general population with normal kidney function, the survival benefit from a CIED appears less for those with chronic kidney disease and ESRD. However, ICDs confer a significant survival benefit when comparing ESRD patient with and without an ICD for the treatment of ventricular fibrillation and sudden cardiac death syndrome. One, 2, and 3-year unadjusted survival for dialysis patients receiving an ICD after cardiac arrest was 71%, 53%, and 36% vs. 49%, 33%, and 23% for dialysis patients who did not receive an ICD.3 As the population ages and the volume of patients receiving dialysis continues to increase, so too will the number of comorbidities one must take into consideration. With over 500,000 patients on dialysis and approximately 11% of those with a cardiac implantable device, access management is of concern in these patients. Since 2012, the recommendations have been to place the access on the contralateral side of a CIED. However, this was determined without significant supportive data. The most extensive review has been a review of 19 patients which demonstrated higher failure rates in those with ipsilateral access compared to contralateral (78.9% vs. 35.3%, respectively).8 Due to the current lack of data, we sought to present our experience at a tertiary care system with a large volume of dialysis access. As expected, overall patency rates for contralateral access were superior to that of ipsilateral access. Surprisingly however, when considering primary assisted and secondary patency rates, the 6-month and 1-year patency rates were not significantly different compared to patients with contralateral access. Additionally, the overall patency rate in our study still meets the criteria set forth within the Kidney Disease Outcomes Quality Initiative guidelines, which support the concept that the ipsilateral upper extremity in these patients does not need to be abandoned.
It is well known that any foreign material within the central venous system can lead to venous scarring and obstruction. Implantation of cardiac devices has been associated with stenosis and occlusion of the central veins, ranging from 3.6% to 33% for occlusion and 10% to 65% for central stenosis.5–7 Lead entry through either the subclavian or the cephalic vein does not appear to make a difference. Similarly, no difference has been appreciated on either upper extremities. Symptomatic central venous stenosis can often be treated with angioplasty alone or angioplasty in conjunction with stenting. Unfortunately, these treatments do not routinely have satisfactory results. One retrospective review demonstrated that 50% of stenoses recurred by 26 months.9 In addition to central venous stenosis in patients with ESRD on dialysis, there is also concern that this set of patients has a higher rate of bacteremia, which would warrant lead removal. The one study evaluating the complication rate of CIEDs in ESRD patients demonstrated increased rate of device-related complications, such as lead dislodgement and hematoma formation at the insertion site. There was a nonsignificant trend toward a higher incidence of pocket infections.10
In the general population, clinical symptoms occur in only a minority of patients with central stenosis (1–5%). In one series of non-ESRD patients followed after 6 months of having a CIED inserted, 64% were noted to have developed central venous stenosis, while only 2.6% of that group was symptomatic.11 However, with a need for elevated velocities and flow rate that is adequate for dialysis, the implications of central stenosis in the ESRD patients are far greater. An AVF can increase the venous return by adding 1,000–2,000 mL/min mean blood flow, unmasking a clinically significant stenosis. There have been very few reports looking at how a CIED affects the patency of dialysis access. In one study looking at patients with CIEDs and their patency rates of ipsilateral or contralateral AV access, primary failure rate was 78.9% for ipsilateral access compared to 35.5% in patients with contralateral access (P = 0.02).8 Based on the limited research that has been performed, the majority of patients with an ipsilateral CIED have symptomatic central stenosis leading to poor patency and need for either ligation of the access or new access altogether. This study demonstrates similar findings in decreased patency rates; however, we witnessed that the 6-month and 1-year data were not significantly different. Because this subset of patients has limited access options, abandoning an entire upper extremity for access possibilities is not ideal. Contemporary data demonstrate that approximately 50% of all constructed fistulas fail to attain suitability for hemodialysis.12 Removing half of a patient’s access options limits their chance at AV access even further. For this reason, a catheter-based or computed tomography venogram is performed at our institution prior to access construction in patients with a CIED. This allows for evaluation of both the ipsilateral and contralateral access options. By performing this study prior, it confirms if there is central venous stenosis and assists in the decision process of where to place an access.
Despite the effort over the years to improve the utilization of permanent dialysis access, a significant portion of the population is still using a tunneled dialysis catheter. About 80.3% of patients in the United States initiate dialysis through a catheter, and 20% are still using a catheter at 1 year. With the high complication rates already seen in tunneled catheters, the addition of cardiac leads increases this risk even more.13 Previous literature has documented that 1.3% of patients have both a CIED and a tunneled dialysis catheter.8 The combination of both a catheter and cardiac leads likely increases the risk of central venous stenosis, which will then lead to complications with permanent access down the road. There is also a high incidence of blood-stream infection associated with tunneled catheters. This ranges from 0.20 to 0.55 episodes per 100 catheter days.14 CIED-led infection has also been previously reported to be as high as 8%. The single most important variable found in association with CIED-led infection was the presence of a tunneled venous catheter, increasing the relative risk to 1.52.15 This evidence proves the importance of avoiding central venous catheters when possible. It also brings into consideration the use of epicardial leads instead of transvenous to avoid these types of complications.
Letourneau et al.16 reported that >50% of patients aged >75 years died <2 years after starting dialysis, with a mean survival of 31 months, while Joly et al.17 found that the median survival of octogenarians undergoing dialysis was 28 months. With such a limited life expectancy in this group of patients, all access options should be used. This article continues to demonstrate the superiority of hemodialysis access in the contralateral limb of patients with a CIED; however, it also demonstrates significantly better patency rates than previously described. Its our recommendation that providers continue to use the contralateral upper extremity when possible, but that ipsilateral limb dialysis options should not be abandoned and should still be used before a temporary tunneled dialysis catheter or lower extremity fistula.
The limitations to our study include it being a retrospective review with limited follow-up and being underpowered. Retrospective review will inevitably increase the chance for bias. Additionally, even though the majority of the interventions on these patients were done at our institution, there may be other interventions that were performed and unknown to our team. Finally, despite this being one of the largest published studies on the topic, the sample size is limited which leads to an under-powered study.
CONCLUSION
ESRD patients in need of a CIED must receive a multidisciplinary approach to their care when it comes to their dialysis access. Based on the results of this study, it is still recommended to place the AV access in the upper extremity contralateral to a CIED as this has shown to have longer overall patency rates. However, it is our recommendation that the ipsilateral limb not be abandoned as this side too still demonstrates statistically equivalent functional patency rates, with the understanding that more secondary interventions will be necessary.
REFERENCES
- 1.Saad TF, Weiner HL. Venous hemodialysis catheters and cardiac implantable electronic devices: avoiding a high-risk combination. Semin Dial 2017;30:187–92. [DOI] [PubMed] [Google Scholar]
- 2.Saad TF, Hentschel DM, Koplan B, et al. , ASDIN Clinical Practice Committee Workgroup. Cardiovascular implantable electronic device leads in CKD and ESRD patients: review and recommendations for practice. Semin Dial 2013;26: 114–23. [DOI] [PubMed] [Google Scholar]
- 3.Herzog CA, Li S, Weinhandl ED, et al. Survival of dialysis patients after cardiac arrest and the impact of implantable cardioverter defibrillators. Kidney Int 2005;68:818–25. [DOI] [PubMed] [Google Scholar]
- 4.Sakhuja R, McLaughlin GC, Thakur R, et al. Meta-analysis of mortality in dialysis patients with an implantable cardioverter defibrillator. Am J Cardiol 2009;103:735–41. [DOI] [PubMed] [Google Scholar]
- 5.Oginosawa Y, Abe H, Nakashima Y. The incidence and risk factors for venous obstruction after implantation of transvenous pacing leads. Pacing Clin Electrophysiol 2002;25: 1605–11. [DOI] [PubMed] [Google Scholar]
- 6.Korkelia P, Nyman K, Ylitalo A, et al. Venous obstruction after pacemaker implantation. Pacing Clin Electrophysiol 2007;30:199–206. [DOI] [PubMed] [Google Scholar]
- 7.Goto Y, Abe T, Sekine S, et al. Long-term thrombosis after transvenous permanent pacemaker implantation. Pacing Clin Electrophysiol 1998;21:1192–5. [DOI] [PubMed] [Google Scholar]
- 8.Tan CS, Jie C, Joe J, et al. The impact of transvenous cardiac devices on vascular access patency in hemodialysis patients. Semin Dial 2013;26:728–32. [DOI] [PubMed] [Google Scholar]
- 9.Kalman PG, Lindsay TF, Clarke K, et al. Management of upper extremity central venous obstruction using interventional radiology. Ann Vasc Surg 1998;12:202–6. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta A, Montalvo J, Medendorp S, et al. Increased complication rates of cardiac rhythm management devices in ESRD patients. Am J Kidney Dis 2007;49:656–63. [DOI] [PubMed] [Google Scholar]
- 11.Da Costa SSD, Neto AS, Costa R, et al. Incidence and risk factors of upper extremity deep vein lesions after permanent transvenous pacemaker implant: a 6-month follow-up prospective study. Pacing Clin Electrophysiol 2002;25:1301–6. [DOI] [PubMed] [Google Scholar]
- 12.Dember LM, Beck GJ, Allon M, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 2008;299:2164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States Renal Data System. 2016 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 2016. [Google Scholar]
- 14.Saad TF. Central venous dialysis catheters: catheter associated infection. Semin Dial 2001;14:446–51. [DOI] [PubMed] [Google Scholar]
- 15.Guha A, Maddox WR, Colombo R, et al. Cardiac implantable electronic device infection in patients with end-stage renal disease. Heart Rhythm 2015;12:2395–401. [DOI] [PubMed] [Google Scholar]
- 16.Letourneau I, Ouimet D, Dumont M, et al. Renal replacement in end-stage renal disease patients over 75 years old. Am J Nephrol 2003;23:71–7. [DOI] [PubMed] [Google Scholar]
- 17.Joly D, Anglicheau D, Alberti C, et al. Octogenarians reaching end-stage renal disease: cohort study of decision-making and clinical outcomes. J Am Soc Nephrol 2003;14:1012–21. [DOI] [PubMed] [Google Scholar]