Structured Abstract
Objective
To determine which initial surgical treatment results in the lowest rate of death or neurodevelopmental impairment (NDI) in premature infants with necrotizing enterocolitis (NEC) or isolated intestinal perforation (IP).
Summary Background Data:
The impact of initial laparotomy versus peritoneal drainage for NEC or IP on the rate of death or NDI in extremely low birth weight infants is unknown.
Methods:
We conducted the largest feasible randomized trial in 20 US centers, comparing initial laparotomy versus peritoneal drainage. The primary outcome was a composite of death or NDI at 18–22 months corrected age, analyzed using prespecified frequentist and Bayesian approaches.
Results:
Of 992 eligible infants, 310 were randomized and 96% had primary outcome assessed. Death or NDI occurred in 69% of infants in the laparotomy group versus 70% with drainage (adjusted relative risk [aRR] = 1.0; 95% confidence interval [CI]: 0.87–1.14). A preplanned analysis identified an interaction between preoperative diagnosis and treatment group (p = 0.03). With a preoperative diagnosis of NEC, death or NDI occurred in 69% after laparotomy versus 85% with drainage (aRR=0.81; 95% CI: 0.64 to 1.04). The Bayesian posterior probability that laparotomy was beneficial (risk difference <0) for a preoperative diagnosis of NEC was 97%. For preoperative diagnosis of IP, death or NDI occurred in 69% after laparotomy versus 63% with drainage (aRR, 1.11; 95% CI: 0.95 to 1.31); Bayesian probability of benefit with laparotomy = 18%.
Conclusions:
There was no overall difference in death or NDI rates at 18–22 months corrected age between initial laparotomy versus drainage. However, the preoperative diagnosis of NEC or IP modified the impact of initial treatment.
Mini abstract
In a randomized clinical trial at 20 U.S. centers, there was no difference in the rates of death or neurodevelopmental impairment at 18–22 months in extremely low birthweight infants randomized to initial laparotomy versus peritoneal drain placement. The surgeon’s preoperative diagnosis of necrotizing enterocolitis (NEC) versus isolated intestinal perforation (IP) significantly modified the overall treatment effect. The data suggest that initial laparotomy reduces the rate of death or neurodevelopmental impairment in infants with a preoperative diagnosis of NEC but not IP.
INTRODUCTION
Surgically treated necrotizing enterocolitis (NEC) and isolated intestinal perforation (IP) occur most often in extremely low birth weight (ELBW; <1,000 grams birthweight) infants. Nearly half of these infants die,1,2 approximately 60% of survivors have neurodevelopmental impairment (NDI), and 20% develop cerebral palsy.3,4 Although they may present similarly, NEC and IP constitute two distinct conditions. NEC involves a variable extent of necrotic or perforated bowel, usually with extensive peritoneal contamination.1 IP involves a small, focal perforation with otherwise normal appearing intestine.5 Although the accuracy of preoperative diagnoses is unknown, many surgeons use this distinction in surgical decision making, often preferring laparotomy for presumed NEC and drain placement for infants with presumed IP.6,7
Initial surgical treatment for NEC and IP has been either laparotomy with bowel resection or bedside peritoneal drainage with subsequent laparotomy if needed. The novel therapy of peritoneal drainage was introduced in the 1970’s, primarily due to the vulnerability of the premature infants with these conditions.8 High-quality comparative studies of the new therapy versus the prior standard have been slow in coming.13 Two randomized trials comparing laparotomy versus drainage identified no significant difference in mortality but did not assess NDI.9,10 Neither prior trial enrolled their planned sample size, demonstrating the difficulty of completing such trials. A systematic review identified one prospective observational study and two randomized clinical trials (RCT) comparing initial laparotomy versus peritoneal drainage.11 These authors called for larger multi center trials in which infants are stratified based on the preoperative diagnosis of NEC versus IP, as well as the need for longer term neurodevelopmental assessment.
The best treatment is unknown and difficult to assess because of the need to accurately assess effects on NDI, the challenge of conducting randomized trials of emergency surgical therapies, and the rarity of ELBW infants with NEC or IP.11–13 The Necrotizing Enterocolitis Surgery Trial (NEST) was designed to be the largest feasible trial evaluating the impact of initial laparotomy versus drainage on the rates of death or NDI and whether the preoperative diagnosis of NEC versus IP affects the outcomes. The primary hypothesis was that laparotomy would result in a lower rate of death or NDI at 18–22 months corrected age. We also hypothesized there would be no significant interaction between treatment and preoperative diagnosis, given our limited sample size.
METHODS
Trial Design and Participants
This was a prospective randomized trial conducted at 20 U.S. centers within the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network from January 2010 to March 2017 with follow up completed in August 2019. Infants were randomized in a 1:1 ratio to initial laparotomy versus peritoneal drainage using variable block sizes. The trial was approved by the institutional review board at each center and infants were enrolled and randomized after written informed parental consent was obtained. Eligible infants that were not randomized were offered enrollment into a prospective observational cohort with surgical treatment decided by surgeon and the same outcomes were measured. Enrollment into this parallel cohort required consent and was discontinued February 14, 2013. The treatment and outcome of eligible non-randomized infants will be addressed in a separate manuscript. The trial was registered with ClinicalTrials.gov as NCT01029353.
Inclusion criteria were birth weight ≤ 1,000 grams, age ≤8 weeks and 0 days, a decision to perform surgery for suspected NEC or IP, and hospital capability to perform both laparotomy and drain placement. Exclusion criteria included a major congenital anomaly affecting surgical considerations or the likelihood of the primary outcome; congenital nonbacterial infection; prior NEC or IP; prior laparotomy or drain placement; or parental or attending physician refusal. We excluded infants for whom full support was not provided or for whom follow-up was deemed unlikely, which was assessed by study coordinators at each site.
Randomization and Masking
Infants were stratified by center and estimated baseline risk of death or NDI (≤ 0.74; >0.74) using a formula derived from our prior observational study that included gestational age, birth weight, vasopressor administration at enrollment, high-frequency ventilation at enrollment, FiO2 at enrollment, pH prior to and closest to randomization, and the surgeon’s preoperative diagnosis (NEC or IP).2 The randomization sequence was computer-generated by the data coordinating center and was concealed to all trial personnel. Eligibility criteria were entered into the Neonatal Research Network (NRN) telephone randomization system by study coordinators. If all inclusion criteria were met the infant was randomized and the coordinator received the randomization number and treatment group assignment. Masking of the care providers, study coordinators entering data until discharge, and families of the study subjects was not thought to be feasible. Examiners for the standardized neurologic examination and the Bayley Scales of Infant & Toddler Development, Third Edition (Bayley-III) at 18–22 months corrected age were not told of the treatment group assignment.
Procedures
Peritoneal drainage involved placement of a ¼ inch Penrose drain in the lower abdomen in the neonatal intensive care unit (NICU) with local anesthesia and sedation. Laparotomy was performed under general anesthesia in the operating room or NICU. Surgeons recorded details of either initial surgical procedure, including location of peritoneal drain, intraoperative findings at laparotomy, and occurrence of intraoperative complications in both treatment groups. Operative details and the decision to perform a subsequent laparotomy were at the discretion of the attending pediatric surgeon. Subsequent laparotomy was discouraged during the first 12 hours after initial drainage and the indications for all subsequent laparotomies were recorded. Operative reports and pathology reports for resected specimens were uploaded to the trial database. Trained study coordinators collected data at Randomization, for all operations, and at 120 days in hospital, transfer to another healthcare facility, death, or discharge (whichever came first). Criteria for the surgeon’s preoperative diagnosis of NEC or IP were based on surgeon judgment and pre-trial discussions. Infants were assessed at 18–22 months corrected age according to standard practices within the NRN.14
Outcomes
The primary outcome was a composite of death or NDI at 18–22 months corrected age. NDI was defined as any of the following: moderate to severe cerebral palsy (CP) with Gross Motor Function Classification System level ≥ 2, Bayley-III cognitive composite score <85, severe bilateral visual impairment consistent with vision <20/200, or permanent hearing loss despite amplification that prevents communication or understanding the examiner. Prespecified secondary outcomes included intraoperative complications (hemorrhage, hypothermia, coagulopathy, cardiopulmonary resuscitation, death), post-operative surgical complications (wound dehiscence, intestinal stricture or fistula, other), number of surgical procedures for each infant, sepsis episodes, duration of parenteral nutrition, development of parenteral nutrition-associated cholestasis, length of hospital stay, rehospitalizations, and each component of the composite primary outcome. Ostomy closure was excluded in reporting subsequent laparotomy after either initial surgical treatment.
Statistical analyses
Partly based on our observational study,2 we hypothesized that 80% of infants randomized to initial drain and 65% randomized to laparotomy would die or develop NDI. To achieve 80% power with a two-sided alpha of 0.05, 150 randomized infants per treatment group with primary outcome data were required.
The primary analysis assessed the effect of treatment (laparotomy or drainage) on death or NDI at 18–22 months. Intent-to-treat analyses were performed. Robust Poisson regression models were used to determine the adjusted relative risk (aRR) and adjusted absolute risk difference (aRD) for the primary outcome, including baseline risk of death or NDI as a covariate and center as a clustering effect in a generalized estimating equation (GEE) framework. Primary analyses also included a prespecified assessment of interaction between treatment and preoperative diagnosis (NEC or IP). Although there was some prior evidence that laparotomy may be preferable for NEC2 and drainage better for IP,6,7 we hypothesized that no significant interaction between treatment and preoperative diagnosis would be identified in our trial due to the fairly limited sample size. In accordance with best practices for conducting and reporting subgroup effects,22–25 this single subgroup analysis was prespecified in the trial protocol, was hypothesis-testing not hypothesis-generating, and is consistent with prior publications.6,7
Preplanned secondary analyses aimed at enhancing the interpretation of study findings included Bayesian analyses assessing the adjusted relative risk (aRR), adjusted risk difference (aRD), and posterior probability of benefit for the primary and secondary outcomes.15–20 Neutral prior probabilities (centered at an aRR of 1.0 and aRD of 0) were used with a 95% credible interval (Crl) that included the treatment effects on important clinical outcomes identified in almost all neonatal trials (aRR of 0.33–3.00).21 The agreement between the preoperative and intraoperative diagnosis in infants who had an initial laparotomy was assessed with kappa values. Analyses were performed with SAS, version 9.4 (SAS Institute) and R, version 3.6.1 (R Project for Statistical Computing). Additional information about the analyses is provided in the Supplement.
RESULTS
Between January 2010 and March 2017, 310 infants were randomized to initial laparotomy (n=148) or initial drainage (n=162), Figure 1. Seven infants did not receive the assigned treatment and two were withdrawn. Complete primary outcome data at 18–22 months were available for 96% (295/308). The numbers of subjects assessed for different outcomes varied slightly depending on missing data and exact numbers are shown in Tables 3–5. Treatment groups were comparable at baseline except that more drain infants (29%) than laparotomy infants (19%) received postnatal steroids (Tables 1 and 2).
Figure 1.
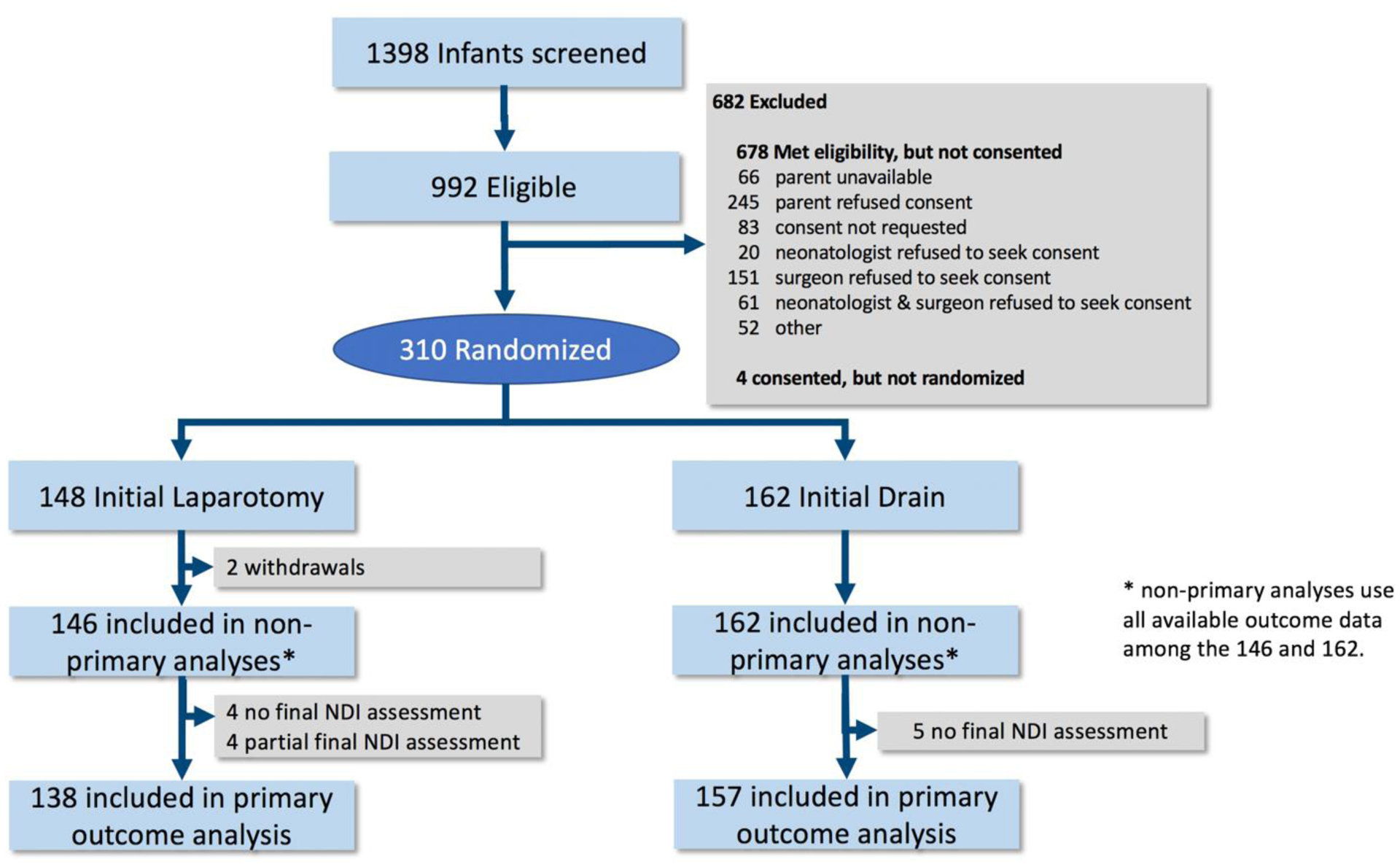
Participant flow
Table 3.
Primary and major secondary outcomes by preoperative diagnosis
| Outcome | Initial Laparotomy (N = 146) n/N (%) | Initial Drainage (N = 162) n/N (%) | Frequentist Adjusted Relative Risk (95% CI) | Bayesian Adjusted Relative Risk (95% CrI) | Bayesian Adjusted Risk Difference (95% CrI) | Bayesian Posterior Probability (%) of RD <0 with Laparotomy |
|---|---|---|---|---|---|---|
| Death or NDI | ||||||
| NEC | 29/42 (69%) | 44/52 (85%) | 0.81 (0.64, 1.04) | 0.81 (0.63, 1.00) | −14% (−31, 1) | 97 |
| IP | 68/99 (69%) | 64/102 (63%) | 1.11 (0.95, 1.31) | 1.09 (0.90, 1.33) | 6% (−7, 18) | 18 |
| Death | ||||||
| NEC | 17/42 (40%) | 27/53 (51%) | 0.77 (0.52, 1.13) | 0.82 (0.53, 1.20) | −11% (−30, 8) | 87 |
| IP | 24/104 (23%) | 21/109 (19%) | 1.28 (0.79, 2.06) | 1.18 (0.74, 1.87) | 5% (−7, 20) | 20 |
| Survival with NDI | ||||||
| NEC | 12/42 (29%) | 17/52 (33%) | 0.73 (0.42, 1.27) | 0.76 (0.48, 1.18) | −19% (−45, 7) | 92 |
| IP | 44/99 (44%) | 43/102 (42%) | 1.11 (0.85, 1.45) | 1.08 (0.83, 1.42) | 5% (−10, 20) | 26 |
| Death or moderate to severe cerebral palsy | ||||||
| NEC | 22/42 (52%) | 38/52 (73%) | 0.70 (0.47, 1.05) | 0.73 (0.52, 0.96) | −18% (−38, −2) | 99 |
| IP | 36/100 (36%) | 41/105 (39%) | 0.95 (0.76, 1.19) | 0.95 (0.68, 1.32) | −2% (−16, 12) | 62 |
| Death or Bayley cognitive composite score < 85 | ||||||
| NEC | 28/42 (67%) | 41/50 (82%) | 0.81 (0.61, 1.06) | 0.80 (0.61, 1.01) | −13% (−32, 2) | 96 |
| IP | 67/99 (68%) | 59/101 (58%) | 1.17 (1.01, 1.37) | 1.15 (0.94, 1.42) | 9% (−4, 22) | 8 |
Denominators in each cell reflect the number of infants with complete data for each outcome reported
Table 5.
Other secondary outcomes by preoperative diagnosis
| Initial Laparotomy (N = 146) n/N (%) | Initial Drainage (N = 162) n/N (%) | Frequentist Adjusted Relative Risk (95% CI) | Frequentist Adjusted Risk Difference (95% CI) | |
|---|---|---|---|---|
| Subsequent laparotomy * | ||||
| NEC | 12/41 (29.3%) | 30/53 (56.6%) | 0.53 (0.31, 0.89) | −26% (−40, −11) |
| IP | 23/104 (22.1%) | 51/109 (46.8%) | 0.44 (0.29, 0.66) | −26% (−36, −15) |
| Any intraoperative complication | ||||
| NEC | 8/41 (19.5%) | 7/53 (13.2%) | 1.44 (0.58, 3.57) | 6% (−8, 19) |
| IP | 21/104 (20.2%) | 14/109 (12.8%) | 1.63 (1.06, 2.50) | 8% (1, 14) |
| Wound dehiscence | ||||
| NEC | 6/42 (14.3%) | 3/53 (5.7%) | 2.52 (0.84, 7.55) | 8% (0, 17) |
| IP | 5/104 (4.8%) | 5/109 (4.6%) | 1.05 (0.38, 2.88) | 0% (−4, 5) |
| Intra-abdominal abscess | ||||
| NEC | 2/42 (4.8%) | 1/53 (1.9%) | 1.78 (0.82, 3.86) | Not Estimable |
| IP | 3/104 (2.9%) | 3/109 (2.8%) | 1.16 (0.42, 3.20) | Not Estimable |
| Intestinal stricture | ||||
| NEC | 4/42 (9.5%) | 3/53 (5.7%) | 1.68 (0.35, 8.05) | Not Estimable |
| IP | 3/103 (2.9%) | 6/109 (5.5%) | 0.50 (0.10, 2.44) | Not Estimable |
| Sepsis | ||||
| NEC | 7/42 (16.7%) | 7/53 (13.2%) | 1.29 (0.62, 2.69) | 4% (−6, 14) |
| IP | 16/104 (15.4%) | 27/107 (25.2%) | 0.60 (0.27, 1.32) | −10% (−23, 4) |
| PN-associated cholestasis | ||||
| NEC | 11/42 (26.2%) | 19/53 (35.9%) | 0.75 (0.41, 1.36) | −9% (−28, 10) |
| IP | 25/104 (24.0%) | 27/109 (24.8%) | 0.99 (0.69, 1.42) | 0% (−9, 8) |
| Severe IVH | ||||
| NEC | 1/39 (2.6%) | 3/51 (5.9%) | 0.43 (0.04, 4.45) | Not Estimable |
| IP | 17/101 (16.8%) | 22/106 (20.8%) | 0.81 (0.44, 1.49) | Not Estimable |
Denominators in each cell reflect the number of infants with complete data for each outcome reported.
Table 1.
Baseline infant characteristics (information prior to or on day of enrollment)
| Characteristic | Initial Laparotomy Group (n=146) | Initial Drainage Group (n = 162) |
|---|---|---|
| Infant | ||
| Gestational age, mean (SD), wk | 25.1 (1.7) | 24.9 (1.7) |
| Birth weight, mean (SD), g | 721.2 (138.4) | 711.4 (135.9) |
| Small for gestational age, No. (%) | 15 (10.3) | 16 (9.9) |
| Male, No. (%) | 80 (54.8) | 98 (60.9) |
| Inborn status, No. (%) | 82 (56.2) | 90 (55.6) |
| Apgar score at 1 minute, median (min, max) | 3 (0, 9) | 3 (0, 9) |
| Apgar score at 5 minutes, median (min, max) | 6 (0, 9) | 6 (1, 10) |
| PDA present, No. (%) | 59 (43.4) | 62 (42.2) |
| Received postnatal steroids, No. (%) | 27 (18.5) | 47 (29.0) |
| Received indomethacin, No. (%) | 74 (51.8) | 82 (52.6) |
| Received enteral feedings, No. (%) | 90 (79.7) | 95 (76.6) |
| Early onset sepsis, No. (%) | 1 (0.69) | 6 (3.8) |
| Late onset sepsis, No. (%) | 37 (25.3) | 47 (29.4) |
| Severe Intraventricular hemorrhage (Grade III or IV), No. (%) | 17 (12.1) | 23 (14.7) |
Table 2.
Baseline characteristics at time of randomization
| Characteristic | Initial Laparotomy Group (n=146) | Initial Drainage Group (n = 162) |
|---|---|---|
| Age, mean (SD), d | 11.6 (10.0) | 12.1 (9.9) |
| On vasopressors, No. (%) | 41 (28.1) | 59 (36.4) |
| Receiving HFOV or HFJV, No. (%) | 32 (21.9) | 48 (29.6) |
| pH, mean (SD) | 7.25 (0.1) | 7.23 (0.1) |
| FiO2, mean (SD) | 44.8 (26.3) | 45.9 (24.1) |
| Mean blood pressure, mean (SD), mmHg | 37.1 (10.3) | 37.0 (11.5) |
| Lowest platelet count, mean (SD) | 155.7 (96.0) | 150.4 (80.9) |
| Surgeon’s preoperative diagnosis, No. (%) | ||
| Necrotizing enterocolitis | 42 (28.8) | 53 (32.7) |
| Isolated intestinal perforation | 104 (71.2) | 109 (67.3) |
| Baseline predicted risk of death or NDI | ||
| Baseline Risk, mean (SD) | 0.62 (0.27) | 0.68 (0.24) |
| Baseline Risk, median (p25, p75) | 0.63 (0.42, 0.88) | 0.71 (0.51, 0.89) |
Primary outcome
Death or NDI at 18–22 months corrected age (primary outcome) occurred in 69% of infants (97/141) after initial laparotomy and 70% (108/154) after drainage (aRR = 1.0, 95% CI: 0.87–1.14). Mortality at 18–22 months was 29% (89/308) overall; 41/146 (28%) with initial laparotomy and 48/162 (30%) with initial drainage (aRR = 1.0; 95% CI: 0.69 – 1.45). The effect of initial treatment on the primary outcome differed according to the preoperative diagnosis of NEC versus IP (p=0.03 for an interaction for aRR and p=0.04 for aRD).
Due to the significant interaction between initial treatment and the preoperative diagnosis, the relationship of initial treatment to primary and major secondary outcomes is reported separately by preoperative diagnosis according to accepted statistical recommendations (Table 3).22,23 Among 94 infants assessed at 18–22 months with a preoperative diagnosis of NEC, 29/42 (69%) died or had NDI after initial laparotomy versus 44/52 (85%) after drainage. The frequentist aRR for initial laparotomy versus drainage was 0.81 (95% CI: 0.64–1.04); the Bayesian aRR = 0.81 (95% CrI: 0.63–1.00); and the Bayesian aRD = −14% (95% CrI: −31,1) favoring laparotomy. The Bayesian posterior probability that laparotomy resulted in a lower rate of death or NDI than did drainage (aRD< 0) was 97% (Figure 2). The posterior probabilities that laparotomy reduced death or NDI by at least 1, 2, and 3% were 95%, 94%, and 92%, respectively. Among 201 infants with a preoperative diagnosis of IP, death or NDI occurred in 68/99 (69%) with initial laparotomy and in 64/102 (63%) with initial drainage; frequentist aRR = 1.11 (95% CI: 0.95–1.31); the Bayesian aRR = 1.09 (95% CrI: 0.90–1.33); Bayesian aRD = 6% (95% CrI: −7, 18). The Bayesian posterior probability of a lower rate of death or NDI with initial laparotomy than drainage (aRD < 0) was 18%. Mortality at 18–22 months occurred in 44/95 (46%) with preoperative diagnosis of NEC versus 45/213 (21%) with a preoperative diagnosis of IP. Among infants with a preoperative diagnosis of NEC, mortality was 40% (17/42) with initial laparotomy versus 51% (27/53) with initial drainage. The aRR was 0.77 (95% CI: 0.52–1.13) and the Bayesian aRD for mortality was −11% (95%CrI: −30,8) favoring laparotomy; the probability of a lower death rate (aRD<0) with laparotomy was 87% (Table 2). Among infants with a preoperative diagnosis of IP, 23% (24/104) died after initial laparotomy versus 19% (21/109) with initial drainage. The aRR was 1.28 (95% CI: 0.79–2.06); the Bayesian aRD for mortality was 5% (95% CrI: −7,20); and the probability of a lower death rate (aRD<0) with laparotomy was 20%. Infants with the same preoperative diagnosis in the two treatment groups were comparable at baseline (Supplemental Table 2).
Figure 2. Posterior distribution of the effect of initial surgical treatment (Initial Laparotomy vs. Initial Drainage) on Death or Neurodevelopmental Impairment (NDI) at 18–22 months corrected age.
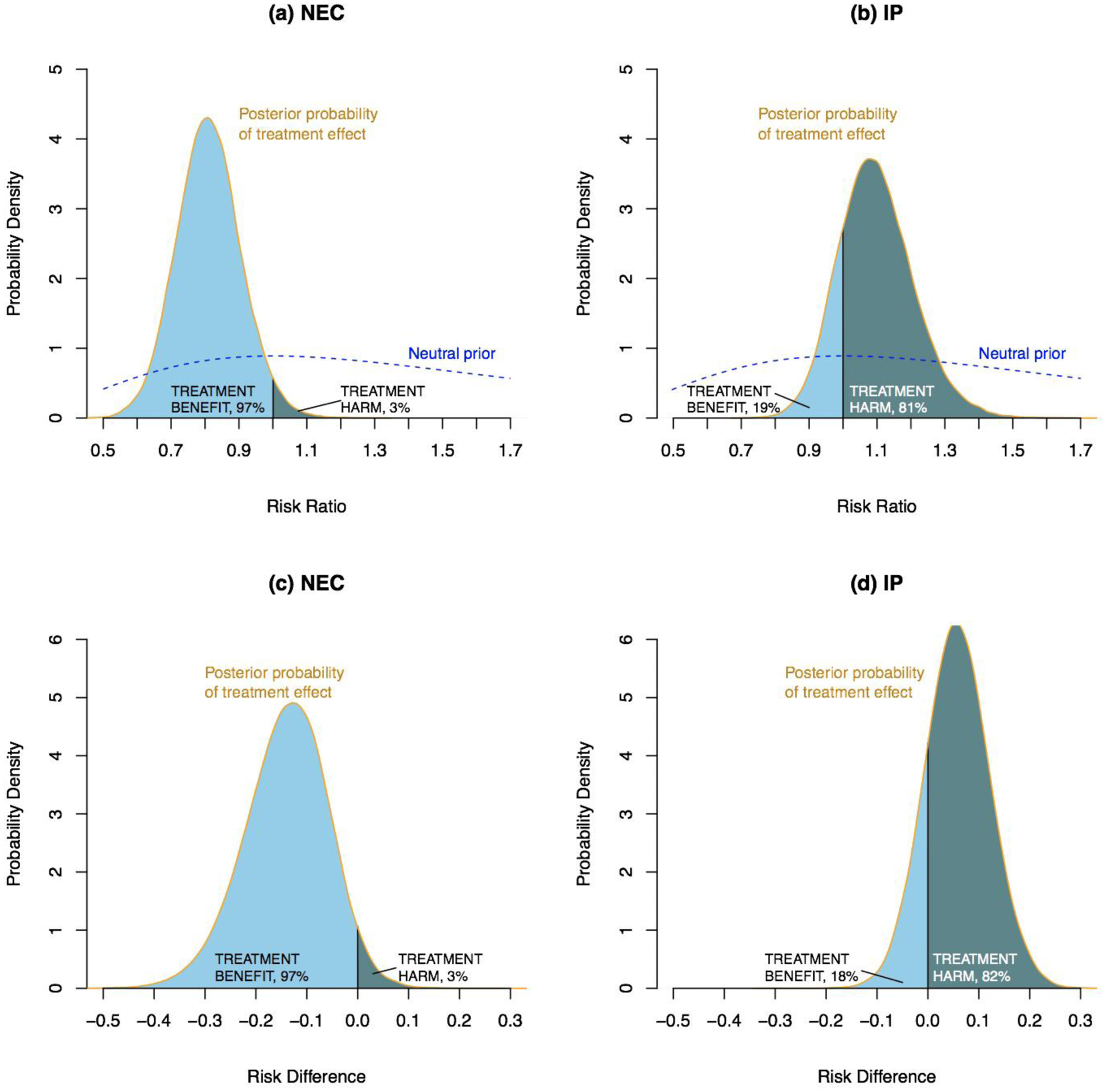
Posterior probability distributions of the adjusted risk ratio and risk difference (Initial Laparotomy minus Initial Drainage) derived by combining a prior distribution with trial results. The dashed blue line plots a neutral prior distribution centered at a risk ratio of 1.0, indicating that a priori an equal number of infants would be expected to benefit by either laparotomy or drainage. Light blue shaded areas indicate the posterior probability of reduced death or disability with initial laparotomy (benefit). Dark blue shaded areas indicate the posterior probability of increased death or disability with initial laparotomy (harm). The total area under each probability density curve equals 1. Among infants with a preoperative diagnosis of NEC (a and c), light blue areas indicate a 97% probability that death or NDI in infants treated with Laparotomy is less likely than in infants treated with initial drainage (benefit). Among infants with a pre-operative diagnosis of IP (b and d), the dark blue areas indicate the probability of death or disability among infants treated with Laparotomy is higher than for infants treated with Initial Drainage (harm).
Secondary outcomes
Findings similar to those for death or NDI and death were observed for other major follow-up outcomes (Table 3). The probability of benefit (aRD<0) with laparotomy relative to drainage with a preoperative diagnosis of NEC ranged from 92–99% for all major follow-up outcomes, including death or moderate to severe cerebral palsy, death or blindness, and death or hearing loss. In contrast, the probability of benefit with laparotomy relative to drainage for infants with a preoperative diagnosis of IP ranged from 8–26%, except for one outcome (62% for death or moderate to severe cerebral palsy). Supplemental Table 3 provides follow-up outcomes for survivors.
Initial laparotomy did not increase the time to full feedings or duration of mechanical ventilation, parenteral nutrition, or hospitalization (Table 4). The total number of operations was similar for treatment groups (mean of 2.0 [SD 1.3] with initial laparotomy versus 2.1 [SD 1.5] with initial drainage). Subsequent laparotomy was performed more often after initial drain (81/162, 50%) than after initial laparotomy (35/145, 24%) (Table 5). Among initial drainage infants that had a subsequent laparotomy, 61/79 (77%) died or had NDI at 18–22 months and 25/81 (31%) died. Of the 53 infants with a preoperative diagnosis of NEC and initial drainage, 23 received drain only and 11 survived (48% survival). Of the 109 infants with a preoperative diagnosis of IP and initial drainage, 58 were treated with drain only and 47 survived (81%).
Table 4.
Other secondary outcomes by preoperative diagnosis
| Outcome | Initial Laparotomy (N = 146) mean (SD) | Initial Drainage (N = 162) mean (SD) | Frequentist Mean Difference (95% CI) |
|---|---|---|---|
| Duration of mechanical ventilation, days | |||
| NEC | 31.0 (22.6) | 31.7 (22.5) | −1.27 (−14.10, 11.6) |
| IP | 34.1 (26.6) | 42.2 (28.0) | −7.85 (−15.84, 0.13) |
| Duration of parenteral nutrition, days | |||
| NEC | 53.5 (31.3) | 65.3 (42.0) | −11.57 (−27.15, 4.01) |
| IP | 56.3 (35.2) | 63.6 (33.7) | −7.63 (−17.46, 2.20) |
| Final bowel length, cm | |||
| NEC | 62.7 (25.0) | 64.0 (44.2) | 4.00 (−18.84, 26.83) |
| IP | 83.1 (20.9) | 74.7 (22.4) | 9.05 (−13.29, 31.38) |
| Time to full feeds, days | |||
| NEC | 29.6 (18.9) | 32.3 (21.3) | −3.13 (−24.62, 18.36) |
| IP | 52.4 (50.4) | 56.3 (27.1) | −2.39 (−14.42, 9.63) |
| Length of hospital stay, days | |||
| NEC | 80.2 (77.8) | 99.3 (87.7) | −18.43 (−48.41, 11.55) |
| IP | 109.6 (67.0) | 121.3 (64.7) | −11.95 (−31.96, 8.06) |
Denominators in each cell reflect the number of infants with complete data for each outcome reported.
Compared to infants with a preoperative diagnosis of IP, those with a preoperative diagnosis of NEC were older at initial operation (mean 21 versus 8 days for IP); more often had pneumatosis on imaging (36% versus 5%), as well as portal vein air (19% versus 2%), and more commonly received postnatal steroids (36% versus 19%) or enteral feedings prior to enrollment (93% versus 72%); all p≤0.001 (Supplemental Table 4). Infants with a preoperative diagnosis of IP more commonly had pneumoperitoneum (93% versus 51%) and were smaller at initial operation (mean 707 versus 900 grams); p<0.001, (Supplemental Table 4).
Among infants who underwent an initial laparotomy and had intraoperative diagnostic data (n=138), the intraoperative diagnosis was concordant with the preoperative diagnosis in 64% (89/138) of cases; kappa = 0.33. With a preoperative diagnosis of NEC, the concordance rate was 78% and with IP it was 59%. In 7% of infants (10/138) an intraoperative diagnosis other than NEC or IP was made at initial laparotomy, including two cases of intestinal volvulus, two gastric perforations, and six other diagnoses.
Intraoperative complications occurred in 20% of infants (29/145) with initial laparotomy and 13% (21/162) with drainage (Table 5). The most common intraoperative complication with initial laparotomy was liver hemorrhage (7/145, 5%); mortality was 43% (3/7) in these patients. Four infants had other hemorrhage, three had intraoperative CPR and there were no intraoperative deaths.
DISCUSSION
Our trial was powered to test the hypothesis that initial laparotomy would result in a 15% absolute lower incidence of death or NDI at 18–22 months corrected age compared to initial drainage. We found no overall difference in death or NDI rates in the initial laparotomy versus drainage groups (aRR = 1.0, 95% CI: 0.87–1.14). Our analysis plan included a prespecified assessment of an interaction between treatment and preoperative diagnosis (NEC or IP) that identified preoperative diagnosis as an effect modifier for death or NDI (p= 0.03). Two prior randomized trials found no overall difference in mortality between laparotomy and drain groups but did not assess whether the overall treatment effect was modified by the preoperative diagnosis and did not assess neurodevelopmental outcomes.9,10
Asking whether the overall treatment effect in a RCT applies to subjects with different baseline characteristics and estimating the treatment effect in subgroups is relevant to most RCTs, although it is not without controversy, and caution is warranted.25 As a result, there are explicit guidelines informing the conduct of such analyses.22–25 While the interaction we identified might be due to chance, such interactions are most credible when, as in our study, they address the primary outcome and are prespecified in the protocol, hypothesis-testing, limited in number, assessed in proper statistical analyses of interaction, and biologically plausible.22–25 It is plausible that initial laparotomy would more likely benefit infants with NEC, especially those with multiple perforations and extensive intestinal necrosis and peritonitis whereas infants with IP and a single and often small perforation may require only a peritoneal drain. Multiple pediatric surgery groups currently use clinical practice guidelines that advise treating infants with NEC with laparotomy and those with IP with initial peritoneal drainage.6,7,30
Despite the fact that we prespecified the interaction testing and planned to estimate the treatment effect in each diagnostic subgroup, due to sample size constraints our treatment effect estimates in these subgroups should be considered as secondary findings. In accordance with the American Statistical Association and others, we utilized multiple statistical approaches to augment the interpretation of our trial.16,20,26,27 We used Bayesian analyses to estimate the probability of treatment benefit, a quantity that cannot be assessed with traditional frequentist analyses.15–20 Bayesian analyses have been strongly recommended for analyzing trials with limited statistical power, those involving rare diseases,15,20 and for subgroup analyses like ours.16–19 Bayesian analyses of our trial provides needed information for providers and other stakeholders that must act with limited information that frequentist analyses do not clearly provide, which has also been shown in other trials.29
The p value ordinarily designated for statistical significance (<0.05) was not met in assessing the benefit of initial laparotomy for ELBW infants with NEC, and the 95% confidence interval does not exclude the possibility initial laparotomy is harmful. However, as emphasized by the American Statistical Association, a P value greater than .05 does not necessarily imply evidence in favor of the null hypothesis; many other hypotheses may be equally or more consistent with the data, and scientific conclusions should not be based on whether a P value passes a specific threshold.26,27 For these reasons, a recent commentary in Nature with more than 800 signatories called for avoiding dichotomous thinking, eliminating the use of statistical significance from the medical literature, and promoting more nuanced conclusions.27 While our findings favoring initial laparotomy are not definitive, it is reassuring that our Bayesian analyses identified a 97% posterior probability of benefit with laparotomy. With a preoperative diagnosis of IP, the differences between treatment groups are smaller and more uncertainty remains
Limitations of our trial include a limited sample size and randomization of 31% of eligible infants. Treatment effects may differ in eligible, non-randomized infants and will be addressed in future analyses. Excluding infants because follow up was considered very unlikely (n=28) could introduce selection bias, although including them may increase the proportion of infants that do not have primary outcome data. By chance, treatment groups differed by 10% in baseline postnatal corticosteroid treatment. It is unclear whether such treatment increases or decreases the risk death or NDI among high-risk infants like those enrolled.28,29 The correlation between preoperative and intraoperative diagnosis, assessable only in the laparotomy group, was not high. This was especially true when the preoperative diagnosis was IP, which surgeons should carefully consider when applying the results of our trial. The lack of a true reference standard for these diagnoses is likely partly responsible and should be addressed in future studies. We infer that the preoperative diagnosis, based on physician judgement, is more predictive of outcome than intraoperative findings. Future investigations might include the impact of the rapidity of disease progression, the overall severity of disease, and individual prognostic factors on outcomes.
Important considerations for our findings are the rarity of the conditions studied, the difficulty of conducting trials of emergency surgical therapies, and the slim possibility that new RCTs will compare laparotomy and peritoneal drainage in the foreseeable future. Although peritoneal drainage was introduced in 1977, there have only been two prior RCTs comparing drainage to laparotomy.8–11 Our trial was powered for the overall treatment groups and was conducted within a large, well established research network experienced in conducting difficult neonatal RCTs with robust follow-up to identify effects on NDI. Yet, the trial still required almost 10 years to achieve its sample size and complete the neurodevelopmental assessments for 96% of participants. Our trial highlights questions about the level of evidence that can realistically be expected or required for treatment recommendations for rare and difficult to study diseases.15,18,19
Conclusions
In ELBW infants with NEC or IP, there is no difference in the rates of death or NDI at 18–22 months corrected age when compared in overall treatment groups of initial laparotomy versus initial peritoneal drainage. Preoperative diagnosis (NEC versus IP) is an effect modifier, and our data suggest that initial laparotomy is more likely than initial drainage to reduce death or NDI among infants with a preoperative diagnosis of NEC.
Supplementary Material
Acknowledgements
Participating NRN sites collected data and transmitted it to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this trial. One behalf of the NRN, RTI International had full access to all of the data in the trial, and with the NRN Center Principal Investigators, take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this trial. The following investigators, in addition to those listed as authors, participated in this trial:
NRN Steering Committee Chair: Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006-2011).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (UG1 HD27904) - Martin Keszler, MD; Angelita M. Hensman, PhD RNC-NIC; Barbara Alksninis, RNC PNP; Kristin Basso, MaT RN; Carmena Bishop; Robert T. Burke, MD MPH; Melinda Caskey, MD; Laurie Hoffman, MD; Katharine Johnson, MD; Mary Lenore Keszler, MD; Teresa M. Leach, MEd CAES; Emily Little BSN RN; Elisabeth C. McGowan, MD; Bonnie E. Stephens, MD; Lucille St. Pierre, BS; Elisa Vieira, BSN RN; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (UG1 HD21364) - Anna Maria Hibbs, MD MSCE; Nancy S. Newman, RN; Edward M. Barksdale, Jr., MD.
Children’s Mercy Hospital (UG1 HD68284) - Eugenia K. Pallotto, MD MSCE; Cheri Gauldin, RN BS CCRC; George M. Holcomb III, MD MBA; Dan Ostlie, MD; Anne Holmes, RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC; Allison Scott, RNC-NIC BSN CCRC; Prabhu S. Parimi, MD; Lisa Gaetano, RN MSN.
Cincinnati Children’s Hospital Medical Center (UG1 HD27853, UL1 TR77) - Kurt Schibler, MD; Michael A. Helmrath, MD MS; Stephanie L. Merhar, MD MS; Tanya E. Cahill, MD; Teresa L. Gratton, PA; Cathy Grisby, BSN CCRC; Kristin Kirker, CRC; Sandra Wuertz, RN BSN CLC.
Duke University School of Medicine, University Hospital, University of North Carolina, Duke Regional Hospital, and WakeMed Health and Hospitals (UG1 HD40492, UL1 TR1117) - Ronald N. Goldberg, MD; William Adamson, MD; Obinna O. Adibe, MD; Joanne Finkle, RN JD; William F. Malcolm, MD; Patricia L. Ashley, MD PhD; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandra Grimes, RN BSN; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Janice Wereszczak, CPNP-AC/PC; Stephen D. Kicklighter, MD; Ginger Rhodes-Ryan, ARNP MSN, NNP-BC.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (UG1 HD27851, UL1 TR454) - Ira Adams-Chapman, MD (deceased); David P. Carlton, MD; Ellen C. Hale, RN BS CCRC; Yvonne Loggins, RN; Richard Ricketts, MD; Diane I. Bottcher, RN MSN; Sheena L. Carter, PhD; Salathiel Kendrick-Allwood, MD; Maureen Mulligan LaRossa, RN; Colleen Mackie, RRT; Gloria Smikle, PNP MSN; Lynn C. Comerford, NNP.
Eunice Kennedy Shriver National Institute of Child Health and Human Development - Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (UG1 HD27856, UL1 TR6) - Brenda B. Poindexter, MD MS; Scott A. Engum, MD; Charles M. Leys, MD MSCI; Lu-Ann Papile, MD; Heidi Harmon, MD MS; Dianne E. Herron, RN CCRC; Carolyn Lytle, MD MPH; Lucy C. Miller, RN BSN CCRC; Heike M. Minnich, PsyD HSPP; Lucy Smiley, CCRC; Leslie Dawn Wilson, BSN CCRC.
McGovern Medical School at The University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, and Memorial Hermann Southwest Hospital (UG1 HD21373) - Georgia McDavid, RN; Emily K. Stephens, RN BSN-NIC; KuoJen Tsao, MD; Elizabeth Allen, MS; Julie Arldt-McAlister, RN BSN; Katrina Burson, RN BSN; Allison G. Dempsey, PhD; Andrea F. Duncan, MD MSc; Elizabeth Eason, MD; Patricia W. Evans, MD; Carmen Garcia, RN CCRP; Donna Hall, RN; Beverly Harris, RN BSN; Margarita Jiminez, MD MPH; Janice John, CPNP; Patrick M. Jones, MD MA; Amir Khan, MD; M. Layne Lillie, RN BSN; Karen Martin, RN; Carrie M. Mason, MA LPA; Kimberly Rennie, PhD; Sara C. Martin, RN BSN; Shannon McKee, EdS; Michelle Poe, PhD; Shawna Rodgers, RN BSN; Saba Khan Siddiki, MD; Daniel Sperry, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP).
Nationwide Children’s Hospital, Abigail Wexner Research Institute at Nationwide Children’s Hospital, Center for Perinatal Research, The Ohio State University College of Medicine, and The Ohio State University Wexner Medical Center (UG1 HD68278) -Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, RN, PhD; R. Lawrence Moss, MD; Keith O. Yeates, PhD; Julie Gutentag, RN, BSN; Christopher J. Timan, MD; Kristi L. Small, BS; Rox Ann Sullivan, RN, BSN; Jacqueline McCool; Melanie Stein, RRT, BBA; Erin Ferns; Lorraine Kelly Quan, MD; Rajan Thakkar, MD; Cole Hague, BA, MS; Stephanie Burkhardt, BS, MPH; Jessica Purnell, BS, CCRC; Mary Ann Nelin, MD; Helen Carey, PT, DHSc, PCS; Lindsay Pietruszewski, PT, DPT
RTI International (UG1 HD36790) - Dennis Wallace, PhD; Marie G. Gantz, PhD; Carla M. Bann, PhD; Jeanette O’Donnell Auman, BS; Margaret Crawford, BS; Jenna Gabrio, MPH; Jamie E. Newman, PhD MPH; Carolyn M. Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University and Lucile Packard Children’s Hospital (UG1 HD27880, UL1 TR93) - M. Bethany Ball, BS CCRC; Gary E. Hartman, MD; Barbara Bentley, PhD; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Lynne C. Huffman, MD; Jean G. Kohn, MD MPH; Casey Krueger, PhD; Melinda S. Proud, RCP; Nicholas H. St. John, PhD; Hali Weiss, MD.
Tufts Medical Center (U10 HD53119) - John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Anne Kurfiss, MPH; Ellen Nylen, RN BSN; Paige T. Church, MD.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (UG1 HD34216) - Namasivayam Ambalavanan, MD; Mike K. Chen, MD MBA; Carroll M. Harmon, MD PhD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Kirstin J. Bailey, PhD; Fred J. Biasini, PhD; Stephanie A. Chopko, PhD; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Mary Beth Moses, PT MS PCS; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Julie Preskitt, MSOT MPH; Richard V. Rector, PhD; Sally Whitley, MA OTR-L FAOTA.
University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (UG1 HD68270) - Uday Devaskar, MD; Meena Garg, MD; Stephen B. Shew, MD; Teresa Chanlaw, MPH.
University of Iowa and Mercy Medical Center (UG1 HD53109, UL1 TR442) - John A. Widness, MD; Jane E. Brumbaugh, MD; Heidi M. Harmon, MD; Dan L. Ellsbury, MD; Karen J. Johnson, RN BSN; Jacky R. Walker, RN; Claire A. Goeke, RN; Mendi L. Schmelzel, MSN RN; Diane L. Eastman, RN CPNP MA; Donia B. Bass, MS ARNP NNP-BC; Tracy L. Tud, RN; Graeme J. Pitcher, MBBCh FCS(SA) FACS; Julia S. Shelton, MD MPH.
University of New Mexico Health Sciences Center (UG1 HD53089, UL1 TR41) - Conra Backstrom Lacy, RN; Sandra Brown, BSN; Cynthia Reyes, MD; Carol Hartenberger, BSN MPH; Jean R. Lowe, PhD; Sandra Sundquist Beauman, MSN RNC-NIC; Mary Ruffner Hanson, RN BSN.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (UG1 HD68244) - Eric C. Eichenwald, MD; Haresh Kirpalani, MB MSc; Aasma S. Chaudhary, BS RRT; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD; Jonathan Snyder, RN BSN.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University at Buffalo Women’s and Children’s Hospital of Buffalo (UG1 HD68263, UL1 TR42) - Dale L. Phelps, MD; Mary Rowan, RN; Christopher A. Gitzelmann, MD; Ronnie Guillet, MD PhD; Gary J. Myers, MD; Anne Marie Reynolds, MD MPH; Ann Marie Scorsone, MS CCRC; Kyle Binion, BS; Constance Orme, BA; Holly I.M. Wadkins, MA; Michael G. Sacilowski, MAT CCRC; Rosemary L. Jensen; Joan Merzbach, LMSW; William A. Zorn, PhD; Osman Farooq, MD; Deanna Maffett, RN; Ashley Williams, MSEd; Julianne Hunn, MSHCM; Stephanie Guilford, BS; Karen Wynn, RN; Michelle E. Hartley-McAndrew, MD; Caitlin Fallone, MA; Premini Sabaratnam, MPH; Emily Li, BA; Jennifer Donato, BS; Kimberly G. McKee, BS; Rachel Jones; Timothy P. Stevens, MD MPH; Derek Wakeman, MD; Kelly R. Coleman, PsyD; Jayasree Nair, MD.
University of Texas Southwestern Medical Center, Parkland Health & Hospital System, and Children’s Medical Center Dallas (UG1 HD40689) - Luc P. Brion, MD; Pablo J. Sánchez, MD; Diana M. Vasil, MSN BSN RNC-NIC; Sally S. Adams, MS RN CPNP; Lijun Chen, RN PhD; Maria M. De Leon, RN BSN; Francis Eubanks, RN BSN; Alicia Guzman; Elizabeth T. Heyne, MS MA PA-C PsyD; Lizette E. Lee, RN; Linda A. Madden, BSN RN CPNP; Nancy A. Miller, RN; Janet S. Morgan, RN; Lara Pavageau, MD; Pollieanna Sepulveda, RN BSN; Catherine Twell Boatman, MS CIMI; Joseph T. Murphy, MD; Li Ern Chen, MD MSCS; Anne C. Fischer, MD PhD; Julie R. Fuchs, MD.
University of Utah University Hospital, Intermountain Medical Center, McKay-Dee Hospital, Utah Valley Hospital, and Primary Children’s Medical Center (UG1 HD87226, U10 HD53124, UL1 RR25764) - Mariana Baserga, MD MSCI; Roger G. Faix, MD; Stephen D. Minton, MD; Carrie A. Rau, RN BSN CCRC; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN MS; Shawna Baker, RN; Jill Burnett, RNC; Michael Steffen, PhD; Kathryn D. Woodbury, RN BSN; Susan T. Schaefer, RRT RN BSN; Jennifer O. Elmont, RN BSN; D. Susan Christensen, RNC BSN; Earl Maxson, BSN.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (UG1 HD21385) - Beena G. Sood, MD MS; Steven Bruch, MD; Athina Pappas, MD; Sanjay Chawla, MD; Monika Bajaj, MD; Rebecca Bara, RN BSN; Kirsten Childs, RN BSN; Bogdan Panaitescu, MD PhD; Mary E. Johnson, RN BSN; Laura A. Goldston, MA; Stephanie A. Wiggins, MS; Mary K. Christensen, BA RRT; Martha D. Carlson, MD PhD; John Barks, MD.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1 TR142) - Richard A. Ehrenkranz, MD (deceased); Harris Jacobs, MD; Patricia Cervone, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Joanne Williams, RN BSN.
Data and Safety Monitoring Committee - Christine A. Gleason, MD, chair, University of Washington; Marilee C. Allen, MD, Johns Hopkins University School of Medicine; Robert J. Boyle, MD, University of Virginia Health System; Traci Clemons, PhD, The EMMES Corporation; Mary E. D’Alton, MD, Columbia Ob/Gyn Midtown; Abhik Das (ex officio), PhD, RTI International; Martin Keszler MD, Georgetown University Hospital; Menachem Miodovnik, MD, Washington Hospital Center; T. Michael O’Shea, MD MPH, Wake Forest University School of Medicine; Michael G. Ross, M.D., M.P.H., Harbor-UCLA Medical Center; Robin Steinhorn, MD, The George Washington University; Steven J. Weiner, MS, The George Washington University; Marian Willinger (ex officio), MD, Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Funding for trial:
The following grants from the NICHD supported this trial: UG1 HD27904 (Brown University), UG1 HD21364 (Case Western), UG1 HD68284 (Children’s Mercy), UG1 HD27853, UL1 TR77 (Cincinnati Children’s), UG1 HD40492, UL1 TR1117 (Duke and UNC), UG1 HD27851, UL1 TR454 (Emory), UG1 HD27856, UL1 TR6 (Indiana U), UG1 HD21373 (UT Houston), UG1 HD68278 (Nationwide Children’s), UG1 HD36790 (RTI International), UG1 HD27880, UL1 TR93 (Stanford), U10 HD53119 (Tufts), UG1 HD34216 (UAB), UG1 HD68270 (UCLA), UG1 HD68270 (Iowa), UG1 HD53089, UL1 TR41 (U New Mexico), UG1 HD68244 (U Pennsylvania), UG1 HD68263, UL1 TR42 (Rochester), UG1 HD40689 (UT Southwestern), UG1 HD87226, U10 HD53124, UL1 RR25764 (U Utah), UG1 HD21385 (Wayne State), U10 HD27871, UL1 TR142 (Yale)
References
- 1.Neu J, Walker WA. Necrotizing Enterocolitis. N Engl J Med 2011; 364: 255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blakely ML, Tyson JE, Lally KP, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis or isolated intestinal perforation in extremely low birth weight infants: outcomes through 18 months adjusted age. Pediatrics 2006; 117(4): e680–687. [DOI] [PubMed] [Google Scholar]
- 3.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics 2005; 115(3): 696–703. [DOI] [PubMed] [Google Scholar]
- 4.Wadhawan R, Oh W, Hintz SR, et al. , Neurodevelopmental outcomes of extremely low birth weight infants with spontaneous intestinal perforation or surgical necrotizing enterocolitis. J Perinatol 2014; 34(1): 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pumberger W, Mayr M, Kohlhauser C, et al. Spontaneous localized intestinal perforation in very low birth weight infants: a distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg 2002; 195: 796–803. [DOI] [PubMed] [Google Scholar]
- 6.Cass DL, Brandt ML, Patel DL, et al. Peritoneal drainage as definitive treatment for neonates with isolated intestinal perforation. J Pediatr Surg 2000; 35(11): 1531–1536. [DOI] [PubMed] [Google Scholar]
- 7.Jakaitis BM, Bhatia AM. Definitive peritoneal drainage in the extremely low birth weight infant with spontaneous intestinal perforation: predictors and hospital outcomes. J Perinatol 2015; 35(8): 607–611. [DOI] [PubMed] [Google Scholar]
- 8.Ein SH, Marshall DG, Girvan D. Peritoneal drainage under local anesthesia for perforations from necrotizing enterocolitis. J Pediatr Surg 1977; 12(6): 963–967. [DOI] [PubMed] [Google Scholar]
- 9.Moss RL, Dimmitt RA, Barnhart DC, et al. Laparotomy versus peritoneal drainage for necrotizing enterocolitis and perforation. N Engl J Med 2006; 354: 2225–2234. [DOI] [PubMed] [Google Scholar]
- 10.Rees CM, Eaton S, Kiely EM, et al. Peritoneal drainage or laparotomy for neonatal bowel perforation? A randomized controlled trial. Ann Surg 2008; 248: 44–51. [DOI] [PubMed] [Google Scholar]
- 11.Rao SC, Basani L, Simmer K, et al. Peritoneal drainage versus laparotomy as initial surgical treatment for perforated necrotizing enterocolitis or spontaneous intestinal perforation in preterm low birth weight infants. Cochrane Database Syst Rev 2011June15; (6): CD006182. [DOI] [PubMed] [Google Scholar]
- 12.Flake AW. Necrotizing enterocolitis in preterm infants - is laparotomy necessary? N Engl J Med 2006; 354(21): 2275–2276. [DOI] [PubMed] [Google Scholar]
- 13.McCulloch P, Altman DG, Campbell WB, et al. No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009; 374: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 14.Newman JE, Bann CM, Vohr BR, et al. Improving the Neonatal Research Network annual certification for neurologic examination of the 18–22 month child. J Pediatr 2012;161(6):1041–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legakos SW. Clinical trials and rare diseases. N Engl J Med 2003;348:2455–2456. [DOI] [PubMed] [Google Scholar]
- 16.Wijeysundera DN, Austin PC, Hux JE, et al. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol 2009; 62:13–21. [DOI] [PubMed] [Google Scholar]
- 17.Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian approaches to clinical trials and health care evaluation. John Wiley & Sons Ltd., Chichester, West Sussex, England and Hoboken, NJ, USA. 2004. [Google Scholar]
- 18.Lilford RJ, Thornton JG, Braunholtz D. Clinical trials and rare diseases: a way out of a conundrum. BMJ 1995; 311:1621–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edwards SJL, Lilford RJ, Braunholtz D, et al. Why “underpowered” trials are not necessarily unethical. Lancet 1997;350:804–807. [DOI] [PubMed] [Google Scholar]
- 20.Yarnell CJ, Abrams D, Baldwin MR, et al. , Clinical trials in critical care: can a Bayesian approach enhance clinical and scientific decision making? Lancet Respir Med 2020. doi: 10.1016/S2213-2600(20)30471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sinclair JC, Haughton DE, Bracken MB, et al. Cochrane neonatal systematic reviews: a survey of the evidence for neonatal therapies. Clin Perinatol 2003June;30(2):285–230 [DOI] [PubMed] [Google Scholar]
- 22.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine - reporting subgroup analyses in clinical trials. N Engl J Med 2007;357:2189–2194. [DOI] [PubMed] [Google Scholar]
- 23.Schandelmaier S, Briel M, Varadhan R, et al. , Development of the instrument to assess the credibility of effect modification analyses (ICEMAN) in randomized controlled trials and meta-analyses. CMAJ 2020;192:E901–906.- - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothwell PM. Subgroup analysis in randomized controlled trials: importance, indications, and interpretation. Lancet 2005;365:176–186. [DOI] [PubMed] [Google Scholar]
- 25.Assmann SF, Pocock SJ, Enos LE, et al. Subgroup analysis and other (mis)uses of baseline data in clinical trials. Lancet 2000;355:1064–1069. [DOI] [PubMed] [Google Scholar]
- 26.Wasserstein RL, Schirm AL, Lazar NA. Moving to a world beyond “p < 0.05”. The American Statistician 2019;73: No. Sup1, 1–19. [Google Scholar]
- 27.Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature 2019; 567:305–307 [DOI] [PubMed] [Google Scholar]
- 28.Doyle LW, Cheong JL, Ehrenkranz RA, et al. Late (>7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2017October24;10(10):CD001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doyle LW, Halliday HL, Ehrenkranz RA, et al. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. J Pediatr 2014;165:1258–1260. [DOI] [PubMed] [Google Scholar]
- 30.Quiroz HJ, Rao K, Brady AC, et al. , Protocol-driven surgical care of necrotizing enterocolitis and spontaneous intestinal perforation. J Surg Res 2020;255:396–404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


