Abstract
MicroRNAs are small single-stranded, non-coding RNAs which have a known role in post-transcriptional regulation of gene expression. Recent studies have reported that extracellular vesicles are capable of specific delivery of miRNAs to a target cell or tissue from a host cell. MiRNAs are generated by host cells, selectively packaged into EVs, and then delivered to nearby target cells with full functionality. After delivery to the target cells, these EV-packaged miRNAs regulate the translation of their target genes. Thus, EV transported miRNAs have become a newly understood method for intercellular communication. In this review, we summarize the novel findings of EV-miRNA transfer in acute lung injury, chronic obstructive pulmonary disease, bronchopulmonary dysplasia, asthma, and idiopathic pulmonary fibrosis.
Keywords: Extracellular Vesicle (EV), Inflammation, Inflammatory Lung Responses, Acute Lung Injury (ALI), Acute Respiratory Distress Syndrome (ARDS), Alveolar Macrophage, Lung Epithelial Cell
INTRODUCTION
MiRNA Function
Since the first discovery of non-coding RNAs (ncRNAs) in 1993, researchers have worked to uncover the role ncRNAs play in cells [8]. MicroRNAs (miRNAs), which are a type of ncRNA, are small (about 22 nucleotides) single stranded RNAs, which play a critical role in posttranscriptional regulation of protein expression [1]. MiRNAs are encoded and transcribed inside the nucleus, and are then transported to the cytoplasm where they are incorporated into the ribonucleoprotein-silencing machinery [3]. Through this regulatory role, miRNAs target messenger RNAs (mRNAs) for either suppression or degradation, which leads to an overall decrease in the expression of the gene translated from that mRNA [2]. In recent years, studies have proved that miRNAs are likely involved in most cell processes, and have a major impact in many diseases, including vascular inflammation [4,9].
EV Function
Extracellular vesicles (EVs) are membranous structures which consist of exosomes and macrovesicles originating from the endosomal system, as well as apoptotic bodies originating from apoptotic cells [5]. EVs are established as a method of cell to cell communication, which allows for the passing cellular material between host and target cell [6]. Release of EVs was originally thought to be a method of discarding membranous proteins from host cells, however, recent studies have reported evidence that intercellular communication through EVs plays an important role in the physiological and pathological processes of multiple diseases [7]. The type and amount of EVs released vary based on the status of the disease, and therefore, EVs could serve as novel biomarkers for various lung diseases [40]. With the vital discovery of EVs and research into their role in multiple diseases, EVs have the potential to become a new drug delivery system and a novel diagnostic / therapeutic target [41].
EVs are known to contain DNA, RNA, and proteins, which are passed from host cell to target cell [42]. Despite the various components found in EVs, which may all play a regulatory role on disease biogenesis, we will focus specifically only on EV-miRNAs in this review.
Discovery of EV-miRNA
EV miRNAs are considered to be possible diagnostic markers and therapeutic targets of multiple pulmonary diseases [17]. Previous studies have shown that not all miRNAs are transferred via exosomes, and that there is a selectivity mechanism which determines the regulatory miRNAs chosen for export [16]. The first to report of EV mediated miRNA transfer between cells was in 2007 by Valadi et al. [21]. Following this report, in 2010, three independent studies reported that these EV transferred miRNAs created an RNA interference effect in the recipient cell [22,23,24]. Since these findings, there have been multiple papers suggesting EV-containing miRNAs play crucial regulatory roles in many pulmonary diseases including ALI, COPD, asthma, pulmonary arterial hypertension (PAH), and pulmonary fibrosis [7,31].
Current Studies of EV-miRNAs in Lung Inflammation
Major categories of EVs
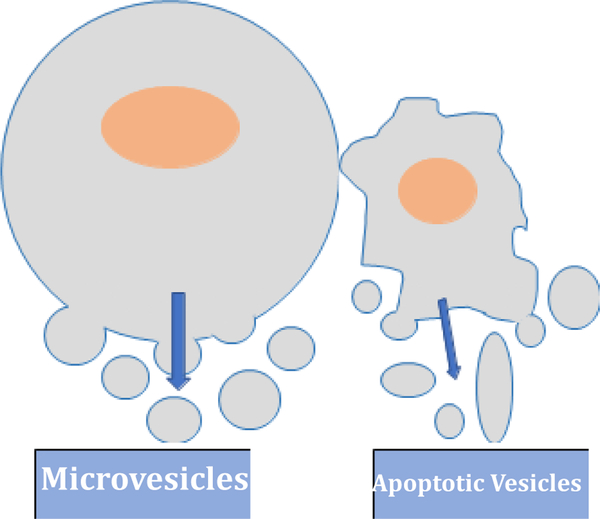
The three major categories of EVs are commonly referred to as; exosomes, microvesicles, and apoptotic vesicles [10,14]. Exosomes have an endosomal origination, are 40–150nm in size, and are involved in intercellular communication [11]. Exosomes are formed by the invagination of the cells lipid bilayer, followed by the release from within the cell after acquiring proteins, DNA, and RNA from their cell of origin [11,12]. Exosome release occurs by the fusion of the late exosome with the cell membrane [12]. Microvesicles originate from the plasma membrane, are 50–2000nm in size, and transfer proteins and nucleic acids to nearby cells [25]. Unlike exosomes, microvesicles are formed by the outward budding and splitting of the plasma membrane [25,26]. Apoptotic vesicles are released by the disassembly of apoptotic cells, and are 1,000–5,000 nm in size [10]. In recent years, there has been increasing evidence which suggests miRNA-containing EVs produced during apoptosis play a significant immune regulatory role in multiple diseases [27].
EV miRNAs in Acute Lung Injury
Acute Respiratory Distress Syndrome (ARDS) is a devastating syndrome responsible for significant morbidity and mortality. Its mild form is known as Acute Lung Injury (ALI). Non-cardiogenic pulmonary edema, vascular leakage, inflammation, lung epithelial cell injury and dysfunction play important roles in the pathogenesis of ARDS. To date, very few studies have been conducted to study the transfer of miRNAs by EVs in the pathophysiology of lung inflammation. In ALI, the EV transfer of miR-146a and miR-155 have both been reported in cells derived from dendritic cells [13,15]. In the study of the EV transfer of miR-146a and miR-155 by Alexander et al., it was found that miR-146a inhibits endotoxin induced inflammation, while miR-155 promotes this inflammation [13,15]. In our previous study, we found that Microvesicles (MVs) were the main type of EV found in the early stages of hyperoxia [28]. In this study, we also identified the induction of both miR-320a and miR-221 in epithelial-derived MVs released in response to hyperoxia [28]. The delivery of these specific miRNAs (miR-320a and miR-221) via MVs to target cells promotes macrophage-mediated pro-inflammatory effects [28]. Another report, which studied EVs isolated from Broncho-Alveolar Lavage Fluid (BALF) of patients with influenza virus-derived ARDS, reported the upregulation of EVs containing miRNA-17–5p [42]. The study concluded that the EV delivered miR-NA-17–5p lead to a downregulation of antiviral factor M×1 and enhanced viral replication in influenza virus-derived ARDS patients [42].
EV miRNAs in COPD
Chronic obstructive Pulmonary Disease (COPD) is a respiratory disease characterized by inflammation of the airways leading to the lungs [18]. To date, there is major lack in reports studying EV-miR-NAs in COPD. A study by Fuijita et al. found that the EV transfer of HBEC-derived miR-210 plays a role in the biogenesis of COPD [19]. This study found that specifically, miRNA-210 regulates autophagy processes by targeting ATG7, and that miRNA-210 expression levels were inversely correlated with ATG7 expression in lung fibroblasts (LFs). An additional study found that cigarette smoke induced the EV transfer of miR-191, miR-126, and miR-125a via ceramide-synthesis enzyme acid sphingomyelinase (aSMase) [20]. These miR-NAs were transferred to macrophages, leading to the promotion of apoptotic cells [20].
EV miRNAs in Bronchopulmonary dysplasia
Bronchopulmonary Dysplasia (BPD) is a form of chronic lung disease which is commonly seen in premature babies [32]. Despite the increase in study of the disease, the rate of complications is still remarkably high, and no safe therapy developed has had a major impact on the rate of incidence and severity of BPD [36]. One study, which focused on characterizing the change in EV miRNA expression in BPD patients, tested human samples to observe the different expressions of EV miRNAs in human mesenchymal stem cell-derived extracellular vesicles (mEVs) vs fibroblast EVs (fEVs) [37]. This study discovered 30 miRNAs that were expressed notably greater in mEVs than fEVs [37]. Of these thirty EV miRNAs, the study concluded miR-1246, miR-6511a-5p, and miR-22–3p as the top 3 differentially expressed in mEVs, and the top 3 miRs from mesenchymal stem cells (MSCs) sorted into mEVs were miR-630, miR-4286, and miR-4454+7975 [37]. MSC-derived mEVs have been reported to reduce tissue fibrotic responses [38]. Further investigation into the regulatory mechanisms of EV miRNAs in BPD could offer a reliable therapy to treating this poorly understood disease.
EV miRNAs in Asthma
Asthma is a chronic inflammatory disease of the airways, and patients who have asthma usually show symptoms of wheezing, dyspnea, chest tightness, and cough [34]. Further studies into asthma will be required to broaden our knowledge of the disease and understand the complex mechanisms involved in the biogenesis [33]. The first investigation of EV miRNAs in asthma studied the expression levels of a wide range of miRNAs from BALF-EVs isolated in asthmatic patients [35]. This study by Levanen et al. identified 24 miRNAs (let-7c, let-7b, 141, 200b, let-7d, let-7a, 21, 27, let-7e, 34c-5p, 34b-5p, 19b, 1972, 665, 658, 483–5p, 0022, 0024, 0026a, 0099a, 0200c, 1268, 0203, 0130a) were significantly altered between control and asthma patients [35]. This investigation did not look further into the mechanisms of these EV miRNAs in asthmatic patients, but concluded that these findings suggest some, if not all of these reported EV miRNAs play a role in the regulation of asthma pathology in the lung [35].
EV miRNAs in Idiopathic Pulmonary Fibrosis
Idiopathic Pulmonary Fibrosis (IPF) is a pulmonary disease which leads to progressive decline of lung function, characterized by chronic inflammation [29]. Despite previous success in the molecular diagnostics and pathobiology of IBF, the biogenesis of IPF is still unclear, and requires further study [30]. One study published in 2016, identified the promotion of serum EV-containing miRNA-21–5p expression in IPF mice and patients [31]. The study did not map the direct regulatory effect of EV miRNA-21–5p, however they did suggest a novel biomarker, EV miRNA-21–5P, which could be used clinically to distinguish patients who require intensive IBF therapy [31]. Another study, which studied mEV miRNAs in human bone marrow-derived MSCs, which target profibrotic genes in IPF fibroblasts discovered the upregulation of mEV packaged miR-199a-3p, 21–5p, 630, 22–3p, 196–5p, 199b-5p, 34a-5p and 148a-3p [37]. Previous studies have identified the roles of these miRNAs in IPF fibroblasts: serum miR-21 in EV is related to poor prognosis in IPF, and miR-21 activates myofibroblasts in vitro; miR-199 is associated in liver fibrosis, and is also upregulated in IPF and activates myofibroblasts; miR-22 suppresses cardiac fibrogenesis and cirrhosis; miR-196–5p suppresses renal fibrosis; and miR-34–5p is pro-fibrogenic in the heart and controls pneumocyte senescence in IPF [37]. This study also reports that miR-630, which the study suggests may regulate adherens-junction dependent cell migration and fibroblast attack, is the most upregulated mEV packaged miRNA in their study [37]. These Thy-1-mediated Mev-containing miRNAs seem to work in unison to induce an anti-myofibroblastic effect in IPF fibroblasts [37].
Pitfalls of Current EV-miRNA Research and Future Directions
In addition to the lack of reports studying EV-miRNAs in lung inflammation, the papers which have been published, do possess clear pitfalls. The majority of published reports focus on the potential of EVs or EV-miRNAs as biomarkers, however, the physiological and pathological functions these EV-miRNAs are still unexplored. Additionally, of those reports which do study the physiological functions of these EV-miRNAs, there are still critical issues which need further clarification. The concentration of each specific miRNA in the EVs must be determined to understand the critical threshold necessary to trigger a downstream function in the recipient cell. Moreover, given that each EV may only carry a limited number of miRNAs, it should be determined how many EVs are required to trigger the functional effects in the recipient cells. It also is critical to determine if there is a mechanism which guides EVs to specific recipient cells. For example, in our studies, all the macrophage-derived or epithelial cell-derived EVs target macrophages, followed by the same cells with their “parent” cells [28]. Interestingly, only macrophages engulf the EVs, but not so much by polymorphonuclear cell family (PMNs). Both macrophages and PMNs are phagocytes, therefore, phagocytosis cannot explain the uptake entirely and some other mechanisms must exist. Lastly, there is no consistent manner to block EV generation. All the functional studies in the above published work have some degree of “artifacts” which likely deliver an extreme (over-dosed) amount of EVs and EV-miR-NAs when doing in vivo functional studies. All of the above pitfalls should be corrected and studied in future studies of EV-miRNAs.
Future studies into EV-miRNAs will help to uncover the unclear role of EVs and EV-miRNAs in the pathogenesis of many diseases. Firstly, future studies should look to reveal the detailed mechanisms of how miRNAs are selectively encapsulated into EVs. Secondly, work is necessary to further understand the detailed signaling pathways of EV-miRNAs after being taken by the recipient cells. Additionally, it is critical for the development of a consistent and fast method to detect EV-miRNAs in each disease models, using body fluids. Lastly, a classification system should be created for microvesicles, exosomes and apoptotic bodies; specifically, by their function, generation, markers and physiological significance.
Figure 1:
Schema of the three major categories of Extracellular vesicles (EVs)
Figure 2:
Schema of the function of EV-miRNAs.
Table 1:
Currently reported EV-miRNAs involved in lung inflammation.
| Disease | EV-miRNA | Origin | Type of EV | Author |
|---|---|---|---|---|
| ALI | miR-146a, 155 | Dendritic cells | Exosome | Zeng 2013, Alexander 2015 |
| ALI | miR-320a, 221 | Epithelial cells | Microvesicles | Lee 2016 |
| ALI | miRNA-17-5p | Broncho-alveolar lavage fluid | Total EVs | Scheller 2018 |
| COPD | miR-210 | Human bronchial epithelial cells (HBEC) | Total EVs | Fujita 2015 |
| COPD | miR-191, 216, 215a | Lung endothelial cells | Exosome | Serban 2016 |
| BPD | miR-1246, 6511a5p, 22-3p, 21-5p, 296-5p, 196a-5p, 708-5p, 4707, 1244-5p, 20a/b5p, 214-3p, 148a3p, 199b-5p, 196-3p+6732-5p, 199a/b-3p, 6715p, 34a-5p, 648, 515-5p, 181a-23p, 630, 518e-3p, 6503-3p, 887-3p, 10b-5p, 1269a, 1915-3p, 193a/ b5p, 644a, 518d-3p | Mesenchymal | Total EVs | Shentu 2017 |
| Asthma | miR-let-7c, let-7b, 141, 200b, let-7d, let-7a, 21, 27, let-7e, 34c-5p, 34b-5p, 19b, 1972, 665, 658, 483-5p, 0022, 0024, 0026a, 0099a, 0200c, 1268, 0203, 0130a | BALF | Exosome | Levanen 2013 |
| IPF | miR-21-5p | Serum | Total EVs | Makiguchi 2016 |
| IPF | miR-199a-3p, 215p, 630, 22-3p, 196-5p, 199b-5p, 34a-5p, 148a-3p | Bone marrowde-rived MSCs | Total EVs | Shentu 2017 |
Acknowledgments
This work was supported by NIH grants: R01HL102076, R21AI121644, R33 AI121644, R01GM111313, R01GM127596, Wing Tat Lee award (to Y.J.)
REFERENCES
- 1.Hammond SM An overview of microRNAs. Advanced drug delivery reviews 2015, 87, 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarnieri DJ; DiLeone RJ MicroRNAs: a new class of gene regulators. Annals of medicine 2008, 40, 197–208. [DOI] [PubMed] [Google Scholar]
- 3.Fabian MR; Sonenberg N. The mechanics of miRNA-mediated gene silencing: a look under the hood of miRISC. Nature structural & molecular biology 2012, 19, 586–593. [DOI] [PubMed] [Google Scholar]
- 4.Ardekani AM; Naeini MM The Role of MicroRNAs in Human Diseases. Avicenna journal of medical biotechnology 2010, 2, 161–179. [PMC free article] [PubMed] [Google Scholar]
- 5.van Niel G; D’Angelo G; Raposo G. Shedding light on the cell biology of extracellular vesicles. Nature reviews. Molecular cell biology 2018, 19, 213–228. [DOI] [PubMed] [Google Scholar]
- 6.Bang C; Thum T. Exosomes: new players in cell-cell communication. The international journal of biochemistry & cell biology 2012, 44, 2060–2064. [DOI] [PubMed] [Google Scholar]
- 7.Chen J; Hu C; Pan P. Extracellular Vesicle MicroRNA Transfer in Lung Diseases. Frontiers in physiology 2017, 8, 1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almeida MI; Reis RM; Calin GA MicroRNA history: discovery, recent applications, and next frontiers. Mutation research 2011, 717, 1–8. [DOI] [PubMed] [Google Scholar]
- 9.Jamaluddin MS; Weakley SM; Zhang L; Kougias P; Lin PH; Yao Q; Chen C. miRNAs: roles and clinical applications in vascular disease. Expert review of molecular diagnostics 2011, 11, 79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortiz A. Not all extracellular vesicles were created equal: clinical implications. Annals of translational medicine 2017, 5, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalluri R. The biology and function of exosomes in cancer. The Journal of clinical investigation 2016, 126, 1208–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hessvik NP; Llorente A. Current knowledge on exosome biogenesis and release. Cellular and molecular life sciences : CMLS 2018, 75, 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexander M; Hu R; Runtsch MC; Kagele DA; Mosbruger TL; Tolmachova T; Seabra MC; Round JL; Ward DM; O’Connell RM Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nature communications 2015, 6, 7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanez-Mo M; Siljander PR; Andreu Z; Zavec AB; Borras FE; Buzas EI; Buzas K; Casal E; Cappello F; Carvalho J, et al. Biological properties of extracellular vesicles and their physiological functions. Journal of extracellular vesicles 2015, 4, 27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Z; Gong H; Li Y; Jie K; Ding C; Shao Q; Liu F; Zhan Y; Nie C; Zhu W, et al. Upregulation of miR-146a contributes to the suppression of inflammatory responses in LPS-induced acute lung injury. Experimental lung research 2013, 39, 275–282. [DOI] [PubMed] [Google Scholar]
- 16.Nolte-’t Hoen EN; Buermans HP; Waasdorp M; Stoorvogel W; Wauben MH; t Hoen PA Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic acids research 2012, 40, 9272–9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pattarayan D; Thimmulappa RK; Ravikumar V; Rajasekaran S. Diagnostic Potential of Extracellular MicroRNA in Respiratory Diseases. Clinical reviews in allergy & immunology 2018, 54, 480–492. [DOI] [PubMed] [Google Scholar]
- 18.Vestbo J; Hurd SS; Agusti AG; Jones PW; Vogelmeier C; Anzueto A; Barnes PJ; Fabbri LM; Martinez FJ; Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American journal of respiratory and critical care medicine 2013, 187, 347–365, doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 19.Fujita Y; Araya J; Ito S; Kobayashi K; Kosaka N; Yoshioka Y; Kadota T; Hara H; Kuwano K; Ochiya T. Suppression of autophagy by extracellular vesicles promotes myofibroblast differentiation in COPD pathogenesis. Journal of extracellular vesicles 2015, 4, 28388, doi: 10.3402/jev.v4.28388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serban KA; Rezania S; Petrusca DN; Poirier C; Cao D; Justice MJ; Patel M; Tsvetkova I; Kamocki K; Mikosz A, et al. Structural and functional characterization of endothelial microparticles released by cigarette smoke. Scientific reports 2016, 6, 31596, doi: 10.1038/srep31596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valadi H; Ekstrom K; Bossios A; Sjostrand M; Lee JJ; Lotvall JO Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology 2007, 9, 654–659, doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 22.Kosaka N; Iguchi H; Yoshioka Y; Takeshita F; Matsuki Y; Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. The Journal of biological chemistry 2010, 285, 17442–17452, doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pegtel DM; Cosmopoulos K; Thorley-Lawson DA; van Eijndhoven MA; Hopmans ES; Lindenberg JL; de Gruijl TD; Wurdinger T; Middeldorp JM Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences 1. of the United States of America 2010, 107, 6328–6333, doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y; Liu D; Chen X; Li J; Li L; Bian Z; Sun F; Lu J; Yin Y; Cai X, et al. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Molecular cell 2010, 39, 133–144, doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Akers JC; Gonda D; Kim R; Carter BS; Chen CC Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. Journal of neuro-oncology 2013, 113, 1–11, doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tricarico C; Clancy J; D’Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases 2017, 8, 220–232, doi: 10.1080/21541248.2016.1215283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caruso S; Poon IKH Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Frontiers in immunology 2018, 9, 1486, doi: 10.3389/fimmu.2018.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H; Zhang D; Zhu Z; Dela Cruz CS; Jin Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Scientific reports 2016, 6, 35250, doi: 10.1038/srep35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hewlett JC; Kropski JA; Blackwell TS Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix biology : journal of the International Society for Matrix Biology 2018, 71–72, 112–127, doi: 10.1016/j.matbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L; Huang W; Zhang L; Chen Q; Zhao H. Molecular pathogenesis involved in human idiopathic pulmonary fibrosis based on an integrated microRNAmRNA interaction network. Molecular medicine reports 2018, 10.3892/mmr.2018.9456, doi: 10.3892/mmr.2018.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Makiguchi T; Yamada M; Yoshioka Y; Sugiura H; Koarai A; Chiba S; Fujino N; Tojo Y; Ota C; Kubo H, et al. Serum extracellular vesicular miR-21–5p is a predictor of the prognosis in idiopathic pulmonary fibrosis. Respiratory research 2016, 17, 110, doi: 10.1186/s12931-016-0427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papagianis PC; Pillow JJ; Moss TJ Bronchopulmonary dysplasia: Pathophysiology and potential anti-inflammatory therapies. Paediatric respiratory reviews 2018, 10.1016/j. prrv.2018.07.007, doi: 10.1016/j.prrv.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Ober C; Yao TC The genetics of asthma and allergic disease: a 21st century perspective. Immunological reviews 2011, 242, 10–30, doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carr TF; Kraft M. Management of Severe Asthma before Referral to the Severe Asthma Specialist. The journal of allergy and clinical immunology. In practice 2017, 5, 877–886, doi: 10.1016/j.jaip.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levanen B; Bhakta NR; Torregrosa Paredes P; Barbeau R; Hiltbrunner S; Pollack JL; Skold CM; Svartengren M; Grunewald J; Gabrielsson S, et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. The Journal of allergy and clinical immunology 2013, 131, 894–903, doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ee MT; Thebaud B. Therapeutic Potential of Stem Cells for Bronchopulmonary Dysplasia: “It’s about time” or “Not so fast”? Current pediatric reviews 2018, 10.2174/1573396314666180911100503, doi: 10.2174/1573396314666180911100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shentu TP; Huang TS; Cernelc-Kohan M; Chan J; Wong SS; Espinoza CR; Tan C; Gramaglia I; van der Heyde H; Chien S, et al. Thy-1 dependent uptake of mesenchymal stem cell-derived extracellular vesicles blocks myofibroblastic 2. differentiation. Scientific reports 2017, 7, 18052, doi: 10.1038/s41598-017-18288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phinney DG; Di Giuseppe M; Njah J; Sala E; Shiva S; St Croix CM; Stolz DB; Watkins SC; Di YP; Leikauf GD, et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nature communications 2015, 6, 8472, doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubo H. Extracellular Vesicles in Lung Disease. Chest 2018, 153, 210–216, doi: 10.1016/j.chest.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 40.Vader P; Mol EA; Pasterkamp G; Schiffelers RM Extracellular vesicles for drug delivery. Advanced drug delivery reviews 2016, 106, 148–156, doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 41.Scheller N; Herold S; Kellner R; Bertrams W; Jung AL; Janga H; Greulich T; Schulte LN; Vogelmeier CF; Lohmeyer J, et al. Pro-viral miRNAs detected in BALF extracellular vesicles of patients with influenza virus-induced ARDS. The Journal of infectious diseases 2018, 10.1093/infdis/jiy554, doi: 10.1093/infdis/jiy554. [DOI] [PubMed] [Google Scholar]
- 42.Zaborowski MP; Balaj L; Breakefield XO; Lai CP Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797, doi: 10.1093/biosci/biv084. [DOI] [PMC free article] [PubMed] [Google Scholar]