Abstract
Introduction
Exercise and nutrition are the best targets to tackle mobility issues in community-dwelling older adults. As exercise response relies on multiple factors, improving the understanding of their interactions is a necessity to tailor effective preventive strategies. Based on a prevention care path designed for community-dwelling older adults with mobility disability risk, our main goal was to determine the predictive factors of the response to a multimodal intervention, combining structured exercise training and nutritional counselling. Thus, this study aimed to tailor prevention programs for non-responder participants.
Methods
We analyzed the response of participants to a prevention program and built a multivariate predictive model to highlight the profile of the best responders. The model was based on the likelihood of at least 1 point of short physical performance battery (SPPB) score gain. Inclusion criteria were being aged ≥70 years and having completed a multicomponent group-based supervised training consisting of 20 sessions (10 weeks).
Results
A total of 103 participants were included, their mean ± SD age was 81.9 ± 5.7 years. The model demonstrated interactions between baseline SPPB score (OR=0.42; p < 0.001), body mass index (BMI; OR=0.82; p=0.003), and grip strength value (OR=1.15; p=0.008). The highest probability of response was found for participants with low SPPB, normal BMI (21 kg/m2), and high grip strength (27 kg).
Conclusion
This study demonstrated that the response to a multimodal intervention in community-dwelling older adults with mobility disability risk was influenced by the baseline SPPB score, BMI, and grip strength value. To increase the proportion of responders, strategies that could be more effective include constituting more homogenous group, and implementing a specific approach for obese sarcopenic older adults and those with low grip strength by increasing the dose of physical activity and monitoring endurance and mobility activities between sessions. Our results provide important consideration for the development of targeted-interventions.
Keywords: exercise, nutrition, prevention, responders profiles
Introduction
The proportion of older adults is growing rapidly in the global population, while the health span only improves slowly.1 Therefore, one of the main challenges of aging is to prevent the onset of mobility disability and the associated syndromes. Sarcopenia stands out among the prognosis factors for mobility disability.2 This pathologic condition associates loss of strength and muscle mass.3 Recent studies including large population samples have demonstrated that a substantial proportion of community-dwelling older adults have sarcopenia.4 Advancing age also leads to the development of sedentary behaviors with an increase of functional limitation,5 which favor mobility disability,6 sarcopenia7 and consequently a worsening of the quality of life.8
Screening the risk of mobility disability in community-dwelling older adults is a necessity to develop preventive strategies and to detect early stages of sarcopenia.4,9 A sustained intervention integrating multidisciplinary actions and combining physical exercise and nutrition has been recognized as an effective strategy for the care of sarcopenic older adults in loss of mobility.10–12 Designing this specific intervention in prevention care paths remains essential as it can be transposed into the daily life of older adults in community-dwelling.13–15 These lifestyle programs also aim to engage the participant in health-related behaviors to preserve mobility, reduce fall incidence, and increase walking time.13,14,16
Although strong evidence has been reported suggesting the benefits of exercise to struggle sarcopenia and mobility disability, the variability of responses to exercise remains unclear.17–19 As a result, it is essential to determine the predictive factors of exercise responders in order to orientate patients towards appropriate protocols. Establishing responder profiles could guide clinicians to optimize individual responses and adaptations. These issues represent a deep concern for developing preventive strategies through targeted-interventions.
Based on a pre-existing preventive care path experienced in daily life by community-dwelling older adults, the main goal was to determine the predictive factors of the response to a multimodal intervention, combining structured exercise training and nutritional counselling. Thus, this study aimed to tailor prevention programs for non-responder participants. We hypothesized that baseline physical function and other baseline characteristics are prone to predict a functional response to a structured and supervised exercise intervention.
Study Design and Methods
Prevention Care Path
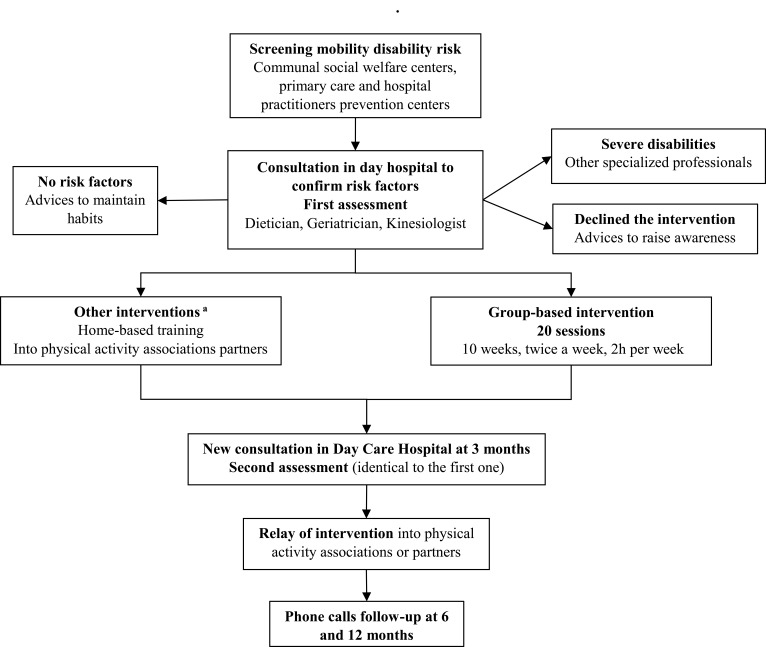
“Comfortable on my legs” is a primary and secondary prevention care path designed for community-dwelling older adults since 2014 (Figure 1). This care path aims to reduce the risk of mobility disability by restoring functional and physical capacities of older adults over 70 years old. The screening of the older adults at risk of mobility disability was managed by communal social welfare centers, primary care practitioners, and prevention centers. To this end, they used 11 criteria based on the SARC-F questionnaire (fear of falling, at least one fall within the last 12 months, difficulty to raise from a chair, difficulty to climb one floor or 10 steps, walking disorder, feeling of low strength, physical activity <30 minutes per day, involuntary loss of weight, BMI <21 kg/m2, fatigue during moderate physical activities, inability to walk 400 meters without stopping).20 Meeting at least one screening criterion was sufficient for a visit in day-hospital (Hôpital Lyon Sud, Hospices Civils de Lyon, France) to be proposed, in order to accurately assess the risk of mobility disability. This risk was assessed during a multidimensional consultation (medical, physical, and nutritional) with 3 professionals (geriatrician, dietician, and kinesiologist). Each participant received nutritional counselling from a trained dietician. Participants diagnosed with at least one mobility disability risk factor were orientated to a group-based structured exercise intervention (8 participants per group maximum) consisting of 20 sessions (two 1-hour sessions per week) supervised by trained kinesiologists. Participants with severe disabilities (ie, short physical performance battery [SPPB] score <5, high cardiovascular risk, heavy locomotive handicap, high cognitive impairment, or dementia) were orientated to other specialized professionals (such as physiotherapists). At the end of interventions, a reassessment was made in day-hospital. A referral to partner structures, as well as a provided individualized booklet of exercises, allowed participants to maintain an appropriate level of physical activities after interventions, according to ACSM recommendations.21 The care path is composed of both nutritional and physical dimensions, which is very close to the Integrate Care for Older People (ICOPE) recommendations for older adults physical autonomy promoted by the WHO.22
Figure 1.
Care path description. aWhen the participant could not attend the collective sessions.
Participants
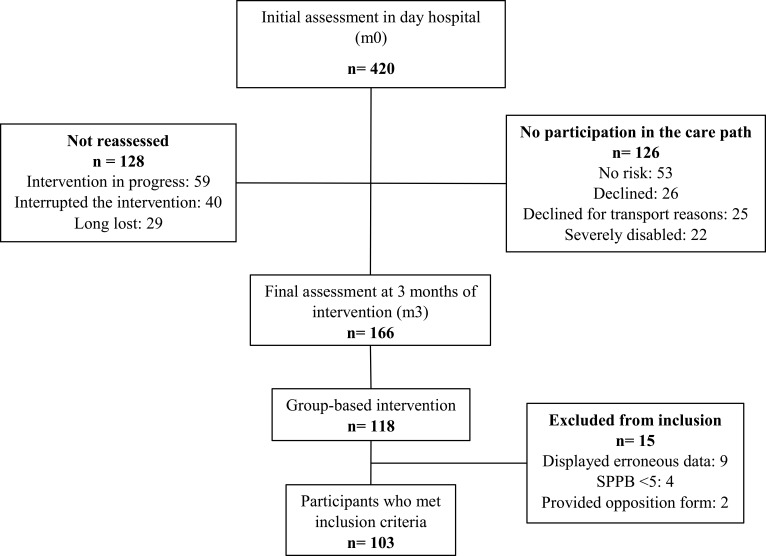
Participants were recruited from a cohort of 420 older adults living in Lyon urban area (France) between 2016 and 2020. The inclusion criteria were: being aged ≥70 years, having been diagnosed with mobility disability risk at initial assessment, having been reassessed 3 months after intervention, and having completed the 20 collective sessions (Figure 2). Among the participants who completed the collective sessions, participants not meeting the inclusion criteria, having an SPPB score <5, displaying erroneous values after assessments, or providing an opposition form were excluded. This study was approved by the scientific and ethical committee of the Hospices Civils de Lyon (France) in January 2021 (n°21_051, NCT 04798404). This study complies with the Helsinki Declaration. After receiving detailed information, all study participants gave their informed consent.
Figure 2.
Participants flow.
Exercise Intervention
The intervention conducted was a progressive multicomponent training protocol. Sessions involved functional exercises, muscular reinforcement performed at body-weight or with small materials (elastic bands or dumbbells), balance exercises, and adapted sport and physical activities. Progression was based on an increase in workload (or in muscle strains). The workload was considered as the interaction between intensity and volume of exercise (set x repetitions). Intensity was based on effort tolerance, modulate by contraction regimen, velocity, and load (elastic band stiffness). Participants were encouraged to reproduce exercises at home once they were correctly performed in supervised collective sessions.
Trained kinesiologists supervised the collective training sessions. As most of the participants were poly-pathological, the adaptability of sessions was essential. Therefore, the goal of the kinesiologist was to provide educative instructions in order to accompany participants towards a safe and autonomous practice of adapted physical activities. The equipment required for the sessions was simple to use and affordable (elastic band or dumbbells) and could easily be used at home.
The sessions were organized as follows: [1] 10 minutes of general warm-up of the body, involving joint mobilization, [2] 30 minutes of combined resistance and balance exercises, [3] 15 minutes of static and dynamic balance exercises, based on adapted physical and sport activities (basketball, badminton, …), and [4] 5 minutes of stretching or cooling down. Exercises were performed sitting or standing near a chair to avoid imbalance.
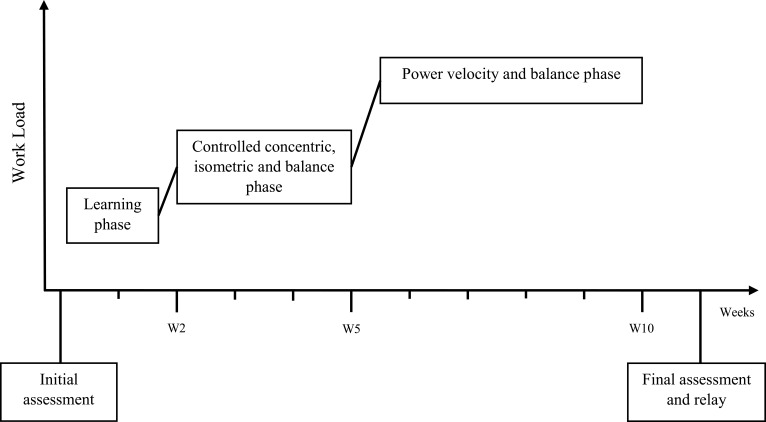
The training followed a 3-phase chronology (Figure 3) as it is generally admitted that nervous and morphological training adaptations have different temporalities.23–26
Figure 3.
Training phases.
The 1st Phase Took Place During the 1st and 2nd Weeks
The aim was to get participants back in motion as most of them were sedentary. During this phase, kinesiologists provided close attention to participant posture and technique. Exercises involved mostly functional exercises or global multi-joint movements. The intensity targeted at this stage was low to moderate, and exercises were performed using body weight or light loads. The volume of each exercise was around 2 to 3 series for 6 to 10 repetitions.
The 2nd Phase Took Place During the 3rd, 4th, and 5th Weeks
Sessions were intensified with a progressive increase in both intensity and volume, and consequently in the muscular constraints. A close attention was paid to exertion tolerance. The exercises consisted of 2 to 3 series of 8 to 15 repetitions with a recovery time of about 30 seconds to 1 minute between each series. Isometric work lasted 30 seconds with a recovery time of 15 seconds. In order to begin dynamic balance tasks, 15 minutes of adapted physical activities were added.
The 3rd Phase Started on the 6th Week
Exercises involved high velocity concentric contractions during the beginning of the movement, then slowed and controlled eccentric contractions at the end of the movement. At the beginning of this phase, the training volume was slightly reduced to fit with the effort tolerance of participants. This work was also supplemented with dual-task exercises containing a cognitive component.
Assessments
Main Outcome
The primary outcome was assessed using the SPPB score, evaluating the physical function of the lower limb in older adults.27 It was assessed during both visits at the day-hospital. The functional response to exercise was defined as the change in SPPB score. One point of positive change in this score was considered as clinically significant to prove a change in the functional status.27 Participants were asked to complete three tests supervised by a doctor: a static balance test, a 4-meter gait speed test, and the time required to perform 5 sit-to-stands as quickly as possible. Depending on the results of each subtest, a score was calculated and compared to a scale from 0 to 12. A score ≤8 was considered as a high risk of mobility disability and as a factor of severe sarcopenia according to European Working Group of Sarcopenia in Older People 2 (EWGSOP 2) criteria.3
Secondary Outcomes
Secondary outcome assessments were collected at initial and final assessments in day-hospital.
The gait speed was assessed with the 4-meter walking test. A gait speed ≤0.8 m/s was considered as a factor of severe sarcopenia.3
The Timed-up-and-Go (TUG) test was used to evaluate the functional capacity of the older adult. A time ≥20 seconds was considered as a factor of severe sarcopenia.3
The Grip Strength (GS) test was used to estimate the strength of the upper limbs. The GS was assessed using a hydraulic hand dynamometer model SH5001 (SAEHAN Corporation, Yangdeok-Dong, North Gyeongsang, South Korea), with the dominant arm placed at 90° and the elbow at the side of the body.28 Three measures were performed to determine the mean GS. For women and men, a score <16 kg and <27 kg, respectively, were considered as a low strength of the upper limb and a criterion of probable sarcopenia.3
The 5-repetition Chair Stand Test (CST) from the SPPB was used to estimate the lower limb strength among older adults.3,29 For women and men, a score >15 seconds was considered as low strength of the lower limb and a criterion of probable sarcopenia.
The dietician assessed the Body Mass Index (BMI) of each participant.
Additional Assessments
The risk of mobility disability was confirmed when at least one of these factors was diagnosed: sarcopenia at least probable, report of at least one fall within the last 12 months prior to the initial assessment, usual gait speed ≤0.8 m/s, or score <6 on the Rapid Assessment of Physical Activity (RAPA) questionnaire.
The diagnosis of sarcopenia was made using the EWGSOP2 algorithm.3 Low muscle strength and low muscle mass were criteria to confirm the presence of sarcopenia. Additionally, low physical performance was the criterion to diagnose a severe sarcopenia case. A sarcopenia case at least probable, with only low muscle strength assessed, was considered sufficient to trigger the intervention.
Skeletal Muscle Mass measure was derived by Bio Impedance Analysis (BIA), (Bodystat, QuadScan 4000, Isle of Man, British Isles) and was managed by the trained dietician at each visit. Muscle mass estimation was defined using the Skeletal Muscle Index (SMI; using Janssen equation, dividing the adjusted appendicular muscle mass by the height squared).30 Cut-off points were defined as an estimated SMI ≤6.42 kg·m2 for women and ≤8.87 kg·m2 for men.31
The level of physical activity was assessed using the RAPA score.32 Kinesiologists managed the filling of the questionnaire for participants. Participants with a score ≥6 were considered as regularly active, participants with a score ranging from 3 to 5 were considered as underactive, and participants with a score <3 were considered as sedentary.
Statistical Analysis
Variables were expressed as mean ± standard deviation (SD) or count (percentage). The means before and after the exercise intervention were compared using paired t-tests for each analyzed variable. Then, a logistic regression model was used in which a positive functional response to exercise (gain of at least one point of SPPB score) was regressed on the following baseline characteristics: SPPB score, age, sex, BMI, GS, gait speed, TUG, and CST. A multivariate model was selected using a forward selection procedure based on the likelihood ratio test (LRT): starting from the null model, the most statistically significant covariate according to LRT was added to the current model. When a covariate was not statistically significant (p < 0.05), the procedure ended. Interaction terms between selected covariates were also considered for entering the final model (Table 1). The model interpretation was based on 3 stratifications by GS and BMI. GS was stratified with the thresholds defined in the EWGSOP2 algorithm, ie, 27 for men and 16 for women, and 10 for the weakest participants (in the lowest tertile of functional outcomes according to Taekema et al).33 BMI was stratified into 3 different values: 21 kg/m2 for normal-weight participants, 25 kg/m2 for overweight participants, and 30 kg/m2 for stage-1-obese participants. All statistical tests and analyses were performed using R software.34
Table 1.
Odds Ratio of the Selected Model
| Predictors | OR a | CI b | p |
|---|---|---|---|
| (Intercept) c | 0.80 | [0.44; 1.43] | 0.459 |
| SPPB d | 0.42 | [0.28; 0.58] | <0.001 |
| BMI e | 0.82 | [0.72; 0.93] | 0.003 |
| Grip Strength | 1.15 | [1.04; 1.29] | 0.008 |
| BMI * Grip Strength | 0.98 | [0.96; 1.00] | 0.075 |
Notes: aOdds Ratio. bConfidence Interval 95%. cOdds. dShort Physical Performance Battery. eBody Mass Index. Significant interactions (p<0.05) are presented in bold.
Results
Baseline Characteristics
The population study was composed of 103 participants, their mean ± SD age was 81.9 ± 5.7 years, and there were 71 female and 32 male participants (Table 2). All participants presented mobility disability risk factors after initial assessment. Among the 76 participants who met the diagnostic criteria for sarcopenia, 2 (2.6%) had probable sarcopenia, 11 (14.5%) had sarcopenia, and 25 (32.9%) had severe sarcopenia (Table 2). The remaining 38 (50.0%) non-sarcopenic participants reported at least one fall in the last 12 months, or were scored as underactive using the RAPA questionnaire, or had a low gait speed. Overall, the population was considered as sedentary regarding the RAPA questionnaire (2.7 ± 1.4). There were 52 (50.2%) fallers (ie, report of a fall during the last 12 months prior to the initial assessment), which corresponded to a mean ± SD of 2.1 ± 2.2 falls per fallers. Protein intake was insufficient for 61 (75.3%) participants regarding baseline needs, ie, for 18 (75%) male participants and 43 (61.4%) female participants.
Table 2.
Population Characteristics at Initial Assessment (n=103)
| Total n=103 | Women n=71 | Men n=32 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | 81.9 ± 5.7 | 81.7 ± 6.2 | 82.3 ± 4.5 | |||||||||
| Weight, kg | 70.8 ± 15.0 | 62.3 ± 14.0 | 81.0 ± 11.9 | |||||||||
| Height, cm | 159 ± 17.4 | 157.3 ± 5.8 | 162.9 ± 30.3 | |||||||||
| BMI, kg/m2 a | 27.2 ± 4.8 | 26.8 ± 5.1 | 28.3 ± 4.0 | |||||||||
| SMI, kg/m2 b | – | 6.09 ± 1.05 | 8.43 ± 1.25 | |||||||||
| RAPA (/10) c | 2.7 ± 1.4 | 2.5 ± 1.4 | 3.1 ± 1.4 | |||||||||
| Fallers, n (%) d | 52 (50.5%) | 35 (49.3%) | 17 (53.1%) | |||||||||
| Number of falls per faller | 2.1 ± 2.2 | 2.3 ± 2.6 | 1.6 ± 1.3 | |||||||||
| Grip strength, kg | – | 15.8 ± 4.2 | 27.6 ± 7.3 | |||||||||
| Chair Stand Test, s | 14.3 ± 4.6 | 14.9 ± 4.8 | 12.8 ± 3.8 | |||||||||
| Gait speed, m/s | 0.91 ± 0.27 | 0.86 ± 0.28 | 1.03 ± 0.21 | |||||||||
| TUG, s | 14.5 ± 6.1 | 15.1 ± 6.8 | 13.3 ± 4.0 | |||||||||
| SPPB, /12 | 9.5 ± 1.9 | 9.1 ± 2.0 | 10.2 ± 1.6 | |||||||||
| Protein needs, g/per day | 74.7 ± 11.4 | 70.2 ± 9.6 | 85.4 ± 7.7 | |||||||||
| Protein intake, g/per day | 64.1 ± 13.1 | 60.5 ± 10.1 | 72.6 ± 15.4 | |||||||||
| Sarcopenia, n (%) e | NS | PS | S | SS | NS | PS | S | SS | NS | PS | S | SS |
| 38 (50.0%) | 2 (2.6%) | 11 (14.5%) | 25 (32.9%) | 23 (49.4%) | 1 (1.9%) | 7 (13.2%) | 22 (41.5%) | 15 (65.2%) | 1 (4.3%) | 4 (17.4%) | 3 (13.0%) | |
Notes: Quantitative variables are expressed as mean ± standard deviation, qualitative variables are expressed as count (percentage). aBody Mass Index. bEstimated Skeletal Muscle Index by bio impedance analysis (BIA). cRapid Assessment of Physical Activity questionnaire. dAt least one fall (traumatic or not) within the last 12 months. eDiagnosis was possible for 76 participants regarding EWGSOP2 algorithm; diagnosis was not possible when BIA was dysfunctional (n=15), and for participants with both BMI >31 and low physical performance (n=9).
Abbreviations: NS, no sarcopenia; PS, probable sarcopenia; S, sarcopenia; SS, severe sarcopenia.
Changes in Physical Performance and Functional Status After the Group-Based Exercise Intervention
Significant improvements were observed after the intervention for SPPB score (p < 0.001), gait speed (p < 0.001), TUG performance (p < 0.001), and GS in women (p < 0.001). The baseline GS of women was 15.8 ± 4.2 kg (Table 2), which were indicative of low performances in terms of upper body strength. A significant increase (p < 0.01) was observed in GS after the intervention in women (17.00 ± 4.6 kg) who were then above the threshold (16 kg). GS values for men did not change significantly (p=0.856) and remained stable (27.6 ± 7.3 kg at baseline and 27.4 ± 6.4 kg after the intervention) and above the threshold of low upper body strength (27 kg). Overall, there was no statistical difference in the time required in CST (p=0.697) between initial and final assessment. Nevertheless, this value remained stable (14.3 ± 4.6 s at baseline and 14.08 ± 4.9 s after the intervention) and still below the threshold of diminished lower limb strength (15 s).
Changes in SPPB Score
After excluding the 15 participants who had a maximal SPPB score, 49/88 (56%) participants increased their SPPB score. Among the 39/88 participants who did not increase the SPPB score, 27 (69%) had a stable score, and 12 (31%) had a decreased score.
Predictive Model
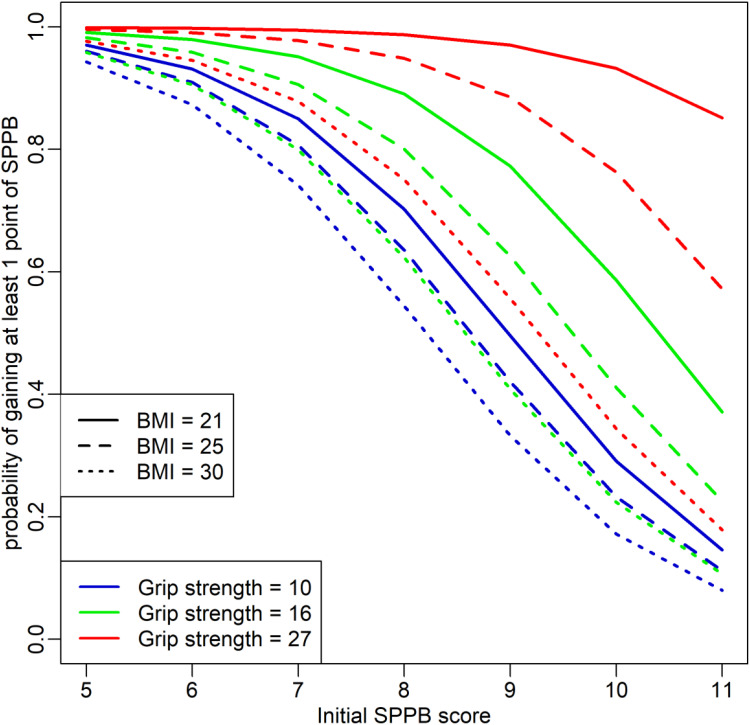
The covariates SPPB, BMI, and GS were centered on their median values (10, 26 kg/m2, and 17 kg respectively). In the final model (Figure 4), odds ratio is reported in Table 1. Besides these odds ratios, the model predicts an odd of 0.80 for an individual with an initial SPPB score of 10, BMI of 26 kg/m2, and GS of 17 kg to gain at least one point in the SPPB score, which corresponds also to 44% probability. For a median value of GS of 17 kg, when the initial SPPB score increased by one point, the odds of gain were multiplied by 0.42, whereas when BMI increased by one point, the odds were multiplied by 0.82 (Table 1). Due to the interaction term, however (Table 1), the effect of BMI slightly weakened for the highest GS values.
Figure 4.
Prediction related to the selected model.
The probability of gain decreased for higher BMI values. In contrast, a higher initial GS resulted in a higher probability of gain. The probability of gain diminished with a higher initial SPPB score. However, this effect was almost imperceptible in thinner and stronger individuals (BMI = 21 kg/m2 and GS = 27 kg, Figure 4).
Discussion
These results provided the profiles of responders to a structured and supervised exercise intervention within a preventive care path designed for community-dwelling older adults at risk of mobility disability. The model introduced demonstrated that the gain in SPPB score was influenced by the baseline BMI and GS value.
An association was found between the baseline SPPB score and BMI, which highlights a negative effect of a high BMI on the probability of improvement in the SPPB score. High adiposity may have blunted the positive effects of the exercise intervention. These findings are consistent with studies whose evidence has emphasized that a high BMI (≥30 kg/m2) was associated with a poor probability of change in physical function.18,35 Besides, low GS value and high BMI have also been associated with an increased risk of functional decline,36 but their relationship with exercise responsiveness remains to be investigated.
Further analyses showed a surprising positive effect of baseline GS on the change in SPPB. In other words, higher GS values led to a higher probability of gain in SPPB score, whereas lower values led to a lower probability of gain. Additionally, higher baseline BMI and low GS value were associated with a greater decrease in probability of gain, while the adverse effect of a high BMI was less pronounced in participants with higher GS. Mechanisms explaining the influence of baseline GS value in exercise responsiveness remain uncertain. Grip strength is a maximal isometric voluntary contraction, and it is well known that the loss of maximal strength with age is mainly caused by neuromuscular remodeling within the muscle.37 Thus, a high GS value may reflect, in a way, neuromuscular effectiveness. Indeed, GS value has been found moderately associated with overall muscle strength in older adults.3,31,38 Therefore, one potential and physiological cause of this GS positive effect could be a preserved neuromuscular system that could provide better adaptations to exercise. Indeed, improvements induced by short-term resistance and functional training in older adults are mainly resulting from neuromuscular adaptations.17,39 To confirm these assumptions, it would also be interesting to test the maximal voluntary strength of the lower limb to potentially highlight the same positive effect as demonstrated by Porto et al.38 Deeper investigations are needed to explore the influence of the neuromuscular system on the response to exercise.
Regarding the baseline profiles of participants, the model stressed paradoxes. Participants with poor physical function but high GS value might present mobility, neurosensory, or balance impairments that might explain their low SPPB score. On the opposite, some participants had high physical function and low grip strength. Although these profiles appear to be controversial, their poor chance of response can be explained mainly by the ceiling effect of the baseline SPPB score. Conversely to baseline GS value, higher SPPB scores exhibited a negative effect on the chance of response. The SPPB score represents a more complex set of elements, which are not limited to the quality of the neuromuscular system and may consequently be related to less adaptations to exercise. Interest towards GS for mobility disability management is growing up constantly,40 and further studies will be needed to pursue its role in older adults at risk of mobility disability.41
Other factors may also be related to the probability of response to the structured exercise intervention conducted. Lavin et al have demonstrated that the heterogeneity of response to resistance exercise highly depends on modifiable factors, such as functional capacity, and non-modifiable factors, such as age.17 They have shown that octogenarians often displayed limited adaptations to resistance training. However, in this model and despite a mean age above 80 years, age was not significantly associated with the change in SPPB score, meaning that the gain was possible regardless of age.
The response to exercise is also highly dependent on the type of training conducted. Several literature reviews and meta-analyses have identified different training methodologies to be applied for older adults.39,41–45 Progressive resistance training represents an effective strategy to elicit gain in lean body mass,46 improve physical function,47 and enhance muscular strength.48 Overall, the main differences between these training programs lie in the workload, the intensity, and sometimes in the contraction regimes.39,49,50 As a result, the present study focused on those 3 determinants to monitor training progression.
The present study showed that at the end of the exercise intervention, the highest probability of improvement in the SPPB score was obtained in participants with a higher level of GS and normal BMI at baseline. This better understanding of factors involved in the response to a functional and resistance exercise intervention could lead to improve training adaptations to obtain the best individual results in older adults at risk of mobility disability. The type and dose of physical activity are the best targets to improve individual responses, particularly among participants with low GS value and high BMI.
It may be beneficial to adapt the type of physical activity while using endurance sessions as a complement of resistance training to improve adaptations.39 The endurance component, combined with different types of exercises (resistance, functional, power, balance), enhances the response to training on mobility, balance, strength, power, muscular hypertrophy, cardiorespiratory function, and reduces the risk of fall.42,51–54 To add an endurance component, authors have proposed to walk (minimum 30 to 35 minutes 3 times per week49 or 30 minutes of brisk walking once a week55) or to practice biking on cycle-ergometer during 13 minutes at 60% to 85% of the reserve heart rate 3 times per week.56 In obese sarcopenic older adults who participated in a controlled weight-loss program, Villareal et al's findings have demonstrated that a greater increase in physical function was observed after a combination of endurance and resistance training.57
The dose of physical activity is considered as the absolute level of physical activity practiced. Participants who did not increase their performance may need a greater dose of physical activity. This hypothesis is supported by Fielding et al who have highlighted a dose-dependent effect of physical activity in the change of SPPB score, as well as in the onset of mobility disability risk at 24 months of follow-up.58 Consequently, a sustained follow-up of endurance activities (walking, biking) should be proposed to reduce sedentary behavior between training sessions with a follow-up of daily mobility exercises performed at home and behavioral strategies. Additionally and because of heterogeneity within groups, the dose of exercise performed may have been insufficient for participants with high baseline physical function. Thereby, the constitution of groups should be homogenized by taking into account various physical parameters (RAPA score, physical activity experiences, physical performance …) and functional limitations (pain, injuries …) in order to optimize the dose of exercise required for each one. As stated previously, the volume and intensity of exercises are essential components to enable adaptations, as long as they are adapted to the needs, abilities, and effort tolerance of each individual. Thus, forming homogeneous groups could enable participants to reach a sufficient and adapted workload per session to induce adapted muscular constraints and thus promote greater neuromuscular and architectural adaptations to training.
In summary, combining these different adaptations may increase the proportion of responders and at the same time improve the response to exercise for participants.
Nutrition also plays a major role in a complementary way. Indeed, the present study clearly underlined the negative impact of obesity on exercise response. Although this intervention was well tolerated, it may not be adequate in the first place for participants with BMI of 30 or more. Although authors have suggested that a controlled weight loss may be an adjuvant therapy for improving responsiveness to exercise,11,36 this may not be appropriate for obese older adults and in particular for obese sarcopenic patients. Indeed, energy deficits by acute calorie restriction may worsen the loss of muscle mass induced by sarcopenia.36,59 Therefore, it is crucial to strictly adjust protein supplementation in obese sarcopenic patients undergoing restricted caloric intake programs.36 It demands sustained and pragmatic strategies, which require additional clinical evidence to prove its efficacy.36 Moreover, we were able to show that the protein intake was insufficient in about three-quarter of participants, and it is now currently accepted that the combination of protein intake and exercise acts synergistically and allows a better response to training.36,60,61
Clearly, our objective was not to show the effectiveness of our program that was inspired by models that have already proven their effectiveness but to objectivize the factors that lead to a better response to exercise based on a prevention program implemented in real-life settings. Several limits, however, should be underlined. The number of participants who were reassessed remains limited and the results come from a single center, which makes it difficult to generalize the results. There was no significant interaction between sex and the change in the SPPB score, despite the fact that higher GS values concerned men. This result remains unclear and requires further investigations. The SPPB score has limits as the ceiling effect does not take into account the progress made by participants with a maximal score at baseline. Sayers et al have advocated to rather assess the 400-m walking test for high functioning patients as they have shown less physical performance discrimination.62 Notwithstanding, participants with high baseline physical function (SPPB 10–12) were included because they presented other mobility disability risk factors, such as falls or a sedentary lifestyle.
In conclusion, the present study demonstrated that the response to a structured and supervised multimodal intervention in community-dwelling older adults with mobility disability risk was influenced by the baseline SPPB score, the baseline BMI, and the baseline GS value. Participants who presented low SPPB score, normal BMI, and high GS value had the best likelihood of response. The relationship between GS value and its effect on exercise response remains unclear. These results provide important conclusions for the development of targeted-interventions in prevention programs. To increase the proportion of responders, strategies that could be more effective include constituting more homogenous group and implementing a specific approach for obese sarcopenic older adults and those with low grip strength by increasing the dose of physical activity and monitoring endurance and mobility activities between sessions. Preventive care paths for mobility disability risk represent a major challenge in primary and secondary care. Further investigations to complete the responder profiles will improve the effectiveness of these interventions promoted by the WHO.
Acknowledgments
The authors acknowledge geriatricians, dieticians, medical secretaries and clinical research assistants from Hôpital Lyon Sud, Hospices Civils de Lyon, for their involvement in the program, their participation in assessments and their help to collect data. Authors acknowledge municipalities of Métropole de Lyon for their collaboration to propose the care path. Authors acknowledge financial partners such as the Agence Régionale de la Santé (Auvergne Rhône Alpes), Conférence des Financeurs de la métropole de Lyon et du Rhône, and the Mutuelle des Travailleurs de la Région Lyonnaise (MTRL) for their support and their confidence. Also, authors acknowledge the Caisse d’Assurance Retraite et la Santé au Travail (CARSAT) for their implication as the main relay for participants. The authors thank Hélène Boyer (Hospices Civils de Lyon) for help in manuscript preparation.
Disclosure
The authors report no conflicts of interest related to this work.
References
- 1.Merchant RA, Morley JE, Izquierdo M. Exercise, aging and frailty: guidelines for increasing function. J Nutr Health Aging. 2021;25(4):405–409. doi: 10.1007/s12603-021-1590-x [DOI] [PubMed] [Google Scholar]
- 2.Janssen I. Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J Am Geriatr Soc. 2006;54(1):56–62. doi: 10.1111/j.1532-5415.2005.00540.x [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. 2017;16(1):1–10. doi: 10.1186/s40200-017-0302-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leirós-Rodríguez R, Romo-Pérez V, García-Soidán JL, Soto-Rodríguez A. Prevalence and factors associated with functional limitations during aging in a representative sample of Spanish population. Phys Occup Ther Geriatr. 2018;36(2–3):156–167. doi: 10.1080/02703181.2018.1449163 [DOI] [Google Scholar]
- 6.Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D. Age-related change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol. 2016;71(9):1184–1194. doi: 10.1093/gerona/glw043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buford TW, Anton SD, Judge AR, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing Res Rev. 2010;9(4):369–383. doi: 10.1016/j.arr.2010.04.004.Models [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oh B, Cho B, Choi HC, et al. The influence of lower-extremity function in elderly individuals’ quality of life (QOL): an analysis of the correlation between SPPB and EQ-5D. Arch Gerontol Geriatr. 2014;58(2):278–282. doi: 10.1016/j.archger.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 9.Sobestiansky S, Michaelsson K, Cederholm T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85–89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr. 2019;19(1):1–13. doi: 10.1186/s12877-019-1338-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hassan EB, Duque G. Osteosarcopenia: a new geriatric syndrome. Aust Fam Physician. 2017;46(11):849–853. [PubMed] [Google Scholar]
- 11.Anton SD, Hida A, Mankowski R, et al. Nutrition and exercise in sarcopenia. Curr Protein Pept Sci. 2018;19(7):649–667. doi: 10.2174/1389203717666161227144349 [DOI] [PubMed] [Google Scholar]
- 12.Billot M, Calvani R, Urtamo A, et al. Preserving mobility in older adults with physical frailty and sarcopenia: opportunities, challenges, and recommendations for physical activity interventions. Clin Interv Aging. 2020;15:1675–1690. doi: 10.2147/CIA.S253535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opdenacker J, Boen F, Coorevits N, Delecluse C. Effectiveness of a lifestyle intervention and a structured exercise intervention in older adults. Prev Med. 2008;46(6):518–524. doi: 10.1016/j.ypmed.2008.02.017 [DOI] [PubMed] [Google Scholar]
- 14.Clemson L, Fiatarone Singh MA, Bundy A, et al. Integration of balance and strength training into daily life activity to reduce rate of falls in older people (the LiFE study): randomised parallel trial. BMJ. 2012;345(7870):1–15. doi: 10.1136/bmj.e4547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Dongen EJI, Haveman-Nies A, Doets EL, Dorhout BG, de Groot LCPGM. Effectiveness of a diet and resistance exercise intervention on muscle health in older adults: proMuscle in practice. J Am Med Dir Assoc. 2020;21(8):1065–1072.e3. doi: 10.1016/j.jamda.2019.11.026 [DOI] [PubMed] [Google Scholar]
- 16.Groessl EJ, Kaplan RM, Rejeski WJ, et al. Physical activity and performance impact long-term quality of life in older adults at risk for major mobility disability. Am J Prev Med. 2019;56(1):141–146. doi: 10.1016/j.amepre.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavin KM, Roberts BM, Fry CS, Moro T, Rasmussen BB, Bamman MM. The importance of resistance exercise training to combat neuromuscular aging. Physiology. 2019;34(2):112–122. doi: 10.1152/physiol.00044.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chmelo EA, Crotts CI, Newman JC, et al. Heterogeneity of physical function responses to exercise training in older adults. J Am Geriatr Soc. 2015;63(3):1759–1765. doi: 10.1111/jgs.13322.Heterogeneity [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE Study randomized clinical trial. J Am Med Assoc. 2014;10(4):591–598. doi: 10.17267/2238-2704rpf.v10i4.3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36. doi: 10.1002/jcsm.12048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U.S Department of Health and Human Services. Physical Activity Guidelines for Americans; 2018. [Google Scholar]
- 22.Tavassoli N, Piau A, Berbon C, et al. Framework Implementation of the INSPIRE ICOPE-CARE program in collaboration with the World Health Organization (WHO) in the Occitania Region. J Frailty Aging. 2020;2:1–7. doi: 10.14283/jfa.2020.26 [DOI] [PubMed] [Google Scholar]
- 23.Kamen G, Knight CA. Training-related adaptations in motor unit discharge rate in young and older adults. J Gerontol. 2004;59(12):1334–1338. doi: 10.1093/gerona/59.12.1334 [DOI] [PubMed] [Google Scholar]
- 24.Patten C, Kamen G, Rowland DM. Adaptations in maximal motor unit discharge rate to strength training in young and older adults. Muscle Nerve. 2001;24(4):542–550. doi: 10.1002/mus.1038 [DOI] [PubMed] [Google Scholar]
- 25.Folland JP, Williams AG. The adaptations to strength training: morphological and neurological contributions to increased strength. Sport Med. 2007;37(2):145–168. doi: 10.2165/00007256-200737020-00004 [DOI] [PubMed] [Google Scholar]
- 26.Baroni BM, Rodrigues R, Franke RA, Geremia JM, Rassier DE, Vaz MA. Time course of neuromuscular adaptations to knee extensor eccentric training. Int J Sports Med. 2013;34(10):904–911. doi: 10.1055/s-0032-1333263 [DOI] [PubMed] [Google Scholar]
- 27.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.M85 [DOI] [PubMed] [Google Scholar]
- 28.Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. doi: 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 29.Mentiplay BF, Clark RA, Bower KJ, Williams G, Pua YH. Five times sit-to-stand following stroke: relationship with strength and balance. Gait Posture. 2020;78(January):35–39. doi: 10.1016/j.gaitpost.2020.03.005 [DOI] [PubMed] [Google Scholar]
- 30.Janssen I, Heymsfield SB, Baumgartner RN, Ross R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89(2):465–471. doi: 10.1152/jappl.2000.89.2.465 [DOI] [PubMed] [Google Scholar]
- 31.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Topolski TD, LoGerfo J, Patrick DL, Williams B, Walwick J, Patrick MB. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3(4):1–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Taekema DG, Gussekloo J, Maier AB, Westendorp RGJ, De Craen AJM. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39(3):331–337. doi: 10.1093/ageing/afq022 [DOI] [PubMed] [Google Scholar]
- 34.Ihaka R, Gentleman R. R: a language and environment for statistical computing. 2020.
- 35.Layne AS, Hsu FC, Blair SN, et al. Predictors of change in physical function in older adults in response to long-term, structured physical activity: the LIFE Study. Arch Phys Med Rehabil. 2017;98(1):11–24.e3. doi: 10.1016/j.apmr.2016.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018;14(9):513–537. doi: 10.1038/s41574-018-0062-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duchateau J, Klass M, Baudry S. Évolution Et Adaptations À L’Entraînement Du Système Neuromusculaire Au Cours Du Vieillissement [Development and training adaptations of the neuromuscular system during aging]. Sci Sport. 2006;21(4):199–203. France. doi: 10.1016/j.scispo.2006.03.006 [DOI] [Google Scholar]
- 38.Porto JM, Midori Nakaish AP, Cangussu-Oliveira LM, Renato CFJ, Sallua BS, Daniela Cristina CDA. Relationship between grip strength and global muscle strength in community-dwelling older people. Arch Gerontol Geriatr. 2019;82:273–278. doi: 10.1016/j.archger.2019.03.005 [DOI] [PubMed] [Google Scholar]
- 39.Fragala MS, Cadore EL, Dorgo S, et al. Resistance training for older adults: position statement from the national strength and conditioning association. J Strength Cond Res. 2019;33(8):2019–2052. doi: 10.1519/jsc.0000000000003230 [DOI] [PubMed] [Google Scholar]
- 40.Cawthon PM, Travison TG, Manini TM, et al. Establishing the link between lean mass and grip strength cut points with mobility disability and other health outcomes: proceedings of the sarcopenia definition and outcomes consortium conference. J Gerontol. 2020;75(7):1317–1323. doi: 10.1093/gerona/glz081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bohannon RW. Grip strength: an indispensable biomarker for older adults. Clin Interv Aging. 2019;14:1681–1691. doi: 10.2147/CIA.S194543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas E, Battaglia G, Patti A, et al. Physical activity programs for balance and fall prevention in elderly. Medicine. 2019;98(27):1–9. doi: 10.1097/MD.0000000000016218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macdonald SHF, Travers J, Shé ÉN, et al. Primary care interventions to address physical frailty among community-dwelling adults aged 60 years or older: a meta-analysis. PLoS One. 2020;15:2. doi: 10.1371/journal.pone.0228821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y, Zhang Y, Du S, Wang Q, Xia H, Sun R. Exercise interventions for improving physical function, daily living activities and quality of life in community-dwelling frail older adults: a systematic review and meta-analysis of randomized controlled trials. Geriatr Nurs. 2020;41(3):261–273. doi: 10.1016/j.gerinurse.2019.10.006 [DOI] [PubMed] [Google Scholar]
- 45.Law TD, Clark LA, Clark BC. Resistance exercise to prevent and manage sarcopenia and dynapenia. Annu Rev Gerontol Geriatr. 2016;63(8):1–18. doi: 10.1891/0198-8794.36.205.Resistance [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sport Exerc. 2011;43(2):249–258. doi: 10.1249/MSS.0b013e3181eb6265.Influence [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papa EV, Dong X, Hassan M. Resistance training for activity limitations in older adults with skeletal muscle function deficits: a systematic review. Clin Interv Aging. 2017;12:955–961. doi: 10.2147/CIA.S104674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9(3):226–237. doi: 10.1016/j.arr.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsekoura M, Billis E, Tsepis E, et al. The effects of group and home-based exercise programs in elderly with sarcopenia: a randomized controlled trial. J Clin Med. 2018;7(12):480. doi: 10.3390/jcm7120480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lichtenberg T, Von Stengel S, Sieber C, Kemmler W. The favorable effects of a high-intensity resistance training on sarcopenia in older community-dwelling men with osteosarcopenia: the randomized controlled frost study. Clin Interv Aging. 2019;14:2173–2186. doi: 10.2147/CIA.S225618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cadore EL, Izquierdo M. How to simultaneously optimize muscle strength, power, functional capacity, and cardiovascular gains in the elderly: an update. Age. 2013;35(6):2329–2344. doi: 10.1007/s11357-012-9503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cadore EL, Casas-Herrero A, Zambom-Ferraresi F, et al. Multicomponent exercises including muscle power training enhance muscle mass, power output, and functional outcomes in institutionalized frail nonagenarians. Age. 2014;36(2):773–785. doi: 10.1007/s11357-013-9586-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao W, Sun Y, Zhang T, et al. Exercise programs for muscle mass, muscle strength and physical performance in older adults with sarcopenia: a systematic review and meta-analysis. Aging Dis. 2020;11(4):863–873. doi: 10.14336/AD.2019.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blain H, Bloch F, Borel L, et al. Activité Physique et Prévention Des Chutes Chez Les Personnes Âgées. [Physical Activity and Fall Prevention in the Elderly]. Collection Expertise Collective. France: Les éditions INSERM; 2014. [Google Scholar]
- 55.Sousa N, Mendes R, Silva A, Oliveira J. Combined exercise is more effective than aerobic exercise in the improvement of fall risk factors: a randomized controlled trial in community-dwelling older men. Clin Rehabil. 2017;31(4):478–486. doi: 10.1177/0269215516655857 [DOI] [PubMed] [Google Scholar]
- 56.Ansai JH, Aurichio TR, Gonçalves R, Rebelatto JR. Effects of two physical exercise protocols on physical performance related to falls in the oldest old: a randomized controlled trial. Geriatr Gerontol Int. 2016;16(4):492–499. doi: 10.1111/ggi.12497 [DOI] [PubMed] [Google Scholar]
- 57.Villareal DT, Aguirre L, Gurney B, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017;376(20):1943–1955. doi: 10.1056/NEJMoa1616338.Aerobic [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fielding RA, Guralnik JM, King AC, et al. Dose of physical activity, physical functioning and disability risk in mobility-limited older adults: results from the LIFE study randomized trial. PLoS One. 2017;12(8):1–20. doi: 10.1371/journal.pone.0182155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12(7):487–491. doi: 10.1007/BF02982710 [DOI] [PubMed] [Google Scholar]
- 60.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the arthritis, diet, and activity promotion trial. Arthritis Rheum. 2004;50(5):1501–1510. doi: 10.1002/art.20256 [DOI] [PubMed] [Google Scholar]
- 61.Cermak NM, Res PT, De Groot LCPGM, Saris WHM, Van Loon LJC. Protein supplementation augments the adaptive response of skeletal muscle to resistance-type exercise training: a meta-analysis. Am J Clin Nutr. 2012;96(6):1454–1464. doi: 10.3945/ajcn.112.037556 [DOI] [PubMed] [Google Scholar]
- 62.Sayers SP, Guralnik JM, Newman AB, Brach JS, Fielding RA. Concordance and discordance between two measures of lower extremity function: 400 meter self-paced walk and SPPB. Aging Clin Exp Res. 2006;18(2):100–106. doi: 10.1007/BF03327424 [DOI] [PubMed] [Google Scholar]