Abstract
Background
Back and lower limb pain have a major impact on physical function and quality of life. While obesity is a modifiable risk factor for musculoskeletal pain, the role of adiposity is less clear. This systematic review aimed to examine the relationship between both adiposity and its distribution and back and lower limb pain.
Methods
A systematic search of electronic databases was conducted to identify studies that examined the association between anthropometric and/or direct measures of adiposity and site specific musculoskeletal pain. Risk of bias was assessed and a best evidence synthesis was performed.
Results
A total of 56 studies were identified which examined 4 pain regions, including the lower back (36 studies), hip (two studies), knee (13 studies) and foot (eight studies). 31(55%) studies were assessed as having low to moderate risk of bias. 17(30%) studies were cohort in design. The best evidence synthesis provided evidence of a relationship between central adiposity and low back and knee pain, but not hip or foot pain. There was also evidence of a longitudinal relationship between adiposity and the presence of back, knee and foot pain, as well as incident and increasing foot pain.
Conclusions
This systematic review provides evidence of an association between both body fat and its central distribution and low back and knee pain, and a longitudinal relationship between adiposity and back, knee and foot pain. These results highlight the potential for targeting adiposity in the development of novel treatments at these sites.
Introduction
Musculoskeletal conditions are a leading disease burden worldwide. They are not only the second most common cause of global disability, but disability-adjusted life years (DALYs) for musculoskeletal conditions have increased alarmingly, with a rise of up to 62% between 1990 and 2016 [1]. One in three people worldwide live with a musculoskeletal condition, which is characterised by pain and disability, leads to reduced quality of life, and results in a huge economic burden [2]. Back and lower limb pain are highly prevalent musculoskeletal conditions and make a major contribution to their increasing burden at an individual, familial and societal level. Current efforts to reduce the profound impact of these conditions have focussed on determining modifiable risk factors for management and prevention.
Obesity is an escalating, global epidemic. The 2016 Global Burden of Disease Study showed that the prevalence of obesity is not only increasing, but obese people are actually living longer, which allows for the development of co-existing conditions, such as musculoskeletal pain [3]. There is growing evidence to indicate that obesity is a modifiable risk factor for musculoskeletal pain at different sites. A meta-analysis by Shiri and colleagues reported overweight and obesity, measured by weight and body mass index (BMI), to be risk factors for low back pain [4], while a systematic review by Butterworth et al. found increased BMI to be strongly associated with non-specific foot pain in the general population [5]. While these reviews provide evidence for a relationship between obesity, measured by body weight or BMI, and musculoskeletal pain, they do not account for body composition and thus don’t consider the individual contributions of fat mass and lean tissue mass (or muscle mass). This is of particular importance given there is evidence to show that fat mass or adiposity and muscle mass have different roles in the pathogenesis of musculoskeletal disease [6,7].
There is growing evidence to show that adiposity plays an important role in musculoskeletal pain. Adipose tissue acts as an endocrine organ, releasing a host of pro-inflammatory cytokines and adipokines [8], which can heighten inflammatory changes leading to destruction of tissue [9] and increasing pain and disability. A single systematic review has examined the relationship between body fat and musculoskeletal pain [10], reporting a positive cross-sectional association between higher body fat and single-site joint pain in the low back, knee and foot. However, no conclusions could be drawn from longitudinal data regarding the role of adiposity in back and lower limb pain, as there was a lack of available high quality, cohort studies. Moreover, the review focussed on studies that used direct measures of body fat, such as fat mass and percentage of body fat, and excluded those that examined anthropometric measures, such as waist circumference and waist hip ratio [11], thus limiting the opportunity to examine role of fat distribution, particularly central adiposity.
Understanding the role of adiposity in musculoskeletal pain, particularly back and lower limb pain, has huge potential to inform the development of novel prevention and treatment approaches, as well as further our understanding of mechanisms underlying the relationship between obesity and musculoskeletal pain. The aims of this systematic review were to: (i) examine the relationship between central adiposity and back and lower limb pain and (ii) investigate the longitudinal association between adiposity and both the presence, incidence and progression of pain at these sites.
Methods
This systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (see S1 Checklist) [12].
Data sources and searches
We performed electronic searches of six databases, including MEDLINE, Embase, CINAHL, Cochane Central Register of Controlled Trials, Scopus and Web of Science from database inception to February 2, 2021. Our initial search for studies was conducted using text words and subject terms on three key databases and then based on this, we developed the search strategy, with subject classification systems investigated for each database and expanded our data sources to include all six databases for our final search. The final searches of all six databases, covering the key concepts of adiposity and musculoskeletal pain, were performed using the appropriate specifications for each database. The comprehensive search strategy for OVID Medline is provided (see S1 Medline Database search strategy in S1 Text). The searches were restricted to adult human studies but not limited based on language. To identify grey literature, we searched Google scholar, using key terms such as ‘adiposity’ and ‘musculoskeletal pain’, from 2011 to 14 February 2021, and Scopus, using our Scopus search strategy and selecting for conference proceedings, from inception to 14 February 2021. In addition, reference lists of reviews and key papers were searched to identify relevant literature.
Inclusion and exclusion criteria
Studies were included if they investigated the relationship between adiposity and low back or lower limb pain, using at least one measure of adiposity and reporting pain as an outcome measure. Studies that examined adiposity using: (i) anthropometric measures, including waist circumference, hip circumference, waist-hip ratio, waist-height ratio, and skin folds, and (ii) direct fat measures, such as fat mass and body fat percentage, using dual-energy X-ray absorptiometry (DXA) and bioelectrical impedance, were included.
For the purposes of this review, waist circumference was defined as a measurement around the trunk at the midpoint between the lower margin of the least palpable rib and the top of the iliac crest [11]. Hip circumference was considered to be a measure around the hips at the maximum posterior extension of the buttocks, while waist-hip ratio and waist-height ratio were calculated by dividing waist circumference by hip circumference measures, and waist circumference by height, respectively [11]. Skinfold measures assessed the subcutaneous fat thickness and were measured by skinfold calipers [13], while fat mass and body fat percentage were defined as the total mass of adipose tissue or percentage of total adipose tissue of the whole body mass respectively. Central adiposity, an accumulation of both subcutaneous and visceral fat in the lower torso around the abdominal area, was assessed by waist circumference or waist-hip or waist-height ratio measures, which are recommended by the World Health Organization [11].
Data on the presence, incidence and progression of pain in each region was recorded from the included studies where possible. The presence of pain, which was reported from cross-sectional, case-control and cohort studies, was defined as pain recorded at one point in time. For a cohort study, this could have been where adiposity was assessed at baseline and pain was measured at follow-up. Incident pain was defined as where pain was assessed at both baseline and follow-up in a cohort study, with pain absent at baseline and present at follow-up. Moreover, the progression of pain was described where pain was present at both baseline and follow-up in a cohort study and was assessed as increasing, decreasing or not changing over the study period There was no hierarchy given to these pain outcomes, however data from cohort studies were considered the highest level of evidence, followed by case-control studies and then cross-sectional studies.
We excluded studies that: (1) reported BMI or weight only; (2) examined only intramuscular fat; (3) reported pain in the head, neck or upper limb; (4) investigated pain other than musculoskeletal pain, i.e. abdominal pain, cardiac pain; and (5) examined multisite musculoskeletal pain where data specific to the back or lower limb were not reported separately.
Study selection
Titles and abstracts were assessed by two investigators (WP and TR) for relevance and the full texts were retrieved for relevant studies.
Data extraction
Data were extracted and tabulated by two reviewers (WP and TR) independently. Studies were categorized based on: (i) the site of pain investigated (low back, hip, knee and foot), (ii) their study design (cross-sectional, case-control or cohort) and (iii) the type of adipose measure reported (anthropometric versus direct fat measures). Data extracted from the studies included (1) author and year of publication, (2) study population characteristics (number of study participants, gender (% women), mean (SD) age, recruitment source), (3) assessment method and measure for adiposity and pain, (4) results (OR/RR, 95%CI) and (5) conclusions.
Risk of bias assessment
To assess the risk of bias of the included studies, two reviewers (TR and JF) independently assessed the included studies using the Cochrane risk of bias assessment [14]. The Cochrane risk of bias assessment examines the internal and external validity of the included studies, based on four items for cross-sectional studies and five items for cohort studies, with each item scored as low, moderate or high risk of bias. An overall assessment was then given for each study; low if every individual item scored low, moderate if all items scored low except either one high or two moderate, or high if individual items scored more than one high or more than two moderate.
Best evidence synthesis
A best evidence synthesis was used to summarise the data. It was not possible to perform a meta-analysis as there was substantial clinical and methodological heterogeneity across the studies, including differences in the clinical populations investigated, risk factors and outcomes measured, and statistical data and analyses performed. Based on the study design, the number of studies, the risk of bias rating, and consistency of the results of the studies, levels of evidence for the association between adiposity and pain was determined for each region. The studies were ranked according to their design, with cohort studies considered the highest level of evidence, followed by case-control studies and then cross-sectional studies. Studies were classified as having an association (“positive” or “negative”) if the association reported was statistically significant according to the authors’ predetermined alpha value (or p < .05 where this was not reported) or where the confidence interval for an odds ratio did not cross one.
The levels of evidence used were adapted from the Lievense’s standardized criteria [15], which have been used previously in observational studies of musculoskeletal conditions [16]. They included: evidence of an association, conflicting evidence, limited evidence or no evidence. ‘Evidence of an association’ was defined as consistent findings in multiple, cohort studies, while ‘conflicting evidence’ was defined as inconsistent findings across the number and types of studies. ‘Limited evidence’ was defined as consistent findings in a small number of studies, including a single cohort study or one or two case-control or cross-sectional studies, and ‘no evidence’ was used when there are no studies that provided any evidence.
Results
Identification of studies
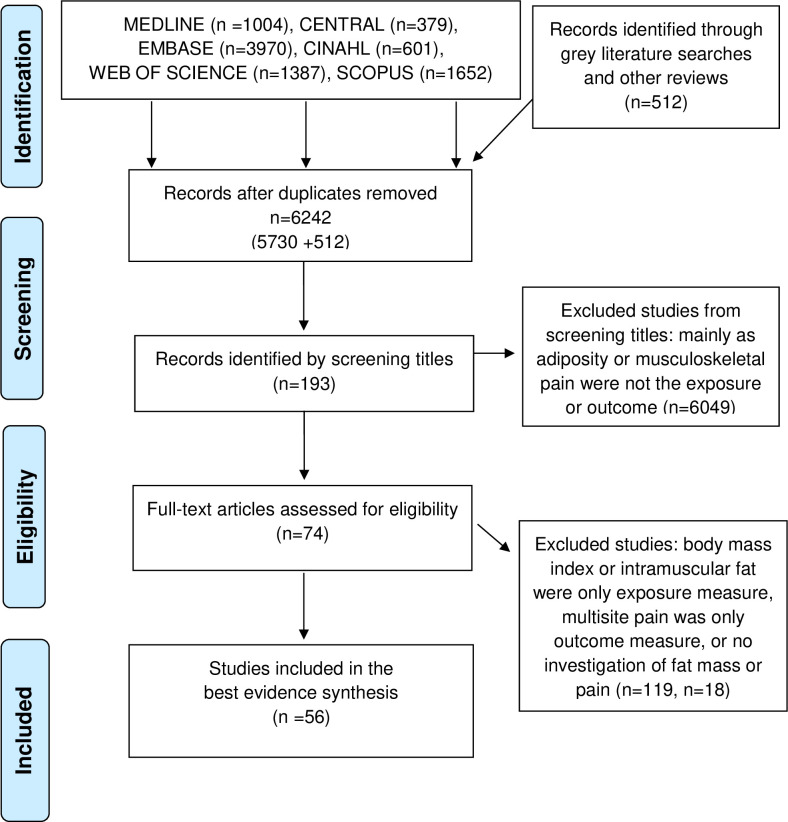
After removal of the duplicates, 6,242 records remained (Fig 1). A total of 6,049 studies were excluded based on the screening of titles and abstracts, leaving 193 studies for full text analysis. A further 74 studies were excluded as they did not meet the review’s inclusion criteria: 37 studies only included BMI as their measure of obesity [17–51], 18 studies did not examine any associations between fat mass and pain [50,52–67], nine studies examined adiposity within a specific muscle [68–75], five studies did not specify a site of pain [76–79], two studies only examined multisite pain [80,81], and three studies examined pain in children [82–84].
Fig 1. PRISMA flow diagram.
Characteristics of the included studies
A total of 56 studies were included in this review (Table 1). Of the included studies, 17 were cohort [85–101], ten were case-control [102–111] and 29 were cross-sectional [6,7,112–138] studies. Twenty one studies were conducted in Australia [6,7,85,89,90,92–94,97–99,101,102,105,106,117,119,122,126,129,134], seven in Japan [95,107,118,121,131,132,136], five in Finland [87,96,123,124,127], four in the USA [86,91,100,112], two in Brazil [128,130], Turkey [109,120], The Netherlands [113,115], India [114,135], Nigeria [116,133], and China [104,110], and one each in, Korea [125], Slovenia [103], Norway [88], United Kingdom [100], Sweden [111], Mexico [137], Egypt [138] and Greece [108]. Of the 56 studies, 36 examined low back pain [6,7,87,88,92,93,95–97,100,103–105,107,108,111–118,121–123,127,130–138], two examined hip pain [94,102], 13 examined knee pain [86,89,91,94,101,109,110,120,124,125,128,129,132], and eight examined foot pain [85,90,94,98,99,106,119,126].
Table 1. Characteristics of studies investigating the relationship between adiposity and back and lower limb pain.
| Author (Country, year) | Study population | No. of participants (%women) Age (years): Mean (SD) | Method of measuring adiposity | Measure of adiposity | Measure of pain | Risk of bias rating |
|---|---|---|---|---|---|---|
| Low back pain | ||||||
| Cross-sectional studies | ||||||
| Anthropometric fat measurement | ||||||
| Yoshimoto (Japan, 2019) [136] | Participants who attended an annual health check-up by the ‘All Japan Labour Welfare Foundation’ were recruited. | 45,192 (32.1) 50.5 (7.1) |
NA | WC | Single question: “Do you have LBP under treatment including follow-up?” | Moderate |
| Hussien (Egypt, 2019) [138] | Participants examined and diagnosed by their physician and referred for physical therapy. | 132 (38) 33.1 (9.23) |
Flexible tape measure | WC HC WHR |
VAS | Moderate |
| Kulandaivelan (2018, India) [135] | Participants recruited from a survey of an urban geographical area. | 1503 (54.2) 48.2 (13.1) |
Non-elastic inch tape | WC | Pain lasting > 1 day in the past 12 months | Moderate |
| Brady (Australia, 2018) [33] | Participants from a randomised controlled trial of vitamin D in community-based overweight/obese individuals |
62 (37.1) 31.3 (8.5) |
NA | WC | Single question “Have you had back pain in the past month?” | Moderate |
| Machado (Brazil, 2018) [130] | Participants from PAINEL study | 268 (70.9) 75.5 (6.1) |
Not stated | WC | Interview question “pain in last 6 months that did not disappear for at least 30 consecutive days” | High |
| Ogwumike (Nigeria, 2016) [133] | Participants were post-menopausal women recruited from government secretariats, schools, and hospitals in the local government area of Oyo State. |
310 (100) 41–50 yo: 30.3% 51–60 yo: 64.8% 60–65 yo: 4.8% |
NA | WC WHR WHtR |
Standardized Nordic Musculoskeletal Questionnaire: prevalence of back pain over 12 months. |
Low |
| Chou (Australia, 2016) [7] | Participants from the Geelong Osteoporosis Study, who were randomly recruited from the electoral roll | 820 (100) No LBP: 58.1 (17.1) LBP: 62.9 (14.0) |
Tape measure | WHR | Chronic Pain Grade Questionnaire | Moderate |
| Frilander (Finland 2015) [123] | Register of the Finnish Defence Forces | 1385 (0) 40.2 |
Not stated | WC, WHR | Three questions: “Have you ever had LBP?” “Have you had LBP during the preceding 20 days?” “If you had LBP, did it radiate?” | Moderate |
| Muramoto (Japan, 2014) [132] | Healthy Japanese volunteers who attended a basic health check-up (Yakumo study in 2011–12) | 217 (100) 68.3 (5.0) |
Non-stretchable measuring tape | WC HC WHR |
VAS | Low |
| Briggs (United States, 2013) [112] | Participants from the NHANES data base (1999–2004) | 14206 (52.2) Not specified |
Measuring tape | WC | Two specific questions of back pain in the NHANES questionnaire | High |
| Ojoawo (Nigeria, 2011) [116] | Patients referred from an orthopaedic clinic diagnosed with LBP and no serious complications | 64 (100) 52.33(10.24) |
Inelastic flexible tape | WHR, WC, HC | VAS Semantic differential scale |
High |
| Perry (Australia, 2009) [117] | Adolescents from the Western Australian Pregnancy Cohort “Raine” study | 1608 (48.7) 14.06 (0.20) |
Cloth tape | WC | Questionnaire including 3 back pain questions regarding having back pain: ever, in the last month and longer than 3 months | Moderate |
| Shiri (Finland, 2008) [127] | Participants of the Cardiovascular Risk in Young Finns Study (1980–2001) | 2620 (51.8) 31.2 (5.0) |
Tape measure | WC, HC, WHR | Structured interview | High |
| Toda (Japan, 2000) [118] | Japanese participants with chronic low back pain, with or without positive straight leg raise | 330 (62) Cases: 59.7(8.7) Controls: 57.6 (8.7) |
Segmental bioelectrical impedance | WHR | Not reported | High |
| Han (The Netherlands, 1997) [113] | Subjects from the MORGAN project randomly recruited from three towns: Amsterdam, Maastricht and Doetinchem. | 12,905 (54.4) 42.9 (10.7) |
Tape measure | WC, WHR | Questionnaire | High |
| Direct fat measurement | ||||||
| Endo (Japan, 2019) [131] | Participants recruited from CoHRE study | 1314 (59.4) Female No LBP: 72.5 (6.6) LBP: 73.5 (6.5) Male No LBP: 72.9 (6.6) LBP: 72.8 (6.4) |
Bioelectrical impedance analysis | Fat mass | Single question: Do you have low back pain at present? | Moderate |
| Brady (Australia, 2018) [33] | Participants from a randomised controlled trial of vitamin D in community-based overweight/obese participants |
62 (37.1) 31.3 (8.5) |
Dual x-ray absorptiometry | Fat mass Body fat % |
Single question “Have you had back pain in the past month?” | Moderate |
| Nava-Bringas (Mexico, 2018) [137] | Patients receiving care in the Spinal Rehabilitation Dept of National Rehabilitation Institute | 27 (66.7) 58.6 (6.98) |
Bioelectric impedance analysis | Fat mass Body fat % |
Numerical scale (0–10) | High |
| Brooks (Australia, 2016) [122] | Recruited through media advertising and leaflet drops | 70 (57) Range: 18–76 |
Tape measure Bioelectrical impedance analysis |
Abdominal to lumbar fat mass ratio | VAS | Low |
| Chou (Australia, 2016) [7] | Participants from the Geelong Osteoporosis Study, who were randomly recruited from the electoral roll | 820 (100) No LBP: 58.1 (17.1) LBP: 62.9 (14.0) |
Dual x-ray absorptiometry | Fat mass | Chronic Pain Grade Questionnaire | Moderate |
| Iizuka (Japan, 2015) [121] | Participants were recruited from an annual medical examination concerning life threatening diseases | 273 (65.6) 64.3 (13.2) |
Bioelectrical impedance analysis | Total body fat mass | Questionnaire regarding presence of LBP and chronic LBP with aid of VAS | Moderate |
| Bihari (India, 2011) [114] | All age groups and both sexes from Gurgaon and NOIDA in the National Capital Region | 2086 (48.4) Range: 10–70 |
Bioelectric impedance analysis | Total body fat mass | Structured interview | High |
| Ojoawo (Nigeria, 2011) [116] | Patients referred from an orthopaedic clinic diagnosed with LBP and no serious complications | 64 (100) 52.33(10.24) |
Mathematical calculations | Body fat % | VAS Semantic differential scale |
High |
| Urquhart (Australia, 2011) [6] | Participants ranging from normal weight to obese from community weight loss clinics or recruited by local media | 135(83.1) 47.4 (9.0) |
Dual x-ray absorptiometry | Total body fat mass, upper and lower limb fat mass | Chronic Pain Grade Questionnaire | Moderate |
| Hodselmans (The Netherlands, 2010) [115] | Outpatients diagnosed with nonspecific chronic low back pain | 101 (46) 39.2(9.6) |
Skin fold calipers | Body fat % | Patient included if complained of LBP for >3 months | High |
| Toda (Japan, 2000) [118] | Japanese participants with chronic low back pain, with or without a positive straight leg raise | 330 (62) Cases: 59.7(8.7) Controls: 57.6 (8.7) |
Segmental bioelectrical impedance | Body fat % | Not reported | High |
| Case control studies | ||||||
| Anthropometric fat measurement | ||||||
| Dario (Australia 2016) [105] | Population based Murcia Twin Registry | 1128 (100) Cases: 53.59 (7.38) Controls: 53.23 (7.38) |
Inelastic tape measure | WC, WHR | Single question “Have you ever had chronic LBP, with chronic defined as greater than 6 months?” | High |
| Yip (China, 2001) [104] | Recruited from University Family Medical Clinic or from previous population-based study | 417 (100) NA |
Measuring tape | WC, HC, WHR | Back pain for more than one day | High |
| Hultman (Sweden, 1993) [111] | Recruited from a metropolitan industrial company and the Karolinska Hospital Dept of Orthopaedic Surgery outpatient clinic. | 168 (0) Group 1: 50 (3) Group 2: 50 (3) Group 3: 49 (6) |
Skin fold calipers | Skin fold measures at the biceps, triceps, subscapular, and supra iliaca crest sites were used to calculate % fat (volume). |
3 groups: Group 1: never had LBP or slight LBP impairment Group 2: had several or at least one episode of LBP (but no LBP for 2 months pre-study) Group 3: ≥3 years of chronic LBP, > 3months of sick leave in the previous year |
High |
| Direct fat measurement | ||||||
| Sakai (Japan, 2017) [107] | Participants recruited from the orthopaedic surgery outpatient department | Cases: 100 (55) Controls: 256 (45) Cases: 74.4 (6.0) Controls: 73.2 (7.6) |
Dual x-ray absorptiometry | Fat mass, body fat % | Persistent back pain for 3 months | High |
| Dario (Australia, 2016) [105] | Population based Murcia Twin Registry | 1128 (100) Cases: 53.59 (7.38) Controls: 53.23 (7.38) |
Bioelectrical impedance analysis | Body fat % | Single question “Have you ever had chronic LBP, with chronic defined as greater than 6 months” | High |
| Spyropoulos (Greece, 2008) [108] | Participants selected from previous survey of office workers, who were randomly recruited from 3000 employees from 4 of 18 government offices | 60 (100) Cases: 41.7 (7.3) Controls: 42.2 (7.3) |
Skin fold calipers | Body fat % | Cases of chronic LBP were considered if pain persisted for a minimum of 15 months. | High |
| Celan (Slovenia, 2005) [103] | Bus drivers recruited from a municipal transport company | 112 (0) 44.2 (5.6) |
Lorenz’ constitutional index | Body fat % | Single question about previous LBP, with one follow up question regarding number of episodes if yes (no duration specified) | High |
| Cohort studies | ||||||
| Anthropometric fat measurement | ||||||
| Muthuri (UK, 2020) [100] | Participants recruited from the MRC National Survey of Health and Development (British cohort study from midlife to age 69 yo). |
3426 (49.7) 36, 43, 53, 60–64, 68–69 years |
Not stated | WC | All ages (except 68 yo): single question about whether they had sciatica, lumbago or recurring/ severe backache all or most of the time (ever at ages 36 and 43 and in the previous 12 months at ages 53 and 60–64). Age 68: single question about whether they had experienced any ache or pain in the previous month which had lasted for 1 day or longer. |
Moderate |
| Shiri (Finland, 2019) [96] | Participants from Finnish population based surveys, Health 2000 and Health 2001 | 1850 (55.0) Over 30 |
Not stated | WC | Participants asked how many days of back pain they have had in the past 12 months. | Moderate |
| Dario (Australia, 2017) [93] | Participants recruited from Murcia Twin Registry | 1098 (47.3) 53.7 (7) |
Inelastic tape measure | WC, WHR | Single question “Have you ever suffered from chronic LBP?” | Moderate |
| Hussain (Australia, 2017) [92] | Participants recruited from AusDiab study | 4986 (55.7) | Metal anthropometric tape | WC | Chronic Pain Grade Questionnaire | Moderate |
| Heuch (Norway, 2015) [88] | Participants recruited from the Nord-Trondelag Health Study (HUNT) | 25329 (55) 30–69 |
Not stated | WC, WHR | Two questions regarding presence and area of pain | High |
| Shiri (Finland, 2013) [87] | Participants of the Cardiovascular Risk in Young Finns Study (1980–2007) based on the 2001–2007 follow up | 1224(52.5) 31.4(5.0) |
Tape measure | WC | Single question “Have you had low back trouble (pain, ache, or unpleasant sensations) during the preceding 12 months?” with follow up questions regarding radiating pain. | High |
| Direct fat measurement | ||||||
| Muthuri (UK, 2020) [100] | Participants recruited from the MRC National Survey of Health and Development (British cohort study from midlife to age 69 yo). |
3426 (49.7) 36, 43, 53, 60–64, 68–69 years |
Dual X-ray absorptiometry | Fat mass FMI |
60–64 years: single question about whether they had sciatica, lumbago or recurring/severe backache all or most of the time in the previous 12 months. 68 years: single question about whether they had experienced any ache or pain in the previous month which had lasted for 1 day or longer. |
Moderate |
| Brady (Australia, 2019) [97] | Participants from local media and public, private and community health clinics | 123 (78) 48.6 (8.5) |
Dual X-ray absorptiometry | Fat mass | Chronic Pain Grade Questionnaire | Moderate |
| Dario (Australia, 2017) [93] | Participants recruited from Murcia Twin Registry | 1098 (47.3) 53.7 (7) |
Bioelectric impedance analysis |
Body fat % | Single question “Have you ever suffered from chronic LBP?” | Moderate |
| Hashimoto (Japan, 2017) [95] | Participants were employees of companies based in the greater Tokyo metropolitan area |
1152 (0) 28.0 (4.6) |
Skin fold using subcutaneous fat thickness-measuring device | Body fat % | Presence of LBP was obtained using questionnaire with options (none, in the past, present) | High |
| Hussain (Australia, 2017) [92] | Participants recruited from AusDiab study | 4986 (55.7) | Bioelectric impedance analysis | Body fat % Fat mass |
Chronic Pain Grade Questionnaire | Moderate |
| Hip pain | ||||||
| Case control studies | ||||||
| Anthropometric fat measurement | ||||||
| Fearon (Australia, 2012) [102] | Recruited from private healthcare providers. Participants either had a gluteal tendon reconstruction, hip osteoarthritis or no hip pain. |
102 (100) 62(13.3) |
Non stretch tape measure | WC, HC, greater trochanter circumference |
Trochanteric pain was used to identify hip pain. | High |
| Cohort | ||||||
| Direct fat measurement | ||||||
| Pan (Australia 2017) [94] | Tasmania Older Adult Cohort Study | 768 (50) 67.1 (7.3) |
Dual x-ray absorptiometry | Fat mass, FMI | Presence of pain (yes/no) | Moderate |
| Knee pain | ||||||
| Cross-sectional studies | ||||||
| Anthropometric fat measurement | ||||||
| Lee (Korea, 2016) [125] | Fifth Korean National Health and Nutrition Examination Survey | 1664 (67.6) 66.99 (0.33) |
Dual x-ray absorptiometry | WC | Presence of pain for 30 days from last 3 months and knee pain intensity measured on a scale of 1–10. | Low |
| Frilander (Finland, 2016) [124] | Register of the Finnish Defence Forces | 1913 (0) No knee pain: 34.6 Knee pain: 37.5 |
Not stated | WC | Three questions: “Have you ever had LBP?” “Have you had LBP during the preceding 20 days?” “If you had LBP, did it radiate?” | High |
| Muramoto (Japan, 2014) [132] | Healthy Japanese volunteers who attended a basic health check-up (Yakumo study in 2011–12) | 217 (100) 68.3 (5.0) |
Non-stretchable measuring tape | WC HC WHR |
VAS | Low |
| Direct fat measurement | ||||||
| Alfieri (Brazil, 2017) [128] | Patients referred to the physical therapy department of a private university in São Paulo |
107 (87) 61.8 (10.1) |
Bioelectric impedance analysis | Fat mass % | WOMAC | Moderate |
| Lee (Korea, 2016) [125] | Fifth Korean National Health and Nutrition Examination Survey | 1664 (67.6) 66.99 (0.33) |
Dual x-ray absorptiometry | Leg to whole body fat mass | Presence of pain for 30 days from last 3 months and knee pain intensity measured on a scale of 1–10. | Low |
| Ozer Kaya (Turkey, 2014) [120] | Volunteers applying at a sports centre for an exercise consultation | 149 (100) 42.6 (4.1) |
TANITA Body composition analyser | Body fat %, fat mass | VAS | Moderate |
| Scott (Australia, 2012) [129] | Study conducted within the Tasmania Older Adult Cohort Study, a population based study | 357 (50) Males no knee pain: 63.0 (7.3) Males with knee pain: 62.0 (7.2) Females no knee pain: 62.0 (7.0) Females with knee pain: 61.7 (7.5) |
Dual x-ray absorptiometry | Body fat % | Single question: Do you have pain at any of these sites today? with a list to choose from including “any knee pain” | Low |
| Case-control studies | ||||||
| Anthropometric fat measurement | ||||||
| Li (China, 2016) [110] | Participants attending 2nd Xiangya Hospital for total knee arthroplasty. | Cases: 70 (82.9) Controls: 81 (80.2) Cases: 63.6 (range: 50–75) Controls 64.1 (range: 50–80) |
NA | Waist circumference | VAS | Moderate |
| Sutbeyaz (Turkey, 2007) [109] | Cases were recruited from the musculoskeletal rehabilitation outpatient clinic of Ankara Physical Medicine and Rehabilitation Education and Research Hospital. Controls were nurses, physiotherapist, secretaries, nurse-aids, and maintenance workers. |
Cases: 16 (57.1) Controls: 16 (57.1) Cases: 43.96 (10.29) Controls: 43.71 (10.02) |
Measuring tape | WHR | WOMAC | High |
| Direct fat measurement | ||||||
| Sutbeyaz (Turkey, 2007) [109] | Cases were recruited from the musculoskeletal rehabilitation outpatient clinic of Ankara Physical Medicine and Rehabilitation Education and Research Hospital. Controls were nurses, physiotherapist, secretaries, nurse-aids, and maintenance workers. |
Cases: 16 (57.1) Controls: 16 (57.1) Cases: 43.96 (10.29) Controls: 43.71 (10.02) |
Skin fold callipers | Fat mass | WOMAC | High |
| Cohort studies | ||||||
| Anthropometric fat measurement | ||||||
| Pan (Australia, 2020) [101] | Participants recruited from the Tasmanian Older Adult Cohort Study (TASOAC) |
Minimal pain: n = 512 (48), 62.9 (7.4) Mild pain: n = 328 (51), 63.0 (7.6) Moderate pain: n = 145 (57), 62.8 (7.2) |
NA | WC | WOMAC | Low |
| Jin (Australia. 2016) [89] | Participants selected from electoral roll | Increase in pain: 175 (54) 62.4 (7.16) No increase in pain: 591 (48) 61.9 (6.97) |
Measuring tape | WC, WHR | WOMAC | Low |
| Batsis (USA, 2014) [91] | Participants recruited form Osteoarthritis Initiative | 2182 (60–71 across all groups) 67.5–68.7 across all groups |
Measuring tape | WC | WOMAC | Moderate |
| Direct fat measurement | ||||||
| Pan (Australia 2017) [94] | Tasmania Older Adult Cohort Study | 768 (50) 67.1 (7.3) |
Dual x-ray absorptiometry | Fat mass, FMI | Presence of pain (yes/no) | Moderate |
| Jin (Australia. 2016) [89] | Participants selected from electoral roll | Increase in pain: 175 (54) 62.4 (7.16) No increase in pain: 591 (48) 61.9 (6.97) |
Dual X-ray absorptiometry |
Body fat % | WOMAC | Low |
| Barber (United States, 2012) [86] | Female basketball players from a single country public school district in Kentucky | 248 (100) 12.76 (1.13) |
Not specified | Body fat % | Anterior knee pain scale (AKPS) questionnaire | Moderate |
| Foot pain | ||||||
| Cross-sectional studies | ||||||
| Anthropometric fat measurement | ||||||
| Butterworth (Australia, 2016) [126] | Individuals selected at random from the electoral roll |
796 Foot pain: 68 (IQR: 24–90) No foot pain: 57 (IQR 25–98) |
Measuring tape | WHR | MFPDI | Moderate |
| Direct fat measurement | ||||||
| Butterworth (Australia, 2016) [126] | Individuals selected at random from the electoral roll |
796 Foot pain: 68 (IQR: 24–90) No foot pain: 57 (IQR 25–98) |
Dual x-ray absorptiometry | Total fat mass FMI |
MFPDI | Moderate |
| Tanamas (Australia, 2012) [119] | From weight loss clinics who range from normal weight to obese | 137 (83.2) 47.5 (9.0) |
Dual x-ray absorptiometry | Total body, trunk, android & gynoid fat mass, FMI | MFPDI | Moderate |
| Case control studies | ||||||
| Direct fat measurement | ||||||
| Walsh (Australia, 2017) [54] | Participants recruited from advertisements placed in newspapers, local general practitioner clinics and online via social media. | 88 (100) Cases: 56.6 (10.3) Controls: 56.7 (6.5) |
Dual X-ray absorptiometry | Total body fat mass | MFPDI | Moderate |
| Cohort studies | ||||||
| Anthropometric fat measurement | ||||||
| Laslett (Australia, 2018) [98] | Participants from Tasmanian Older Adult Cohort study | Foot pain: 227 (55) No foot pain: 333 (49) Foot pain: 63.1 (7.6) No foot pain: 63.0 (7.4) |
Not stated | WC | Single question “Do you have pain at any of these sites” | Moderate |
| Direct fat measurement | ||||||
| Laslett (Australia, 2018) [98] | Participants from Tasmanian Older Adult Cohort study | Foot pain: 227 (55) No foot pain: 333 (49) Foot pain: 63.1 (7.6) No foot pain: 63.0 (7.4) |
Dual X-ray absorptiometry | FMI | Single question “Do you have pain at any of these sites” | Moderate |
| Walsh (Australia, 2018) [99] | Recruited from surgical waiting lists at 2 tertiary hospitals | 38 (84) 45.7 (9.4) |
Dual X-ray absorptiometry | FMI | Manchester Oxford foot questionnaire | High |
| Pan (Australia, 2017) [94] | Tasmania Older Adult Cohort Study | 768 (50) 67.1 (7.3) |
Dual x-ray absorptiometry | Fat mass, FMI | Presence of pain (yes/no) | Moderate |
| Walsh (Australia, 2016) [90] | The North West Adelaide Health Study (NWAHS) | 1462 (53.4) 64.99 (10.58) |
Dual x-ray absorptiometry | FMI | Single question “On most days, do you have pain, aching, or stiffness in either of your feet?” |
High |
| Butterworth (Australia, 2013) [85] | Participants from a larger study of obesity and musculoskeletal disease who did not have foot pain at base line | 51 (73) 49.2(8.1) |
Dual X-ray absorptiometry | FMI & total body fat mass | MFPDI | Moderate |
Body fat % = body fat percentage, FMI = Fat mass index, HC = Hip Circumference, LBP = Low Back Pain, MFPDI = Manchester Foot Pain and Disability Index, NHANES = National Health and Nutrition Examination Survey, WC = Waist Circumference, WHR = Waist-Hip Ratio, WHtR = Waist-height Ratio, WOMAC = Western Ontario and McMaster Universities Arthritis Index. VAS = visual analogue scale
Study populations
A total of 39 studies recruited both male and female participants [6,85,87–94,96–101,107,109,110,112–115,117–119,121,122,125,127–131,134–138], while eleven studies included female participants only [7,86,102,104–106,108,116,120,132,133], five studies included male participants only [95,103,111,123,124] and one study did not specify the gender of their participants [126] (Table 1). The mean age of the participants in 41 studies was above 40 years [6,7,85,89–94,97–99,101–111,113,116,118–121,123,125,126,128–133,135–137], while six studies had a mean participant age between 20–40 years [95,115,124,127,134,138], and three studies had a mean age below 18 years [86,117,123]. Bihari et al. [114] included participants from 10 to 70 years of age, Brooks et al. [122] included participants from 18–76 years, Shiri et al. [96] included participants over the age of 30, Heuch et al. [88] included participants from 30–69 years and Muthuri et al. [100] followed participants over 32 years, collecting data at the age of 36, 43, 53, 60–64 and 68–69 years. One study did not specify the age of their participants [112].
Participant data were obtained from 12 existing databases or studies; including the Osteoarthritis Initiative [91], Australian Diabetes, Obesity and Lifestyle Study [92], National Health And Nutrition Examination Study [112], Western Australian Pregnancy Cohort [117], Young Finns Study [87,127], Morgan project [113], Nord-Trøndelag Health Study [88], Hong Kong Department of Community and Family Medicine study [104], North West Adelaide Health Study [90], Tasmania Older Cohort Study [94,98,129], PAINEL study [130], CoHRE study [131], Tasmanian Older Adult Cohort Study (TASOAC) [101], a clinical trial of vitamin D in overweight/obese individuals [134], Yakumo study [132] and a British cohort study based on the MRC National Survey of Health and Development [100]. Participants were also recruited from local GP or health care clinics in eight studies [6,102,106,116,118–120,138] and from hospitals, and outpatient and rehabilitation clinics in seven studies [107,109–111,115,136,137] and registries in four studies [93,105,123,124]. Three studies recruited from electoral role [7,89,126], three studies recruited from media advertising and leaflet drops [85,97,122], and three studies from surveys [96,125,135], two studies recruited from government offices and schools [108,133], and single studies recruited from a physical therapy department [128], companies in the metropolis area [95], the national capital region [114], an annual medical examination [121], surgical waiting list [99], a municipal transport company [103] and a country public school [86].
Assessment of adiposity
Adiposity was assessed using various methods; 16 studies used dual energy X-ray absorptiometry [6,7,85,89,90,94,97–100,106,107,119,125,126,129], 11 studies used bioelectric impedance analysis [92,93,105,114,118,120–122,128,131,137], 20 studies used a tape measure to determine waist and hip circumference [87,89,91–93,102,104,105,109,112,113,116,117,122,126,127], two studies used mathematical calculations [103,116,132,135,137,138], and five studies used skin fold callipers [95,108,109,111,115] (Table 1). Twelve studies did not specify how adiposity was measured [86,88,96,100,101,110,123,124,130,133,134,136].
Different adiposity measures were reported across the studies, with 18 studies measuring body fat percentage [86,89,92–95,105,107,108,114–116,118,120,128,129,134,137], 20 studies measuring fat mass [6,7,85,90,92,97–100,107,109,119–122,125,126,131,134,137], 29 studies measuring waist and/or hip circumference [87–89,91–93,96,98,100–105,110,112,113,116,117,123,124,127,130,132–136,138], 13 studies measuring waist-hip ratio [7,87,93,105,109,113,116,118,122,127,132,133,138], two studies measuring waist height ratio [46,133] and one study measuring percentage of body fat volume [111].
Assessment of pain
A range of measures were used to assess pain (Table 1). While the Western Ontario and McMaster Universities Arthritis Index (WOMAC) and visual analogue scale (VAS) were the most commonly used validated tools, a large number of studies used structured interviews or self-administered questionnaires. Low back pain was examined using the visual analogue scale [116,121,122,132,137,138], Chronic Pain Grade scale [6,7,92,97], NHANES general wellbeing index [112], Nordic Musculoskeletal Questionnaire [133], questions regarding the history of low back pain (e.g. Have you ever had back pain? (“yes” or “no”)) [88,93,95,96,100,104,105,107,108,111,113,115,117,123,130,131,134–136] and structured interviews [103,114,127]. Hip pain was assessed by asking about the presence of pain (yes/no) [94] and any history of hip pain [102]. Knee pain was assessed using WOMAC Index [89,91,101,109,128], questions regarding the presence of pain (yes or no) [94,129], anterior knee pain scale [86] and visual analogue scale [110,120,132] and self-administered questionnaires [124,125]. Foot pain was measured using the Manchester Foot Pain and Disability Index [85,106,119,126], Manchester-Oxford foot questionnaire [99] and asking about the presence of pain (yes/no) [94,98], or the history of foot pain (Over the past month, have you had pain, aching, or stiffness in either of your feet on most days?) [90].
The follow-up periods between baseline and the assessment of pain varied between the cohort studies. Of the 8 cohort studies of back pain [87,88,92,93,95–97,100], the follow-up time ranged from 2 to 20 years, with half of the studies investigating time periods less than 10 years and half of the studies examining time periods over 10 years. The single cohort study of hip pain followed up participants over 5 years [94], while the 5 cohort studies of knee pain ranged from 2 to 10.7 years [86,89,91,94,101], with 3 studies examining time periods of 5 or 6 years. Moreover, the 5 studies examining foot pain had follow-up periods ranging from 4 to 20 years [85,90,94,98, 99], with 4 of the 5 studies focusing on a 3–5 year follow-up.
Risk of bias assessment
Of the 56 studies included in the review, 24 had a high risk of bias [86–88,90,95,99,102–105,107–109,111–116,118,124,127,130,137], 24 had a moderate risk of bias [6,7,85,91–94,96–98,100,106,110,117,119–121,123,128,131,134–136,138], and eight had a low risk of bias [89,101,122,125,126,129,132,133] (Table 1). Of the 17 cohort studies, the risk of bias was rated as high for six studies [86–88,95,99,106,111,137] and low to moderate for eleven studies [85,89,91–94,96–98,100,101]. For these cohort studies, the criteria ‘assessment of exposure’ and ‘assessment of outcome’ more frequently scored a high risk than the other Cochrane criteria. Eight of the ten case-control studies were assessed as having a high risk of bias [102–105,107–109,111], and two a moderate risk of bias [106,110]. The criteria ‘assessment of exposure’ and ‘assessment of outcome’ were most frequently associated with high risk of bias when assessing the case-control studies. Of the 29 cross sectional studies, ten had a high risk of bias [112–116,118,124,127,130,137], 13 had a moderate risk of bias [6,7,117,119–121,123,128,131,134–136,138], and six had a low risk of bias [122,125,126,129,132,133]. The criteria associated with the ‘assessment of the outcome’ were most frequently associated with a high risk of bias for cross-sectional studies.
Relationship between adiposity and low back pain
Anthropometric fat measures
Waist circumference. Twenty one studies examined the association between waist circumference and low back pain (Table 2). Of these studies, 13 were cross sectional studies [112,113,116,117,123,127,130,132–136,138], two were case control studies [104,105] and six were cohort studies [87,88,92,93,96,100]. Eight of the 13 cross-sectional studies found significant associations between waist circumference and low back pain [112,113,117,127,132,134–136], with two studies reporting an association in females only [113,127], two studies finding a relationship in males only [117,136] and the remaining 4 studies finding an association in both males and females [112,132,134,135]. Five studies did not find an association between waist circumference and radiating and non-specific low back pain [123], presence of low back pain [130,133] or low back pain intensity [116,138]. Of the two case-control studies, one study found greater waist circumference was associated with less low back pain (lasting 14 days or greater) in middle age women [104], while the other study found no association between waist circumference and chronic low back pain [105].
Table 2. Results of the studies investigating the relationship between adiposity and low back pain.
| First author (year) | Definition of pain | Variables adjusted for | Main findings (OR/RR/β coefficient (95% CI)) | Conclusions |
|---|---|---|---|---|
| Low back pain | ||||
| Cross-sectional studies | ||||
| Anthropometric fat measurement | ||||
| Yoshimoto (2019) [136] | Responded yes to the question, “Do you have LBP under treatment including follow-up?” | Adjustment for age, smoking habits, alcohol intake, and physical activity. |
Men: Abdominal obesity: LBP vs no LBP: 335 (52.3) vs 13,709 (45.6) No abdominal obesity: LBP vs no LBP: 306 (47.7) vs 56,345 (54.4) Pearson’s chi square: p < 0.001. OR: 1.34 (1.02, 1.76) Women: Abdominal obesity: LBP vs no LBP: 52 (21.1) vs 1749 (12.3) No abdominal obesity: LBP vs no LBP: 194 (78.9) vs 12,502 (87.7) Pearson’s chi square: p < 0.001. OR: 1.70 (0.94, 3.08) |
The proportion of abdominal obesity was significantly higher in participants with LBP than in those without LBP for each sex. The presence of abdominal obesity was significantly associated with LBP among men, but not among women. |
| Hussien (Egypt, 2019) [138] | Continuous or recurrent localised LBP ≥ 3 months. Pain intensity rating on the VAS of ≥ 1. |
No adjustments made. | WC: Tb = -0.02, p = 0.7 HC: Tb = 0.04, p = 0.6 WHR: Tb = -0.04, p = 0.5 |
There were no associations between the anthropometric measures and pain intensity. |
| Kulandaivelan (2018) [135] | Pain lasting > 1 day in the past 12 months | NA |
Presence of pain: OR: 1.39 (1.08, 1.81) |
Abdominal obesity increases the risk of low back pain. |
| Brady (2018) [33] | Responded yes to LBP in the past month. | Age, sex |
Presence of pain WC 109.6 ± 16.8 vs 101.0 ± 9.3 cm OR: 1.1 (1.0, 1.1) |
Participants who had back pain in the past month had a higher waist circumference compared to those without back pain. |
| Machado (2018) [130] | Disabling pain in the last 1 year | Gender, BMI, WC, self-rate health, multi-morbidity, chronic musculoskeletal pain other than LBP, frequent LBP, physical activity, low gait speed, fatigue, sitting, sleep, depression symptoms, depression diagnosis, fear beliefs |
Presence of disabling LBP WC (male ≥102 cm, female ≥88) OR 0.47 (0.11–2.14) |
WC, dichotomised into high and low, was not associated with LBP. |
| Ogwumike (Nigeria, 2016) [133] | Presence of back pain in the past year. | Age |
Presence of pain WC: 1.51 (0.94, 2.40) WHtR: 1.70 (1.07, 2.75) WHR: 1.04 (0.66, 1.67) |
Waist height ratio (WHtR) was found to be associated with LBP in post-menopausal women. |
| Chou (2016) [7] | Cohort split into two groups: 1. No pain/disability or low intensity pain (<50) and low disability (<3) 2. High intensity pain (≥50) or high disability (≥3) |
Age, emotional disorder, education and mobility |
High-intensity pain and/or disability vs low-intensity pain and/or disability (Estimated marginal means) WHR (SD): 0.96 (0.006) vs 0.97 (0.006), p = 0.04 |
WHR was higher in those with either high intensity pain or high disability compared to those with no or low intensity pain or no or low disability. |
| Frilander (2015) [123] | Yes response to chronic LBP and radiating LBP (0 = no, 1 = below knee, 2 = above knee) | Age, smoking, education |
Chronic LBP (WC, <94 cm ref) 94–101.9cm: OR 1.04 (0.63–1.73) ≥102cm: OR 1.24 (0.75–2.03) Radiating LBP (WC <94 cm ref) 94–101.9cm: OR 1.03 (0.69–1.53) ≥102cm: OR 1.31 (0.88–1.96) |
WC was not associated with incident, chronic LBP. WC was not associated with incident, radiating LBP. |
|
Chronic LBP (Waist-to-height ratio, ≤0.5 ref) >0.5: OR 1.33 (0.75–1.72) Radiating LBP (Waist-to-height ratio, ≤0.5 ref) >0.5: OR 1.44 (1.02–2.04) | ||||
| Muramoto (2014) [132] | Pain intensity rating on the VAS of ≥ 1. | Age | WC: r = 0.2, p<0.005 HC: r = 0.2, p<0.01 WHR: r = 0.2, p<0.01 Multivariate analyses: WC: significant association reported. Data not provided. p<0.05. |
Central obesity was associated with LBP intensity. |
| Briggs (2013) [112] | Positive response to “during the past 3 months, did you have LBP?” | NA |
LBP vs no LBP Men (WC, <102 cm ref) vs women (WC, <88 cm ref) Chi-squared; p<0.001 |
A larger WC increased the odds of reporting LBP. |
| Ojoawo (2011) [116] | Pain rating between 1 and 10. | NA |
Pain intensity HC: r = 0.41, p<0.05 WC: r = 0.24, p>0.05 WHR: r = 0.18, p>0.05 |
Increased HC, but not WC or WHR had a significant relationship with the intensity of pain experienced in women with low back pain. |
| Perry (2009) [117] | Positive response to back pain ever, back pain in past month or chronic back pain lasting >3 months. | Physical characteristics |
Prevalent LBP Male (IQR ref) Univariate Low WC: OR 0.45 (0.23–0.86) High WC: OR 1.12 (0.67–1.86) Multivariate WC: OR 2.20 (1.11, 4.36) |
An increased likelihood of low back pain was associated with greater central adiposity in adolescent males but not females. |
|
Prevalent LBP Female (IQR ref) Univariate: Low WC: OR 1.14 (0.68–1.93) High WC: OR 1.06 (0.62–1.81) WC: OR Data not provided. | ||||
| Shiri (2008) [127] | Dichotomous variable of LBP. Those who recovered in one month, had recurrent or continuous back pain compared with those who recovered in one week or had no back pain. | Age, educational status, occupational status and smoking |
Pain intensity Male (WC, <94.0cm ref) 94.0–101.9cm: OR 1.1 (0.7–1.6) ≥102.0cm: OR 0.7 (0.4–1.1) Female (WC, <80.0cm ref) 80.0–87.9cm: OR 1.3 (0.9–1.8) ≥88.0cm: OR 1.8 (1.3–2.4) |
WC, HC and WHR were significantly associated with LBP in females, but not in males. |
|
Pain intensity Male (HC, lowest tertile ref) Middle tertile: OR = 1.3 (0.9–1.9) Highest tertile: OR = 1.0 (0.6–1.4) Female (HC, lowest tertile ref) Middle third: OR = 1.0 (0.7–1.4) Highest third: OR = 1.6 (1.1–2.1) | ||||
|
Pain intensity Male (WHR, <0.9 ref) 0.9–1.0: OR 0.9 (0.5–1.8) >1.0: OR 0.9 (0.5–1.8) Female (WHR, <0.8 ref) 0.8–0.9: OR 1.2 (0.8–1.5) >0.9: OR 2.3 (1.3–3.9) | ||||
| Toda (2000) [118] | Responded ‘yes’ to duration of current episode of LBP > 3 months or recurrent LBP compared to responded ‘no’ to LBP or low back problems in past 10 years. | NA |
Presence of pain Female (WHR) Control vs negative straight leg raise 86.5 (5.3) vs 90.8 (6.4), p<0.001 Control vs positive straight leg raise 86.5 (5.3) vs 87.3 (6.3), p>0.05 |
Central adiposity may be a risk factor for chronic low back pain with a negative straight leg raise test result in women, but not in men. Positive straight leg raise was not associated with central adiposity. |
|
Presence of pain Male (WHR) Control vs negative straight leg raise 90.2 (4.4) vs 90.5 (4.8), p>0.05 Control vs positive straight leg raise 91.9 (4.0) vs 90.5 (4.8), p>0.05 | ||||
| Han (1997) [113] | Responded yes to LBP in past 12 months. Chronic LBP defined as responded yes to a total of twelve weeks or more. | Age, smoking, education |
WCtertiles 86.9cm and 95.9cm for males, 75.0cm and 84.0cm for females, lowest tertile ref. Male (chronic LBP) Middle tertile: OR 0.94 (0.78–1.14) Highest tertile: OR 1.13 (0.94–1.37) Male (LBP past 12 months) Middle tertile: OR 0.89 (0.78–1.02) Highest tertile: OR 0.97 (0.85–1.12) Female (chronic LBP) Middle tertile: OR 1.26 (1.08–1.48) Highest tertile: OR 1.49 (1.27–1.75) Female (LBP past 12 months) Middle tertile: OR 1.12 (1.00–1.27) Highest tertile: OR 1.21 (1.06–1.37) |
Women with a large waist (increased central adiposity) have a significantly increased likelihood of low back pain. There was no association for men. |
|
WHRtertiles 0.872 and 0.936 for males, 0.756 and 0.815 for females, lowest tertile Male (chronic LBP) Middle tertile: OR 0.93 (0.77–1.13) Highest tertile: OR 0.98 (0.80–1.19) Male (LBP past 12 months) Middle tertile: OR 0.97 (0.85, 1.11) Highest tertile: OR 1.00 (0.79–1.06) Female (chronic LBP) Middle tertile: OR 1.27 (1.09–1.50) Highest tertile: OR 1.35 (1.15–1.58) Female (LBP past 12 months) Middle tertile: 1.02 (0.91–1.15) Highest tertile: 1.14 (1.01–1.30) | ||||
| Direct fat measurement | ||||
| Endo (2019) [131] | Responded yes to “Do you have any low back pain at present?” | NA |
Female (No LBP vs LBP) Fat mass, kg (SD): 15.2 (6.4) vs 15.4 (6.2), p = 0.55 Male (No LBP vs LBP) Fat mass, kg (SD): 11.7 (5.0) vs 11.9 (5.1), p = 0.51 |
There were no significant differences in fat mass between participants with LBP and without LBP, in either female or male groups. |
| Brady (2018) [33] | Responded yes to LBP in the past month. | Age, sex |
Presence of pain Fat mass 39.9 ± 12.3 vs. 33.9 ± 9.8%, p = 0.04 OR: 1.1 (1.0, 1.1) |
Participants who reported having back pain in the past month had higher fat-mass compared to those without back pain. |
| Nava-Bringas (2018) [137] | Chronic low back pain (>3 months) and radiographic evidence of lumbar osteoarthritis (including facet joint osteoarthritis and disc degeneration). Pain score of ≥1 over the past 7 days. |
No adjustments made. |
Pain intensity Fat mass rho: -0.239 p = 0.2 % body fat rho: 0.09 p = 0.7 |
There was no correlation between fat mass or percentage body fat and back pain. |
| Brooks (2016) [122] | VAS score, minimum of 2.0 and maximum of 10.0. | NA |
Pain intensity Abdominal to lumbar fat mass ratio r = 0.32, p = 0.007 |
Abdominal adiposity was associated with chronic LBP. |
| Chou (2016) [7] | Cohort split into two groups: 1. No pain/disability or low intensity pain (<50) and low disability (<3) 2. High intensity pain (≥50) or high disability (≥3) |
Age, emotional disorder, education and mobility |
No or Low-Intensity Pain/Disability vs High-Intensity Pain and/or Disability (Estimated marginal means) Fat mass, kg (SD): 23.2 (0.3) vs 24.5 (0.7), p = 0.10 FMI, kg/m2 (SD): 7.6 (0.1) vs 8.0 (0.2), p = 0.08 |
There were no significant differences in fat mass or FMI between those with no or low intensity pain/disability compared with those with high intensity pain/disability. |
| Iizuka (2015) [121] | Incident pain defined as responded yes to “Do you have low back pain at present?” Chronic pain defined as responded yes to “Have you had chronic low back pain persisting for three months or more?” | Age, gender |
Presence of pain (total body fat mass) OR 1.02 (0.94, 1.02) Chronic pain (total body fat mass) OR 0.98 (0.93, 1.03) Presence of pain plus intensity (total body fat mass) β -0.05 (-0.07, 0.03) |
Total body fat mass did not have a significant relationship with incidence, chronicity or intensity of present LBP. |
| Bihari (2011) [14] | Not specified | No adjustments | Backache vs no musculoskeletal disorders (total body fat mass) OR 1.2 (0.9–1.7) |
Higher body fat percentage was not associated with back pain. |
| Ojoawo (2011) [116] | Pain rating between 1 and 10. | NA |
Pain intensity (body fat %) r = 0.67, p<0.01 |
Increased body fat percentage has a significant relationship with the intensity of pain experienced in women with low back pain. |
| Urquhart (2011) [6] | Pain intensity measured on the Chronic Pain Grade Questionnaire, 0–100. Low pain intensity <50 High pain intensity ≥50 |
Age, sex, height, physical activity, fat or lean tissue |
Pain intensity (total fat mass) OR 1.19 (1.04–1.38) Pain intensity (upper limb fat mass) OR 1.18 (0.93–1.50) Pain intensity (lower limb fat mass) OR 1.51 (1.04–2.20) |
Greater total and lower limb fat mass were associated with higher levels of low back pain intensity. |
| Hodselmans (2010) [115] | Responded yes to LBP for >3 months. | NA |
Predicted normative data vs LBP participants (fat mass %) Mean (SD): 26.4 (6.1) vs 30.4 (8.2) p<0.001 |
Patients with chronic LBP have an increased body fat percentage. |
| Toda (2000) [118] | Responded ‘yes’ to duration of current episode of LBP > 3 months or recurrent LBP compared to responded ‘no’ to LBP or low back problems in past 10 years. | NA |
Presence of pain Female (body fat %) Control vs negative straight leg raise 27.9 (6.7) vs 30.5 (6.5), p = 0.03 Control vs positive straight leg raise 28.6 (7.0) vs 27.9 (6.7), p>0.05 |
Body fat mass percentage may be a risk factor for chronic LBP without a positive straight leg raise test result in women, but in not men. Positive straight leg raise was not associated with central adiposity. |
|
Presence of pain Male (body fat %) Control vs negative straight leg raise 22.6 (5.7) vs 22.3 (6.1), p>0.05 Control vs positive straight leg raise 24.9 (4.4) vs 22.3 (6.1), p>0.05 | ||||
| Case control studies | ||||
| Anthropometric fat measurement | ||||
| Dario (2016) [105] | Responded yes to “Have you ever suffered from chronic LBP?” with chronic defined as lasting at least 6 months. | Smoking, leisure physical activity |
Presence of pain Chronic LBP (WC) OR 1.06 (0.93–1.22) Chronic LBP (WHR) OR 1.02 (0.89–1.17) |
WC and WHR are not associated with chronic LBP. |
| Yip (2001) [104] | Not specified | Source of recruitment (random subjects from population-based study vs family clinic subjects), menopausal status |
LBP ≥ 1 day WC: OR 0.67 (0.41–1.09) HC: OR 0.80 (0.49–1.31) WHR: OR 0.72 (0.47–1.11) LBP ≥ 14 days WC: OR 0.52 (0.29–0.92) HC: OR 1.10 (0.62–0.70) WHR: OR 0.43 (0.26–0.70) |
High WHR ratios was inversely associated with the risk of severe LBP in middle-aged women. |
| Hultman (1993) [111] | 3 groups: Group 1: never had LBP or slight LBP impairment Group 2: had several or at least one episode of LBP, no LBP for 2 months pre-study Group 3: ≥3 years of chronic LBP, > 3 months of sick leave in the previous year |
NA |
Presence of pain Fat volume % (skin folds) Group 1: 30 (6) Group 2: 28 (6) Group 3: 28 (6) Data not provided. |
There were no differences in fat volume between those with no, intermittent and chronic LBP. |
| Direct fat measures | ||||
| Sakai (2017) [107] | Persistent moderate to severe LBP for minimum previous 3 months | NA |
Male (LBP vs no LBP) Upper limb fat mass (g) 1781.18 ± 728.75 vs 1655.43 ± 656.38, p = 0.24 Lower limb fat mass (g) 4509.52 ± 1530.68 vs 4054.76 ± 1391.11, p = 0.05 Body fat % 35.77 ± 6.71 vs 27.69 ± 7.57, p<0.001 |
Body fat percentage was significantly higher in participants with LBP in both male and females. Additionally, lower limb fat mass was significantly greater in males with LBP. |
|
Female (LBP vs no LBP) Upper limb fat mass 1978.41 ± 553.97 vs 2053.18 ± 998.24, p = 0.59 Lower limb fat mass 4902.61 ± 1338.75 vs 4861.08 ± 1826.70, p = 0.87 Body fat % 41.05 ± 4.09 vs 34.25 ± 8,84, p<0.001 | ||||
| Dario (2016) [105] | Responded yes to “Have you ever suffered from chronic LBP?” with chronic defined as lasting at least 6 months. | Smoking, leisure physical activity |
Presence of pain Chronic LBP (body fat %) OR 1.15 (1.01–1.32) |
Body fat percentage was associated with LBP prevalence in women. |
| Spyropoulos (2008) [108] | Complained about LBP symptoms for a minimum of 15 months. | NA |
Healthy women vs women with chronic LBP (body fat %) 31.3 (5.2) vs 34.7 (5.1), p = 0.035 |
Body fat percentage was significantly higher in women working in offices with chronic LBP compared to healthy controls. |
| Celan (2005) [103] | Responded yes to previous LBP and also responded yes to having 3 times or more previous episodes. | NA |
No low back problems vs recurrent low back problems (body fat %) 25.54 vs 26.39, p = 0.43 |
There were no significant differences in body fat percentage between those with and without low back pain. |
| Cohort | ||||
| Anthropometric fat measures | ||||
| Muthuri (2020) [100] | All ages (except 68 yo): Responded yes to the question about whether they had sciatica, lumbago or recurring/severe backache all or most of the time (ever at ages 36 and 43 and in the previous 12 months at ages 53 and 60–64). Age 68: Responded yes to the question about whether they had experienced any ache or pain in the previous month which had lasted for 1 day or longer. |
Age, BMI, sex, education, occupational class and time-varying covariates (height, cigarette smoking status, physical activity and symptoms of anxiety and depression). |
Follow-up 36 to 43 yo: 7 years 43 to 53 yo: 10 years 53 to 60–64 yo: 7–10 years 60–64 to 68 yo: 4–8 years Presence of pain 36 years: 1.08 (0.97, 1.21) 43 years: 1.14 (1.02, 1.26) 53 years: 1.23 (1.07, 1.40) 60–64 years: 1.06 (0.92, 1.21) |
Higher WC was associated with increased odds of back pain between the ages 36 and 68. |
| Shiri (2019) [96] | Assessed by the number of days of LBP in the last 12 months | Age, gender, BMI, physical activity, walking or cycling to work, depression, strenuous physical work, using vibrating tools, keeling or squatting, standing or leaning forward, LBP past 30 days |
11 year follow-up Presence of pain LBP > 7 days (WC, normal ref) Increased: OR 1.07 (0.88–1.31) Obese: OR 1.40 (1.16–1.68) LBP > 30 days (WC, normal ref) Increased: OR 0.98 (0.77–1.26) Obese: OR 1.41 (1.13–1.76) |
Individuals with an obese WC were at a higher risk of larger number of days of LBP than those with a normal WC. |
| Dario (2017) [93] | Responded yes to “Have you ever suffered from chronic LBP?” | Age, gender |
2 to 4 year follow-up: Incident chronic LBP Total sample (WC, no pain ref) OR 0.98 (0.74–1.30) Within MZ and DZ twins (WC, no pain ref) OR 0.48 (0.16–1.50) Total sample (WHR, no pain ref) OR 1.05 (0.81–1.36) Within MZ and DZ twins (WHR, no pain ref) OR 0.47 (0.18–1.21) |
Risk of chronic back pain was no higher for individuals with an increased WC or WHR. |
| Hussain (2017) [92] | Pain categorised into 3 groups from the Chronic Pain Grade Questionnaire; no pain (0), low pain (<50), high pain (≥50) | Age, education, smoking status, socio-economic indexes for areas, mental component score of SF-36 |
12 year follow-up: Male (WC, no pain ref) Low intensity LBP OR 1.11 (0.98–1.27) High intensity LBP OR 1.25 (1.07–1.46) |
A larger WC was significantly associated with high intensity LBP compared to no pain in both male and females. A larger WC was also significantly associated with low intensity LBP in females, but not in males. When WC was split into quartiles, each quartile had significantly higher pain levels compared to the lowest WC quartile in both males and females, except for quartile 2 in females. |
| Female (WC, no pain ref) Low intensity LBP OR 1.13 (1.03–1.24) High intensity LBP OR 1.36 (1.22–1.52) | ||||
|
12 year follow-up: Pain intensity Male (WC, quartile 1 ref) Quartile 2: OR 1.43 (1.10–1.84) Quartile 3: OR 1.78 (1.36–2.34) Quartile 4: OR 1.50 (1.12–2.00) | ||||
|
Pain intensity Female (WC, quartile 1 ref) Quartile 2: OR 1.20 (0.96–1.50) Quartile 3: OR 1.42 (1.13–1.78) Quartile 4: OR 2.09 (1.65–2.65) | ||||
| Heuch (2015) [88] | Responded yes to “During the last year, have you had pain and/or stiffness in your muscles and limbs that has lasted for at least 3 consecutive months?” and responded lower back to “Where did you have pain and/or stiffness?” | Age, education, work status physical activity, smoking, HDL-cholesterol, triglycerides, blood pressure, body weight, BMI, WC, HC |
11 year follow-up: Female Incident LBP WC: RR 1.08 (1.03–1.13) WHR: RR 1.03 (0.99–1.08) HC: 1.07 (1.02, 1.12) Recurrent or persistent LBP WC: RR 1.07 (1.04–1.10) WHR: RR 1.02 (0.99–1.05) HC: 1.07 (1.04, 1.10) |
WC was associated with recurrence/persistence and incidence of pain in women but not in men. WHR was not associated with LBP in women or men. |
| Male Incidence LBP WC: RR 1.06 (1.00–1.13) WHR: RR 1.04 (0.98–1.10) HC: 1.06 (1.00, 1.12) Recurrence or persistent LBP WC: RR 1.02 (0.97–1.07) WHR: RR 1.01 (0.97–1.06) HC: 1.02 (0.97, 1.06) | ||||
| Shiri (2013) [87] | Responded yes to “Have you had low back trouble (pain, ache, or unpleasant sensations) during the preceding 12 months?” and responded greater than 7 days to “What is the total length of time you have had low back trouble during the preceding 12 months?” | Age, gender, educational status, occupational status, smoking |
6 year follow-up: Presence of non-specific LBP > 7 days (WC baseline, normal ref) Male Increased: OR 1.1 (0.6–2.0) Obese: OR 0.9 (0.5–1.8) 1cm increase: OR 1.00 (0.98–1.03) Female Increased: OR 1.7 (0.9–2.8) Obese: OR 1.1 (0.6–2.0) 1cm increase: OR 1.01 (0.99–1.03) |
Baseline WC and average WC over 7 years were not associated with non-specific LBP in males or females, with the exception of an obese WC in females. |
|
Presence of non-specific LBP (WC 7 year average, normal ref) Male Increased: OR 0.8 (0.5–1.5) Obese: OR 0.8 (0.4–1.6) 1cm increase: OR 1.00 (0.98–1.02) Female Increased: OR 1.5 (0.9–2.6) Obese: OR 1.7 (1.0–3.0) 1cm increase: OR 1.01 (0.99–1.04) | ||||
| Direct fat measurement | ||||
| Muthuri (2020) [100] | 60–64 years: Responded yes to the question about whether they had sciatica, lumbago or recurring/severe backache all or most of the time in the previous 12 months. 68 years: Responded yes to the about whether they had experienced any ache or pain in the previous month which had lasted for 1 day or longer. |
Sex, lean mass index, fat mass index, education at age 26, occupational class at age 53 and the following covariates (assessed at age 60–64): height, cigarette smoking status, physical activity and symptoms of anxiety and depression |
Follow-up: 60–64 to 68 yo: 4–8 years Presence of pain: FMI OR: 1.24 (1.04, 1.45) |
Higher fat mass index was associated with higher odds of back pain at age 68. |
| Brady (2019) [97] | High pain intensity >50 out of 100 | Age, gender, strenuous physical activity, mental health component score, total lean tissue mass |
3 year follow-up: Pain intensity Fat mass: OR 1.05 (1.01–1.09) |
Individuals with greater fat mass had a greater risk of high intensity LBP. |
| Dario (2017) [93] | Responded yes to “Have you ever suffered from chronic LBP?” | Age, gender |
2–4 year follow-up: Incident chronic LBP Total sample (percent fat, no pain ref) OR 0.87 (0.66–1.14) Within MZ and DZ twins (percent fat, no pain ref) OR 1.00 (0.35–2.85) |
Percentage fat mass was not predictive of LBP in adult twins. |
| Hashimoto (2017) [95] | Did not have LBP in the past or the present at baseline. | Maximal oxygen uptake, age, drinking, smoking |
20 year follow-up: Incident LBP Persistent LBP per 10,000 men years (body fat percentage quartiles) Q1: reference Q2: OR 0.86 (0.43–1.71) Q3: OR 1.46 (0.79–2.72) Q4: OR 2.12 (1.13–3.98) |
Individuals within the highest quartile of body fat mass were more likely to develop LBP compared to those in the lowest quartiles. |
| Hussain (2017) [92] | Pain categorised into 3 groups from the Chronic Pain Grade Questionnaire; no pain (0), low pain (<50), high pain (≥50) | Age, education, smoking status, socio-economic indexes for areas, mental component score of SF-36 |
12 year follow-up: Pain intensity Male (no pain ref) Low intensity LBP (percent fat) OR 1.28 (1.09–1.51) High intensity LBP (percent fat) OR 1.45 (1.19–1.77) Low intensity LBP (fat mass) OR 1.11 (0.97–1.27) High intensity LBP (fat mass) OR 1.23 (1.05–1.44) |
Both males and females with a higher percentage fat mass and total fat mass were at higher risk of high intensity LBP compared to individuals with no pain. Individuals with a higher percentage fat mass were at higher risk of low intensity LBP compared to those with no pain. Females, but not males, with larger total fat mass were at higher risk of low intensity LBP compared to no pain. |
|
12 year follow-up: Pain intensity Female (no pain ref) Low intensity LBP (percent fat) OR 1.41 (1.25-.1.59) High intensity LBP (percent fat) OR 1.39 (1.22–1.57) Low intensity LBP (fat mass) OR 1.28 (1.16–1.41) High intensity LBP (fat mass) OR 1.27 (1.15–1.40) | ||||
DZ = dizygotic, HC = hip circumference, IQR = inter-quartile range, LBP = low back pain, MZ = monozygotic, NA = not available, OR = odds ratio, RR = relative risk, SD = standard deviation WC = waist circumference, WHR = waist-hip ratio.
Of the six cohort studies, five studies found significant associations [87,88,92,96,100]. Three studies found a significant relationship between waist circumference and the presence of low back pain [87,88,100], with two studies reporting obese waist circumference to be associated with a larger number of days of low back pain [114,117] and one study finding waist circumference to be associated with high intensity low back pain [92]. The other two studies examined the relationship between waist circumference and incident low back pain and reported conflicting results [93], with one of the studies also examining recurrent and persistent low back pain and finding an association with waist circumference [59].
Hip circumference. Six studies examined the association between hip circumference and low back pain. Of the six studies, three cross-sectional studies found significant associations between hip circumference and low back pain intensity [116,132], but one study reported it in females only [127]. However, one cross-sectional study reported no significant association between hip circumference and low back pain intensity, a case-control study found no significant association between hip circumference and low back pain defined as pain for one or more days or 14 or more days [104] and one cohort study found no association between hip circumference and incident or recurrent/persistent low back pain [88].
Waist-hip ratio and waist-to-height ratio. Twelve studies, including eight cross sectional, two case-control and two cohort studies, examined the association between waist-hip ratio and low back pain. Of the eight cross-sectional studies [7,86,113,118,127,132,133,138], {], five found an association between waist-hip ratio and low back pain [7,113,118,127,132], while three did not find a relationship [86,133,138]. One case control study found waist-hip ratio was significantly associated with low back pain for 14 or more days [104], while another case control study found no association [105]. Both cohort studies found no association between waist-hip ratio and incident low back pain, with one study investigating this relationship in twins [93], and the other study examining females and males separately [88].
With respect to waist-to-height ratio, two studies examined the relationship between this adiposity measure and low back pain, finding an association with the presence of pain in post-menopausal women [133], and with radiating low back pain, but not chronic low back pain [123].
Direct fat measures
Body fat mass. Twelve studies, including eight cross sectional, one case-control and three cohort studies, examined the association between body fat mass and low back pain (Table 2). Of the eight cross sectional studies, one study found an association between abdominal to lumbar fat mass ratio and low back pain [122], and three studies found an association between total body fat mass and pain intensity [6,7] and the presence of low back pain [134]. The remaining four studies found no association between total body fat mass and chronic low back pain [114,121,131,137]. The case control study found an association between fat mass and presence of pain in males, but not females [107]. While one cohort study found associations between fat mass and high intensity pain in females and males, and fat mass and low intensity pain in females only [92], the remaining two cohort studies found greater fat mass was associated with a higher risk of the presence of pain [100] and high pain intensity [97].
Body fat percentage. Twelve studies, including five cross sectional, four case-control and three cohort studies, examined the association between body fat percentage and low back pain. Three cross sectional studies found associations between body fat percentage and low back pain [115,116,118], while two found no association [134,137]. Three case control studies found significant associations between body fat percentage and chronic low back pain [105,108] and presence of pain [107], while the remaining case control study found no association between body fat percentage and recurrent low back pain [103]. One cohort study found percentage fat mass to be significantly associated with high intensity pain in both females and males [92]. The second cohort study found those in the highest quartile of body fat mass were significantly more likely to develop low back pain than those in the lowest quartile [95] and the third study found no associations between percentage fat mass and incident low back pain in twins [93].
Summary of the evidence
Overall there was evidence of an association between adiposity and low back pain from 26 of the 36 identified studies (Table 3). Specifically, there was evidence from 5 of 6 cohort studies and 12 of 15 cross-sectional studies to indicate that there is a positive relationship between central adiposity and low back pain. There was also evidence provided by six of six cohort studies for a longitudinal relationship between adiposity and presence of low back pain, but conflicting evidence for a relationship between adiposity and incident low back pain (two of three studies) and limited evidence for a relationship with increasing low back pain (one of one study) (Table 4).
Table 3. Summary of the evidence examining the relationship between any and central adiposity and back and lower limb pain.
| No. of studies using direct and anthropometric measures: Any adiposity | No of studies using anthropometric measures: Central adiposity^ | ||||||
|---|---|---|---|---|---|---|---|
| Conducted | Association | No association | Association | No association | |||
| Low back pain | Evidence | Evidence | |||||
| Cohort | 8 | 7 | 1 | 5 | 1 | ||
| Case Control^^ | 6 | 4 | 2 | -1* | 2 | ||
| Cross-sectional | 22 | 15 | 7 | 12** | 3 | ||
| Hip pain | Limited evidence | Limited evidence | |||||
| Cohort | 1 | 1 | 0 | 0 | 0 | ||
| Case Control^^ | 1 | 0 | 1 | 0 | 1 | ||
| Cross-sectional | 0 | 0 | 0 | 0 | 0 | ||
| Knee pain | Evidence |
Evidence |
|||||
| Cohort | 5 | 4 | 1 | 3 | 0 | ||
| Case Control^^ | 2 | 1 | 1 | 1 | 1 | ||
| Cross-sectional | 6 | 4 | 2 | 2 | 1 | ||
| Foot pain | Evidence | Limited evidence | |||||
| Cohort | 5 | 5 | 0 | 1 | 0 | ||
| Case Control^^ | 1 | 0 | 1 | 0 | 0 | ||
| Cross-sectional | 2 | 2 | 0 | 0 | 1 | ||
^ These studies reported anthropometric measures of adiposity, such as waist circumference, waist-hip ratio, and hip circumference, to measure central adiposity.
^^ All case control studies were cross-sectional in design.
# These studies used direct measures of adiposity, such as fat mass and percentage total body fat, to measure total body adiposity.
* A study by Yip and colleagues found an inverse relationship between central adiposity and low back pain (included here).
** A study by Ojoawo et al reported a relationship for hip circumference, but not waist circumference or waist-hip ratio, and low back pain (included here).
Table 4. Summary of evidence from cohort studies examining the longitudinal relationship between adiposity and the presence of pain, incident pain and progression of pain.
| Region and adiposity measurement | Presence of pain No of cohort studies | Summary of Evidence | Incident pain No of cohort studies | Summary of Evidence | Progression of pain No of cohort studies | Summary of Evidence | |||
|---|---|---|---|---|---|---|---|---|---|
| Association | No association | Association | No association | Association | No association | ||||
| Low back pain | Evidence | Conflicting evidence | Limited evidence | ||||||
| Anthropometric | 4 | 0 | 1 | 1 | 1 | 0 | |||
| Direct | 3 | 0 | 1 | 1 | 0 | 0 | |||
| Both | 5 * | 0 | 2 | 1* | 1 | 0 | |||
| Hip pain | Limited evidence | No evidence | No evidence | ||||||
| Anthropometric | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Direct | 1 | 0 | 0 | 0 | 0 | 0 | |||
| Both | 1 | 0 | 0 | 0 | 0 | 0 | |||
| Knee pain | Evidence | Limited evidence | Limited evidence | ||||||
| Anthropometric | 2 | 0 | 0 | 0 | 2 | 0 | |||
| Direct | 2 | 0 | 0 | 1 | 1 | 0 | |||
| Both | 3* | 0 | 0 | 1 | 2 *¥ | 0 | |||
| Foot pain | Evidence | Evidence | Evidence | ||||||
| Anthropometric | 0 | 0 | 1 | 0 | 1 | 0 | |||
| Direct | 2 | 0 | 2 | 1 | 2 | 0 | |||
| Both | 2 | 0 | 3 | 1 | 2* | 0 | |||
*The same study provided results relating to anthropometric and direct measures of adiposity.
¥ One study examined trajectories of pain, while the other study reported an increase in pain.
Relationship between adiposity and hip pain
Anthropometric fat measures
One case control study found no significant difference in waist circumference, hip circumference and waist-hip ratio between individuals with greater trochanteric pain and controls [102] (Table 5).
Table 5. Results of the studies investigating the relationship between adiposity and hip and knee pain.
| Hip pain | ||||
| Case control studies | ||||
| Anthropometric fat measurement | ||||
| Fearon (2012) [102] | Participants diagnosed with trochanteric pain | NA |
Control vs trochanteric pain (WC) Mean (95% CI)– 83.0cm (78.9–87.1) vs 88.4cm (82.9–93.9), p = 0.42 Control vs trochanteric pain (HC) Mean (95% CI) 102.8cm (99.4–106.2) vs 109.1cm (104.9–113.2), p = 0.09 Control vs trochanteric pain (WHR) Mean (95% CI) 0.81 (0.783–0.837) vs 0.79 (0.75–0.83), p = 0.884 |
Those with trochanter pain did not have significantly larger WC, HC or WHR compared to controls. |
| Cohort studies | ||||
| Direct fat measurement | ||||
| Pan (2017) [94] | Responded yes to the presence of hip pain. | Age, sex, height, smoking history, physical activity, emotional problems, education level and employment |
5 year follow-up: Presence of pain (fat mass) OR 1.38 (1.13–1.70) |
Hip pain was significantly associated with high fat mass. |
|
Presence of pain (FMI) OR 1.42 (1.13–1.79) | ||||
| Knee pain | ||||
| Cross-sectional studies | ||||
| Anthropometric fat measurement | ||||
| Lee (2016) [125] | Pain intensity was reported on a scale of 1–10. Pain was categorised as mild (1–3), moderate (4–6) or severe (7–10). | NA |
Knee pain vs no knee pain (WC) Mean (SD) = 85.92 (0.50) vs 85.14 (0.34), p = 0.19 |
Participants with knee pain did not have significantly higher WC compared to participants with no knee pain. |
| Frilander (2016) [124] | Responded yes to “Have you during the previous 30 days had pain, ache or motion soreness?” | No adjustments |
Presence of pain WC (continuous) OR 1.15 (0.99–1.33) Presence of pain WC (<94cm ref) 94–101.9cm: OR 1.16 (0.86–1.55) ≥101.9cm: OR 1.38 (1.04–1.82) |
Knee pain was significantly associated with WC > 101.9cm among men who served in the Finnish military. |
| Muramoto (2014) [132] | Pain intensity of ≥ 1 on the VAS. | Age | WC: r = 0.2, p<0.005 HC: r = 0.2, p<0.01 WHR: r = 0.2, p<0.01 Multivariate analyses: WC: significant association reported. Data not provided. p<0.01. |
Central obesity was associated with knee pain intensity. |
| Direct fat measurement | ||||
| Alfieri (2017) [128] | WOMAC, score of ≥ 1 scale of 0–100 | NA |
Adequate adiposity vs excessive adiposity Mean (SD) = 53.6 (25.6) vs 59.9 (16.8), p<0.05 |
Participants with excessive adiposity (in accordance with the American College of Sports Medicine (ACSM) recommendation) had a higher pain score on the WOMAC than those with adequate adiposity. |
| Lee (2016) [125] | Pain intensity was reported on a scale of 1–10. Pain was categorised as mild (1–3), moderate (4–6) and severe (7–10). | Age, sex, physical activity, BMI |
Pain intensity; Severe knee pain (≥7) Leg to whole body fat mass: OR 1.01 (0.98–1.05) |
Fat mass in the leg, relative to whole body, was not correlated with knee symptoms. |
| Ozer Kaya, (2014) [120] | Pain was graded on the VAS, 0-100mm. Scores of ≥70 were excluded. | NA |
Knee pain vs no knee pain (body fat percentage) Mean (SD) = 39.29 (7.86) vs 38.13 (7.67), p>0.05 Knee pain vs no knee pain (fat mass, kg) Mean (SD) = 30.46 (11.77) vs 28.64 (9.59), p>0.05 |
There were no significant differences in body fat percentage or fat mass between knee pain and non-knee pain subjects. |
| Scott (2012) [129] | Responded yes to the question “Do you have pain at any of these sites today?” (for knee pain). |
NA |
Males Knee pain vs no knee pain (fat mass %) % (SD) = 28.0 (5.2) vs 27.2 (4.4), p = 0.073 |
Percentage fat mass was significantly higher in those with knee pain among females, but not males. |
|
Females Knee pain vs no knee pain (fat mass %) % (SD) = 40.1 (5.5) vs 39.0 (5.0), p = 0.046 | ||||
| Case-control studies | ||||
| Anthropometric fat measurement | ||||
| Li (2016) [110] | Pain intensity score ≥1 on the VAS | NA |
Pain intensity Knee OA vs controls 6.82+/-1.07, 5.93+/-0.88, p = 0.005 |
A greater WC was associated with increased pain intensity. |
| Sutbeyaz (2007) [109] | Participants had knee pain most days of the month | NA |
Pain most days vs no pain (WHR) Mean (SD) = 0.89 (0.08) vs 0.90 (0.08), p = 0.80 |
WHR was not found to be significantly associated with the presence of knee pain. |
| Direct fat measurement | ||||
| Sutbeyaz (2007) [109] | Participants had knee pain most days of the month | NA |
Pain most days vs no pain (fat mass, kg) Mean (SD) = 29.40 (7.16) vs 33.60 (7.52), p = 0.06 |
Total fat mass measured by skin fold was not found to be significantly associated with the presence of knee pain. |
| Cohort studies | ||||
| Anthropometric fat measurement | ||||
| Pan (2020) [101] | Minimal pain’ group: consistently low level of pain Mild pain’ group: a mild level of pain consistent throughout the follow-up. Moderate pain: relatively high level of pain consistent throughout the follow-up. |
Age, sex, physical activity, smoking history, unemployment, education level and radiographic knee osteoarthritis. |
10.7 year follow-up: Mild vs minimal pain trajectory (WC): RR: 1.68 (1.25, 2.25) Moderate vs minimal pain trajectory (WC): RR: 3.19 (2.12, 4.80) |
Central obesity increased risk of ‘Mild pain’ and ‘Moderate pain’ rather than ‘Minimal pain’. |
| Jin (2016) [89] | Knee pain was defined as a score of ≥1 on a scale of 0–10. Consistent pain was assessed as any pain at baseline and follow up. Fluctuating pain was assessed as pain in any one or two time-points. | Age, gender, height, radiographic OA |
Average 5.1 year follow-up: Consistent pain vs no pain (WHR) RR 1.25 (0.98–1.59) Fluctuating pain vs no pain (WHR) RR 1.46 (1.18–1.80) |
WHR and WC was found to be a significant predictor of increasing knee pain and more consistently associated with non-weight bearing knee pain. Similarly WHR and WC were significantly associated with an increase in knee pain, however no significant relationship was found with total knee pain. |
|
Consistent pain vs no pain (WC) RR 1.46 (1.18–1.80) Fluctuating pain vs no pain (WC) RR 1.55 (1.27–1.89) | ||||
|
Average 5.1 year follow-up: Increase in knee pain (WC) OR 1.37 (1.18–1.59) Total knee pain (WC) OR 1.38 (0.97–1.80) | ||||
|
Increase in knee pain (WHR) OR 1.23 (1.03–1.47) Total knee pain (WHR) OR 1.36 (0.83–1.90) | ||||
| Batsis (2014) [91] | Pain as assessed on the WOMAC- 5 point Likert scale. | Age, sex, education level, race, cohort type (incident, progression and control), Charlson co-morbidity score, current smoking status, baseline scores where available |
6 year follow-up: Right knee pain intensity (WC, quartiles) Mean (SD) = 8.4 (11.9) vs 10.8 (13.4) vs 12.3 (14.3) vs 14.2 (15.3): ANOVA, p<0.01 Left knee pain intensity (WC, quartiles) Mean (SD) = 7.8 (12.6) vs 10.8 (14.6) vs 12.0 (15.3) vs 13.8 (16.1): ANOVA, p<0.01 |
WOMAC scores were significantly higher in the higher quartile WC groups compared to the lower quartiles. |
| Direct fat measurement | ||||
| Pan (2017) [94] | Yes response to presence of pain | Age, sex, height, smoking history, physical activity, emotional problems, education level and employment |
5 year follow-up: Presence of pain (fat mass) OR 1.99 (1.59–2.49) |
Knee pain was significantly associated with fat mass and FMI in an older cohort. |
|
Presence of pain (FMI) OR 2.06 (1.60–2.64) | ||||
| Jin (2016) [89] | Knee pain was defined as a score of ≥1 on a scale of 0–10. Consistent pain was assessed as any pain at baseline and follow up. Fluctuating pain was assessed as pain in any one or two time-points. | Age, gender, height, radiographic OA |
Average 5.1 year follow-up: Consistent pain vs no pain (fat mass) RR 1.89 (1.43–2.51) Fluctuating pain vs no pain (fat mass) RR 1.78 (1.41–2.25) |
Body fat mass was found to be a significant predictor of increasing knee pain and more consistently associated with non-weight bearing knee pain. |
|
Increase in knee pain (fat mass) OR 1.36 (1.20–1.55) Total knee pain (fat mass) OR 1.17 (0.76–1.59) | ||||
| Barber (2012) [86] | Pain was assessed on a 0–100 scale. Scores of 100 represented no pain. | NA |
2 year follow-up: Incident patellofemoral pain vs no patellofemoral pain Mean body fat % (95% CI): 22.2 (19.4–24.9) vs 22.9 (21.8–24.1), p>0.05 |
No significant difference in body fat percentage was found between middle school female basketball players who developed patellofemoral pain and those who did not. |
FMI = fat mass index, HC = hip circumference, NA = not available, OR = odds ratio, RR = relative risk, WC = waist circumference, WOMAC = Western Ontario and McMaster Universities Arthritis Index, WHR = waist-hip ratio.
Direct fat measures
Body fat mass. A single cohort study found greater body fat mass was associated with the presence of hip pain among older individuals [94].
Overall there was limited evidence for an association between adiposity and hip pain based on two studies with conflicting results (Table 3). There was limited evidence to suggest central adiposity is not a risk factor for hip pain (one case-control study) and limited or no evidence that there is a longitudinal relationship between adiposity and the presence (one cohort study), incidence (no studies) or progression of hip pain (no studies) (Table 4).
Relationship between adiposity and knee pain
Anthropometric fat measures
Waist circumference. Seven studies, including three cross-sectional, one case-control and three cohort studies, examined the association between waist circumference and knee pain (Table 5). While one cross-sectional study found a significant difference in knee pain between those with a waist circumference <94cm and those with waist circumference ≥101.9cm [124] and a second cross-sectional study found an association between waist circumference and knee pain intensity [132], a third cross sectional study found no differences in waist circumference between those with and without knee pain [125]. Moreover, a case-control study found that a greater waist circumference was associated with increased pain intensity [110]. Of the three cohort studies, one reported a significant association between waist circumference and consistent and fluctuating knee pain [89], and the other two found a significant relationship between waist circumference and knee pain intensity [91,101].
Waist-hip ratio. One cross-sectional study and one case-control study found significant associations between waist-hip ratio and pain intensity [132] and the presence of knee pain on most days [109], while a cohort study reported an association between waist-hip ratio and fluctuating knee pain, but not consistent knee pain [89].
Direct fat measures
Body fat mass. Four studies examined the relationship between body fat mass and knee pain. Two cross sectional studies found no significant association between fat mass and knee pain [120,125], while one case-control study found no association between fat mass and presence of knee pain on most days [109] (Table 5). The single cohort study found greater body fat mass was associated with the presence of knee pain among older individuals [94].
Body fat percentage. Three cross sectional and two cohort studies examined the association between body fat percentage and knee pain. Two cross sectional studies found an association between body fat percentage and knee pain [128], however one found this association only in females [129], while the remaining cross-sectional study found no association between body fat percentage and knee pain [120]. Of the two cohort studies, one study found an association between body fat percentage and consistent and fluctuating knee pain [89], while the other found no association between body fat percentage and incident patellofemoral pain [86].
Summary of the evidence
Overall there was evidence from nine of the 13 identified studies for an association between adiposity and knee pain (Table 3). There was evidence to indicate that central adiposity is a risk factor for knee pain (six of 8 studies) and there is a longitudinal relationship between adiposity and the presence of knee pain (three of three cohort studies) (Table 4). However, there was limited evidence for a relationship between adiposity and incident and increasing knee pain with a limited number of cohort studies identified in each case.
Relationship between adiposity and foot pain
Anthropometric fat measures
Waist circumference. One cohort study found that individuals with a larger waist circumference were at greater risk of incident and increasing foot pain [98] (Table 6).
Table 6. Results of the studies investigating the relationship between adiposity and foot pain.
| Foot pain | ||||
| Cross-sectional studies | ||||
| Anthropometric fat measurement | ||||
| Butterworth (2016) [126] | Foot pain was defined as a score of ≥1 with minimum and maximum scores ranging from 0–19 | Age, depression, mobility and education. |
Foot pain vs no foot pain (WHR) OR 1.02 (0.99–1.06) |
Presence of foot pain was not associated with WHR. |
| Direct fat measurement | ||||
| Butterworth (2016) [126] | Foot pain was defined as a score of ≥1 with minimum and maximum scores ranging from 0–19 | Age, depression, mobility, education, residual of weight on fat mass or BMI on FMI respectively. |
Foot pain vs no foot pain (total fat mass) OR 1.02 (1.003–1.05) Foot pain vs no foot pain (FMI) OR 1.08 (1.01–1.15) |
In men, fat mass, but not WHR, was associated with having foot pain. |
| Tanamas (2012) [119] | Foot pain was classified as having current foot pain and pain in the last month as well as a score of ≥1 with minimum and maximum scores ranging from 0–19 | Total fat mass: age, sex, skeletal muscle mass FMI: age, sex, FFMI All others: age, sex |
Foot pain vs no foot pain (total fat mass) OR 1.05 (1.02–1.09) |
The effect of obesity on foot pain was related to an increase in adiposity, particularly in the android distribution of fat. In contrast, the gynoid distribution of fat was found to have a beneficial effect. |
|
Foot pain vs no foot pain (FMI) OR 1.16 (1.06–1.28) | ||||
|
Foot pain vs no foot pain (total body fat %) OR 1.10 (1.05–1.14) | ||||
|
Foot pain vs no foot pain (android/total body fat ratio) OR 1.42 (1.11–1.83) | ||||
|
Foot pain vs no foot pain (gynoid/total body fat ratio) OR 0.83 (0.73–0.93) | ||||
|
Foot pain vs no foot pain (trunk/total body fat ratio) OR 1.05 (0.98–1.12) | ||||
| Case control studies | ||||
| Direct fat measurement | ||||
| Walsh (2017) [54] | Score of ≥ 1 on the VAS | N/A |
Foot pain group vs control group (fat mass, kg) 33.1 vs 31.5 p = 0.578 |
There was no significant difference in total fat mass between the foot pain and control groups. |
| Cohort studies | ||||
| Anthropometric fat measurement | ||||
| Laslett (2018) [98] | Pain (yes/no) | Age, gender |
5 year follow-up: Incident foot pain (WC) RR 1.22 (1.01–1.49) Change in foot pain (WC) RR 1.26 (1.19–1.34) |
Individuals with a greater WC were at higher risk of incident foot pain and an increase in foot pain. However, individuals with greater WHR were only at risk of an increase in foot pain. |
|
Incident foot pain (WHR) RR 1.23 (0.95–1.61) Change in foot pain (WHR) RR 1.27 (1.16–1.39) | ||||
| Direct fat measurement | ||||
| Laslett (2018) [98] | Pain (yes/no) | Age, gender |
5 year follow-up: Incident foot pain Total fat mass RR 0.92 (0.71–1.19) FMI RR 1.16 (0.93–1.46) |
Individuals with greater total fat mass and FMI were at higher risk of increases in foot pain. However, greater total fat mass and FMI were not associated with incident foot pain. |
|
5 year follow-up: Change in foot pain Total fat mass RR 1.28 (1.18–1.40) FMI RR 1.21 (1.18–1.24) | ||||
| Walsh (2018) [99] | Assessed by Manchester-Oxford foot questionnaire and converted to 100 scale | Age, gender, depression, treatment group |
4–20 year follow-up: Change in foot pain (FMI) β coefficient 1.5 (0.2–2.8) |
FMI was a predictor of change in foot pain. |
| Pan (2017) [94] | Presence of pain (yes/no) | Age, sex, height, smoking history, physical activity, emotional problems, education level and employment |
5 year follow-up: Presence of pain (fat mass) OR 1.87 (1.51–2.32) |
Foot pain was associated with fat mass and FMI in an older cohort. |
|
Presence of pain (FMI) OR 1.99 (1.57–2.53) | ||||
| Walsh (2016) [90] | Prevalent (presence of) pain defined as responding yes to “On most days, do you have pain, aching, or stiffness in either of your feet?” Future foot pain was defined as responding yes to “Over the past month, have you had pain, aching, or stiffness in either of your feet on most days?” |
BMI, FFMI, WHR, age, IL-6 level, TNF level |
3–4 year follow-up: Presence of foot pain vs no foot pain (FMI) OR 1.08 (1.04–1.12) |
FMI was positively associated with both the presence of foot pain and future foot pain. |
|
3-4year follow-up: Future foot pain vs no foot pain (FMI) OR 1.06 (1.02–1.11) | ||||
| Butterworth (2013) [85] | Foot pain was defined as a score of ≥1 with minimum and maximum scores ranging from 0–19 | Age, gender, mental component summary, total fat-free mass/FFMI respectively |
3 year follow-up Incident foot pain vs no foot pain (total fat mass) OR 1.11 (1.03–1.20) |
Total fat mass was found to be a predictor of incident foot pain. |
|
Incident foot pain vs no foot pain (FMI) OR 1.28 (1.04–1.57) | ||||
BMI = body mass index, FFMI = fat free mass index, FMI = fat mass index, OR = odds ratio, WHR = waist-hip ratio, WHtR = Waist-height ratio.
Waist-hip ratio. One cross-sectional study, which examined the association between waist-hip ratio and foot pain, found no significant association [126].
Direct fat measures
Body fat mass. Seven studies, including two cross-sectional, one case control and five cohort studies, examined the association between fat mass measures and foot pain (Table 6). Both cross -sectional studies found significant associations between fat mass and foot pain [119,126], while the case-control study did not find any significant difference between total fat mass in individuals with foot pain compared to those without [106]. Five cohort studies found significant positive relationships between direct fat measures and foot pain, with two studies reporting an association with the presence of foot pain [90,94], three studies finding a relationship with incident foot pain [85,90,99] and two studies reporting an association with progression of foot pain [98,99]. While Laslett and colleagues reported a relationship between fat measures and increasing foot pain, no association was found for incident foot pain [98].
Summary of the evidence
Overall there was evidence from seven of the eight identified studies for an association between adiposity measures and foot pain (Table 3). While there was limited evidence for a relationship between central adiposity and foot pain (two conflicting studies), there was evidence for a longitudinal relationship between adiposity and the presence of pain (two of two studies), incident foot pain (three of four studies) and progression of foot pain from 6 months to 5 years (two of two studies) (Table 4).
Discussion
This systematic review found that both body fat and its central distribution are associated with musculoskeletal pain. There was evidence of a relationship between central adiposity and low back and knee pain, but limited or conflicting evidence for hip and foot pain. There was also evidence of a longitudinal relationship between adiposity and the presence of low back, knee and foot pain, as well as both incident and increasing foot pain. Taken together, these findings further our understanding of the mechanisms underlying obesity-related musculoskeletal pain and highlight adiposity as a potential therapeutic target in the management of back and lower leg pain.
This systematic review is the first to examine the relationship between fat distribution and musculoskeletal pain. We found evidence that central adiposity, defined as the accumulation of extra subcutaneous and visceral fat concentrated just above or around the waistline, was associated with pain in the lower back and knee. This was based on evidence of a significant, positive association in 16 of the 22 studies of low back pain, including five of six cohort studies, and 6 of 8 studies of knee pain, including 3 of 3 cohort studies. This finding is consistent with evidence that central adiposity is associated with a greater risk of major public health conditions, such as cardiovascular disease and diabetes, which are associated with huge socioeconomic burdens globally [11]. Given visceral fat associated with central adiposity is an important correlate of metabolic disturbances [139], and the cells in central, visceral fat have a much higher turnover than subcutaneous fat cells in other regions of the body [140], central adiposity may be an important way to target obesity. For instance, management strategies targeted to enhance weight loss around the abdominal region may be particularly beneficial. Overall, the evidence for an association between central adiposity and low back and knee pain indicates that it is not just extra body fat that contributes to poor health and chronic pain, but also the distribution of the fat.
This review found evidence of a longitudinal relationship between adiposity and the presence of low back, knee and foot pain, as well as incident and increasing foot pain. The findings suggest that increased adiposity can lead to back and lower limb pain in the future, and in the case of foot pain, the development or increasing intensity of pain. Our results, which take into account 17 cohort studies, build on the conclusions of a previous review of seven cohort studies [10], which suggested that such associations may exist, but was limited by a lack of high quality studies. Our results highlight the need for high quality clinical trials to examine the efficacy of approaches that target weight loss, be it through physical activity, diet and/or medical options, in the management of back and lower limb pain in overweight and obese individuals. They also suggest that investigating the efficacy of the targeted interventions, such as exercise programs that focus on reducing adipose tissue and nutrition plans that optimize health but minimize fat intake. Moreover, given current evidence collectively indicates that musculoskeletal pain has an important systemic inflammatory component, there is an exciting opportunity to examine the efficacy of pharmaceutical and complementary medicines as potential treatment targets to reduce inflammation in individuals with musculoskeletal pain with a specific overweight/obese profile.
Moreover, evidence from this review informs our knowledge of the mechanisms that underlie obesity-related musculoskeletal pain. Several mechanisms, including increased physical loading and systemic metabolic processes, have been proposed to explain the role of obesity in musculoskeletal pain. In overweight or obese individuals, excess adipose tissue may result in increased load on a region and subsequently, altered posture and abnormal movement patterns resulting in pain and disability. There is also growing evidence to support systemic metabolic processes, with evidence that adiposity is associated with pain in non-weight-bearing regions, such as the hand [94]. Adipose tissue is metabolically active, releasing a multitude of pro-inflammatory cytokines and adipokines, which may potentiate inflammatory changes in a region resulting in pain [141]. Moreover, inflammation has been shown to alter the excitation thresholds and responses to stimuli of peripheral nerves, subsequently leading to peripheral and central sensitisation [142,143]. Our findings provide evidence that both mechanical and metabolic mechanisms may be at play in lower back, knee and foot pain, with the potential for total body fat and central obesity to load these regions and increased visceral and subcutaneous fat to alter metabolic processes. However, preliminary evidence from studies that reported adiposity to be associated with pain in non-weight bearing regions, such as the neck and hand, suggest that future research examining these regions may further our understanding of the pathogenesis of obesity-related musculoskeletal pain.
This systematic review has several important strengths, including conducting a comprehensive, systematic search of the literature based on six electronic databases, performing a risk of bias assessment of studies using the Cochrane risk of bias assessment, and conducting a best evidence synthesis to summarise the strength of the available evidence. Moreover, this review is novel, as it is the first to provide evidence of the role of central adiposity in site-specific musculoskeletal pain, as well as an updated summary of the evidence examining the longitudinal association between adiposity and back and lower limb pain. While this review was not registered a priori with an international prospective register, we have provided a detailed description of our review methodology from development of our search strategy to the assigning of levels of evidence and documented any changes in our initial methodology in this publication. Furthermore, while the review was limited by the paucity of high quality cohort studies, as well as significant heterogeneity in the identified studies, which meant a meta-analysis could not be performed, we used established levels of evidence to summarise the data for each musculoskeletal pain region
This systematic review found that both body fat and its distribution are associated with site-specific musculoskeletal pain. There was evidence of a positive relationship between central adiposity and low back and knee pain and a longitudinal association between adiposity and the presence of back, knee and foot pain, as well as incident and worsening foot pain. These findings are not only important in understanding the mechanisms which underlie chronic, musculoskeletal pain, but in the development of innovative treatment approaches for these debilitating conditions.
Supporting information
(DOC)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
FMC: National Health and Medical Research Council Investigator Grant (#1194829) SMH: National Health and Medical Research Council Early Career Fellowship (#1142198) MME: Bangabandhu Science and Technology Fellowship, Ministry of Science and Technology, Government of the People’s Republic of Bangladesh TAR: Australian Government Research Training Program Scholarship DMU: National Health and Medical Research Council/Medical Research Future Fund Career Development Fellowship (Clinical Level 2 #1142809). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017; 390(10100): 1211–59. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Briggs AM, Cross MJ, Hoy DG, Sanchez-Riera L, Blyth FM, Woolf AD, et al. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. The Gerontologist. 2016; 56Suppl 2: S243–55. doi: 10.1093/geront/gnw002 [DOI] [PubMed] [Google Scholar]
- 3.Gregg E, Shaw J. Global health effects of overweight and obesity. N Engl J Med. 2017; 377(1): 80–1. doi: 10.1056/NEJMe1706095 [DOI] [PubMed] [Google Scholar]
- 4.Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The Association Between Obesity and Low Back Pain: A Meta-Analysis. American Journal of Epidemiology. 2010; 171(2): 135–54. doi: 10.1093/aje/kwp356 [DOI] [PubMed] [Google Scholar]
- 5.Butterworth P, Landorf K, Smith S, Menz H. The association between body mass index and musculoskeletal foot disorders: a systematic review. Obes Rev. 2012; 13(7): 630–42. doi: 10.1111/j.1467-789X.2012.00996.x [DOI] [PubMed] [Google Scholar]
- 6.Urquhart DM, Berry P, Wluka AE, Strauss BJ, Wang Y, Proietto J, et al. 2011 Young Investigator Award winner: Increased fat mass is associated with high levels of low back pain intensity and disability. Spine (Phila Pa 1976). 2011; 36(16): 1320–5. doi: 10.1097/BRS.0b013e3181f9fb66 [DOI] [PubMed] [Google Scholar]
- 7.Chou L, Brady SR, Urquhart DM, Teichtahl AJ, Cicuttini FM, Pasco JA, et al. The association between obesity and low back pain and disability is affected by mood disorders: A population-based, cross-sectional study of men. Medicine. 2016; 95(15): e3367. doi: 10.1097/MD.0000000000003367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coppack SW. Pro-inflammatory cytokines and adipose tissue. The Proceedings of the Nutrition Society. 2001; 60(3): 349–56. doi: 10.1079/pns2001110 [DOI] [PubMed] [Google Scholar]
- 9.Willard F. 8—Neuroendocrine—immune network, nociceptive stress and the general adaptive response. In: Everett T, Dennis M, Ricketts E, editors. Physiotherapy in Mental Health: Butterworth-Heinemann; 1995. p. 102–26. [Google Scholar]
- 10.Walsh TP, Arnold JB, Evans AM, Yaxley A, Damarell RA, Shanahan EM. The association between body fat and musculoskeletal pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2018; 19(1): 233. doi: 10.1186/s12891-018-2137-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Waist Circumference and Waist-hip Ratio: Report of a WHO Expert Consultation. Geneva; 2011.
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annals of Internal Medicine. 2009; 151(4): 264–9. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 13.Duren DL, Sherwood RJ, Czerwinski SA, Lee M, Choh AC, Siervogel RM, et al. Body composition methods: comparisons and interpretation. J Diabetes Sci Technol. 2008; 2(6): 1139–46. doi: 10.1177/193229680800200623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lievense AM, Bierma-Zeinstra SM, Verhagen AP, van Baar ME, Verhaar JA, Koes BW. Influence of obesity on the development of osteoarthritis of the hip: a systematic review. Rheumatology (Oxford, England). 2002; 41(10): 1155–62. doi: 10.1093/rheumatology/41.10.1155 [DOI] [PubMed] [Google Scholar]
- 16.Urquhart D, Zheng Y, Cheng A, Rosenfeld J, Chan P, Liew S, et al. Could low grade bacterial infection contribute to low back pain? A systematic review. BMC Med. 2015; 13: 13. doi: 10.1186/s12916-015-0267-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacLellan G, Dunlevy C, E OM, Blake C, Breen C, Gaynor K, et al. Musculoskeletal pain profile of obese individuals attending a multidisciplinary weight management service. Obesity Facts. 2017; 10 (Supplement 1): 193. doi: 10.1097/j.pain.0000000000000918 [DOI] [PubMed] [Google Scholar]
- 18.Dufour AB, Losina E, Menz HB, LaValley MP, Hannan MT. Obesity, foot pain and foot disorders in older men and women. Obesity Research and Clinical Practice. 2017; 11(4): 445–53. doi: 10.1016/j.orcp.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Najafipour H, Sadeghigoghari M, Kordestani Z, Tahami AN, Ghavipisheh M. Prevalence of the musculoskeletal pain syndrome and its associated factors in people between 15 and 80 years in kerman: A population-based study on 1700 individuals. Iranian Red Crescent Medical Journal. 2017; 19(4). [Google Scholar]
- 20.Okamoto CS, Dunn AS, Green BN, Formolo LR, Chicoine D. Correlation of Body Composition and Low Back Pain Severity in a Cross-Section of US Veterans. Journal of Manipulative and Physiological Therapeutics. 2017; 40(5): 358–64. doi: 10.1016/j.jmpt.2017.03.003 [DOI] [PubMed] [Google Scholar]
- 21.Peng T, Perez A, Pettee Gabriel K. The Association Among Overweight, Obesity, and Low Back Pain in U.S. Adults: A Cross-Sectional Study of the 2015 National Health Interview Survey. Journal of Manipulative and Physiological Therapeutics. 2018. [DOI] [PubMed] [Google Scholar]
- 22.Higgins D, Buta E, Heapy A, Driscoll M, Kerns R, Masheb R, et al. The relationship among BMI, pain intensity, and musculoskeletal diagnoses. Journal of Pain. 2016; 1): S28. [Google Scholar]
- 23.DePalma MJ, Ketchum JM, Kouchouk A, Powell D, Ruchala MD. Multivariate analyses of age, gender, and BMI on the source of low back pain. PM and R. 2010; 1): S62. [Google Scholar]
- 24.Segar AH, Urban JPG, Fairbank JCT, Judge A. The association between body mass index (BMI) and back or leg pain in patients with spinal conditions results from the genodisc study. Spine. 2016; 41(20): E1237–E43. doi: 10.1097/BRS.0000000000001606 [DOI] [PubMed] [Google Scholar]
- 25.Aoyagi K, Ross PD, Okano K, Hayashi T, Moji K, Kusano Y, et al. Association of body mass index with joint pain among community-dwelling women in Japan. Aging—Clinical and Experimental Research. 2002; 14(5): 378–81. doi: 10.1007/BF03324465 [DOI] [PubMed] [Google Scholar]
- 26.Brooks C, Siegler JC, Cheema BS, Marshall PWM. No relationship between body mass index and changes in pain and disability after exercise rehabilitation for patients with mild to moderate chronic low back pain. Spine. 2013; 38(25): 2190–5. doi: 10.1097/BRS.0000000000000002 [DOI] [PubMed] [Google Scholar]
- 27.Hagen K, Heuch I, Nygaard O, Zwart JA. The impact of body mass index on the prevalence of low back pain: The HUNT study. Spine. 2010; 35(7): 764–8. doi: 10.1097/BRS.0b013e3181ba1531 [DOI] [PubMed] [Google Scholar]
- 28.Macfarlane GJ, De silva V, Jones GT. The relationship between body mass index across the life course and knee pain in adulthood: Results from the 1958 birth cohort study. Rheumatology. 2011; 50(12): 2251–6. doi: 10.1093/rheumatology/ker276 [DOI] [PubMed] [Google Scholar]
- 29.Ozkaya DB, Onsun N, Topukcu B, Su O, Bahali AG, Dizman D, et al. The relationship between body mass index, waist circumference and psoriatic arthritis in the Turkish population. Postepy Dermatologii i Alergologii. 2016; 33(3): 219–23. doi: 10.5114/ada.2016.60615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sajan CK, Malik C, Srikrishnan MT. The association between body mass index and low back pain on the quality of life. PM and R. 2011; 1): S217. [Google Scholar]
- 31.Singh JA, Gabriel SE, Lewallen DG. Higher Body Mass Index Is Not Associated With Worse Pain Outcomes After Primary or Revision Total Knee Arthroplasty. Journal of Arthroplasty. 2011; 26(3): 366–74.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cadish LA, Hacker MR, Dodge LE, Dramitinos P, Hota LS, Elkadry EA. Association of body mass index with hip and thigh pain following transobturator midurethral sling placement. American Journal of Obstetrics and Gynecology. 2010; 203(5): 508.e1–.e5. doi: 10.1016/j.ajog.2010.07.023 [DOI] [PubMed] [Google Scholar]
- 33.Brady S, Hussain SM, Brown W, Heritier S, Urquhart D, Wang Y, et al. The course and contributors to back pain in middle-aged women over nine years: Data from the australian longitudinal study on women’s health. Internal Medicine Journal. 2018; 48 (Supplement 4): 10. [DOI] [PubMed] [Google Scholar]
- 34.Cooper L, Ells L, Ryan C, Martin D. Perceptions of adults with overweight/obesity and chronic musculoskeletal pain: An interpretative phenomenological analysis. Journal of clinical nursing. 2018; 27(5–6): e776–e86. doi: 10.1111/jocn.14178 [DOI] [PubMed] [Google Scholar]
- 35.Li J, Chen J, Qin Q, Zhao D, Dong B, Ren Q, et al. Chronic pain and its association with obesity among older adults in China. Archives of Gerontology & Geriatrics. 2018; 76: 12–8. doi: 10.1016/j.archger.2018.01.009 [DOI] [PubMed] [Google Scholar]
- 36.Su CA, Kusin DJ, Li SQ, Ahn UM, Ahn NU. The Association between Body Mass Index and the Prevalence, Severity, and Frequency of Low Back Pain. Spine. 2018; 43(12): 848–52. doi: 10.1097/BRS.0000000000002601 [DOI] [PubMed] [Google Scholar]
- 37.Angst F, Angst J, Ajdacic-Gross V, Aeschlimann A, Rossler W. Epidemiology of Back Pain in Young and Middle-Aged Adults: A Longitudinal Population Cohort Survey From Age 27–50 Years. Psychosomatics. 2017. doi: 10.1016/j.psym.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 38.Baek SR, Lim JY, Lim JY, Park JH, Lee JJ, Lee SB, et al. Prevalence of musculoskeletal pain in an elderly Korean population: Results from the Korean Longitudinal Study on Health and Aging (KLoSHA). Archives of Gerontology and Geriatrics. 2010; 51(3): e46–e51. doi: 10.1016/j.archger.2009.11.011 [DOI] [PubMed] [Google Scholar]
- 39.Borg JH, Westerstahl M, Lundell S, Madison G, Aasa U. Longitudinal study exploring factors associated with neck/shoulder pain at 52 years of age. Journal of Pain Research. 2016; 9: 303–10. doi: 10.2147/JPR.S93845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernandez-De-Las-Penas C, Alonso-Blanco C, Hernandez-Barrera V, Palacios-Cena D, Jimenez-Garcia R, Carrasco-Garrido P. Has the prevalence of neck pain and low back pain changed over the last 5 years? A population-based national study in Spain. Spine Journal. 2013; 13(9): 1069–76. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez-De-Las-Penas C, Hernandez-Barrera V, Alonso-Blanco C, Palacios-Cena D, Carrasco-Garrido P, Jimenez-Sanchez S, et al. Prevalence of neck and low back pain in community-dwelling adults in spain: A population-based national study. Spine. 2011; 36(3): E213–E9. doi: 10.1097/BRS.0b013e3181d952c2 [DOI] [PubMed] [Google Scholar]
- 42.Gandhi R, Perruccio AV, Rizek R, Dessouki O, Evans HMK, Mahomed NN. Obesity-related adipokines predict patient-reported shoulder pain. Obesity Facts. 2013; 6(6): 536–41. doi: 10.1159/000357230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellsing AL, Bryngelsson IL. Predictors of musculoskeletal pain in men: A twenty-year follow-up from examination at enlistment. Spine. 2000; 25(23): 3080–6. doi: 10.1097/00007632-200012010-00016 [DOI] [PubMed] [Google Scholar]
- 44.Honda R, Noborisaka Y, Ishida M, Ishizaki M, Yamada Y. Impact of obesity on musculoskeletal pain and difficulty of daily movements in Japanese middle-aged women. Maturitas. 2002; 42(1): 23–30. doi: 10.1016/s0378-5122(02)00025-7 [DOI] [PubMed] [Google Scholar]
- 45.Kaaria S, Laaksonen M, Rahkonen O, Lahelma E, Leino-Arjas P. Risk factors of chronic neck pain: A prospective study among middle-aged employees. European Spine Journal. 2012; 21 (5): 1022. doi: 10.1002/j.1532-2149.2011.00065.x [DOI] [PubMed] [Google Scholar]
- 46.Mork PJ, Vik KL, Moe B, Lier R, Bardal EM, Nilsen TI. Sleep problems, exercise and obesity and risk of chronic musculoskeletal pain: the Norwegian HUNT study. European Journal of Public Health. 2014; 24(6): 924–9. doi: 10.1093/eurpub/ckt198 [DOI] [PubMed] [Google Scholar]
- 47.Peltonen M, Lindroos AK, Torgerson JS. Musculoskeletal pain in the obese: A comparison with a general population and long-term changes after conventional and surgical obesity treatment. Pain. 2003; 104(3): 549–57. doi: 10.1016/S0304-3959(03)00091-5 [DOI] [PubMed] [Google Scholar]
- 48.Tsuritani I, Honda R, Noborisaka Y, Ishida M, Ishizaki M, Yamada Y. Impact of obesity on musculoskeletal pain and difficulty of daily movements in Japanese middle-aged women. Maturitas. 2002; 42(1): 23–30. doi: 10.1016/s0378-5122(02)00025-7 [DOI] [PubMed] [Google Scholar]
- 49.Brooks JM, Deiches J, Xiaoling X, Batsis JA, Fong C, DiMilia P, et al. Differences in Self-Reported Physical Activity, Exercise Self-Efficacy and Outcome Expectancies, and Health Status by Body Mass Index Groups in People with Chronic Pain. Journal of Rehabilitation. 2018; 84(4): 46–52. [PMC free article] [PubMed] [Google Scholar]
- 50.Lee SH, Son C, Yeo S, Ha IH. Cross-sectional analysis of self-reported sedentary behaviors and chronic knee pain among South Korean adults over 50 years of age in KNHANES 2013–2015. BMC Public Health. 2019; 19(1): 1375. doi: 10.1186/s12889-019-7653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwarze M, Hauser W, Schmutzer G, Brahler E, Beckmann NA, Schiltenwolf M. Obesity, depression and hip pain. Musculoskeletal Care. 2019; 17(1): 126–32. doi: 10.1002/msc.1380 [DOI] [PubMed] [Google Scholar]
- 52.Noormohammadpour P, Mansournia MA, Koohpayehzadeh J, Asgari F, Rostami M, Rafei A, et al. Prevalence of chronic neck pain, low back pain, and knee pain and their related factors in community-dwelling adults in Iran: A population-based national study. Clinical Journal of Pain. 2017; 33(2): 181–7. doi: 10.1097/AJP.0000000000000396 [DOI] [PubMed] [Google Scholar]
- 53.Suri P, Boyko EJ, Smith NL, Jarvik JG, Williams FMK, Jarvik GP, et al. Modifiable risk factors for chronic back pain: insights using the co-twin control design. Spine Journal. 2017; 17(1): 4–14. doi: 10.1016/j.spinee.2016.07.533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Walsh TP, Butterworth PA, Urquhart DM, Cicuttini FM, Landorf KB, Wluka AE, et al. Increase in body weight over a two-year period is associated with an increase in midfoot pressure and foot pain. Journal of foot and ankle research. 2017; 10: 31. doi: 10.1186/s13047-017-0214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goulston L, D’Angelo S, Sanchez M, Spector T, Hart D, Arden N. Is waist circumference a better predictor of incident symptomatic radiographic knee osteoarthritis, radiographic knee osteoarthritis and knee pain than body mass index over 10 years? Osteoarthritis and Cartilage. 2016; 24: S206–S7. [Google Scholar]
- 56.Yang L, Mu L, Huang K, Zhang T, Mei Z, Zeng W, et al. Abdominal adipose tissue thickness measured using magnetic resonance imaging is associated with lumbar disc degeneration in a Chinese patient population. Oncotarget. 2016; 7(50): 82055–62. doi: 10.18632/oncotarget.13255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ruhdorfer AS, Wirth W, Eckstein F. Decline of thigh muscle cross-sectional areas in chronically painful vs. matched painless knees: Data from the osteoarthritis initiative. Osteoarthritis and Cartilage. 2014; 1): S327–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jentzsch T, Geiger J, Slankamenac K, Wanner GA, Simmen HP, Werner CML. Obesity measured by outer abdominal fat may cause facet joint arthritis at the lumbar spine. Swiss Medical Weekly. 2014; 204): 30S. [DOI] [PubMed] [Google Scholar]
- 59.Segar A, Urban J, Fairbank J, Judge A. The influence of obesity on back and leg pain in spinal patients: A study of 2,636 patients. Osteoarthritis and Cartilage. 2015; 2): A378–A9. [Google Scholar]
- 60.Arranz L, Canela MA, Rafecas M. Relationship between body mass index, fat mass and lean mass with SF-36 quality of life scores in a group of fibromyalgia patients. Rheumatology International. 2012; 32(11): 3605–11. doi: 10.1007/s00296-011-2250-y [DOI] [PubMed] [Google Scholar]
- 61.Jespersen E, Verhagen E, Holst R, Klakk H, Heidemann M, Rexen CT, et al. Total body fat percentage and body mass index and the association with lower extremity injuries in children: a 2.5-year longitudinal study. British journal of sports medicine. 2014; 48(20): 1497–502. doi: 10.1136/bjsports-2013-092790 [DOI] [PubMed] [Google Scholar]
- 62.Hashem LE, Roffey DM, Alfasi AM, Papineau GD, Wai DC, Phan P, et al. Exploration of the Inter-Relationships Between Obesity, Physical Inactivity, Inflammation, and Low Back Pain. Spine (03622436). 2018; 43(17): 1218–24. [DOI] [PubMed] [Google Scholar]
- 63.Cooper DJ, Scammell BE, Batt ME, Palmer D. Factors associated with pain and osteoarthritis at the hip and knee in Great Britain’s Olympians: a cross-sectional study. British Journal of Sports Medicine. 2018; 52(17): 1101–8. doi: 10.1136/bjsports-2017-098315 [DOI] [PubMed] [Google Scholar]
- 64.Quittner M, Rantalainen T, Ridgers ND, Trudel G, Sheikh A, Connell D, et al. Intervertebral disc status is associated with vertebral marrow adipose tissue and muscular endurance. European Spine Journal. 2018; 27(8): 1704–11. doi: 10.1007/s00586-018-5567-3 [DOI] [PubMed] [Google Scholar]
- 65.Resnick B, Hebel JR, Gruber-Baldini AL, Hicks GE, Hochberg MC, Orwig D, et al. The impact of body composition, pain and resilience on physical activity, physical function and physical performance at 2 months post hip fracture. Archives of Gerontology & Geriatrics. 2018; 76: 34–40. doi: 10.1016/j.archger.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlaeger S, Inhuber S, Rohrmeier A, Dieckmeyer M, Freitag F, Klupp E, et al. Association of paraspinal muscle water-fat MRI-based measurements with isometric strength measurements. European Radiology. 2019; 29(2): 599–608. doi: 10.1007/s00330-018-5631-8 [DOI] [PubMed] [Google Scholar]
- 67.Goubert D, Meeus M, Willems T, De Pauw R, Coppieters I, Crombez G, et al. The association between back muscle characteristics and pressure pain sensitivity in low back pain patients. Scandinavian Journal of Pain. 2018; 18(2): 281–93. doi: 10.1515/sjpain-2017-0142 [DOI] [PubMed] [Google Scholar]
- 68.Davison M, Keir P, Maly M, Adachi J, Beattie K. Knee pain intensity is associated with muscle adiposity in the whole thigh and hamstrings of women with knee osteoarthritis. Journal of Rheumatology. 2016; 43 (6): 1149–50. [Google Scholar]
- 69.Davison M, Maly MR, Adachi JD, Beattie KA. Calf muscle adiposity is associated with impaired physical performance in knee OA. Arthritis and Rheumatology Conference: American College of Rheumatology/Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP. 2015; 67(SUPPL. 10).
- 70.Dannhauer T, Ruhdorfer A, Sattler M, Wirth W, Eckstein F. Cross sectional and longitudinal relationship of thigh adipose tissue with knee pain, radiographic oa status, and structural progression-data from the osteoarthritis initiative. Osteoarthritis and Cartilage. 2014; 1): S331. [Google Scholar]
- 71.Wang J, Han W, Wang X, Pan F, Liu Z, Halliday A, et al. Mass effect and signal intensity alteration in the suprapatellar fat pad: Associations with knee symptoms and structure. Osteoarthritis and Cartilage. 2014; 22(10): 1619–26. doi: 10.1016/j.joca.2014.05.018 [DOI] [PubMed] [Google Scholar]
- 72.Le Cara EC, Marcus RL, Dempsey AR, Hoffman MD, Hebert JJ. Morphology versus function: The relationship between lumbar multifidus intramuscular adipose tissue and muscle function among patients with low back pain. Archives of Physical Medicine and Rehabilitation. 2014; 95(10): 1846–52. doi: 10.1016/j.apmr.2014.04.019 [DOI] [PubMed] [Google Scholar]
- 73.Dannhauer T, Ruhdorfer A, Wirth W, Eckstein F. Quantitative relationship of thigh adipose tissue with pain, radiographic status, and progression of knee osteoarthritis: Longitudinal findings from the osteoarthritis initiative. Investigative Radiology. 2015; 50(4): 268–74. doi: 10.1097/RLI.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 74.Maly MR, Calder KM, MacIntyre NJ, Beattie KA. Relationship of intermuscular fat volume in the thigh with knee extensor strength and physical performance in women at risk of or with knee osteoarthritis. Arthritis Care and Research. 2013; 65(1): 44–52. doi: 10.1002/acr.21868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Almeida AC, Pedroso MG, Aily JB, Goncalves GH, Pastre CM, Mattiello SM. Influence of a periodized circuit training protocol on intermuscular adipose tissue of patients with knee osteoarthritis: Protocol for a randomized controlled trial. BMC Musculoskeletal Disorders. 2018; 19 (1) (no pagination)(421). doi: 10.1186/s12891-018-2325-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blumel JE, Arteaga E, Mezones-Holguin E, Zuniga MC, Witis S, Vallejo MS, et al. Obesity is associated with a higher prevalence of musculoskeletal pain in middle-aged women. Gynecological Endocrinology. 2017; 33(5): 378–82. doi: 10.1080/09513590.2016.1269741 [DOI] [PubMed] [Google Scholar]
- 77.Seo YI, Kim HA, Cho NH, Yoo JJ. Relationship between body mass index, fat mass and muscle mass withmusculoskeletal pain in community residents. Arthritis and Rheumatism. 2013; 10): S467. [Google Scholar]
- 78.Mundal I, Grawe RW, Bjorngaard JH, Linaker OM, Fors EA. Prevalence and long-term predictors of persistent chronic widespread pain in the general population in an 11-year prospective study: The HUNT study. BMC Musculoskeletal Disorders. 2014; 15 (1) (no pagination)(213). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park IY, Cho NH, Lim SH, Kim HA. Gender-specific associations between fat mass, metabolic syndrome and musculoskeletal pain in community residents: A three-year longitudinal study. PLoS ONE. 2018; 13 (7) (e0200138). doi: 10.1371/journal.pone.0200138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vehmas T, Shiri R, Luoma K, Viikari-Juntura E. The relations of obesity indicators and early metabolic disturbance with upper extremity pain. Pain medicine (Malden, Mass). 2013; 14(7): 1081–7. doi: 10.1111/pme.12132 [DOI] [PubMed] [Google Scholar]
- 81.Brady SR, Mamuaya BB, Cicuttini F, Wluka AE, Wang Y, Hussain SM, et al. Body composition is associated with multisite lower body musculoskeletal pain in a community-based study. The journal of pain: official journal of the American Pain Society. 2015; 16(8): 700–6. [DOI] [PubMed] [Google Scholar]
- 82.Mikkonen PH, Laitinen J, Remes J, Tammelin T, Taimela S, Kaikkonen K, et al. Association between overweight and low back pain: a population-based prospective cohort study of adolescents. Spine (Phila Pa 1976). 2013; 38(12): 1026–33. doi: 10.1097/BRS.0b013e3182843ac8 [DOI] [PubMed] [Google Scholar]
- 83.Bjurvald LM, Morinder G, Janson A. Musculoskeletal pain in obese children and adolescents at a specialized pediatric obesity clinic. Obesity Facts. 2018; 11 (Supplement 1): 189. [Google Scholar]
- 84.Deere KC, Clinch J, Holliday K, McBeth J, Crawley EM, Sayers A, et al. Obesity is a risk factor for musculoskeletal pain in adolescents: Findings from a population-based cohort. Pain. 2012; 153(9): 1932–8. doi: 10.1016/j.pain.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 85.Butterworth PA, Urquhart DM, Cicuttini FM, Menz HB, Strauss BJ, Proietto J, et al. Fat mass is a predictor of incident foot pain. Obesity. 2013; 21(9): E495–9. doi: 10.1002/oby.20393 [DOI] [PubMed] [Google Scholar]
- 86.Barber Foss KD, Hornsby M, Edwards NM, Myer GD, Hewett TE. Is body composition associated with an increased risk of developing anterior knee pain in adolescent female athletes? The Physician and sportsmedicine. 2012; 40(1): 13–9. doi: 10.3810/psm.2012.02.1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shiri R, Solovieva S, Husgafvel-Pursiainen K, Telama R, Yang X, Viikari J, et al. The role of obesity and physical activity in non-specific and radiating low back pain: the Young Finns study. Seminars in arthritis and rheumatism. 2013; 42(6): 640–50. doi: 10.1016/j.semarthrit.2012.09.002 [DOI] [PubMed] [Google Scholar]
- 88.Heuch I, Hagen K, Zwart JA. A comparison of anthropometric measures for assessing the association between body size and risk of chronic low back pain: The HUNT study. PLoS ONE. 2015; 10(10): no pagination. doi: 10.1371/journal.pone.0141268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jin X, Ding C, Wang X, Antony B, Laslett LL, Blizzard L, et al. Longitudinal associations between adiposity and change in knee pain: Tasmanian older adult cohort study. Seminars in arthritis and rheumatism. 2016; 45(5): 564–9. doi: 10.1016/j.semarthrit.2015.10.006 [DOI] [PubMed] [Google Scholar]
- 90.Walsh TP, Gill TK, Evans AM, Yaxley A, Shanahan EM, Hill CL. Association of Fat Mass and Adipokines with Foot Pain in a Community Cohort. Arthritis Care and Research. 2016; 68(4): 526–33. doi: 10.1002/acr.22719 [DOI] [PubMed] [Google Scholar]
- 91.Batsis JA, Zbehlik AJ, Barre LK, Mackenzie TA, Bartels SJ. The impact of waist circumference on function and physical activity in older adults: longitudinal observational data from the osteoarthritis initiative. Nutrition journal. 2014; 13: 81. doi: 10.1186/1475-2891-13-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hussain SM, Urquhart DM, Wang Y, Shaw JE, Magliano DJ, Wluka AE, et al. Fat mass and fat distribution are associated with low back pain intensity and disability: Results from a cohort study. Arthritis Research and Therapy. 2017; 19 (1) (no pagination)(26). doi: 10.1186/s13075-017-1242-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dario AB, Loureiro Ferreira M, Refshauge K, Luque-Suarez A, Ordonana JR, Ferreira PH. Obesity does not increase the risk of chronic low back pain when genetics are considered. A prospective study of Spanish adult twins. Spine Journal. 2017; 17(2): 282–90. doi: 10.1007/s12020-017-1326-1 [DOI] [PubMed] [Google Scholar]
- 94.Pan F, Laslett L, Blizzard L, Cicuttini F, Winzenberg T, Ding C, et al. Associations Between Fat Mass and Multisite Pain: A Five-Year Longitudinal Study. Arthritis care & research. 2017; 69(4): 509–16. doi: 10.1002/acr.22963 [DOI] [PubMed] [Google Scholar]
- 95.Hashimoto Y, Matsudaira K, Sawada SS, Gando Y, Kawakami R, Kinugawa C, et al. Obesity and low back pain: a retrospective cohort study of Japanese males. Journal of physical therapy science. 2017; 29(6): 978–83. doi: 10.1589/jpts.29.978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shiri R, Falah-Hassani K, Heliovaara M, Solovieva S, Amiri S, Lallukka T, et al. Risk Factors for Low Back Pain: A Population-Based Longitudinal Study. Arthritis care & research. 2019; 71(2): 290–9. doi: 10.1002/acr.23710 [DOI] [PubMed] [Google Scholar]
- 97.Brady SRE, Urquhart DM, Hussain SM, Teichtahl A, Wang Y, Wluka AE, et al. High baseline fat mass, but not lean tissue mass, is associated with high intensity low back pain and disability in community-based adults. Arthritis Research and Therapy. 2019; 21 (1) (no pagination)(165). doi: 10.1186/s13075-019-1953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Laslett LL, Menz HB, Otahal P, Pan F, Cicuttini FM, Jones G. Factors associated with prevalent and incident foot pain: data from the Tasmanian Older Adult Cohort Study. Maturitas. 2018; 118: 38–43. doi: 10.1016/j.maturitas.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 99.Walsh TP, Quinn SJ, Evans AM, Yaxley A, Chisholm JA, Kow L, et al. Fat mass, but not fat-free mass, predicts increased foot pain with obesity, independent of bariatric surgery. Surgery for Obesity & Related Diseases. 2018; 14(9): 1389–95. [DOI] [PubMed] [Google Scholar]
- 100.Muthuri S, Cooper R, Kuh D, Hardy R. Do the associations of body mass index and waist circumference with back pain change as people age? 32 years of follow-up in a British birth cohort. BMJ Open. 2020; 10(12): e039197. doi: 10.1136/bmjopen-2020-039197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pan F, Tian J, Cicuttini F, Jones G. Metabolic syndrome and trajectory of knee pain in older adults. Osteoarthritis Cartilage. 2020; 28(1): 45–52. doi: 10.1016/j.joca.2019.05.030 [DOI] [PubMed] [Google Scholar]
- 102.Fearon A, Stephens S, Cook J, Smith P, Neeman T, Cormick W, et al. The relationship of femoral neck shaft angle and adiposity to greater trochanteric pain syndrome in women. A case control morphology and anthropometric study. Br J Sports Med. 2012; 46(12): 888–92. doi: 10.1136/bjsports-2011-090744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Celan D, Turk Z. The impact of anthropometric parameters on the incidence of low back pain. Collegium antropologicum. 2005; 29(1): 101–5. [PubMed] [Google Scholar]
- 104.Yip YB, Ho SC, Chan SG. Tall stature, overweight and the prevalence of low back pain in Chinese middle-aged women. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 2001; 25(6): 887–92. [DOI] [PubMed] [Google Scholar]
- 105.Dario AB, Ferreira ML, Refshauge K, Sanchez-Romera JF, Luque-Suarez A, Hopper JL, et al. Are obesity and body fat distribution associated with low back pain in women? A population-based study of 1128 Spanish twins. European Spine Journal. 2016; 25(4): 1188–95. doi: 10.1007/s00586-015-4055-2 [DOI] [PubMed] [Google Scholar]
- 106.Walsh TP, Arnold JB, Gill TK, Evans AM, Yaxley A, Hill CL, et al. Foot pain severity is associated with the ratio of visceral to subcutaneous fat mass, fat-mass index and depression in women. Rheumatology International. 2017; 37(7): 1175–82. doi: 10.1007/s00296-017-3743-0 [DOI] [PubMed] [Google Scholar]
- 107.Sakai Y, Matsui H, Ito S, Hida T, Ito K, Koshimizu H, et al. Sarcopenia in elderly patients with chronic low back pain. Osteoporosis and Sarcopenia. 2017; 3(4): 195–200. doi: 10.1016/j.afos.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Spyropoulos P, Chronopoulos E, Papathanasiou G, Georgoudis G, Koutis C, Kompoti A. Chronic low back pain and function of Greek office workers.2008. 129–35 p. [Google Scholar]
- 109.Sutbeyaz ST, Sezer N, Koseoglu BF, Ibrahimoglu F, Tekin D. Influence of knee osteoarthritis on exercise capacity and quality of life in obese adults. Obesity. 2007; 15(8): 2071–6. doi: 10.1038/oby.2007.246 [DOI] [PubMed] [Google Scholar]
- 110.Li H, George DM, Jaarsma RL, Mao X. Metabolic syndrome and components exacerbate osteoarthritis symptoms of pain, depression and reduced knee function. Ann Transl Med. 2016; 4(7): 133. doi: 10.21037/atm.2016.03.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hultman G, Nordin M, Saraste H, Ohlsèn H. Body composition, endurance, strength, cross-sectional area, and density of MM erector spinae in men with and without low back pain. J Spinal Disord. 1993; 6(2): 114–23. [PubMed] [Google Scholar]
- 112.Briggs MS, Givens DL, Schmitt LC, Taylor CA. Relations of C-reactive protein and obesity to the prevalence and the odds of reporting low back pain. Arch Phys Med Rehabil. 2013; 94(4): 745–52. doi: 10.1016/j.apmr.2012.11.026 [DOI] [PubMed] [Google Scholar]
- 113.Han TS, Schouten JS, Lean ME, Seidell JC. The prevalence of low back pain and associations with body fatness, fat distribution and height. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1997; 21(7): 600–7. doi: 10.1038/sj.ijo.0800448 [DOI] [PubMed] [Google Scholar]
- 114.Bihari V, Kesavachandran C, Pangtey BS, Srivastava AK, Mathur N. Musculoskeletal pain and its associated risk factors in residents of National Capital Region. Indian journal of occupational and environmental medicine. 2011; 15(2): 59–63. doi: 10.4103/0019-5278.90375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hodselmans AP, Dijkstra PU, Geertzen JH, van der Schans CP. Nonspecific chronic low back pain patients are deconditioned and have an increased body fat percentage. International journal of rehabilitation research Internationale Zeitschrift fur Rehabilitationsforschung Revue internationale de recherches de readaptation. 2010; 33(3): 268–70. doi: 10.1097/MRR.0b013e328335213f [DOI] [PubMed] [Google Scholar]
- 116.Ojoawo A, Oloagun aM, Bamiwoyec bS. Relationship Between Pain Intensity and Anthropometric Indices in Women with low back pain –A Cross-Sectional Study. J Phys Ther. 2011; 3(2): 45–51. [Google Scholar]
- 117.Perry M, Straker L, O’Sullivan P, Smith A, Hands B. Fitness, motor competence, and body composition are weakly associated with adolescent back pain. The Journal of orthopaedic and sports physical therapy. 2009; 39(6): 439–49. doi: 10.2519/jospt.2009.3011 [DOI] [PubMed] [Google Scholar]
- 118.Toda Y, Segal N, Toda T, Morimoto T, Ogawa R. Lean body mass and body fat distribution in participants with chronic low back pain. Archives of internal medicine. 2000; 160(21): 3265–9. doi: 10.1001/archinte.160.21.3265 [DOI] [PubMed] [Google Scholar]
- 119.Tanamas SK, Wluka AE, Berry P, Menz HB, Strauss BJ, Davies-Tuck M, et al. Relationship between obesity and foot pain and its association with fat mass, fat distribution, and muscle mass. Arthritis Care Res (Hoboken). 2012; 64(2): 262–8. [DOI] [PubMed] [Google Scholar]
- 120.Ozer Kaya D, Duzgun I, Baltaci G. Differences in body fat mass, muscular endurance, coordination and proprioception in woman with and without knee pain: a cross-sectional study. Acta orthopaedica et traumatologica turcica. 2014; 48(1): 43–9. doi: 10.3944/AOTT.2014.3135 [DOI] [PubMed] [Google Scholar]
- 121.Iizuka Y, Iizuka H, Mieda T, Tajika T, Yamamoto A, Ohsawa T, et al. Association between neck and shoulder pain, back pain, low back pain and body composition parameters among the Japanese general population. BMC Musculoskeletal Disorders. 2015; 16: 333. doi: 10.1186/s12891-015-0759-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brooks C, Siegler JC, Marshall PW. Relative abdominal adiposity is associated with chronic low back pain: a preliminary explorative study. BMC public health. 2016; 16: 700. doi: 10.1186/s12889-016-3357-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Frilander H, Solovieva S, Mutanen P, Pihlajamaki H, Heliovaara M, Viikari-Juntura E. Role of overweight and obesity in low back disorders among men: A longitudinal study with a life course approach. BMJ Open. 2015; 5(8): no pagination. doi: 10.1136/bmjopen-2015-007805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frilander H, Viikari-Juntura E, Heliövaara M, Mutanen P, Mattila VM, Solovieva S, et al. Obesity in early adulthood predicts knee pain and walking difficulties among men: A life course study. European Journal of Pain. 2016; 20(8): 1278–87. doi: 10.1002/ejp.852 [DOI] [PubMed] [Google Scholar]
- 125.Lee JY, Han K, McAlindon TE, Park YG, Park SH. Lower leg muscle mass relates to knee pain in patients with knee osteoarthritis. International journal of rheumatic diseases. 2016. doi: 10.1111/1756-185X.12896 [DOI] [PubMed] [Google Scholar]
- 126.Butterworth PA, Menz HB, Urquhart DM, Cicuttini FM, Landorf KB, Pasco JA, et al. Fat Mass Is Associated with Foot Pain in Men: The Geelong Osteoporosis Study. Journal of Rheumatology. 2016; 43(1): 138–43. doi: 10.3899/jrheum.141331 [DOI] [PubMed] [Google Scholar]
- 127.Shiri R, Solovieva S, Husgafvel-Pursiainen K, Taimela S, Saarikoski LA, Huupponen R, et al. The association between obesity and the prevalence of low back pain in young adults: the Cardiovascular Risk in Young Finns Study. Am J Epidemiol. 2008; 167(9): 1110–9. doi: 10.1093/aje/kwn007 [DOI] [PubMed] [Google Scholar]
- 128.Alfieri FM, Silva N, Battistella LR. Study of the relation between body weight and functional limitations and pain in patients with knee osteoarthritis. Einstein. 2017; 15(3): 307–12. doi: 10.1590/S1679-45082017AO4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Scott D, Blizzard L, Fell J, Jones G. Prospective study of self-reported pain, radiographic osteoarthritis, sarcopenia progression, and falls risk in community-dwelling older adults. Arthritis Care Res (Hoboken). 2012; 64(1): 30–7. [DOI] [PubMed] [Google Scholar]
- 130.Machado LAC, Viana JU, Da Silva SLA, Couto FGP, Mendes LP, Ferreira PH, et al. Correlates of a recent history of disabling low back pain in community-dwelling older persons the pain in the elderly (PAINEL) study. Clin J Pain. 2018; 34(6): 515–24. doi: 10.1097/AJP.0000000000000564 [DOI] [PubMed] [Google Scholar]
- 131.Endo T, Abe T, Akai K, Kijima T, Takeda M, Yamasaki M, et al. Height loss but not body composition is related to low back pain in community-dwelling elderlies: Shimane CoHRE study. BMC Musculoskeletal Disorders. 2019; 20(1): 207. doi: 10.1186/s12891-019-2580-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Muramoto A, Imagama S, Ito Z, Hirano K, Tauchi R, Ishiguro N, et al. Waist circumference is associated with locomotive syndrome in elderly females. J Orthop Sci. 2014; 19(4): 612–9. doi: 10.1007/s00776-014-0559-6 [DOI] [PubMed] [Google Scholar]
- 133.Ogwumike OO, Adeniyi AF, Orogbemi OO. Musculoskeletal pain among postmenopausal women in Nigeria: Association with overall and central obesity. Hong Kong Physiother J. 2016; 34: 41–6. doi: 10.1016/j.hkpj.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brady SRE, Mousa A, Naderpoor N, de Courten MPJ, Cicuttini F, de Courten B. Adipsin Concentrations Are Associated with Back Pain Independently of Adiposity in Overweight or Obese Adults. Front Physiol. 2018; 9: 93. doi: 10.3389/fphys.2018.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kulandaivelan S, Ateef M, Singh V, Chaturvedi R, Joshi S. One year prevalence of low back pain and its correlates in Hisar urban population. Journal of Musculoskeletal Research. 2018; 21(2). [Google Scholar]
- 136.Yoshimoto T, Ochiai H, Shirasawa T, Nagahama S, Uehara A, Sai S, et al. Sex differences in the association of metabolic syndrome with low back pain among middle-aged Japanese adults: a large-scale cross-sectional study. Biol Sex Differ. 2019; 10(1): 33. doi: 10.1186/s13293-019-0249-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nava-Bringas TI, López-Domínguez L, Macías-Hernández SI, Espinosa-Morales R, Chávez-Arias DD, Coronado-Zarco R. Asociación de la composición corporal total con la fuerza del tronco, el dolor y la discapacidad en pacientes con espondiloartrosis lumbar. Cir Cir. 2018; 86(5): 388–91. doi: 10.24875/CIRU.18000006 [DOI] [PubMed] [Google Scholar]
- 138.Hussien H, Kamel E, Kamel R. Association between pain intensity and obesity in patients with chronic non-specific low back pain. Bioscience Research. 2019; 16(4): 3579–83. [Google Scholar]
- 139.Despres JP. Is visceral obesity the cause of the metabolic syndrome? Annals of medicine. 2006; 38(1): 52–63. doi: 10.1080/07853890500383895 [DOI] [PubMed] [Google Scholar]
- 140.Ness-Abramof R, Apovian CM. Waist circumference measurement in clinical practice. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition. 2008; 23(4): 397–404. doi: 10.1177/0884533608321700 [DOI] [PubMed] [Google Scholar]
- 141.Cao H. Adipocytokines in obesity and metabolic disease. The Journal of endocrinology. 2014; 220(2): T47–59. doi: 10.1530/JOE-13-0339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013; 21(9): 1145–53. doi: 10.1016/j.joca.2013.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hartvigsen J, Natvig B, Ferreira M. Is it all about a pain in the back? Best practice & research Clinical rheumatology. 2013; 27(5): 613–23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.