Abstract
Papillary thyroid cancer (PTC) is the most common form of differentiated thyroid cancer in the pediatric population and represents the second most common malignancy in adolescent females. Historically, PTC has been classified on the basis of histology, however, accumulating data indicate that molecular subtyping based on somatic oncogenic alterations along with gene expression profiling can better predict clinical behavior and may provide opportunities to incorporate oncogene-specific inhibitory therapy to improve the response to radioactive iodine (RAI). In this issue of the JCI, Y.A. Lee, H. Lee, and colleagues showed that oncogenic fusions were more commonly associated with invasive disease, increased expression of MAPK signaling pathway genes (ERK score), and decreased expression of the sodium-iodine symporter, which was restored by RET- and NTRK-inhibitory therapy. These findings lend credence to the idea of reclassifying pediatric thyroid cancers using a three-tiered system, rather than the two-tiered adult system, and open avenues for the treatment of progressive, RAI-refractory PTC in patients.
Papillary thyroid cancer in pediatrics
Papillary thyroid cancer (PTC) is the most common form of differentiated thyroid cancer in the pediatric population and represents the second most common malignancy in adolescent females (1). Traditionally, the majority of children with PTC undergo near-total thyroidectomy due to the high propensity for bilateral disease (1, 2).
Since publication of the 2015 American Thyroid Association’s pediatric guidelines, there has been an increased effort to stratify surgical and medical therapy to reduce complications of therapy while maintaining a low risk for persistent and recurrent disease. In patients with PTC who have a low risk for regional and distant metastasis, lobectomy without radioactive iodine (RAI) therapy is typically sufficient to achieve remission, eliminating the risk for permanent hypoparathyroidism and the need for lifelong thyroid hormone replacement therapy (3–5). Conversely, in patients with widely invasive disease, with an increased risk for distant metastasis, most commonly to the lungs, RAI is the mainstay of treatment after thyroidectomy and complete cervical neck lymph node dissection. Unfortunately, while disease-specific mortality is low even in patients with distant metastasis, fewer than 20% of pediatric patients with pulmonary metastasis achieve complete remission, and approximately 10% to 15% of patients show progression even with repeated RAI (6–9).
The oncogenic driver alterations in pediatric PTC are the same as those in adults, however, gene fusions occur with a higher frequency, 60% to 70% versus 15%, respectively (10, 11). Rearranged during transfection (RET) proto-oncogene fusions are the most common, followed by fusions involving the neurotrophic tyrosine kinase receptors (NTRK), anaplastic lymphoma kinase (ALK), and, less commonly, BRAF kinase and MET tyrosine kinase receptor (c-MET) genes (9–12). Point mutations are found in approximately 30% of pediatric patients compared with 70% in adults, with the BRAF (BRAFV600E) mutation being the most frequent, followed by PTEN, RAS, and DICER1 mutations (10–12). Oncogenic fusions in tumors of children and adolescents are associated with increased invasive behavior and a decreased response to therapy (9, 12). In adults, BRAF mutations are more commonly found in tumors with aggressive clinical behavior and a decreased response to RAI (13).
Oncogenic landscape and behavior of pediatric PTC
In this issue of the JCI, Y.A. Lee, H. Lee, and colleagues expand our understanding of the oncogenic landscape of invasive PTC in pediatrics (14). The authors provide further evidence that, in contrast to adults, pediatric patients with PTC-harboring fusion oncogenes, rather than a BRAF mutation, are more likely to have lateral neck and pulmonary metastases as well as an increased risk for persistent biochemical and structural disease. Interestingly, but previously reported, there is an age-specific distribution of the oncogenic alterations, with younger age (under 10 years) being associated exclusively with oncogenic fusions and an increasing prevalence of BRAF mutations during early (10–14 years) and late (15–19 years) adolescence (12, 14, 15). The authors suggest that thyrocytes from younger children may be more susceptible to ionizing radiation or other mechanisms of DNA damage, with a reduced ability for repair. However, only 9.7% of patients (3 of 31) with fusion alterations had previous exposure to radiation (14). So, although the theory proposed by Y.A. Lee, H. Lee, and co-authors is plausible, the data are insufficient to draw any conclusions. Additional research is needed to identify factors beyond radiation that may increase the risk for formation of oncogenic fusion alterations in PTC as well as to determine why fusions in pediatric PTC are associated with more invasive disease similar to BRAF-associated PTC in adults.
Understanding the oncogenic landscape and predicting clinical behavior
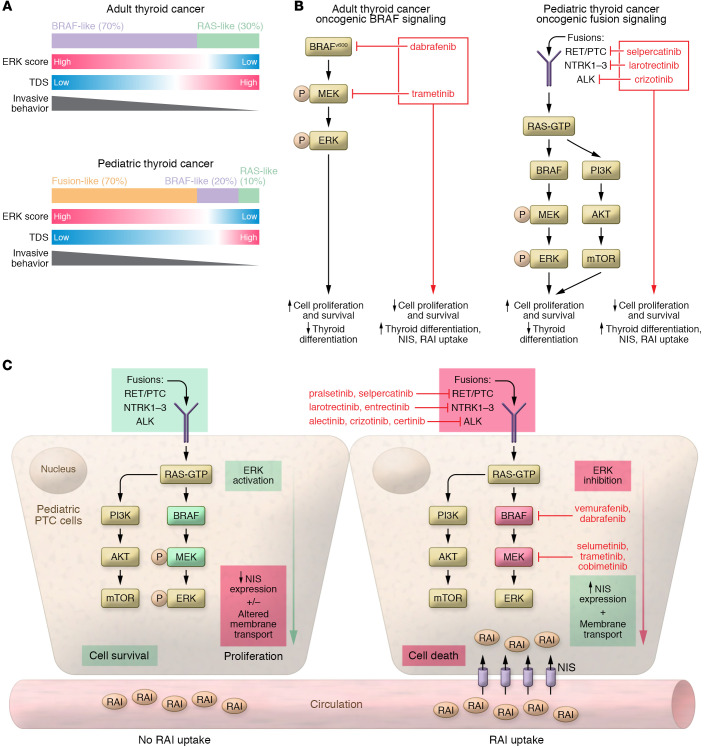
Y.A. Lee, H. Lee, and colleagues expanded the investigation of the genomic landscape of pediatric PTC by analyzing the expression of 16 thyroid-specific genes (established as a thyroid differentiation score [TDS]) and 52 MAPK signaling pathway genes (established as an ERK score) (14). This analysis mirrors the efforts of The Cancer Genome Atlas Research Network (TCGA) report that examined 496 adult PTCs (13). The authors’ preliminary analysis revealed that the gene expression profiles of the pediatric PTCs with fusion alterations closely aligned with the adult BRAF-like group, with higher ERK scores (MAPK activation) and lower TDSs, as well as reduced expression of the sodium-iodide symporter (NIS) gene (SLC5A5) (14). Although the sample size for this analysis was small, these results lend credence to the clinical observation that pediatric PTCs driven by fusion oncogenes more closely align with adult PTCs with BRAF driver mutations in regard to invasive behavior and decreased response to RAI therapy (Figure 1).
Figure 1. PTC signaling in adult and pediatric patients.
(A) Adult and pediatric thyroid cancers are categorized according to different molecular subgroups. TCGA generated a two-tiered classification (BRAF-like vs. RAS-like) in adult PTC, with BRAF-like tumors having a higher ERK score, a lower TDS, and an increased risk for invasive behavior compared with RAS-like tumors. Data from Y.A. Lee, H. Lee, and colleagues (14) show that pediatric tumors harboring kinase fusion oncogenes followed a pattern similar to that seen in adult PTCs with BRAF. Combined with previously published data, a three-tier classification system in pediatric PTC may more accurately correlate with clinical behavior. A three-tiered system would encompass RAS-like, BRAF-like, and fusion-like PTCs and thus reflect an increasing risk of invasive behavior. (B) In adult and pediatric thyroid cancer, signaling pathways are associated with loss of differentiation. In adults, PTCs with a BRAF mutation (and MAPK activation) are associated with a lower TDS (left). In pediatric tumors, PTCs with kinase fusion oncogenes are associated with a lower TDS. The use of MAPK inhibitors to specifically block the oncogenes in these tumors reduces ERK activation and restores the expression of differentiation genes, including the gene that encodes NIS, which correlates with a response to RAI therapy. (C) In pediatric PTC cells, exposure to an oncogene-specific inhibitor results in increased expression of NIS on the basolateral membrane, which sensitizes the cells to RAI.
What is also interesting, but not a focus of the research, is that the presence of a fusion alteration was associated with an increased risk for invasive disease irrespective of histology. In both classic PTC (cPTC) and diffuse sclerosing variant PTC (DSV-PTC), the presence of an oncogenic fusion was associated with an increased rate of lymph node and pulmonary metastasis (14). Somatic driver alteration appeared to better predict invasive disease than histology, supporting the proposal by TCGA to reclassify thyroid cancers into molecular subtypes rather than the traditional pathological classification (13). If we combine the expanded genomic data from Y.A. Lee, H. Lee, and colleagues with previously published somatic oncogene data, a three-tiered molecular classification would reflect an increasing risk for invasive disease, with RAS-like cancers as low risk, followed by BRAF-like and fusion-like cancers as successively higher risk. A three-tier system appears to more closely align with clinical behavior in pediatric patients than does TCGA’s proposed two-tiered, adult-based classification, in which PTC is categorized as RAS-like and BRAF-like (10–12).
Clinical implications and opportunities to improve care and outcomes
Over the past several years, there has been increasing use of systemic chemotherapeutic agents in the treatment of adult patients with advanced, progressive RAI-refractory thyroid cancer that is not amenable to surgery (mostly with pulmonary metastases) (16). Many of the drugs being used in clinical practice and currently undergoing clinical trials have been repurposed from other cancer therapies. Multi-tyrosine kinase inhibitors were the first agents to be used in clinical practice (16). More recently, oncogene-specific targeted inhibitors that block constitutively activated signaling pathways have been added to the repertoire for thyroid cancer therapy. These agents have demonstrated low side-effect profiles with high response rates ranging from 60%–100% in patients with PTC, including reports of resensitization to RAI therapy (17–19).
The patient cases with supportive in vitro data by Y.A. Lee, H. Lee, and colleagues provide a first look into the potential efficacy and mechanism of incorporating oncogene-specific inhibitory therapy in pediatric patients. At baseline, PTC with fusion alterations upregulated ERK signaling, with a concomitant decrease in the TDS, particularly with regard to NIS expression. After treatment with either a RET or NTRK inhibitor, both patients developed increased RAI avidity to regional and/or distant metastatic lesions. Unfortunately, only one of the two patients was retreated with RAI, and, despite an increase in RAI avidity, the patient is reported to have persistent structural disease at the time of publication. Repeating the tests for the ERK score or the TDS in patient samples after oncogene-specific inhibitory therapy would provide a more complete assessment of the mechanistic impact of inhibitor therapy.
The in vitro investigation using the NTRK inhibitor also showed that increased NIS expression correlated with the clinical data (14). However, Y.A. Lee, H. Lee, and colleagues used larotrectinib at much higher concentrations than those used in previous studies in other malignancies, and were approximately 25-fold higher than the peak concentration achieved in clinical practice (20). Future research is needed to determine whether these oncogenic pathways are differentially activated in thyrocytes compared to other epithelial cells harboring identical mutations and whether tailoring tumor-specific oncogene inhibitory therapy for PTC would optimize clinical responses.
Future directions
While more clinical and translational data are needed, the conclusion is clear: It is time for a paradigm shift in the evaluation and management of pediatric thyroid cancer via identification of the somatic driver alterations during the early phases of diagnosis and treatment. Knowledge of the oncogenic alteration increases the diagnostic accuracy of nodules with indeterminate cytology and may help stratify surgery and limit the surgical extent and lymph node dissection for patients with genetic alterations associated with a low risk for invasive disease (RAS-like; i.e., PTEN, RAS, and DICER1 mutations). Conversely, patients with alterations associated with an intermediate (BRAF) or high risk for invasive behavior (fusions involving RET, NTRK, and ALK) would undergo a complete assessment of cervical lymph nodes for dissection (21, 22). The data from Y.A. Lee, H. Lee, and colleagues support and extend our understanding of the genomic landscape and provide an initial insight into the potential efficacy and mechanism of incorporating oncogene-specific inhibitory therapy to improve absorption of RAI (14). The extension of the multiplatform analysis to include micro-RNA and epigenetic alterations will also optimize the molecular classification of pediatric thyroid cancer, allowing for a more complete comparison with TCGA adult PTC data (13).
There is also much to learn about how and when to incorporate oncogene-specific therapy into clinical practice. In addition to salvage therapy, in which oncogene-specific inhibitory therapy is used to treat progressive, RAI-refractory disease, the potential to include these agents to debulk tumors that present with morbidly invasive disease prior to surgery and to consider using these agents to increase the efficacy of the initial RAI treatment in patients with pulmonary metastasis should be explored. We are extremely fortunate that the majority of pediatric patients with PTC experience low disease-specific mortality, however, we are obligated to continue to find opportunities to improve care, to reduce complications, and to strive to achieve a complete response to therapy for as many patients as possible. The study by Y.A. Lee, H. Lee, and colleagues (14) takes us one step closer to achieving these goals.
Acknowledgments
ATF acknowledges support from the NIH (RO1 CA214511). TWL acknowledges support from the Alex’s Lemonade Stand Foundation. ATF, JCRR, TWL, and AJB acknowledge support from the Children’s Hospital of Philadelphia Frontier Program.
Version 1. 09/15/2021
Electronic publication
Footnotes
Conflict of interest: TWL receives research funding from Bayer, Pfizer, and Novartis.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(18):e152696. https://doi.org/10.1172/JCI152696.
Contributor Information
Aime T. Franco, Email: FrancoA1@chop.edu.
Julio C. Ricarte-Filho, Email: jcricarte@gmail.com.
Theodore W. Laetsch, Email: tlaetsch@gmail.com.
Andrew J. Bauer, Email: bauera@chop.edu.
References
- 1.Francis GL, et al. Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid. 2015;25(7):716–759. doi: 10.1089/thy.2014.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baumgarten H, et al. Bilateral papillary thyroid cancer in children: Risk factors and frequency of postoperative diagnosis. J Pediatr Surg. 2020;55(6):1117–1122. doi: 10.1016/j.jpedsurg.2020.02.040. [DOI] [PubMed] [Google Scholar]
- 3.Kluijfhout WP, et al. Is it time to reconsider lobectomy in low-risk paediatric thyroid cancer? Clin Endocrinol (Oxf) 2017;86(4):591–596. doi: 10.1111/cen.13287. [DOI] [PubMed] [Google Scholar]
- 4.Samuels SL, et al. Characteristics of follicular variant papillary thyroid carcinoma in a pediatric cohort. J Clin Endocrinol Metab. 2018;103(4):1639–1648. doi: 10.1210/jc.2017-02454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sugino K, et al. Risk stratification of pediatric patients with differentiated thyroid cancer: is total thyroidectomy necessary for patients at any risk? Thyroid. 2020;30(4):548–556. doi: 10.1089/thy.2019.0231. [DOI] [PubMed] [Google Scholar]
- 6.Alzahrani AS, et al. Lung metastasis in pediatric thyroid cancer: radiological pattern, molecular genetics, response to therapy, and outcome. J Clin Endocrinol Metab. 2019;104(1):103–110. doi: 10.1210/jc.2018-01690. [DOI] [PubMed] [Google Scholar]
- 7.Sugino K, et al. Distant metastasis in pediatric and adolescent differentiated thyroid cancer: clinical outcomes and risk factor analyses. J Clin Endocrinol Metab. 2020;105(11):dgaa545. doi: 10.1210/clinem/dgaa545. [DOI] [PubMed] [Google Scholar]
- 8.Chesover AD, et al. Lung metastasis in children with differentiated thyroid cancer: factors associated with diagnosis and outcomes of therapy. Thyroid. 2021;31(1):50–60. doi: 10.1089/thy.2020.0002. [DOI] [PubMed] [Google Scholar]
- 9.Nies M, et al. Distant metastases from childhood differentiated thyroid carcinoma: clinical course and mutational landscape. J Clin Endocrinol Metab. 2021;106(4):e1683–e1697. doi: 10.1210/clinem/dgaa935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer AJ. Molecular genetics of thyroid cancer in children and adolescents. Endocrinol Metab Clin North Am. 2017;46(2):389–403. doi: 10.1016/j.ecl.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 11.Paulson VA, et al. Thyroid cancer in the pediatric population. Genes (Basel) 2019;10(9):E723. doi: 10.3390/genes10090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pekova B, et al. RET, NTRK, ALK, BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid. 2020;30(12):1771–1780. doi: 10.1089/thy.2019.0802. [DOI] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YA, et al. NTRK and RET fusion–directed therapy in pediatric thyroid cancer yields a tumor response and radioiodine uptake. J Clin Invest. 2021;131:144847. doi: 10.1172/JCI144847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 16.Cabanillas ME, et al. Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endocr Rev. 2019;40(6):1573–1604. doi: 10.1210/er.2019-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaber T, et al. Targeted therapy in advanced thyroid cancer to resensitize tumors to radioactive iodine. J Clin Endocrinol Metab. 2018;103(10):3698–3705. doi: 10.1210/jc.2018-00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groussin L, et al. Larotrectinib-enhanced radioactive iodine uptake in advanced thyroid cancer. N Engl J Med. 2020;383(17):1686–1687. doi: 10.1056/NEJMc2023094. [DOI] [PubMed] [Google Scholar]
- 19.Rothenberg SM, et al. Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin Cancer Res. 2015;21(5):1028–1035. doi: 10.1158/1078-0432.CCR-14-2915. [DOI] [PubMed] [Google Scholar]
- 20.Doebele RC, et al. An oncogenic NTRK fusion in a patient with soft-tissue sarcoma with response to the tropomyosin-related kinase inhibitor LOXO-101. Cancer Discov. 2015;5(10):1049–1057. doi: 10.1158/2159-8290.CD-15-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bauer AJ. Pediatric thyroid cancer: genetics, therapeutics and outcome. Endocrinol Metab Clin North Am. 2020;49(4):589–611. doi: 10.1016/j.ecl.2020.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Krasner JR, et al. Molecular mutations as a possible factor for determining extent of thyroid surgery. J Otolaryngol Head Neck Surg. 2019;48(1):51. doi: 10.1186/s40463-019-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]