Abstract
A 10-year-old, neutered female, French bulldog was presented with a history of dysuria, hematuria, stranguria, and pollakiuria. Diagnostic tests revealed a large mass at the neck of the bladder without any evidence of metastatic disease. Total cystectomy with bilateral cutaneous ureterostomy creation was elected. Histopathology of the bladder mass revealed extraskeletal osteosarcoma, which was resected completely. Neither recurrence nor metastasis had been detected as of 65 months after surgery. Extraskeletal osteosarcomas are typically malignant and carry a poor prognosis. This is the first report of bladder osteosarcoma in a dog treated by total cystectomy and ureterocutaneostomy with excellent long-term control.
Résumé
Issue d’un chien atteint d’un ostéosarcome de la vessie traité par cystectomie totale et urétérocutanéostomie. Une femelle bouledogue français stérilisée âgée de 10 ans a été présentée avec une histoire de dysurie, d’hématurie, de strangurie et de pollakiurie. Les tests diagnostiques ont révélé une large masse au col de la vessie sans aucun signe de maladie métastatique. Une cystectomie totale avec création d’urétérostomie cutanée bilatérale a été choisie. L’histopathologie de la masse vésicale a révélé un ostéosarcome extra-squelettique, qui a été complètement réséqué. Aucune récidive ni métastase n’avait été détectée à 65 mois après la chirurgie. Les ostéosarcomes extra-squelettiques sont généralement malins et ont un mauvais pronostic. Il s’agit du premier rapport d’ostéosarcome de la vessie chez un chien traité par cystectomie totale et urétérocutanéostomie avec une excellente gestion à long terme.
(Traduit par Dr Serge Messier)
Total cystectomy is not commonly performed in veterinary medicine. There are several reports of total cystectomy experimentally and in the treatment of trauma and neoplasia such as transitional cell carcinoma (1–4). The authors (KS, BJS) have also performed total cystectomies (unpublished). Cystectomy is most indicated for neoplasia in the bladder, prostate, and urethra, as well as in complete traumatic or iatrogenic devitalization of the bladder. According to previous reports, ureters have been successfully implanted into the vagina, prepuce, urethra, or colon, although clinical outcomes of ureterocolonic anastomoses were poor (1–4). Major complications following total cystectomy and urinary diversion include permanent urinary incontinence, local tumor recurrence due to incomplete margins, stricture formation, and chronic ascending infection. Hyperammonemia, metabolic acidosis, and uremia have been reported as postoperative complications associated with ureterocolonic anastomosis (1–4).
Extraskeletal osteosarcomas (OSAs) are defined as mesenchymal neoplasms of soft tissue and visceral organs that produce osteoid or bone without the involvement of bone or periosteal tissue (5–8). Extraskeletal OSAs are uncommon in both veterinary medicine and human medicine (7,9–12) and are reported to account for 12.6% of all canine OSAs (13). When mammary glands were excluded, extraskeletal OSAs accounted for only 1.1 to 4.5% of all canine OSAs in previous reports (7,13). Extraskeletal OSAs commonly metastasize to regional lymph nodes and have higher recurrence rates and degree of malignancy and shorter median survival time compared to the other types of OSAs (9,10,13).
The goal of this case report was to describe excellent long-term control of bladder OSA with total cystectomy and bilateral cutaneous ureterostomy in a French bulldog.
Case description
A 10-year-old, neutered female, French bulldog was presented because of an 18-day history of hematuria, dysuria, stranguria, and pollakiuria. The dog was alert on initial presentation and had a history of recurrent allergic skin disease. Physical examination findings were unremarkable. The results of complete blood (cell) count and serum biochemistry profile were insignificant except for mildly elevated alkaline phosphatase [ALP: 377 U/L, reference interval (RI): 47 to 254 U/L]. The results of urinalysis revealed large numbers of red blood cells [> 50/high power field (HPF)], epithelial cells (5 to 6/HPF), amorphous crystals, and a urine specific gravity of 1.015. Thoracic radiography was unremarkable for a French bulldog with no evidence of pulmonary metastasis. Abdominal radiography revealed some mineral opacities in the abdominal cavity and within the ventral abdominal wall, the locations of which were consistent with the ligatures associated with previous ovariectomy performed 9 y before presentation. Abdominal ultrasonography revealed a 2.45 × 2.41 cm, partially hyperechoic, solid mass in the neck of the urinary bladder, causing a partial urethral obstruction. No evidence of hydronephrosis or hydroureter was seen. Enlargement of the lymph nodes was not observed on abdominal ultrasonographic examination.
A transurethral biopsy of the bladder mucosa using endoscopic forceps was performed under ultrasound guidance. Because the dog was severely dysuric due to urethral obstruction and a malignant neoplasm was highly suspected, the owner opted for only cytologic examination and immediate surgical resection. Transitional cell carcinoma was suspected on cytologic examination, thus a radical, en-bloc resection of the mass involving total cystectomy, vaginectomy, and urethral resection with the creation of 2 ureteral stomas in the skin was discussed with the owner. The owner was informed of the surgical procedure, potential postoperative complications including incontinence, and of the long-term commitment required after surgery.
Preoperative contrast-enhanced computed tomography (CT) scan of the thorax and abdomen was performed using iohexol (Moiopamin; HIKARI Pharmaceutical, Tokyo, Japan), 1 mL/kg body weight (BW), omnipaque 300 mg/mL. The CT revealed a partially calcified, 3 × 4 cm mass in the bladder neck. Calcifications were also noted near the caudal poles of both kidneys and several small locations on the abdominal wall. No evidence of tumor metastasis or bone lesions suspicious of primary OSA was found on the CT scan.
The following surgical procedure was carried out. Midazolam (Dormicum injection; Maruishi Pharmaceutical, Osaka, Japan), 0.3 mg/kg BW, SC, was administered as preanesthetic medication. Propofol (Propofol; Pfizer Japan, Tokyo, Japan), 5 mg/kg BW, IV, was infused to effect to induce general anesthesia, then the dog was intubated. Isoflurane inhalation with oxygen was used to maintain a surgical plane of anesthesia. Cefmetazole (Cefmetazon; Daiichi-Sankyo, Tokyo, Japan), 25 mg/kg BW, IV, was administered q2h during surgery. Morphine (Morphine Hydrochloride; Shionogi, Osaka, Japan), 0.1 mg/kg BW diluted with 0.3 mL/kg BW saline epidural injection, and fentanyl (Fentanyl injection; Daiichi-Sankyo, Tokyo, Japan), 1 to 15 μg/kg BW per hour, IV, constant rate infusion (CRI) were administered as analgesics. A fentanyl patch (Durotep MT patch; Janssen Pharmaceutical K.K., Tokyo, Japan), 1.38 μg/kg BW per hour, was applied on the skin after induction of anesthesia and removed 3 d later.
The ventral abdomen was clipped and prepared for aseptic surgery. A median celiotomy was made from the umbilicus to 3 cm caudal to the pubis. Some white lesions were seen on the mid-abdominal wall, consistent with the calcifications seen on the imaging. Partial pubic ostectomy was performed using an oscillating saw, with cuts made on each pubic brim and mid-pubic symphysis, to allow adequate exposure to the urethra for en-bloc resection.
On exploratory celiotomy, both ureters were normal in diameter, and no adhesions were seen around the bladder. The bladder had a thickened wall and was firm on palpation. A solid tumor was palpated from the bladder, extending to the urethra. The dog also had small, firm lesions at the cranial end of the residual uterine horns consistent with the calcifications seen on imaging, in the area of previous bilateral ovariectomy. The median iliac and hypogastric lymph nodes were observed (~5 × 10 mm).
The broad ligaments were incised using electrocautery to remove the calcification site with the uterus. To prevent hematomas that could cause stricture of the ureterostomy sites, both ureteral arteries were cauterized using fine bipolar electrocautery. The ureters were then transected 10 mm cranial to their entrance into the bladder. A 4 Fr feeding tube was placed in each ureter and held with stay sutures of 4-0 polydioxanone (PDS II; Ethicon, Tokyo, Japan). The ventral and lateral ligaments of the bladder were dissected using electrocautery. Additional hemostasis around the bladder neck was obtained with fine ligatures of 4-0 polydioxanone and titanium surgical clips (Hemoclips; Mizuho, Tokyo, Japan). En-bloc resection of the urinary bladder, urethra, and vagina (down to the level of the vestibule) was performed to obtain adequate margins free of neoplastic cells. In addition, the uterus and calcified foci immediately caudal to the kidneys were resected for histopathological evaluation (Figure 1). The median iliac hypogastric lymph nodes and calcified abdominal wall foci were also resected for histopathological evaluation. All resected organs and tissues were submitted for histopathological examination. A urine culture test was also submitted. The surgical site was liberally lavaged with sterile saline solution (Normal saline; Otsuka Pharmaceutical, Tokyo, Japan) and suctioned. All instruments and gloves were changed, and the surgical site was overdraped.
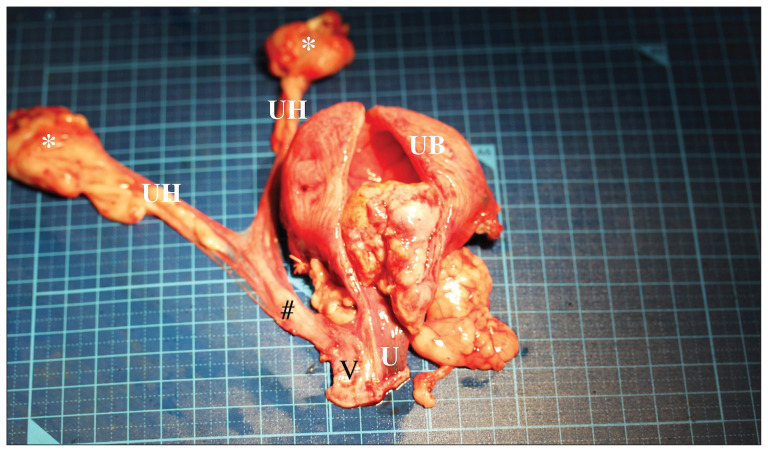
Figure 1.
The uterine horns (UH), calcification areas (*), cervix (#), vagina (V), urinary bladder (UB), and urethra (U) were resected. The tumor was located from the bladder neck to the urethra.
The ureters were gently dissected and mobilized from the retroperitoneal space, being careful not to damage the ureteral blood supply. Fat tissue surrounding the distal ureter was dissected cautiously to prevent subsequent stricture of the stoma. A 4-mm biopsy punch was used to create tunnels through the body wall bilaterally near the 4th nipple. This position of the tunnel was chosen for easier diaper application and to minimize excoriation of the inguinal skin. Each ureter was passed through each tunnel with a 4 Fr feeding tube inserted in retrograde fashion and exited in the subcutaneous tissues. The serosa of each ureter was secured to the abdominal wall with 2 simple interrupted sutures of 5-0 polydioxanone. The abdominal wall was then closed with a 3-0 polydioxanone in a simple continuous pattern. A stoma was created in the skin with a 4-mm biopsy punch and the ureters were passed through the stoma to exit the skin. The subcutaneous midline was closed with a 3-0 polydioxanone in a simple continuous pattern and the midline skin incision was closed with a 3-0 poliglecaprone 25 (Monocryl; Ethicon, Tokyo, Japan) in an intradermal pattern and with skin staples (PreciseVista Disposable Skin Stapler; 3M, Tokyo, Japan). Each ureter was spatulated for 4 to 5 mm and the mucosa was everted slightly and sutured to the skin to make a papillary-shaped fistula, using 4 simple interrupted sutures with 5-0 polydioxanone. The resected pubic portion was not replaced but was also submitted for histopathology. A small amount of white petroleum ointment (White Petroleum; Kenei Pharmaceutical, Osaka, Japan) was applied to the peristomal skin, and disposable baby diaper (Pampers; P&G, Kobe, Japan) was placed.
The dog recovered in an intensive care unit. Intravenous fluids (SOLULACT; Terumo, Tokyo, Japan) (Lactated Ringer’s solution), 3 mL/kg BW per hour, fentanyl CRI, 1 to 5 μg/kg BW per hour, cefmetazole, 25 mg/kg BW, IV, q12h were continued until the next morning. Meloxicam (Metacam; Boehringer-Ingelheim, Tokyo, Japan), 0.2 mg/kg BW, SC was administered immediately after surgery and for the following 3 d at 0.1 mg/kg BW, SC, q24h. Fentanyl CRI was discontinued 12 h after the completion of the surgery. Recovery was uneventful. Postoperative management included diaper changes and urine output measurements by weighing the diapers every 6 to 8 h. The peristomal skin was cleansed with 0.05% chlorhexidine solution (0.05% Hexizac water R; Yoshida Pharmaceutical, Tokyo, Japan) and a thin layer of petroleum ointment was applied twice daily. The dog was hospitalized for 8 d per owner’s request, and the owner came in daily to learn how to care for the stomas. The dog was discharged on amoxicillin (Amoxicillin capsules; NIPRO, Osaka, Japan), 20 mg/kg BW, PO, q12h, based on the urine culture test results and piroxicam (Baxo; FUJIFILM Toyama Chemical, Tokyo, Japan), 0.3 mg/kg BW, PO, q48h. For peristomal skin care, 0.05% chlorhexidine solution and white petroleum ointment (once or twice daily) were used.
Macroscopically, the mass measured 3 × 4 × 2.8 cm and appeared to arise from the trigone. The tumor base was at the membrane of the trigone, and the tumor proliferated along the bladder neck into the urethra for ~1 cm. Microscopic examination of the tumor at the neck of the urinary bladder revealed a poorly demarcated neoplastic focus that extended beneath the urothelium into the tunica muscularis, but not to the serosa. The tumor consisted of solid sheets of cells that varied from pleomorphic to short-spindle or stellate, producing lace-like osteoid matrix and boney trabeculae. The neoplastic cells had pale eosinophilic cytoplasm and an ovoid to polygonal nucleus with finely stippled chromatin and 1 to 2 nucleoli. The histopathologic findings were consistent with osteosarcoma of the urinary bladder (Figure 2). The tumor had not invaded the surrounding tissues and was resected with 2-cm histologic margin and no metastasis was observed in the hypogastric and medial iliac lymph nodes. Granuloma with non-absorbable suture materials was found at the calcification sites, both ovary locations and abdominal wall.
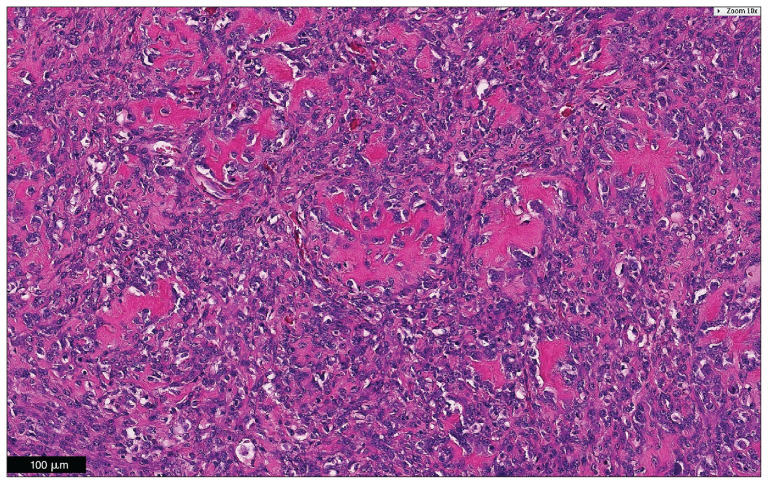
Figure 2.
Microscopic examination of the tumor revealed a poorly demarcated neoplastic focus that extended beneath the urothelium into the tunica muscularis. The tumor produced lace-like osteoid matrix and/or boney trabeculae. The histopathologic findings were consistent with osteosarcoma in the urinary bladder. Hematoxylin and eosin. Bar = 100 μm.
The resected pubis had no evidence of neoplasia. Micrococcus sp. were detected from the urine culture test and were susceptible to amoxicillin.
After the dog was discharged, the owner was compliant with the instructions about home care of the ureterostomies and peristomal skin (Figure 3). Prior to surgery, the dog had been treated for chronic allergic skin inflammation and infection. These conditions persisted after surgery and may have been exacerbated by the continual application of diapers but remained between mild and moderate and were considered acceptable. The owner was satisfied with the dog’s condition and long-term control of the disease despite persistent urinary incontinence.
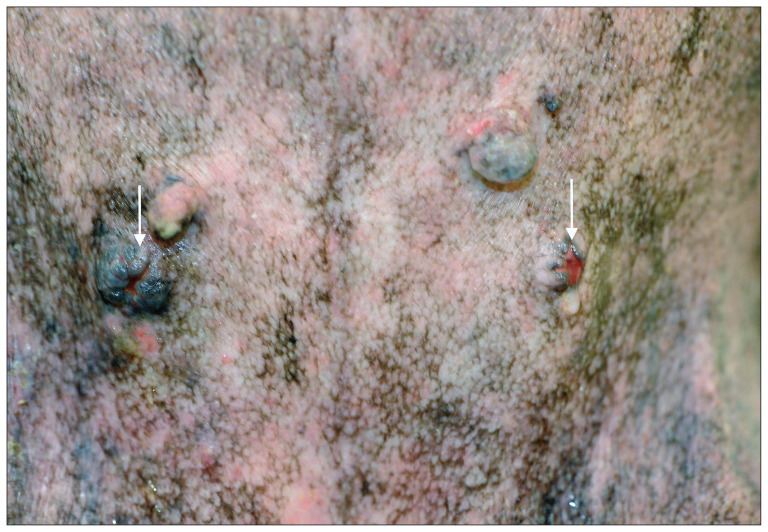
Figure 3.
The image of the surgical site 34 mo after surgery. The upper side of the image is the cranial side of the dog. The stomas (arrows) are located caudal of the 4th nipples. Urine outflow is seen on the right stoma on this image.
The dog did not receive adjuvant chemotherapy and the dog was rechecked with thoracic radiographic and abdominal ultrasonographic examinations every 2 mo for the first 15 mo, then every 3 to 6 mo thereafter. Contrast studies were not repeated, but there were no clinical signs of pyelonephritis, nor ureteral dilation and renal pelvic dilation by abdominal ultrasound. Neither urinalysis nor urine culture was repeated, since drawing a urine sample directly from the renal pelvis is not practical, and inserting ureteral catheter carries risk of ascending infection. Serum alkaline phosphatase concentration remained mildly high, and biliary sludge with diffuse hepatomegaly was observed. The dog was in complete remission for 65 mo at the time of this report.
Discussion
The most common malignant neoplasm of the bladder in dogs is transitional cell carcinoma, which is locally invasive and metastatic in biologic behavior. Other malignant bladder tumors reported in veterinary medicine are squamous cell carcinoma, adenocarcinoma, fibrosarcoma, leiomyosarcoma, and hemangiosarcoma (14).
Extraskeletal OSAs are rare and typically display highly malignant behavior, making any treatment with curative intent challenging (7,9,10,13). En-bloc resection of extraskeletal OSAs in the abdominal cavity can be difficult because of challenges in obtaining clean margins and high chance of recurrence (13). Moreover, due to lack of detectable clinical signs, a definitive diagnosis is often delayed compared to dermal, subcutaneous, and intramuscular extraskeletal OSAs (13).
At the time of writing, this case had been in complete remission for 65 mo. We speculate that there are 3 reasons for this excellent outcome:
Aggressive surgical intervention, involving en-bloc resection of the bladder and urethra and vagina. In the authors’ opinion resection of the entire urethra was indicated to obtain clean margins; this necessitated resection of the vagina to the level of the vestibule.
The surgery was performed promptly when no evidence of regional or distant metastasis was observed.
The tumor was in the bladder neck where clinical signs were manifested before the tumor had grown and invaded to an extent that it may have been unresectable.
It has been reported that a larger tumor size of extraskeletal OSAs is usually associated with a shorter survival time (7,9,10,13). Tumor cells had invaded through the bladder muscle layer but not to the serosa in this dog. No evidence of vascular or lymphatic invasion was observed. The containment of tumor cells by the bladder wall likely prevented dissemination of the neoplastic cells to other visceral components, which typically contributes to a poor prognosis of extraskeletal OSAs.
The dog remained clinically well, and the owner was satisfied with the outcome despite permanent urinary incontinence and need for patient care at home. It is important to note that the owner was extremely vigilant in caring for this dog: taking care of the peristomal skin with petroleum ointment, measuring urine output by weighing diapers 3 to 4 times a day, and observing the stoma sites to confirm continued urine output. Appropriate preoperative counselling should always be provided; this type of consultation and managing owner expectations are critical to successful outcomes in these cases. Surgical technique, patient selection, and the owner’s understanding and cooperation helped achieve an optimal outcome for this dog. Total cystectomy with cutaneous ureterostomy development is a feasible surgical procedure based on the current case and the authors’ (KS, BJS) experience in over 13 successful cases (2010 to 2017, unpublished). Total cystectomy with uretero-urethral/preputial/vaginal anastomosis has been reported by Kadosawa et al (1) and Boston et al (2). Kadosawa et al (1) performed the surgeries with curative intent, whereas Boston et al (2) performed the surgeries as palliative care. Our procedure was more radical compared to these reported procedures and was expected to obtain enough tumor-free margins and to lower the possibility of recurrence. The procedure described in this report was performed with curative intent rather than as a palliative care. Management is slightly more intensive than is the case for an ureterovaginostomy. The development of cutaneous ureterostomies has 2 merits compared to other types of urinary diversion technique: i) the procedure does not require residual urethra or vagina for urinary diversion thus surgery can include resection of the entire urethra, including the papilla, and can extend to the vestibule; and ii) any ureteral urinary diversion procedure (e.g., ureterourethral, uretero-preputial, uretero-vaginal, uretero-cutaneous stomas) is at risk of stricture and obstruction. Only a cutaneous stoma site is easily visible; thus, any issues can be recognized and addressed earlier (e.g., inflammation, stricture, unilateral decreased flow), even by the owner.
Major postoperative complications associated with ureteral stomas are stricture, obstruction, infection, and urine scald. The possible causes of stricture and obstruction include tension on the ureter, hematoma, fibrosis or granuloma formation, inappropriate apposition of the ureter to the skin, and infection. This case had daily inspection of the stomas to ensure that urine was dribbling and the amount of urine output was appropriate. The dog was rechecked every 1 to 2 mo for the first 15 mo, then every 3 to 6 mo thereafter, and never had clinical signs of pyelonephritis. Furthermore, ureter size and renal pelvis were normal in size by abdominal ultrasonographic examinations. As the dog had a pre-existing chronic allergic skin condition, this may have been exacerbated by the continual application of diapers. However, the skin inflammation remained acceptable to the owner and attending veterinarians. No major complications were seen in this case. The authors (KS, BJS) have rarely experienced these complications of cutaneous ureterostoma creation. Thus, total cystectomy with cutaneous ureterostomy should be considered as a feasible procedure for dogs.
Eighty percent of extraskeletal OSAs develop in visceral organs. The reported organs include eyes, meninges, salivary gland, spleen, liver, adrenal glands, thyroid glands, kidneys, bladder, esophagus, stomach, small bowel, mesenteric root, trachea, lungs, retroperitoneal space, muscle and subcutaneous tissue, skin, mammary glands, testes, and vagina (7,9,10,13,15). Treatment options and prognosis of canine bladder osteosarcoma have not been reported in detail. To the best of our knowledge, this is the first report describing complete urethrocystectomy with cutaneous ureterostomy creation in a dog with bladder OSA and good clinical outcomes after surgical resection.
Acknowledgments
We thank the team of veterinarians, veterinary technicians, and students who were involved in this case, including Dr. Daiki Takahashi at Daiki Animal Hospital, Dr. Erika Maeda and Dr. Miki Morita at Nippon Veterinary and Life Science University, and Dr. Kei Harada and Dr. Tetsuya Kobayashi at Japan Small Animal Cancer Center. We also thank Dr. Kim Friesen for proofreading this report and Dr. Tomomi Minamoto at Evergreen Vet Research & Publication for their editing services. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Kadosawa T, Yamashita M, Togeshi E, et al. Total cystectomy and uretero-urethral/preputial/vaginal anastomosis in 14 dogs with transitional cell carcinoma of the bladder. Proc 26th Annual Conf Vet Cancer Soc; October 2006; Callaway Gardens, Georgia. p. 66. (abstr) [Google Scholar]
- 2.Boston S, Singh A. Total cystectomy for treatment of transitional cell carcinoma of the urethra and bladder trigone in a dog. Vet Surg. 2014;43:294–300. doi: 10.1111/j.1532-950X.2014.12104.x. [DOI] [PubMed] [Google Scholar]
- 3.Stone EA, Withrow SJ, Page RL, Schwarz PD, Wheeler SL, Seim HB., 3rd Ureterocolonic anastomosis in ten dogs with transitional cell carcinoma. Vet Surg. 1988;17:147–153. doi: 10.1111/j.1532-950x.1988.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 4.Stone EA, Walter MC, Goldschmidt MH, Biery DN, Bovée KC. Ureterocolonic anastomosis in clinically normal dogs. Am J Vet Res. 1988;49:1147–1153. [PubMed] [Google Scholar]
- 5.Allan CJ, Soule EH. Osteogenic sarcoma of the somatic soft tissues. Clinicopathologic study of 26 cases and review of literature. Cancer. 1971;27:1121–1133. doi: 10.1002/1097-0142(197105)27:5<1121::aid-cncr2820270519>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Lee JS, Fetsch JF, Wasdhal DA, Lee BP, Pritchard DJ, Nascimento AG. A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76:2253–2259. doi: 10.1002/1097-0142(19951201)76:11<2253::aid-cncr2820761112>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Patnaik AK. Canine extraskeletal osteosarcoma and chondrosarcoma. A clinicopathologic study of 14 cases. Vet Pathol. 1990;27:46–55. doi: 10.1177/030098589002700107. [DOI] [PubMed] [Google Scholar]
- 8.Enzinger FM, Weiss SW. Soft Tissue Tumors. 3rd ed. St. Louis, Missouri: Mosby; 1995. pp. 1025–1035. [Google Scholar]
- 9.Thomsen BV, Myers RK. Extraskeletal osteosarcoma of the mandibular salivary gland in a dog. Vet Pathol. 1999;36:71–73. doi: 10.1354/vp.36-1-71. [DOI] [PubMed] [Google Scholar]
- 10.Kuntz CA, Dernell WS, Powers BE, Withrow S. Extraskeletal osteosarcomas in dogs: 14 cases. J Am Anim Hosp Assoc. 1998;34:26–30. doi: 10.5326/15473317-34-1-26. [DOI] [PubMed] [Google Scholar]
- 11.Young RH, Rosenberg AE. Osteosarcoma of the urinary bladder. Report of a case and review of the literature. Cancer. 1987;59:174–178. doi: 10.1002/1097-0142(19870101)59:1<174::aid-cncr2820590133>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Abou Ghaida RR, Saoud RM, Bulbul M. Primary osteosarcoma in a bladder diverticulum. Can J Urol. 2014;21:7393–7395. [PubMed] [Google Scholar]
- 13.Langenbach A, Anderson MA, Dambach DM, Sorenmo KU, Shofer FD. Extraskeletal osteosarcomas in dogs: A retrospective study of 169 cases (1986–1996) J Am Anim Hosp Assoc. 1998;34:113–120. doi: 10.5326/15473317-34-2-113. [DOI] [PubMed] [Google Scholar]
- 14.Norris AM, Laing EJ, Valli VE, et al. Canine bladder and urethral tumors: A retrospective study of 115 cases (1980–1985) J Vet Intern Med. 1992;6:145–153. doi: 10.1111/j.1939-1676.1992.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 15.Ringenberg MA, Neitzel LE, Zachary JF. Meningeal osteosarcoma in a dog. Vet Pathol. 2000;37:653–655. doi: 10.1354/vp.37-6-653. [DOI] [PubMed] [Google Scholar]