Abstract
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by the presence of neuritic plaques and neurofibrillary tangles. The impaired synaptic plasticity and dendritic loss at the synaptic level is an early event associated with the AD pathogenesis. The abnormal accumulation of soluble oligomeric amyloid-β (Aβ), the major toxic component in amyloid plaques, is viewed to trigger synaptic dysfunctions through binding to several presynaptic and postsynaptic partners and thus to disrupt synaptic transmission. Over time, the abnormalities in neural transmission will result in cognitive deficits, which are commonly manifested as memory loss in AD patients. Synaptic plasticity is regulated through glutamate transmission, which is mediated by various glutamate receptors. Here we review recent progresses in the study of metabotropic glutamate receptors (mGluRs) in AD cognition. We will discuss the role of mGluRs in synaptic plasticity and their modulation as a possible strategy for AD cognitive improvement.
Keywords: Alzheimer’s disease, cognition, ionotropic glutamate receptors, long term depression, long term potentiation, metabotropic glutamate receptors, synapse, synaptic plasticity
INTRODUCTION
Glutamate, as a critical excitatory neurotransmitter and primary player in the synaptic plasticity, exerts its physiological functions through binding to its specific receptors [1]. Four classes of receptors have been shown to mediate glutamate transmission: N-methyl-D-aspartate receptors (NMDAs), α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPAs), kainate receptors, and metabotropic glutamate receptors (mGluRs). NMDAs and AMPAs, as well as less abundant kainate receptors, belong to ionotropic glutamate receptors (iGluRs), which are ligand-gated ion channels and require glutamate for conducting rapid excitatory neural transmission [2, 3]. mGluRs require a cascade of signals to transmit a glutamate stimulus from the cell surface to the nucleus [4]. It has been well documented that the levels of glutamate present at any given time in the brain must be optimally maintained for a normal function of the nervous systems [5]; coordination of various iGluRs and mGluRs controls the major and vital functions of glutamate synapses. Because of its indispensable roles in learning and memory as well as synaptogenesis, even the slightest dysregulation in glutamate signaling can inadvertently lead to cognitive dysfunction [6, 7]. Reduced glutamate neurotransmission has been found in association with impaired learning and memory in Alzheimer’s disease (AD) patients [8, 9]. Both iGluRs and mGluRs are found to mediate synaptic failures in AD [10–14]. Memantine, known to modulate NMDA receptor function, is currently prescribed for AD treatment while Ampakines, known as positive allosteric modulators of AMPA receptors, were explored for memory storage and consolidation in AD. In this review, we focus on the action of mGluRs in modulating synaptic transmission in AD, an area that is under investigated.
mGluRs STRUCTURE AND ACTIVATION MECHANISM
mGluRs belong to the class C of G-protein coupled receptors (GPCRs), which have a large extracellular ligand-binding domain, a typical heptahelical membrane structure, and variable lengths of a C-terminal tail. Membrane-bound GPCRs are activated by extracellular or allosteric binding of a ligand following which the coupled G-protein signaling ensues, relaying signals to various downstream effector molecules [15]. Akin to other class C GPCRs, mGluRs structurally comprise of an extracellular domain, bearing the orthosteric binding site for glutamate, and can dimerize constitutively [16, 17]. The conformational changes, occurring in the extracellular domain in response to ligand binding, are transmitted to the helices in the intracellular transmembrane (TM) domain of the receptor that facilitates receptor binding to a G-protein, a process that is popularly known as G-protein coupling of mGluRs [18, 19]. In addition to the orthosteric binding of glutamate, mGluRs can also bind certain molecules allosterically. The allosteric sites are more receptor subtype-specific and can sensitize or desensitize the receptor. Therefore, allosteric binding molecules can be either allosteric agonists, allosteric antagonists, or allosteric modulators [positive allosteric modulators (PAMs) and negative allosteric modulator (NAMs)] [20, 21]. While allosteric agonists can activate the receptor even when there’s no glutamate binding, allosteric modulators fail to do so. Upon binding at the allosteric site of the receptor, PAMs and NAMs indirectly modulate the effects of glutamate transmission [22].
CLASSIFICATION AND EXPRESSIONS OF mGluRs
There are 8 different types of mGluRs, which can be further classified into 3 groups (Table 1) based on their signaling events, sequence similarity, and pharmacological effects [23–25]. The group I mGluRs consist of two members, mGluR1 and mGluR5. The group II also has two members, mGluR2 and mGluR3. The group III has four members: mGluR4, mGluR6, mGluR7, and mGluR8.
Table 1.
Three groups of mGluRs and their signaling pathways
| mGluR group | Members | Brain region | Type of G protein signaling | Key GPCR pathway | Synaptic location |
|---|---|---|---|---|---|
| I | mGluR1 | Broad brain regions; high in Hippocampus | Gq/11 orthe excitatory signaling | Phospholipase C/Inositol-1,4,5-triphosphate/Diacylglycerol (PLC/IP3/DAG) | Postsynaptic |
| mGluR5 | Broad brain regions | ||||
| II | mGluR2 | High in grey matter neurons | Gi/o or the inhibitory signaling | Adenylate cyclase (AC) inhibition | Presynaptic; mGluR3 can also be postsynaptic |
| mGluR3 | Similar to mGluR2 but also prominent in Glia cells | ||||
| III | mGluR4 | High in Cerebellum; broad brain region | Gi/o or the inhibitory signaling | Adenylate cyclase (AC) inhibition; mGlur6 positively couples to cGMP phosphodiesterase | Presynaptic; mGluR6 is postsynaptic |
| mGluR6 | High in retinal neurons | ||||
| mGluR7 | Similar to mGluR4 | ||||
| mGluR8 | Low in most neurons |
Group I mGluRs are predominantly present at the excitatory synapse (Fig. 1), but their locations may vary based on the type of different isoforms [26]. The distribution of mGluR1 in the hippocampus and cerebellum has been extensively studied, and its expression correlates with the establishment of the glutamatergic neurotransmission system and synaptogenesis in these brain regions [27]. mGluR5 receptor is expressed in the hippocampus, amygdala, olfactory bulb, dorsal striatum, nucleus accumbens, and lateral septum [28, 29]. At the synapse, both mGluR1 and mGluR5 appear to be mostly located at postsynaptic terminals, and Homer family proteins regulate the positioning of these receptors close to second messengers [30]. Upon activation, they follow the Gq route of G-protein signaling and activate phospholipase C-β (PLC-β), thus generating inositol triphosphate (IP3) and diacylglycerol (DAG) to trigger intracellular calcium mobilization and PKC activation [31]. Group I mGluRs can sometimes act independent of G-protein-coupled signaling by phosphorylating other target proteins such as transcription factors and ion channels [32]. MAPK/ERK and mTOR/P70 S6 kinase may also be activated by type I receptors and contribute to synaptic plasticity [33].
Fig. 1.
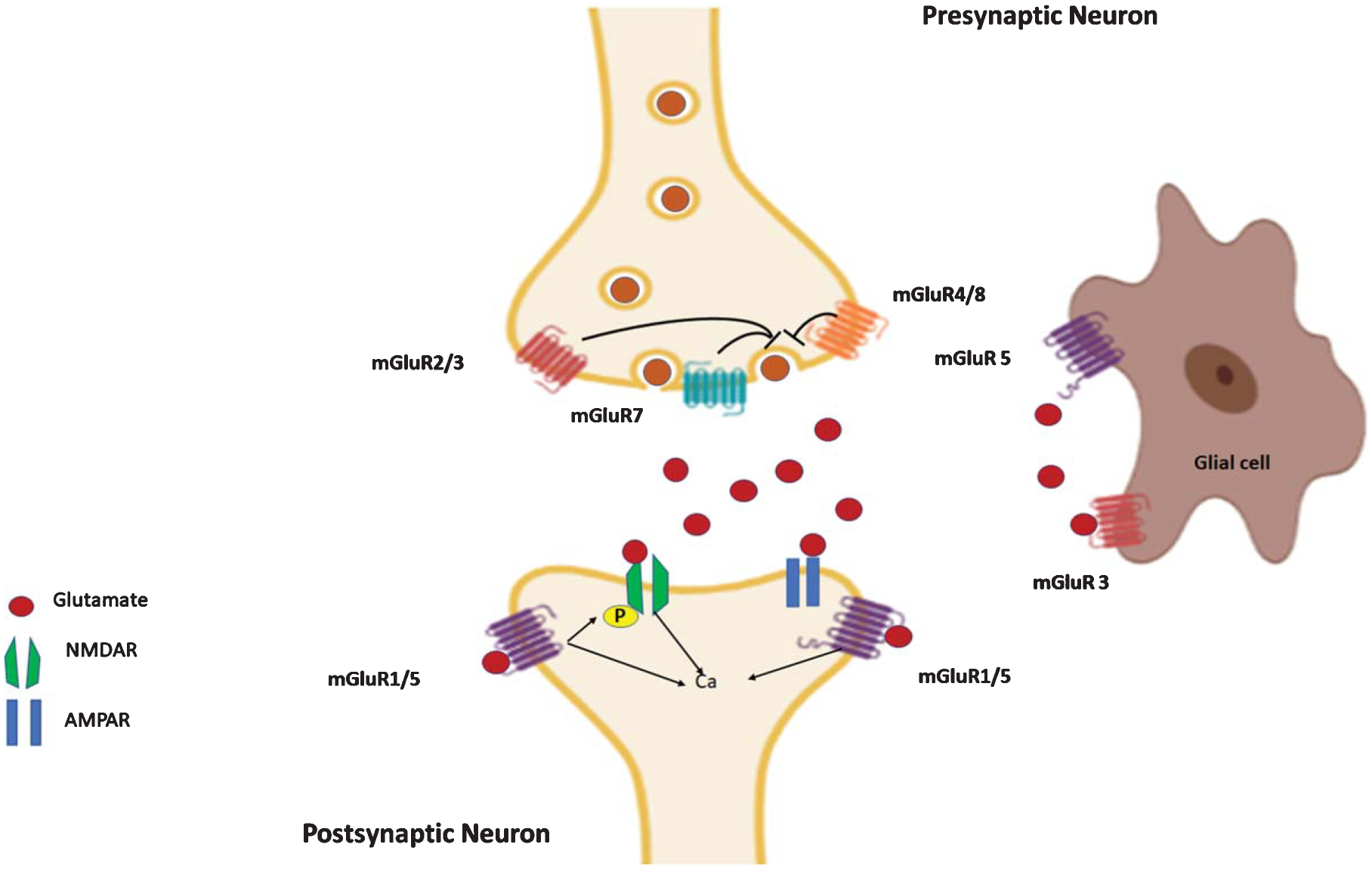
Distribution of different groups of mGluRs at glutamatergic synapse. Group I mGluR1/5 are mostly localized postsynaptically. mGluR2/3 are mainly in presynaptic site, located perisynaptically, but in certain brain regions group II mGluRs are localized in postsynaptic sites. mGluR4/7/8 are found near presynaptic active zones. The presynaptic group II and III mGluRs have an inhibitory effect on the release of glutamate from the synaptic vesicles as well as on calcium signaling. The postsynaptic group I mGluRs promote a rapid calcium influx upon their activation and contribute toward LTP induction. The glial cells (astrocytes, microglia, oligodendrocytes) also reportedly express mGluR1/5, mGluR2/3, and mGluR4/7/8 that play a significant role in glutamate neurotransmission, glial cell proliferation and release of cytokines.
Neuronal group II mGluRs are found both pre- and postsynaptically in broad brain regions including the limbic system, sensory neurons of cortex, dorsal and ventral striatum, amygdala, and thalamus [34–36]. Presynaptic group II mGluRs are often localized to the preterminal region of axons of glutamatergic neurons, away from the active zone, and are viewed as autoreceptors controlling the release of glutamate in response to excessive glutamate release [37, 38]. Presynaptic group II mGluRs have also been found on GABAergic neurons, and their activation negatively feeds back on GABA release [39]. In layer 4 of primary sensory cortical neurons, mGluR2 has a postsynaptic localization and functions as a negative regulator of glutamate transmission [40]. Immuno-electron microscopic results show that most of mGluR3 is located in presynaptic elements and not closely associated with glutamate and GABA release sites, but about 20% neuronal mGluR3 is found in spines of dentate neurons, away from glutamatergic synapses [38].
Group II mGluRs regulate hippocampal synaptic plasticity by inhibiting glutamate release and exerting a suppressive effect on LTP. This attenuation of glutamate release is due to activation of their coupling to Gi/Go proteins, in which the activated receptor acts as a guanine nucleotide exchange factor by replacing GDP with GTP in Gαβγ trimeric complex [41]. GTP-bound Gα, after being dissociated from Gβγ, separately activates their respective downstream signaling enzymes. They negatively regulate the expression of adenylate cyclase (AC) thus reducing intracellular cAMP levels and inhibiting PKA, or decreasing voltage-gated Ca2+ channel activation [15, 42], thereby inhibiting glutamatergic neurotransmission. They are activated by significant synaptic glutamate release or astrocytic glutamate [39].
Group III mGluRs have been found predominantly in presynaptic active zones, with the exception of mGluR6, which is exclusively present at postsynaptic sites in the retina and transmits signals to cGMP phosphodiesterase [43]. Among the group III mGlu Rs, mGluR7 is most prominently expressed in neuronal cells of cortex, olfactory bulb, hippocampus, thalamic nuclei, cerebellum, and is located within the presynaptic active zone of both glutamatergic and GABAergic synapses, acting to inhibit glutamate or GABA release [44, 45]. mGluR7 has 5 spliced forms with mGluR7a most broadly expressed. In globus pallidus, striatal, and retinal synapses, mGluR7 is not only present in pre-synaptic synapses but surprisingly found in postsynaptical sites as well [46, 47]. In the CNS, mGluR4 is mostly present in the cerebellum, but is also detected in the cerebral cortex, olfactory bulb, hippocampus, lateral septum, septofimbrial nucleus, striatum, thalamic nuclei, lateral mammillary nucleus, pontine nuclei, and dorsal horn [48]. mGluR4 shares a similar expression profile to mGluR7, albeit in reduced or comparable levels. Expression of mGluR8 is more restricted in the sense that it is only found in some cortical areas and the thalamus with reduced expression in olfactory bulb, neocortex, hippocampus, amygdala, and cerebellum, and its expression level is normally lower than that of mGluR4 and mGluR7 [49].
Sharing about 70% sequence homology with Group II mGluRs, group III mGluRs also signal through a distinct pathway by coupling to Gi/o proteins [50]. Besides maintaining an equilibrium between their active and inactive states, these mGluRs also exhibit a constitutive activity independent of agonist binding. Consequently, they negatively impact excitatory neurotransmission [51].
While most mGluRs are mainly localized in either pre- or postsynaptic neuronal sites, multiple mGluRs are expressed by non-neuronal cells. For example, immunohistochemical staining shows expression of mGluR1 in astrocytes, microglia, and oligodendrocytes while mGluR5 is mainly found in microglia [52–54] and mGluR5a in developmental oligodendrocyte precursor cells (OPCs). Among group II mGluRs, mGluR3 is more expressed by glial cells than neurons [55]. Early studies showed that mGluR3, but not mGluR2, is found postsynaptically and on astrocytes [38, 56, 57]. Glial mGluR3 activation regulates excitatory amino acid transporters (EAATs) [58], such as increasing the production of glutamate transporters GLAST and GLT-1 [59]; glial mGluR3 also controls the production of neurotrophic factors like TGF-β [60], and is involved in the astrocytic-neuronal communication [55]. More recently, mGluR3 was also detected in microglia, OPCs, and oligodendrocytes [52]. Moreover, low levels of mGluR2 are detected in mature MBP+ oligodendrocytes in early developmental stages but downregulated in the adult [61–63]. Group III mGluR4, mGluR7, and mGluR8 have also been found in microglia, reactive astrocytes, and oligodendrocytes [64, 65]. Functionally, mGluRs expressed by glial cells contribute to synaptic function in the brain, particularly in the context of glial uptake and synthesis of glutamate. It has been shown that the deletion of mGluR5 only in astrocytes impairs high-frequency glutamate uptake [66]. Activation of certain mGluRs in glial cells is shown to alter synaptic function and protect against neurodegeneration [63].
PHYSIOLOGICAL ROLE OF mGluRs
Synaptic plasticity is the neural process in which neuronal networks and their connections are either strengthened or weakened at appropriate synapses through calcium waves. Synaptic strength can be reflected through measures of long-term potentiation (LTP), referring to improved and extended signal transmission, and long-term depression (LTD), which is the inhibition of excitatory neurotransmission [25]. LTP and LTD largely govern motor control, memory, and learning in most of the brain areas studied so far. Acting at either pre- or postsynaptically, mGluRs regulate synaptic plasticity by controlling neuronal excitability or neurotransmitter release (Table 2). For instance, deficiency in mGluR1 in mice decreases LTP and cognitive functions [67–69], while deficiency in mGluR5 decreases CA1 LTP by altering a component of NMDA receptors and restraining synaptic flexibility [70, 71]. mGluR7 is also required for LTP induction at hippocampal SC-CA1 synapses through decreasing GABAergic synaptic transmission in CA1 pyramidal cells [72]. Their sustained effects regulate neuronal plasticity, maintain CNS function, and contribute to pathophysiological conditions related to anxiety, fear extinction, and spatial working memory [73]. It was also noted that mGluR2 is actually required for the development of mossy fiber synapses [74].
Table 2.
mGluRs in the control of synaptic plasticity
| mGluR type | Role in synaptic plasticity | References |
|---|---|---|
| mGluR1 | Modulates NMDAR-LTP; independent LTP induction; LTD induction; its deletion models report LTP deficits; its inhibition reduced microglial phagocytosis of glutamate synapses | [67, 77, 159] |
| mGluR5 | Modulates NMDAR-LTP; independent LTP induction; LTD induction; its ablation in astrocytes impairs high-frequency glutamate uptake and also reduces CA1 LTP | [66, 71, 77] |
| mGluR2 | Stimulates LTD at GABAergic synapses; suppresses synaptic transmission transiently at CA3 synapse of mossy fibers | [48, 194, 195] |
| mGluR3 | Regulates both LTP and LTD based upon its synaptic location; Stimulates chemical LTD while suppressing LTP upon activation in vitro; LTP induction at electrical synapses (eLTP) | [48, 181, 196] |
| mGluR4 | Inhibits LTP; contributes to synaptic transmission inhibition at piriform cortex synapse in L-AP4 challenged olfactory bulb | [90, 197] |
| mGluR7 | Inhibits LTP; known to induce activity regulated plasticity and reported to induce LTD in interneurons of stratum radiatum | [90, 198] |
| mGluR8 | Inhibits LTP; primarily regulates the synaptic transmission in forebrain; KO mice exhibit anxiety-like behavior | [64, 199] |
Postsynaptic functions
By localizing at dendritic fields or peri-synaptic sites surrounding the ionotropic receptors, group I mGluRs (mGluR1/mGluR5) are shown to modulate synaptic strength through redistribution of AMPA and NMDA receptors at excitatory synapses, but they are not normally needed for the initiation of LTP [75]. In subiculum areas, mGluRs are shown to independently induce LTP based on the sliding-threshold model of synaptic plasticity [76]. It is shown that induction of LTP and LTD in CA1-subiculun synapses depends upon the relative activation states of NMDARs and mGluRs. In regular spiking cells, this threshold shifts toward mGluR activation in response to low-frequency afferent stimulation that triggers calcium signaling and masks NMDAR-mediated LTD [76, 77]. Reduced mGluR activity decreases LTP amplitude and prevents neuronal excitotoxicity under normal physiological conditions [78]. Postsynaptically localized mGluRs appear to trigger LTD by enhancing endocytosis of ionotropic AMPA receptor subunits (i.e., GluR1 and 2 in CA1 and GluR2 only in cerebellar Purkinje cells), leading to a long-term reduction in the number of postsynaptic surface AMPAR [79]. In striatal regions, postsynaptically localized type I mGluRs are found to mediate LTD initiation but not its maintenance [80].
Presynaptic functions
At the presynaptic site, mGluRs can regulate synaptic plasticity by affecting intracellular calcium levels to modulate neurotransmitter release, mostly by altering membrane potential and internal storage of calcium ions [81]. In many published studies using mGluR agonists, activation of presynaptic group II or III mGluRs inhibits excitatory synaptic transmission at specific synapses by reducing glutamate release [48]. 4C3HPG, a group II agonist (and group I mGluR antagonist), was found to inhibit LTP expression in the dentate gyrus in vivo [82]. However, transgenic mice and more specific drugs must be used to tease apart the individual contributions of mGluR2 and mGluR3 to synaptic changes. mGluR2 knock-out mice exhibit normal basal synaptic transmission and LTP, but impaired LTD, at mossy fiber-CA3 synapses in the hippocampus [83]. In vitro, activation of mGluR3 receptors by group II agonist NAAG leads to inhibition of hippocampal LTP [84], and induction of chemical LTD [85]. mGluR3 antagonism via β-NAAG blocked LTD but not LTP in the dentate gyrus in vivo. It has been suggested that presynaptic and postsynaptic mGluR3 have distinct roles in synaptic plasticity: postsynaptic mGluR3 are necessary for LTD while presynaptic mGluR3 modulates both LTP and LTD [86].
Activation of group II and III mGluRs (presynaptically localized) can also lead to the development of LTD through G protein-coupled receptors by dissociation of Gαi with Gβγ. The released Gαi and the second messenger nitric oxide are necessary steps in the presynaptic LTD cascade, while Gβγ directly bind voltage-dependent Ca2+ channel to reduce calcium influx to decrease transmitter release [87, 88]. It has also been shown that Gβγ binds directly to the C-terminus region of the SNARE protein SNAP-25, and this binding induces LTD of vesicular glutamate release [89]. On the other hand, inhibition of group III mGluRs promotes NMDA and protein synthesis-dependent induction of long-lasting LTP at the otherwise LTP deficient (SC) CA2 synapses [90]. These receptors can also activate other signaling pathways, including mitogen-activated protein (MAP) kinase and PI-3 (phosphatidylinositol-3) kinase pathways [15, 91, 92].
Group II mGluRs have been shown to associate with a number of interaction partners, including calmodulin [15], β-arrestins [93], glutamate receptor-interacting protein [94], protein interacting with PRKCA 1 [94], Na+/H+ exchanger regulatory factor 1 and 2 [95], protein kinase A [96], protein phosphatase 2C [97], tamalin [98], and Ran binding protein in the microtubule-organizing center [99]. In addition, group II mGluRs directly and indirectly interact with members of other mGluR classes. Recent spectroscopic and biochemical studies show that mGluRs can heterodimerize, which has important functional implications. mGluR2 can form dimers of equal affinity to members of group II mGluRs, and with lower affinity with group III mGluRs [100]. mGluR2/4 heterodimeric complexes are found to differentially regulate the responses to various positive or negative allosteric modulators of mGluRs [101]. In the hippocampus, mGluR2 heterodimerizes with mGluR7, and this complex exhibits both fast kinetics and cooperativity that boosts the affinity and efficacy of each subunit, priming the receptor for activation even in absence of agonist. These properties appear to make mGluR2/7 uniquely suited to activate in response to synaptic glutamate [102]. Finally, mGluR3 and mGluR5 interact synergistically through cross-talk of signaling pathways, where it was found that mGluR3 activation is required for maximal mGluR5 signaling, and mGluR5 activation is required for the induction of mGluR3-dependent LTD in the PFC [103].
Other regulatory functions
In addition to the effect on synaptic plasticity, mGluRs present in the hypothalamus and pituitary can also regulate neuroendocrine functions by controlling the secretion of various hormones [104]. For example, gonadotropin-releasing hormone (GnRH) neurons receive input from GABAergic afferents via GABAA receptors, and presynaptic mGluRs can regulate GABAergic neuronal GABA release to GnRH neurons and control GABAA receptor-mediated postsynaptic currents in GnRH neurons for generation/modulation of rhythmic GnRH release [105, 106]. In the spinal cord, all mGluRs except mGluR6 are expressed within the nociceptive pathways: group I mGluRs are pro-nociceptive while group II and III mGluRs are anti-nociceptive [107]. mGluRs also play a role in the auditory system by enhancing the currents emanating from voltage-gated K+ channels in the neurons responsible for time coding in the auditory brainstem. The cochlear regions show an intense expression of group I mGluRs and a region-specific expression of the other two groups [108]. While many comprehensive reviews have discussed these aspects [109–113], this review will focus on mGluRs in AD cognitive functions.
PIVOTAL ROLE OF mGluRs IN AD PATHOPHYSIOLOGY
The brain usually shrinks to some degree in healthy aging, but surprisingly, does not lose large numbers of neurons under healthy condition [114, 115]. In AD, erroneous synaptic activity precedes memory loss, corroborated by the fact that the synaptic density at hippocampal and cortical regions is reduced. A plethora of literature have documented various synaptic dysfunctions in AD brains [116–121]. Synaptic decline in AD animal studies is often reflected by a significant reduction of LTP or increase in LTD in AD mouse models [122–124]. A remarkable and early synaptic decline in AD has also been associated with altered glutamatergic systems [125]. Glutamatergic synapses, mainly involving NMDA and AMPA receptors, are directly relevant to LTP/LTD changes. Upon LTP induction, AMPA receptors are recruited at synapses, and dendritic spines are increased in number while LTD leads to synaptic loss and spine shrinkage [126, 127].
AD, as one of the most prevalent forms of age-dependent progressive neurodegenerative disorders in patients experiencing cognitive deficits, behavioral changes, and personality alterations, is best recognized as a significant memory loss, occurring even in the mild cognitive impairment stage [128]. Gradual structural and functional damages to the hippocampus and cortical regions, resulting from excessive Aβ aggregation and deposition, neurofibrillary tangles (NFT), and progressive neuronal loss, are believed to cause synaptic dysfunctions in AD brains [14, 129]. Neuronal loss could stem from glutamate excitotoxicity, which is a process by which abnormal glutamate levels exert an excitatory shock to neurons and can cause cell death. When glutamate receptors are over-activated, a massive surge in calcium influx occurs and perturbs normal cellular physiological processes, causing changes in mitochondria function, intracellular and extracellular signaling cascades, and oxidative stress [130, 131]. Depending upon the cellular stage and state of neurons and external factors, the contribution of different types of iGluRs and mGluRs towards excitotoxicity may vary relative to each other [132]. Under normal physiological conditions, excitotoxic levels of glutamate at synaptic clefts can be prevented by rapid conversion of glutamate to glutamine in glial cells. Presynaptic neurons then uptake glutamine, after which it is converted to glutamate by intracellular glutaminase, and normal synaptic signaling events can thus be maintained [133]. Abnormal Aβ levels in the brain have been shown to regulate glutamate levels at synaptic clefts [134, 135].
Aβ peptides, namely Aβ40 and Aβ42, are generated from large transmembrane amyloid-β protein precursor (AβPP), sequentially cleaved by β- and γ-secretases. Aβ exists in the monomeric form, but can self-aggregate to form dimer and oligomers, and even higher order structures called Aβ fibrils with aging [136, 137]. Amyloid plaques mainly consist of Aβ soluble oligomers and insoluble aggregates. Under physiological conditions, Aβ is released at synapses of neurons and may promote normal memory and synaptic plasticity, especially LTP at picomolar levels [138]. Augmentation of LTP by increase of cGMP levels may also require Aβ at physiologically-controlled levels [139]. Aβ peptides are shown to stimulate presynaptic α7 nicotinic receptors to induce synaptic plasticity [140]. APP KO mice exhibit deficits in memory and LTP, emphasizing the fact that Aβ may have a modulatory effect on synaptic plasticity and neurotransmission [141, 142]. On the other hand, under pathological conditions, the soluble form of Aβ oligomers is shown to be highly neurotoxic [143]. This is likely because pathologically increased Aβ oligomers block uptake of glutamate thereby leading to elevated glutamate concentrations in synaptic clefts, and cause neuronal hyper-excitation due to overstimulating NMDA receptors [144]. This event inhibits LTP by desensitizing NMDA receptors, resulting in synaptic depression and the eventual loss of synapses and neuronal death [145]. This Aβ-mediated synaptic loss can occur early in the course of AD and is also accompanied by oxidative stress and tau phosphorylation. Examination of postmortem brains of AD patients reveals excessive free radical generation and altered nitric oxide cascade in correlation with loss of synapses [146–148]. Hence, a precise regulation of Aβ production and release at synapses is required for maintenance of synaptic plasticity [149–152].
Relevance of mGluRs in AD synaptic dysregulation
Active synaptic transmission requires not only functional iGluRs (mostly glutamate and AMPA receptors) but also mGluRs [153]. Aβ oligomers have been shown to contribute to synaptic deficits, either directly or indirectly, by interacting with iGluRs as well as mGluRs [154]. The effect of Aβ oligomers on iGluRs in AD synaptic deficits has been well documented [155–158] and this review focuses recent publications on mGluRs in this aspect. Altered mGluR levels or activities have been associated with AD pathogenesis by changing glutamatergic synaptic functions [10]. Each mGluR has been explored in various conditions and we will dissect their contributions to AD synaptic dysfunctions (Table 3). Following sub-sections will summarize our current knowledge.
Table 3.
mGluRs in Alzheimer’s disease
| mGluR type | Synaptic functions in AD | References |
|---|---|---|
| mGluR1 | Obstructed mGluR1-LTD contribute to the early stage memory and learning deficits in AD patients | [79, 200, 201] |
| mGluR5 | Shifts towards synapse when Aβ oligomers are around and promotes synaptotoxicity; dysregulation of mGluR5 mediated synaptic events; acts as a co-receptor for Aβ oligomers and prion protein (PrPc) interaction | [202, 203, 205] |
| mGluR2 | Increased synaptic Aβ42 generation; its inhibition in AD mice models improves neurogenesis and synaptic transmission while reducing Aβ toxicity | [138, 177] |
| mGluR3 | Enhanced neuronal firing with mGluR3 stimulation; astrocytic mGluR3 protects against NMDAR mediated excitotoxicity | [174, 204] |
| mGluR4 | Knockouts for mGluR4 exhibit improved LTP in hippocampal CA1 region | [185] |
| mGluR7 | mGluR7 activation protects against NMDA induced excitotoxicity at synapse | [187] |
| mGluR8 | Believed to dampen glutamate excitotoxicity and regulate neurotransmitter release | [181] |
Group I mGluRs inAD
In AD research, the role of group I mGluRs (mGluR1 and mGluR5) has been studied to a good extent, and their levels are found to be reduced in AD brains. Studies using postmortem brain samples of AD patients have revealed reduced levels of these two mGluRs in the hippocampus and cortex, and changes are region- and receptor subtype-specific. For example, mGluR1 levels are reduced in the hippocampal CA1 region in AD brains [159]. Interestingly, a study in Japanese population using positron emission tomography (PET) imaging did not find any significant difference in the mGluR1 availability between AD and control groups, especially in the early stages [160]. However, they did not rule out possible changes associated with the progression of AD, which will require further longitudinal follow-up. Indeed, in a separate study, both mGluR1 and mGluR5, quantified by radioligand binding assays, were found to be significantly decreased in pure AD cerebral cortex [161]. In particular, the decrease in mGluR1 levels occurred in cases with early AD changes. Moreover, mGluR1 levels in pure AD cases were lower than those obtained in patients with Lewy body dementia and its reduction is correlated with progression of illness, indicating a strong correlation to neuropathological changes. Expression levels of the phospholipase Cβ1 (PLCβ1) isoform, which acts as the effector of group I mGluRs, is also significantly decreased in AD [161]. mGluR5 levels have been found to be significantly decreased in 16-month-old Tg-ArcSwe (an AD mice model) when compared with control mice [162]. After the compound [18F]FPEB was developed for specific binding to mGluR5, [18F]FPEB-PET was conducted in 16 mild AD patients and 15 age-matched normal controls, and reductions of mGluR5 binding in the hippocampus of early AD was revealed [163]. Aβ oligomers form a complex with cellular prion protein (prp) and mGluR5, and this complex alter signaling involving Fyn and PyK2. Consequently, neuronal functions including reduction of dendritic spines and synaptic memory are disrupted, and blocking this interaction is explored as a potential strategy for AD treatment.
Since Group I mGluRs/PLC signaling is downregulated and desensitized in AD cases and worsen with progression of AD pathologies, it is not surprising to speculate that group I mGluR dysfunction is likely one culprit for the cognitive impairment and dementia in pure AD patients. Activation of group I mGluRs may need to be explored as a potential strategy for reversing pathological changes in these dementia patients. Encouragingly, a couple of studies show that activation of group I mGluRs accelerates nonamyloidogenic processing of AβPP by α-secretase, potentially protecting from Aβ-mediated neurotoxicity [164, 165]. Thus, dysfunction of group I mGluRs is likely associated with AD disease progression and contributes to AD cognitive failure.
Group II mGluRs in AD
In the hippocampus of AD patients, mGluR2 expression is increased in the CA1 and CA3 pyramidal neurons compared to age-matched controls, and immunostaining of mGluR2 exhibited significant overlap with hyperphosphorylated tau-positive neurons [166], implying a role of mGluR2 in the pathogenesis of AD. The dual mGlu2/3 receptor agonist LY379268 was found to induce ERK activation in rat primary cortical neurons [92], and this activation directly increased tau phosphorylation in primary neurons [167]. Since ERK is chronically elevated in AD neurons and is shown to phosphorylate tau in regions of the AD brain that harbors NFT [168], activation of mGlu2/3 receptor may facilitate tau pathology. However, the ERK pathway functions in cell proliferation, differentiation, and survival—indeed, it is commonly implicated in human cancers—and thus while NFT have historically been seen as detrimental to neurons, some authors suggest that tau phosphorylation represents a neuronal compensatory response to stress and is associated with survival [169, 170].
On the flip side, group II mGluR activation is protective for neurons against excitotoxicity via the inhibition of glutamate release [171–173]. Recent efforts have been directed toward teasing apart the individual contributions of mGluR2 and mGluR3 to neuronal survival and synaptic function and dysfunction in the setting of AD. Overall, AD appears to be associated with reductions in mGluR3 but increases in mGluR2 function. activation of mGluR2, but not mGluR3, inhibits GABA release and becomes harmful to neurons subjected to an excitotoxic insult; in mixed cortical cultures, the addition of group II agonist LY379268 reduced K+ depolarization-evoked GABA release in neurons cultured from wildtype or mGluR3 KO, but not mGluR2 KO mice [174]. Also, LY379268 treatment protects cultured neurons from NMDA-mediated excitotoxicity only when astrocytic mGluR3 is present, an effect that can be amplified by the absence of neuronal mGlu2 receptors [174]. Furthermore, the mGluR2 activation by the PAM LY566332 potentiates Aβ toxicity in vitro regardless of whether glial mGlu3 receptors is present, an effect that is abrogated when neurons lack mGlu2 receptors [175]. It is further shown that group II agonist LY379268 is neuroprotective against Aβ toxicity in mixed cultures, likely through a paracrine mechanism mediated by TGF-β1; Anti-TGF-β antibodies also eliminate the neuroprotective action of LY379268 in cultures challenged with Aβ25–35 or Aβ1–42 [175]. LY379268 treatment abrogates its neuronal protection if co-cultured astrocytes lack mGluR3. The selective mGluR3 NAM LY2389575 independently amplifies Aβ toxicity; LY379268, which is otherwise highly protective, becomes neurotoxic in the presence of LY2389575. These findings suggest that mGluR3 is protective against Aβ-induced toxicity, while mGluR2 promotes Aβ toxicity. Hence, the ratio of mGluR3:mGluR2 activity modulates AD risk.
Group II mGluRs have emerged as a possible target for AD therapeutic intervention to reduce Aβ burden at the synapse. This thought is based on the hypothesis that activation of group II mGluRs may trigger synaptic activation of all three secretases, as well as preferential production and release of Aβ42 peptides from nerve terminals [138]. Group II mGluR agonist DCG-IV administration leads to initially increased levels of AβPP CTFs of isolated neuronal synaptosomes taken from TgCRND8 mice. This phase was shortly followed by a CTF cleavage characterized by sustained accumulation of Aβ42 peptides, but surprisingly little effect on the release of Aβ40 peptides [138]. Consequently, the Aβ42:Aβ40 ratio is increased, mimicking a common feature of hereditary AD and animal models of AD [176], which suggests a role of group II mGluRs in amyloidogenic synaptic events. Indeed, when Dutch Aβ-oligomer-forming APP transgenic mice (APP E693Q) were chronically administered the group II mGluR antagonist BCI-838, these mice exhibited a reversal of transgene-related learning and memory impairment, increased hippocampal neurogenesis, reduced anxiety, and reduced Aβ monomers and oligomers in the hippocampus and cortex [177].
Group III mGluRs in AD
The role of group III receptors in AD pathogenesis is still being explored, though it is believed that their activation has neuroprotective effects in the case of AD through regulation of the NMDAR and PI3K pathway [166]. Although less studied than other groups, group III mGluRs play an important role in nervous system disorders and their activation has been found to be beneficial against neurodegeneration [178]. Identification of selective compounds for its subtypes has catapulted studies focusing on the physiological roles and therapeutic potential of group III mGluRs [179]. Group III mGluRs are believed to exert neuroprotective effects by inhibiting glutamate release and dampening its excitotoxicity [180]. In case of AD, they reportedly confer neuroprotection by regulating neurotransmitter release and improving AD symptoms while possessing an improved safety profile. The agonists of group III also reduce the levels of pro-inflammatory cytokines from activated astrocytes, thus preventing cellular demise due to neuroinflammation, a common hallmark of AD [181]. Disruption of calcium homeostasis is one of the primary outcomes of Aβ neurotoxicity. In vitro studies show that a pretreatment of neuronal cells with group III mGluR agonists rescued the cells from Aβ-induced neurotoxicity by reducing the elevated intracellular calcium levels that would otherwise be damaging [182].
mGluR4 agonists have been found to protect degenerating neurons in the caudate nucleus of wildtype mice but not in the mGluR4 knockout mice, suggesting its ability to induce neuroprotection [54]. Its activation also promoted a decline in neurotoxicity associated with excessive activation of microglia by Aβ, a hallmark of AD pathology, suggesting a potential use of mGluR4 agonists in AD therapy [183, 184]. Besides being neuroprotective, mGluR4 also influences LTP at CA1 synapses. The mGluR4−/− mice exhibit improved LTP in the CA1 region of hippocampus, but no such effect has been observed in the prefrontal cortex or dentate gyrus, likely due to the varied expression profile of mGluR4 in these brain regions. Again, the critical involvement of synaptic plasticity at CA1 synapses in AD, mGluR4 inhibition is a potential therapeutic target [185].
Despite a paucity of suitable pharmacological compounds and ligands against mGluR7, studies conducted with knockout mice at the cellular and molecular levels reveal that this particular receptor holds a lot of potential in therapeutic implications against neurodegenerative disorders like AD [186]. Unlike other group III members, mGluR7 is highly abundant in basal forebrain neurons. When this receptor is activated, it suppresses NMDAR currents thus protecting neurons against NMDAR-mediated excitotoxicity. However, Aβ inhibits this protective action of mGluR7, specifically in cholinergic neurons in the basal forebrain area [187]. mGluR7 is also known as the emergency brake system of the cell in the case of abnormal elevation in glutamate levels due to its minimal affinity for glutamate. This was further corroborated by a study involving mGluR7 KO mice that exhibited spontaneous seizures in absence of mGluR7 [15, 188]. Another study reported a reduction in mGluR7 levels in response to elevated miRNA-34a levels in 3×Tg-AD mice models. GRM7, the gene encoding mGluR7, is a target of miR-34a associated with anxiety [189]. The finding corroborated an earlier study involving mGluR7 KO mice exhibiting anxiety-like behavior [190], confirming its role in cognition and behavior.
Mice with targeted mGluR8 gene deletion exhibit subtle behavioral changes, mainly related to a diminished acclimatization to novel surroundings, interrupted context-based freezing response against fear, as well as hyperactivity and anxiety [191]. These changes are likely due to reduced glutamate release along with an abnormal GABA release and lead to the dysfunctional hippocampus. Since hippocampal neurotransmission anomalies are some of the pronounced effects of AD, activation of mGluR8 should be explored as an AD therapeutic target [192].
SUMMARY
Glutamate excitotoxicity occurs when the glutamate receptors are activated beyond a normal threshold, resulting in the loss of dendrites, cell bodies, and other postsynaptic structures. This is one of the major underlying reasons for synaptic failures and neurodegeneration in AD [193]. The ability of mGluRs to modulate glutamate release bodes very well with their contributions to the synaptic failure of AD. Perplexingly, modulation of mGluR activity for AD therapeutic intervention remains underexplored. If research efforts will be growing in this area, including more functional and pharmacological studies as well as pre-clinical explorations, growing knowledge will eventually shed light on novel discoveries of mGluR modulators in AD treatment.
ACKNOWLEDGMENTS
RY is supported by NIH grants (AG025493, NS074256, RFAG058261 and AG046929). Brati Das is a recipient of postdoctoral fellowship from Bright-Focus Foundation (A20201729F).
Footnotes
Authors’ disclosures available online (https://www.j-alz.com/manuscript-disclosures/20-1146r1).
REFERENCES
- [1].Meldrum BS (2000) Glutamate as a neurotransmitter in the brain: Review of physiology and pathology. J Nutr 130, 1007S–1015S. [DOI] [PubMed] [Google Scholar]
- [2].Doumazane E, Scholler P, Fabre L, Zwier JM, Trinquet E, Pin J-P, Rondard P (2013) Illuminating the activation mechanisms and allosteric properties of metabotropic glutamate receptors. Proc Natl Acad Sci U S A 110, E1416–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Reiner A, Levitz J (2018) Glutamatergic signaling in the central nervous system: Ionotropic and metabotropic receptors in concert. Neuron 98, 1080–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marmiroli P, Cavaletti G (2012) The glutamatergic neurotransmission in the central nervous system. Curr Med Chem 19, 1269–1276. [DOI] [PubMed] [Google Scholar]
- [5].Zhou Y, Danbolt NC (2014) Glutamate as a neurotransmitter in the healthy brain. J Neural Transm 121, 799–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zahr NM, Mayer D, Pfefferbaum A, Sullivan EV (2008) Low striatal glutamate levels underlie cognitive decline in the elderly: Evidence from in vivo molecular spectroscopy. Cereb Cortex 18, 2241–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Merritt K, Mcguire P, Egerton A (2013) Relationship between glutamate dysfunction and symptoms and cognitive function in psychosis. Front Psychiatry 4, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Francis PT (2005) Neuroanatomy/pathology and the interplay of neurotransmitters in moderate to severe Alzheimer disease. Neurology 65(6 Suppl 3), S5–S9. [Google Scholar]
- [9].Campos-Peña V, Meraz-Ríos MA (2014) Alzheimer disease: The role of Aβ in the glutamatergic system. In Neurochemistry, Heinbockel T, ed. IntechOpen, doi: 10.5772/57367 [DOI] [Google Scholar]
- [10].Revett TJ, Baker GB, Jhamandas J, Kar S (2013) Glutamate system, amyloid β peptides and tau protein: Functional interrelationships and relevance to Alzheimer disease pathology. J Psychiatry Neurosci 38, 6–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kumar A, Dhull DK, Mishra PS (2015) Therapeutic potential of mGluR5 targeting in Alzheimer’s disease. Front Neurosci 9, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Guntupalli S, Widagdo J, Anggono V (2016) Amyloid-β-induced dysregulation of AMPA receptor trafficking. Neural Plast 2016, 3204519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yan R, Fan Q, Zhou J, Vassar R (2016) Inhibiting BACE1 to reverse synaptic dysfunctions in Alzheimer’s disease. Neurosci Biobehav Rev 65, 326–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu J, Chang L, Song Y, Li H, Wu Y (2019) The role of NMDA receptors in Alzheimer’s disease. Front Neurosci 13, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Niswender CM, Conn PJ (2010) Metabotropic glutamate receptors: Physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol 50, 295–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chun L, Zhang W, Liu J (2012) Structure and ligand recognition of class C GPCRs. Acta Pharmacol Sin 33, 312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Koehl A, Hu H, Feng D, Sun B, Zhang Y, Robertson MJ, Chu M, Kobilka TS, Laeremans T, Steyaert J, Tarrasch J, Dutta S, Fonseca R, Weis WI, Mathiesen JM, Skiniotis G, Kobilka BK (2019) Structural insights into the activation of metabotropic glutamate receptors. Nature 566, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moustaine DE, Granier S, Doumazane E, Scholler P, Rahmeh R, Bron P, Mouillac B, Banères J-L, Rondard P, Pin J-P (2012) Distinct roles of metabotropic glutamate receptor dimerization in agonist activation and G-protein coupling. Proc Natl Acad Sci U S A 109, 16342–16347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jójárt B, Orgován Z, Márki Á, Pándy-Szekeres G, Ferenczy GG, Keserű GM (2019) Allosteric activation of metabotropic glutamate receptor 5. J Biomol Struct Dyn 0, 1–9. [DOI] [PubMed] [Google Scholar]
- [20].Yanamala N, Tirupula KC, Klein-Seetharaman J (2008) Preferential binding of allosteric modulators to active and inactive conformational states of metabotropic glutamate receptors. BMC Bioinformatics 9, S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Feng Z, Ma S, Hu G, Xie X-Q (2015) Allosteric binding site and activation mechanism of class C G-protein coupled receptors: Metabotropic glutamate receptor family. AAPS J 17, 737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zoicas I, Kornhuber J (2019) The role of metabotropic glutamate receptors in social behavior in rodents. Int J Mol Sci 20, 1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Riedel G, Platt B, Micheau J (2003) Glutamate receptor function in learning and memory. Behav Brain Res 140, 1–47. [DOI] [PubMed] [Google Scholar]
- [24].Bennett KA, Doré AS, Christopher JA, Weiss DR, Marshall FH (2015) Structures of mGluRs shed light on the challenges of drug development of allosteric modulators. Curr Opin Pharmacol 20, 1–7. [DOI] [PubMed] [Google Scholar]
- [25].Ménard C, Quirion R (2012) Group 1 metabotropic glutamate receptor function and its regulation of learning and memory in the aging brain. Front Pharmacol 3, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Alvarez FJ, Villalba RM, Carr PA, Grandes P, Somohano PM (2000) Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. J Comp Neurol 422, 464–487. [DOI] [PubMed] [Google Scholar]
- [27].Ryo Y, Miyawaki A, Furuichi T, Mikoshiba K (1993) Expression of the metabotropic glutamate receptor mGluR1α and the ionotropic glutamate receptor GluR1 in the brain during the postnatal development of normal mouse and in the cerebellum from mutant mice. J Neurosci Res 36, 19–32. [DOI] [PubMed] [Google Scholar]
- [28].Swanson CJ, Bures M, Johnson MP, Linden A-M, Monn JA, Schoepp DD (2005) Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov 4, 131–144. [DOI] [PubMed] [Google Scholar]
- [29].Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S (1992) Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem 267, 13361–13368. [PubMed] [Google Scholar]
- [30].Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF (1997) Homer: A protein that selectively binds metabotropic glutamate receptors. Nature 386, 284–288. [DOI] [PubMed] [Google Scholar]
- [31].Maiese K, Chong ZZ, Shang YC, Hou J (2008) Therapeutic promise and principles: Metabotropic glutamate receptors. Oxid Med Cell Longev 1, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Benarroch EE (2008) Metabotropic glutamate receptors: Synaptic modulators and therapeutic targets for neurologic disease. Neurology 70, 964–968. [DOI] [PubMed] [Google Scholar]
- [33].Pandya NJ, Klaassen RV, van der Schors RC, Slotman JA, Houtsmuller A, Smit AB, Li KW (2016) Group 1 metabotropic glutamate receptors 1 and 5 form a protein complex in mouse hippocampus and cortex. Proteomics 16, 2698–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Byrnes KR, Loane DJ, Faden AI (2009) Metabotropic glutamate receptors as targets for multipotential treatment of neurological disorders. Neurotherapeutics 6, 94–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Muguruza C, Meana JJ, Callado LF (2016) Group II metabotropic glutamate receptors as targets for novel antipsychotic drugs. Front Pharmacol 7, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bocchio M, Lukacs IP, Stacey R, Plaha P, Apostolopoulos V, Livermore L, Sen A, Ansorge O, Gillies MJ, Somogyi P, Capogna M (2019) Group II metabotropic glutamate receptors mediate presynaptic inhibition of excitatory transmission in pyramidal neurons of the human cerebral cortex. Front Cell Neurosci 12, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Luján R, Roberts JDB, Shigemoto R, Ohishi H, Somogyi P (1997) Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1α, mGluR2 and mGluR5, relative to neurotransmitter release sites. J Chem Neuroanat 13, 219–241. [DOI] [PubMed] [Google Scholar]
- [38].Tamaru Y, Nomura S, Mizuno N, Shigemoto R (2001) Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: Differential location relative to preand postsynaptic sites. Neuroscience 106, 481–503. [DOI] [PubMed] [Google Scholar]
- [39].Lee CC, Sherman SM (2009) Glutamatergic inhibition in sensory neocortex. Cereb Cortex 19, 2281–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Oldham WM, E Hamm H (2006) Structural basis of function in heterotrimeric G proteins. Q Rev Biophys 39, 117–166. [DOI] [PubMed] [Google Scholar]
- [41].Pin J-P, Duvoisin R (1995) The metabotropic glutamate receptors: Structure and functions. Neuropharmacology 34, 1–26. [DOI] [PubMed] [Google Scholar]
- [42].Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP (2011) Metabotropic glutamate receptors: From the workbench to the bedside. Neuropharmacology 60, 1017–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Klotz L, Wendler O, Frischknecht R, Shigemoto R, Schulze H, Enz R (2019) Localization of group II and III metabotropic glutamate receptors at pre- and postsynaptic sites of inner hair cell ribbon synapses. FASEB J 33, 13734–13746. [DOI] [PubMed] [Google Scholar]
- [44].Dalezios Y, Luján R, Shigemoto R, Roberts JDB, Somogyi P (2002) Enrichment of mGluR7a in the presynaptic active zones of GABAergic and Non-GABAergic terminals on interneurons in the rat somatosensory cortex. Cereb Cortex 12, 961–974. [DOI] [PubMed] [Google Scholar]
- [45].Somogyi P, Dalezios Y, Luján R, Roberts JDB, Watanabe M, Shigemoto R (2003) High level of mGluR7 in the presynaptic active zones of select populations of GABAergic terminals innervating interneurons in the rat hippocampus. Eur J Neurosci 17, 2503–2520. [DOI] [PubMed] [Google Scholar]
- [46].Brandstätter JH, Koulen P, Kuhn R, van der Putten H, Wässle H (1996) Compartmental localization of a metabotropic glutamate receptor (mglur7): Two different active sites at a retinal synapse. J Neurosci 16, 4749–4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kosinski CM, Bradley SR, Conn PJ, Levey AI, Landwehrmeyer GB, Penney JB, Young AB, Standaert DG (1999) Localization of metabotropic glutamate receptor 7 mRNA and mGluR7a protein in the rat basal ganglia. J Comp Neurol 415, 266–284. [PubMed] [Google Scholar]
- [48].Crupi R, Impellizzeri D, Cuzzocrea S (2019) Role of metabotropic glutamate receptors in neurological disorders. Front Mol Neurosci 12, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Ferraguti F, Shigemoto R (2006) Metabotropic glutamate receptors. Cell Tissue Res 326, 483–504. [DOI] [PubMed] [Google Scholar]
- [50].Grueter BA, Winder DG (2005) Group II and III metabotropic glutamate receptors suppress excitatory synaptic transmission in the dorsolateral bed nucleus of the stria terminalis. Neuropsychopharmacology 30, 1302–1311. [DOI] [PubMed] [Google Scholar]
- [51].Wang S, Chen X, Kurada L, Huang Z, Lei S (2012) Activation of group II metabotropic glutamate receptors inhibits glutamatergic transmission in the rat entorhinal cortex via reduction of glutamate release probability. Cereb Cortex 22, 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Luyt K, Váradi A, Durant CF, Molnár E (2006) Oligodendroglial metabotropic glutamate receptors are developmentally regulated and involved in the prevention of apoptosis. J Neurochem 99, 641–656. [DOI] [PubMed] [Google Scholar]
- [53].Jantzie LL, Talos DM, Selip DB, An L, Jackson MC, Folkerth RD, Deng W, Jensen FE (2010) Developmental regulation of group I metabotropic glutamate receptors in the premature brain and their protective role in a rodent model of periventricular leukomalacia. Neuron Glia Biol 6, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ribeiro FM, Vieira LB, Pires RGW, Olmo RP, Ferguson SSG (2017) Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol Res 115, 179–191. [DOI] [PubMed] [Google Scholar]
- [55].Winder DG, Conn PJ (1996) Roles of metabotropic glutamate receptors in glial function and glial-neuronal communication. J Neurosci Res 46, 131–137. [DOI] [PubMed] [Google Scholar]
- [56].Ohishi H, Shigemoto R, Nakanishi S, Mizuno N (1993) Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: An in situ hybridization study. J Comp Neurol 335, 252–266. [DOI] [PubMed] [Google Scholar]
- [57].Petralia RS, Wang Y-X, Niedzielski AS, Wenthold RJ (1996) The metabotropic glutamate receptors, MGLUR2 and MGLUR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience 71, 949–976. [DOI] [PubMed] [Google Scholar]
- [58].Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S (1992) A family of metabotropic glutamate receptors. Neuron 8, 169–179. [DOI] [PubMed] [Google Scholar]
- [59].Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D (2003) Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: Opposite regulation of glutamate transporter proteins. Eur J Neurosci 17, 2106–2118. [DOI] [PubMed] [Google Scholar]
- [60].Bruno V, Battaglia G, Copani A, D’Onofrio M, Di Iorio P, De Blasi A, Melchiorri D, Flor PJ, Nicoletti F (2001) Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab 21, 1013–1033. [DOI] [PubMed] [Google Scholar]
- [61].Luyt K, Varadi A, Molnar E (2003) Functional metabotropic glutamate receptors are expressed in oligodendrocyte progenitor cells. J Neurochem 84, 1452–1464. [DOI] [PubMed] [Google Scholar]
- [62].Deng W, Wang H, Rosenberg PA, Volpe JJ, Jensen FE (2004) Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc Natl Acad Sci U S A 101, 7751–7756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Spampinato SF, Copani A, Nicoletti F, Sortino MA, Caraci F (2018) Metabotropic glutamate receptors in glial cells: A new potential target for neuroprotection? Front Mol Neurosci 11, 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Neugebauer V (2008) Group III metabotropic glutamate receptors (mGlu4, mGlu6, mGlu7, and mGlu8). In The Glutamate Receptors, Gereau RW, Swanson GT, eds. Humana Press, Totowa, NJ, pp. 489–508. [Google Scholar]
- [65].Mao L, Guo M, Jin D, Xue B, Wang JQ (2013) Group III metabotropic glutamate receptors and drug addiction. Front Med 7, 445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Umpierre AD, West PJ, White JA, Wilcox KS (2019) Conditional knock-out of mGluR5 from astrocytes during epilepsy development impairs high-frequency glutamate uptake. J Neurosci 39, 727–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lapointe V, Morin F, Ratté S, Croce A, Conquet F, Lacaille J-C (2004) Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol 555, 125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Naie K, Manahan-Vaughan D (2005) Pharmacological antagonism of metabotropic glutamate receptor 1 regulates long-term potentiation and spatial reference memory in the dentate gyrus of freely moving rats via N-methyl-d-aspartate and metabotropic glutamate receptor-dependent mechanisms. Eur J Neurosci 21, 411–421. [DOI] [PubMed] [Google Scholar]
- [69].Gil-Sanz C, Delgado-García JM, Fairén A, Gruart A (2008) Involvement of the mGluR1 receptor in hippocampal synaptic plasticity and associative learning in behaving mice. Cereb Cortex 18, 1653–1663. [DOI] [PubMed] [Google Scholar]
- [70].Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J (1998) Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem 5, 331–343. [PMC free article] [PubMed] [Google Scholar]
- [71].Lim J, Kim E, Noh HJ, Kang S, Phillips BU, Kim DG, Bussey TJ, Saksida L, Heath CJ, Kim CH (2019) Assessment of mGluR5 KO mice under conditions of low stress using a rodent touchscreen apparatus reveals impaired behavioural flexibility driven by perseverative responses. Mol Brain 12, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Klar R, Walker AG, Ghose D, Grueter BA, Engers DW, Hopkins CR, Lindsley CW, Xiang Z, Conn PJ, Niswender CM (2015) Activation of metabotropic glutamate receptor 7 is required for induction of long-term potentiation at SCCA1 synapses in the hippocampus. J Neurosci 35, 7600–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Chung G, Kim SJ (2017) Sustained activity of metabotropic glutamate receptor: Homer, arrestin, and beyond. Neural Plast 2017, 5125264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Koyama R, Yamada MK, Nishiyama N, Matsuki N, Ikegaya Y (2002) Group II metabotropic glutamate receptor activation is required for normal hippocampal mossy fibre development in the rat. J Physiol 539, 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Gubellini P, Pisani A, Centonze D, Bernardi G, Calabresi P (2004) Metabotropic glutamate receptors and striatal synaptic plasticity: Implications for neurological diseases. Prog Neurobiol 74, 271–300. [DOI] [PubMed] [Google Scholar]
- [76].Fidzinski P, Shor O, Behr J (2008) Target-cell-specific bidirectional synaptic plasticity at hippocampal output synapses. Eur J Neurosci 27, 1111–1118. [DOI] [PubMed] [Google Scholar]
- [77].Wang H, Ardiles AO, Yang S, Tran T, Posada-Duque R, Valdivia G, Baek M, Chuang Y-A, Palacios AG, Gallagher M, Worley P, Kirkwood A (2016) Metabotropic glutamate receptors induce a form of LTP controlled by translation and arc signaling in the hippocampus. J Neurosci 36, 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kang HJ, Wilkins AD, Lichtarge O, Wensel TG (2015) Determinants of endogenous ligand specificity divergence among metabotropic glutamate receptors. J Biol Chem 290, 2870–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lüscher C, Huber KM (2010) Group 1 mGluR-dependent synaptic long-term depression: Mechanisms and implications for circuitry and disease. Neuron 65, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Pinheiro PS, Mulle C (2008) Presynaptic glutamate receptors: Physiological functions and mechanisms of action. Nat Rev Neurosci 9, 423–436. [DOI] [PubMed] [Google Scholar]
- [81].Sherman SM (2014) The function of metabotropic glutamate receptors in thalamus and cortex. Neuroscientist 20, 136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kulla A, Reymann KG, Manahan-Vaughan D (1999) Time-dependent induction of depotentiation in the dentate gyrus of freely moving rats: Involvement of group 2 metabotropic glutamate receptors. Eur J Neurosci 11, 3864–3872. [DOI] [PubMed] [Google Scholar]
- [83].Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, Nakao K, Katsuki M, Nakanishi S (1996) Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science 273, 645–647. [DOI] [PubMed] [Google Scholar]
- [84].Iv PML, Wroblewska B, Sarvey JM, Neale JH (2001) β-NAAG rescues LTP from blockade by NAAG in rat dentate gyrus via the type 3 metabotropic glutamate receptor. J Neurophysiol 85, 1097–1106. [DOI] [PubMed] [Google Scholar]
- [85].Huang L, Rowan MJ, Anwyl R (1999) Induction of long-lasting depression by (+)-α-methyl-4-carboxyphenylglycine and other group II mGlu receptor ligands in the dentate gyrus of the hippocampus in vitro. Eur J Pharmacol 366, 151–158. [DOI] [PubMed] [Google Scholar]
- [86].Pöschel B, Wroblewska B, Heinemann U, Manahan-Vaughan D (2005) The metabotropic glutamate receptor mGluR3 is critically required for hippocampal long-term depression and modulates long-term potentiation in the dentate gyrus of freely moving rats. Cereb Cortex 15, 1414–1423. [DOI] [PubMed] [Google Scholar]
- [87].Anwyl R (1999) Metabotropic glutamate receptors: Electrophysiological properties and role in plasticity. Brain Res Rev 29, 83–120. [DOI] [PubMed] [Google Scholar]
- [88].Cartmell J, Schoepp DD (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75, 889–907. [DOI] [PubMed] [Google Scholar]
- [89].Upreti C, Zhang X, Alford S, Stanton PK (2013) Role of presynaptic metabotropic glutamate receptors in the induction of long-term synaptic plasticity of vesicular release. Neuropharmacology 66, 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dasgupta A, Lim YJ, Kumar K, Baby N, Pang KLK, Benoy A, Behnisch T, Sajikumar S (2020) Group III metabotropic glutamate receptors gate long-term potentiation and synaptic tagging/capture in rat hippocampal area CA2. eLife 9, e55344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Phillips T, Barnes A, Scott S, Emson P, Rees S (1998) Human metabotropic glutamate receptor 2 couples to the MAP kinase cascade in chinese hamster ovary cells. Neuroreport 9, 2335–2339. [DOI] [PubMed] [Google Scholar]
- [92].Ferraguti F, Baldani-Guerra B, Corsi M, Nakanishi S, Corti C (1999) Activation of the extracellular signal-regulated kinase 2 by metabotropic glutamate receptors. Eur J Neurosci 11, 2073–2082. [DOI] [PubMed] [Google Scholar]
- [93].Iacovelli L, Molinaro G, Battaglia G, Motolese M, Menna LD, Alfiero M, Blahos J, Matrisciano F, Corsi M, Corti C, Bruno V, Blasi AD, Nicoletti F (2009) Regulation of group II metabotropic glutamate receptors by G protein-coupled receptor kinases: mGlu2 receptors are resistant to homologous desensitization. Mol Pharmacol 75, 991–1003. [DOI] [PubMed] [Google Scholar]
- [94].Hirbec H, Perestenko O, Nishimune A, Meyer G, Nakanishi S, Henley JM, Dev KK (2002) The PDZ proteins PICK1, GRIP, and syntenin bind multiple glutamate receptor subtypes analysis of PDZ Binding Motifs. J Biol Chem 277, 15221–15224. [DOI] [PubMed] [Google Scholar]
- [95].Ritter-Makinson SL, Paquet M, Bogenpohl JW, Rodin RE, Chris Yun C, Weinman EJ, Smith Y, Hall RA (2017) Group II metabotropic glutamate receptor interactions with NHERF scaffold proteins: Implications for receptor localization in brain. Neuroscience 353, 58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Schaffhauser H, Cai Z, Hubalek F, Macek TA, Pohl J, Murphy TJ, Conn PJ (2000) cAMP-dependent protein kinase inhibits mGluR2 coupling to G-proteins by direct receptor phosphorylation. J Neurosci 20, 5663–5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Flajolet M, Rakhilin S, Wang H, Starkova N, Nuangchamnong N, Nairn AC, Greengard P (2003) Protein phosphatase 2C binds selectively to and dephosphorylates metabotropic glutamate receptor 3. Proc Natl Acad Sci U S A 100, 16006–16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, Nakanishi S (2002) Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J Neurosci 22, 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Seebahn A, Rose M, Enz R (2008) RanBPM is expressed in synaptic layers of the mammalian retina and binds to metabotropic glutamate receptors. FEBS Lett 582, 2453–2457. [DOI] [PubMed] [Google Scholar]
- [100].Levitz J, Habrian C, Bharill S, Fu Z, Vafabakhsh R, Isacoff EY (2016) Mechanism of assembly and cooperativity of homomeric and heteromeric metabotropic glutamate receptors. Neuron 92, 143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Yin S, Noetzel MJ, Johnson KA, Zamorano R, Jalan-Sakrikar N, Gregory KJ, Conn PJ, Niswender CM (2014) Selective actions of novel allosteric modulators reveal functional heteromers of metabotropic glutamate receptors in the CNS. J Neurosci 34, 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Habrian CH, Levitz J, Vyklicky V, Fu Z, Hoagland A, McCort-Tranchepain I, Acher F, Isacoff EY (2019) Conformational pathway provides unique sensitivity to a synaptic mGluR. Nat Commun 10, 5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Di Menna L, Joffe ME, Iacovelli L, Orlando R, Lindsley CW, Mairesse J, Gressèns P, Cannella M, Caraci F, Copani A, Bruno V, Battaglia G, Conn PJ, Nicoletti F (2018) Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 128, 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Durand D, Pampillo M, Caruso C, Lasaga M (2008) Role of metabotropic glutamate receptors in the control of neuroendocrine function. Neuropharmacology 55, 577–583. [DOI] [PubMed] [Google Scholar]
- [105].Chu Z, Moenter SM (2005) Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: A possible local feedback circuit. J Neurosci 25, 5740–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Glanowska KM, Moenter SM (2011) Endocannabinoids and prostaglandins both contribute to GnRH neuron-GABAergic afferent local feedback circuits. J Neurophysiol 106, 3073–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Pereira V, Goudet C (2019) Emerging trends in pain modulation by metabotropic glutamate receptors. Front Mol Neurosci 11, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Tang Z-Q, Lu Y (2018) Anatomy and physiology of metabotropic glutamate receptors in mammalian and avian auditory system. HSOA Trends Anat Physiol 1, 001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Ferraguti F (2017) Metabotropic glutamate receptors in amygdala functions. In mGLU Receptors, Ngomba RT, Di Giovanni G, Battaglia G, Nicoletti F, eds. Springer International Publishing, Cham, pp. 241–277. [Google Scholar]
- [110].Li D-P, Pan H-L (2017) Glutamatergic regulation of hypothalamic presympathetic neurons in hypertension. Curr Hypertens Rep 19, 78. [DOI] [PubMed] [Google Scholar]
- [111].Lian Y-N, Lu Q, Chang J-L, Zhang Y (2018) The role of glutamate and its receptors in central nervous system in stress-induced hyperalgesia. Int J Neurosci 128, 283–290. [DOI] [PubMed] [Google Scholar]
- [112].Caprioli D, Justinova Z, Venniro M, Shaham Y (2018) Effect of novel allosteric modulators of metabotropic glutamate receptors on drug self-administration and relapse: A review of preclinical studies and their clinical implications. Biol Psychiatry 84, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Fan Y, Li C, Huang L, Chen L, Tang Z, Han G, Liu Y (2020) Characterization of group I metabotropic glutamate receptors in rat and human adrenal glands. Front Physiol 11, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Peters R (2006) Ageing and the brain. Postgrad Med J 82, 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM (2009) One-year brain atrophy evident in healthy aging. J Neurosci 29, 15223–15231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298, 789–791. [DOI] [PubMed] [Google Scholar]
- [117].Shankar GM, Walsh DM (2009) Alzheimer’s disease: Synaptic dysfunction and Aβ. Mol Neurodegener 4, 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chakroborty S, Hill ES, Christian DT, Helfrich R, Riley S, Schneider C, Kapecki N, Mustaly-Kalimi S, Seiler FA, Peterson DA, West AR, Vertel BM, Frost WN, Stutzmann GE (2019) Reduced presynaptic vesicle stores mediate cellular and network plasticity defects in an early-stage mouse model of Alzheimer’s disease. Mol Neurodegener 14, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kashyap G, Bapat D, Das D, Gowaikar R, Amritkar RE, Rangarajan G, Ravindranath V, Ambika G (2019) Synapse loss and progress of Alzheimer’s disease -A network model. Sci Rep 9, 6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Song Y, Hu M, Zhang J, Teng Z, Chen C (2019) A novel mechanism of synaptic and cognitive impairments mediated via microRNA-30b in Alzheimer’s disease. EBioMedicine 39, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Yu D, Yan H, Zhou J, Yang X, Lu Y, Han Y (2019) A circuit view of deep brain stimulation in Alzheimer’s disease and the possible mechanisms. Mol Neurodegener 14, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Megill A, Tran T, Eldred K, Lee NJ, Wong PC, Hoe H-S, Kirkwood A, Lee H-K (2015) Defective age-dependent metaplasticity in a mouse model of Alzheimer’s disease. J Neurosci 35, 11346–11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Jang S, Chung HJ (2016) Emerging link between Alzheimer’s disease and homeostatic synaptic plasticity. Neural Plast 2016, 7969272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Li Q, Navakkode S, Rothkegel M, Soong TW, Sajikumar S, Korte M (2017) Metaplasticity mechanisms restore plasticity and associativity in an animal model of Alzheimer’s disease. Proc Natl Acad Sci U S A 114, 5527–5532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Findley CA, Bartke A, Hascup KN, Hascup ER (2019) Amyloid beta-related alterations to glutamate signaling dynamics during Alzheimer’s disease progression. ASN Neuro 11, 1759091419855541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Lüscher C, Malenka RC (2012) NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb Perspect Biol 4, a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Chater TE, Goda Y (2014) The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci 8, 401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors, Elliott C, Masliah E, Ryan L, Silverberg N (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Thome AD, Faridar A, Beers DR, Thonhoff JR, Zhao W, Wen S, Pascual B, Masdeu JC, Appel SH (2018) Functional alterations of myeloid cells during the course of Alzheimer’s disease. Mol Neurodegener 13, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Ong W-Y, Tanaka K, Dawe GS, Ittner LM, Farooqui AA (2013) Slow excitotoxicity in Alzheimer’s disease. J Alzheimers Dis 35, 643–668. [DOI] [PubMed] [Google Scholar]
- [131].Jiang S, Nandy P, Wang W, Ma X, Hsia J, Wang C, Wang Z, Niu M, Siedlak SL, Torres S, Fujioka H, Xu Y, Lee H, Perry G, Liu J, Zhu X (2018) Mfn2 ablation causes an oxidative stress response and eventual neuronal death in the hippocampus and cortex. Mol Neurodegener 13, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Mattson MP (2019) Chapter 11 - Excitotoxicity. In Stress: Physiology, Biochemistry, and Pathology, Fink G, ed. Academic Press, pp. 125–134. [Google Scholar]
- [133].Madeira C, Vargas-Lopes C, Brandão CO, Reis T, Laks J, Panizzutti R, Ferreira ST (2018) Elevated glutamate and glutamine levels in the cerebrospinal fluid of patients with probable Alzheimer’s disease and depression. Front Psychiatry 9, 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Mucke L, Selkoe DJ (2012) Neurotoxicity of amyloid β-protein: Synaptic and network dysfunction. Cold Spring Harb Perspect Med 2, a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Hefendehl JK, LeDue J, Ko RWY, Mahler J, Murphy TH, MacVicar BA (2016) Mapping synaptic glutamate transporter dysfunction in vivo to regions surrounding Aβ plaques by iGluSnFR two-photon imaging. Nat Commun 7, 13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Britschgi M, Olin CE, Johns HT, Takeda-Uchimura Y, LeMieux MC, Rufibach K, Rajadas J, Zhang H, Tomooka B, Robinson WH, Clark CM, Fagan AM, Galasko DR, Holtzman DM, Jutel M, Kaye JA, Lemere CA, Leszek J, Li G, Peskind ER, Quinn JF, Yesavage JA, Ghiso JA, Wyss-Coray T (2009) Neuroprotective natural antibodies to assemblies of amyloidogenic peptides decrease with normal aging and advancing Alzheimer’s disease. Proc Natl Acad Sci U S A 106, 12145–12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Moore BD, Chakrabarty P, Levites Y, Kukar TL, Baine A-M, Moroni T, Ladd TB, Das P, Dickson DW, Golde TE (2012) Overlapping profiles of Aβ peptides in the Alzheimer’s disease and pathological aging brains. Alzheimers Res Ther 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Kim SH, Fraser PE, Westaway D, St George-Hyslop PH, Ehrlich ME, Gandy S (2010) Group II metabotropic glutamate receptor stimulation triggers production and release of Alzheimer’s amyloid(beta)42 from isolated intact nerve terminals. J Neurosci 30, 3870–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Palmeri A, Ricciarelli R, Gulisano W, Rivera D, Rebosio C, Calcagno E, Tropea MR, Conti S, Das U, Roy S, Pronzato MA, Arancio O, Fedele E, Puzzo D (2017) Amyloid-β peptide is needed for cGMP-induced long-term potentiation and memory. J Neurosci 37, 6926–6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Palop JJ, Mucke L (2010) Amyloid-beta-induced neuronal dysfunction in Alzheimer’s disease: From synapses toward neural networks. Nat Neurosci 13, 812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Dawson GR, Seabrook GR, Zheng H, Smith DW, Graham S, O’Dowd G, Bowery BJ, Boyce S, Trumbauer ME, Chen HY, Van der Ploeg LHT, Sirinathsinghji DJS (1999) Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the β-amyloid precursor protein. Neuroscience 90, 1–13. [DOI] [PubMed] [Google Scholar]
- [142].Parihar MS, Brewer GJ (2010) Amyloid-β as a modulator of synaptic plasticity. J Alzheimers Dis 22, 741–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Peric A, Annaert W (2015) Early etiology of Alzheimer’s disease: Tipping the balance toward autophagy or endosomal dysfunction? Acta Neuropathol (Berl) 129, 363–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Anggono V, Tsai L, Götz J (2016) Glutamate receptors in Alzheimer’s disease: Mechanisms and therapies. Neural Plast 2016, 8256196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Esposito Z, Belli L, Toniolo S, Sancesario G, Bianconi C, Martorana A (2013) Amyloid β, glutamate, excitotoxicity in Alzheimer’s disease: Are we on the right track? CNS Neurosci Ther 19, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N (2016) Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: Understanding the therapeutics strategies. Mol Neurobiol 53, 648–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Acquarone E, Argyrousi EK, van den Berg M, Gulisano W, Fá M, Staniszewski A, Calcagno E, Zuccarello E, D’Adamio L, Deng S-X, Puzzo D, Arancio O, Fiorito J (2019) Synaptic and memory dysfunction induced by tau oligomers is rescued by up-regulation of the nitric oxide cascade. Mol Neurodegener 14, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].DeTure MA, Dickson DW (2019) The neuropathological diagnosis of Alzheimer’s disease. Mol Neurodegener 14, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Cirrito JR, Kang J-E, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM (2008) Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron 58, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Abramov E, Dolev I, Fogel H, Ciccotosto GD, Ruff E, Slutsky I (2009) Amyloid-β as a positive endogenous regulator of release probability at hippocampal synapses. Nat Neurosci 12, 1567–1576. [DOI] [PubMed] [Google Scholar]
- [151].van Spronsen M, Hoogenraad CC (2010) Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep 10, 207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Gilbert J, Shu S, Yang X, Lu Y, Zhu L-Q, Man H-Y (2016) [beta]-Amyloid triggers aberrant over-scaling of homeostatic synaptic plasticity. Acta Neuropathol Commun 4, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Marcello E, Epis R, Saraceno C, Di Luca M (2012) Synaptic dysfunction in Alzheimer’s disease. Adv Exp Med Biol 970, 573–601. [DOI] [PubMed] [Google Scholar]
- [154].Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM (2017) Synaptic impairment in Alzheimer’s disease: A dysregulated symphony. Trends Neurosci 40, 347–357. [DOI] [PubMed] [Google Scholar]
- [155].Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, Viola KL, Klein WL (2007) Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci 27, 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Hu N-W, Klyubin I, Anwyl R, Rowan MJ (2009) GluN2B subunit-containing NMDA receptor antagonists prevent Aβ-mediated synaptic plasticity disruption in vivo. Proc Natl Acad Sci U S A 106, 20504–20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, Domercq M, Matute C (2010) Amyloid β oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 47, 264–272. [DOI] [PubMed] [Google Scholar]
- [158].Miñano-Molina AJ, España J, Martín E, Barneda-Zahonero B, Fadó R, Solé M, Trullás R, Saura CA, Rodríguez-Alvarez J (2011) Soluble oligomers of amyloid-β peptide disrupt membrane trafficking of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor contributing to early synapse dysfunction. J Biol Chem 286, 27311–27321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Bie B, Wu J, Foss JF, Naguib M (2019) Activation of mGluR1 mediates C1q-dependent microglial phagocytosis of glutamatergic synapses in Alzheimer’s rodent models. Mol Neurobiol 56, 5568–5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ishibashi K, Miura Y, Toyohara J, Ishiwata K, Ishii K (2019) Unchanged type 1 metabotropic glutamate receptor availability in patients with Alzheimer’s disease: A study using 11C-ITMM positron emission tomography. Neuroimage Clin 22, 101783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Albasanz JL, Dalfó E, Ferrer I, Martín M (2005) Impaired metabotropic glutamate receptor/phospholipase C signaling pathway in the cerebral cortex in Alzheimer’s disease and dementia with Lewy bodies correlates with stage of Alzheimer’s-disease-related changes. Neurobiol Dis 20, 685–693. [DOI] [PubMed] [Google Scholar]
- [162].Fang XT, Eriksson J, Antoni G, Yngve U, Cato L, Lannfelt L, Sehlin D, Syvänen S (2017) Brain mGluR5 in mice with amyloid beta pathology studied with in vivo [11C]ABP688 PET imaging and ex vivo immunoblotting. Neuropharmacology 113, 293–300. [DOI] [PubMed] [Google Scholar]
- [163].Mecca AP, McDonald JW, Michalak HR, Godek TA, Harris JE, Pugh EA, Kemp EC, Chen M-K, Salardini A, Nabulsi NB, Lim K, Huang Y, Carson RE, Strittmatter SM, van Dyck CH (2020) PET imaging of mGluR5 in Alzheimer’s disease. Alzheimers Res Ther 12, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Meziane H, Dodart J-C, Mathis C, Little S, Clemens J, Paul SM, Ungerer A (1998) Memory-enhancing effects of secreted forms of the β-amyloid precursor protein in normal and amnestic mice. Proc Natl Acad Sci U S A 95, 12683–12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Jolly-Tornetta C, Gao ZY, Lee VM, Wolf BA (1998) Regulation of amyloid precursor protein secretion by glutamate receptors in human Ntera 2 neurons. J Biol Chem 273, 14015–14021. [DOI] [PubMed] [Google Scholar]
- [166].Lee H, Zhu X, O’Neill MJ, Webber K, Casadesus G, Marlatt M, Raina AK, Perry G, Smith MA (2004) The role of metabotropic glutamate receptors in Alzheimer’s disease. Acta Neurobiol Exp (Warsz) 64, 89–98. [DOI] [PubMed] [Google Scholar]
- [167].Lee H, Zhu X, Casadesus G, Pallás M, Camins A, O’Neill MJ, Nakanishi S, Perry G, Smith MA (2009) The effect of mGluR2 activation on signal transduction pathways and neuronal cell survival. Brain Res 1249, 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH (2000) Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: Differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogen synthase kinase-3beta. J Neurochem 74, 1587–1595. [DOI] [PubMed] [Google Scholar]
- [169].Morsch R, Simon W, Coleman PD (1999) Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol 58, 188–197. [DOI] [PubMed] [Google Scholar]
- [170].Lee H, Perry G, Moreira PI, Garrett MR, Liu Q, Zhu X, Takeda A, Nunomura A, Smith MA (2005) Tau phosphorylation in Alzheimer’s disease: Pathogen or protector? Trends Mol Med 11, 164–169. [DOI] [PubMed] [Google Scholar]
- [171].Buisson A, Choi DW (1995) The inhibitory mGluR agonist, s-4-carboxy-3-hydroxy-phenylglycine selectively attenuates NMDA neurotoxicity and oxygen-glucose deprivation-induced neuronal death. Neuropharmacology 34, 1081–1087. [DOI] [PubMed] [Google Scholar]
- [172].Buisson A, Yu SP, Choi DW (1996) DCG-IV selectively attenuates rapidly triggered NMDA-induced neurotoxicity in cortical neurons. Eur J Neurosci 8, 138–143. [DOI] [PubMed] [Google Scholar]
- [173].Kingston AE, O’Neill MJ, Bond A, Bruno V, Battaglia G, Nicoletti F, Harris JR, Clark BP, Monn JA, Lodge D, Schoepp DD (1999) Neuroprotective actions of novel and potent ligands of group I and group II metabotropic glutamate receptors. Ann N Y Acad Sci 890, 438–449. [DOI] [PubMed] [Google Scholar]
- [174].Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, Mugnaini M, Nicoletti F, Bruno V (2007) The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci 27, 8297–8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Caraci F, Molinaro G, Battaglia G, Giuffrida ML, Riozzi B, Traficante A, Bruno V, Cannella M, Merlo S, Wang X, Heinz BA, Nisenbaum ES, Britton TC, Drago F, Sortino MA, Copani A, Nicoletti F (2011) Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer’s disease: Selective activation of mGlu2 receptors amplifies β-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol Pharmacol 79, 618–626. [DOI] [PubMed] [Google Scholar]
- [176].Chow VW, Mattson MP, Wong PC, Gleichmann M (2010) An overview of APP processing enzymes and products. Neuromolecular Med 12, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Kim SH, Steele JW, Lee SW, Clemenson GD, Carter TA, Treuner K, Gadient R, Wedel P, Glabe C, Barlow C, Ehrlich ME, Gage FH, Gandy S (2014) Proneurogenic group II mGluR antagonist improves learning and reduces anxiety in Alzheimer Aβ oligomer mouse. Mol Psychiatry 19, 1235–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Flor PJ, Acher FC (2012) Orthosteric versus allosteric GPCR activation: The great challenge of group-III mGluRs. Biochem Pharmacol 84, 414–424. [DOI] [PubMed] [Google Scholar]
- [179].Mercier MS, Lodge D (2014) Group III metabotropic glutamate receptors: Pharmacology, physiology and therapeutic potential. Neurochem Res 39, 1876–1894. [DOI] [PubMed] [Google Scholar]
- [180].Kaur S, DasGupta G, Singh S (2019) Altered neurochemistry in Alzheimer’s disease: Targeting neurotransmitter receptor mechanisms and therapeutic strategy. Neurophysiology 51, 293–309. [Google Scholar]
- [181].Dal Prá I, Armato U, Chiarini A (2019) Family C G-protein-coupled receptors in Alzheimer’s disease and therapeutic implications. Front Pharmacol 10, 1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Zhao L, Zhao ST, Qian ZM, Zhang C, Wu XM, Du F, Ke Y (2009) Activation of group III metabotropic glutamate receptor reduces intracellular calcium in β-amyloid peptide [31–35]-treated cortical neurons. Neurotox Res 16, 174–183. [DOI] [PubMed] [Google Scholar]
- [183].Taylor DL, Diemel LT, Pocock JM (2003) Activation of microglial group III metabotropic glutamate receptors protects neurons against microglial neurotoxicity. J Neurosci 23, 2150–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Célanire S, Campo B (2012) Recent advances in the drug discovery of metabotropic glutamate receptor 4 (mGluR4) activators for the treatment of CNS and non-CNS disorders. Expert Opin Drug Discov 7, 261–280. [DOI] [PubMed] [Google Scholar]
- [185].Iscru E, Goddyn H, Ahmed T, Callaerts-Vegh Z, D’Hooge R, Balschun D (2013) Improved spatial learning is associated with increased hippocampal but not prefrontal long-term potentiation in mGluR4 knockout mice. Genes Brain Behav 12, 615–625. [DOI] [PubMed] [Google Scholar]
- [186].Conn PJ, Niswender CM (2006) mGluR7’s lucky number. Proc Natl Acad Sci U S A 103, 251–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Gu Z, Cheng J, Zhong P, Qin L, Liu W, Yan Z (2014) Aβ selectively impairs mGluR7 modulation of NMDA signaling in basal forebrain cholinergic neurons: Implication in Alzheimer’s disease. J Neurosci 34, 13614–13628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Fisher NM, Seto M, Lindsley CW, Niswender CM (2018) Metabotropic glutamate receptor 7: A new therapeutic target in neurodevelopmental disorders. Front Mol Neurosci 11, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Zhang Y-L, Xing R-Z, Luo X-B, Xu H, Chang RC-C, Zou L-Y, Liu J-J, Yang X-F (2016) Anxiety-like behavior and dysregulation of miR-34a in triple transgenic mice of Alzheimer’s disease. Eur Rev Med Pharmacol Sci 20, 2853–2862. [PubMed] [Google Scholar]
- [190].Zhou R, Yuan P, Wang Y, Hunsberger JG, Elkahloun A, Wei Y, Damschroder-Williams P, Du J, Chen G, Manji HK (2009) Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacology 34, 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Gerlai R, Adams B, Fitch T, Chaney S, Baez M (2002) Performance deficits of mGluR8 knockout mice in learning tasks: The effects of null mutation and the background genotype. Neuropharmacology 43, 235–249. [DOI] [PubMed] [Google Scholar]
- [192].Ferraguti F, Klausberger T, Cobden P, Baude A, Roberts JDB, Szucs P, Kinoshita A, Shigemoto R, Somogyi P, Dalezios Y (2005) Metabotropic glutamate receptor 8-expressing nerve terminals target subsets of GABAergic neurons in the hippocampus. J Neurosci 25, 10520–10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Lewerenz J, Maher P (2015) Chronic glutamate toxicity in neurodegenerative diseases-what is the evidence? Front Neurosci 9, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Nicholls RE, Zhang X, Bailey CP, Conklin BR, Kandel ER, Stanton PK (2006) mGluR2 acts through inhibitory Gα subunits to regulate transmission and long-term plasticity at hippocampal mossy fiber-CA3 synapses. Proc Natl Acad Sci U S A 103, 6380–6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Tang Z-Q, Liu Y-W, Shi W, Dinh EH, Hamlet WR, Curry RJ, Lu Y (2013) Activation of synaptic group II metabotropic glutamate receptors induces long-term depression at GABAergic synapses in CNS neurons. J Neurosci 33, 15964–15977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Wang Z, Neely R, Landisman CE (2015) Activation of group I and group II metabotropic glutamate receptors causes LTD and LTP of electrical synapses in the rat thalamic reticular nucleus. J Neurosci 35, 7616–7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Jones PJ, Xiang Z, Conn PJ (2008) Metabotropic glutamate receptors mGluR4 and mGluR8 regulate transmission in the lateral olfactory tract-piriform cortex synapse. Neuropharmacology 55, 440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ (2005) mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron 46, 89–102. [DOI] [PubMed] [Google Scholar]
- [199].Schmid S, Fendt M (2006) Effects of the mGluR8 agonist (S)-3,4-DCPG in the lateral amygdala on acquisition/expression of fear-potentiated startle, synaptic transmission, and plasticity. Neuropharmacology 50, 154–164. [DOI] [PubMed] [Google Scholar]
- [200].Hovelsø N, Sotty F, Montezinho LP, Pinheiro PS, Herrik KF, Mørk A (2012) Therapeutic potential of metabotropic glutamate receptor modulators. Curr Neuropharmacol 10, 12–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Cheng X, Wu J, Geng M, Xiong J (2014) Role of synaptic activity in the regulation of amyloid beta levels in Alzheimer’s disease. Neurobiol Aging 35, 1217–1232. [DOI] [PubMed] [Google Scholar]
- [202].Wilcox KC, Lacor PN, Pitt J, Klein WL (2011) Aβ oligomer-induced synapse degeneration in Alzheimer’s disease. Cell Mol Neurobiol 31, 939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Piers TM, Kim DH, Kim BC, Regan P, Whitcomb DJ, Cho K (2012) Translational concepts of mGluR5 in synaptic diseases of the brain. Front Pharmacol 3, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Jin LE, Wang M, Galvin VC, Lightbourne TC, Conn PJ, Arnsten AFT, Paspalas CD (2018) mGluR2 versus mGluR3 metabotropic glutamate receptors in primate dorsolateral prefrontal cortex: Postsynaptic mGluR3 strengthen working memory networks. Cereb Cortex 28, 974–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, Kerrisk ME, Vortmeyer A, Wisniewski T, Koleske AJ, Gunther EC, Nygaard HB, Strittmatter SM (2013). Metabotropic glutamate receptor 5 is a co-receptor for Alzheimer Aβ oligomer bound to cellular prion protein. Neuron 79(5), 887–902. [DOI] [PMC free article] [PubMed] [Google Scholar]


