Abstract
Tanycytes are specialized radial glial cells of the hypothalamus that have emerged as important players that sense and respond to fluctuations in whole-body energy status to maintain energy homeostasis. However, the underlying mechanisms by which tanycytes influence energy balance remain incompletely understood. In this issue of the JCI, Lhomme et al. used transgenic mouse models, pharmacological approaches, and electrophysiology to investigate how tanycytes sense glucose availability and integrate metabolic cues into a lactate tanycytic network that fuels pro-opiomelanocortin (POMC) neuronal activity. Notably, the authors found that the tanycytic network relied on monocarboxylate transporters and connexin-43 gap junctions to transfer lactate to POMC neurons. Collectively, this study places tanycytes at the center of the intercellular communication processes governing energy balance.
The role of tanycytes in metabolic control
Proper crosstalk between the periphery and the central nervous system is crucial for an organism to maintain stable energy supplies throughout all tissues. Indeed, impairments in related communication channels lead to metabolic disorders such as obesity and type 2 diabetes (T2D) (1). The arcuate nucleus of the hypothalamus (ARH), which lies just adjacent to the median eminence, plays a prominent role in energy balance control. The strategic location of the hypothalamus provides wide access to nutrient and hormonal signals arriving through the portal capillaries, allowing ARH neurons to monitor and integrate metabolic cues conveying information on systemic energy status (1). While hypothalamic pro-opiomelanocortin (POMC) and agouti-related peptide–expressing (AgRP-expressing) neurons are well-known key elements of the metabolic transponder system, other resident cell types have been recently recognized as active players in this intricate biological process (2). An intriguing case is that of the tanycytes, which have versatile and diverse relevant functions related to energy homeostasis (3).
Tanycytes are specialized radial glial cells that line the third ventricle floor (3V) of the tuberal hypothalamus, forming a physical interface between the cerebrospinal fluid (CSF) and blood. Because of their unique anatomical location, tanycytes are considered nutrient-sensing units capable of perceiving systemic metabolic changes and responding accordingly. Previous studies have shown that the organismal nutritional status directly modulates tanycytic architecture and functionality to facilitate the access of metabolic hormones to hypothalamic neurons (4–6). Tanycytes also respond to glucose fluctuations via dedicated transporters (GLUT1 and GLUT2) and by generating ATP-mediated Ca2+ waves that are thought to influence hypothalamic neuronal activity (7, 8). Importantly, the expression of monocarboxylate transporters (MCTs) by tanycytes led to the speculation that metabolic byproducts of glucose breakdown (e.g., lactate) could act as surrogate signals for nearby neurons (9, 10) in a manner similar to that of the astrocyte-neuron lactate shuttle, whereby astrocytes consume glucose and secrete lactate, which is subsequently taken up by neurons (11). However, the validity of this hypothesis and its potential physiological importance in relation to energy homeostasis have not been formally addressed. In this issue of the JCI, Lhomme et al. show that a network of tanycytes acted collectively to produce and transfer lactate (as a proxy for CSF and blood-borne glucose levels) that ended up fueling the activity of nearby POMC neurons. The POMC neurons, in turn, modulated energy homeostasis (ref. 3 and Figure 1).
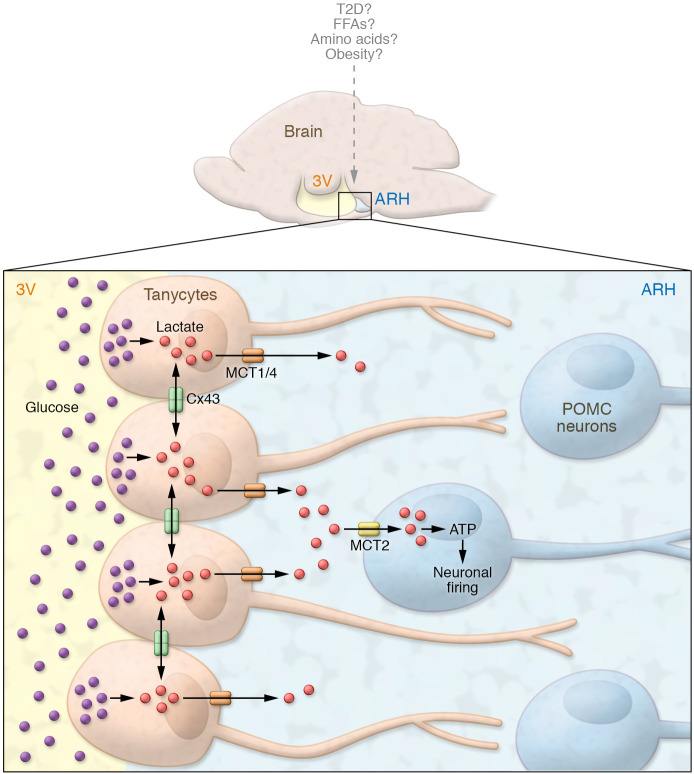
Figure 1. Schematic illustration of the tanycytic lactate shuttle fueling POMC neuronal activity.
Tanycytes lining the 3V wall sense peripheral glucose levels and extend projections to the ARH parenchyma. The study by Lhomme et al. (3) shows that glucose sensing in tanycytes (brown cells) is translated into a lactate signaling pool that travels throughout a tanycytic network via Cx43 gap junctions to magnify metabolic signaling efficiency. Lactate is transmitted via MCTs to POMC neurons, where it is converted to energy (ATP) to induce and sustain POMC neuronal firing. How communication between tanycytes and hypothalamic neurons occurs in the presence of other metabolites (e.g., amino acids and fatty acids) and how the pathway responds under pathophysiological conditions (e.g., obesity and T2D) remain to be elucidated. FFAs, free fatty acids.
Tanycytes sniff glucose status to fuel POMC neurons
In a series of in vitro experiments using primary cultures, Lhomme and collaborators found that tanycytes metabolized glucose in the form of lactate and expressed functional MCTs, which are necessary for lactate release. In addition, POMC neuronal activity was induced and maintained by lactate, rather than glucose, via MCTs. To directly test the hypothesis that tanycytes shuttle lactate to POMC neurons, the authors elegantly dialyzed tanycytes with lactate while simultaneously recording the electrophysiological activity of distant POMC neurons (3). This setup, combined with pharmacological modulation of lactate production or transport, indicated that tanycytic-derived lactate was delivered via MCTs, thereby influencing and sustaining the firing of POMC neurons. Genetic MCT knockdown in tanycytes or POMC neurons impaired energy balance, highlighting the importance of the tanycytic lactate shuttle to maintain POMC neuron activity and whole-body energy balance.
By serendipity, during the paired tanycyte-neuron recordings, Lhomme and colleagues observed that fluorescent dyes diffused from the patched tanycyte, suggesting that this cell type was organized in interconnected functional communities. Indeed, subsequent experiments demonstrated the existence of an intricate gap-junctional tanycytic network mediated by connexin-43 (Cx43). Deletion of Cx43 in hypothalamic tanycytes attenuated the intercellular diffusion of dyes and energy metabolites and decreased spontaneous firing of POMC neurons. From a physiological perspective, mice lacking tanycytic Cx43 had a reduction of ARH lactate content (after peripheral glucose administration) that was associated with hyperphagia and weight gain. These results suggest that the tanycytic connexin–mediated network drives lactate dynamics and the subsequent modulation of POMC neuron function and energy metabolism.
Implications, questions, and future directions
As usually happens, challenging research raises many questions. The study by Lhomme et al. places tanycytes at the center of the intercellular communication processes that interact with the hypothalamic circuits implicated in energy homeostasis control via the sensing of glucose fluctuations and translation of this information into a lactate shuttle that culminates in the modulation of POMC neuronal firing (Figure 1 and ref. 3). A pertinent question is whether this lactate shuttle is an exclusive mechanism for the signaling and fueling of POMC neurons, or whether it also engages other types of neurons. Although this question remains unanswered, a recent report has shown that optogenetic stimulation of tanycytes also activates AgRP neurons, revealing an intimate connection with this neuron type as well (12). This finding suggests that tanycytes are able to establish direct or indirect interactions with diverse types of neurons. Other interesting questions arise regarding the main source of lactate for neurons: the classic astrocyte-neuron shuttle. What is the difference, in terms of neuronal activity modulation, between astrocyte- and tanycyte-mediated processes? Is there a functional compartmentalization or, instead, a synergic tanycytic-astrocytic communication network that strengthens neuronal fueling?
A remarkable finding by Lhomme et al. is that tanycytes are organized in interconnected functional communities (3). This observation prompted the speculation that intercellular tanycytic communication may serve as a mechanism to magnify and synchronize glucose-sensing responses. Similar operational cellular consortiums have also been described to participate in the astrocytic propagation of signal to maintain hippocampal neuronal function (13) or the coordination of electrical activity and insulin release within pancreatic islets (14). In the pancreatic context, it has been proposed that specific cellular hubs initiate and exert control over follower cells (15). Accumulating data support the notion that tanycytes form a heterogeneous population of cells characterized by distinct transcriptomic profiles, neurogenic potential, and marker expression (16). It is therefore likely that this cellular consortium involves diverse subpopulations of tanycytes that may well have defined functions (i.e., hubs) to sense glucose and transmit lactate. Delineating the mechanisms underlying these coordinated behaviors will be crucial to further understand how the brain senses nutrients and engages the appropriate downstream effectors.
It is important to note that tanycytes not only sense glucose, but are also able to detect other molecules including amino acids, fatty acids, hormones, and vitamins (17). Whether the sensing of these factors is also disseminated to other hypothalamic cell types via metabolic proxies and similar gap junction networks remains unknown. Considering that tanycytes are ideally positioned to sense nutrient and hormone variations at the CSF-blood interface, the use of surrogate communication signals is a reasonable strategy to convey the other metabolically relevant substrates.
Finally, the specific physiological and pathophysiological relevance of the findings presented by Lhomme and collaborators remains enigmatic (3). Given the complexity associated with adequate discrimination and monitoring of nutrients, it is likely that the tanycytic networks cooperate with parallel hypothalamic and extrahypothalamic surveillance systems that sense glucose, certain fatty acids, or amino acids. Such redundancy and complementarity would provide robust and precise detection of nutrients. However, can a Western lifestyle affect the function and accuracy of tanycyte nutrient sensing and the lactate shuttle? Excessive high-fat diet consumption damages the blood-brain barrier and triggers inflammatory responses in the hypothalamus (18) that have been associated with an early loss of the structural organization of tanycytes (19). It is therefore plausible that obesogenic diets might disturb tanycyte-mediated nutrient sensing and tanycyte-neuron communication and could actually contribute to the etiology of T2D and obesity. How the tanycytic network responds in such pathophysiological conditions is fundamental to better understanding the role of this cell population in the central control of metabolism.
Lhomme et al. unveiled a tanycytic alliance guided by the axiom that “the whole is greater than the sum of the parts,” which is the basis of a communication mode between hypothalamic tanycytes and POMC neurons for sensing and modulating energy status. This synergy adds an additional layer of sophistication to the mechanisms governing the central control of metabolism and emphasizes, once more, that this biological process is mediated by cooperative and interconnected modules involving diverse cell players rather than single neuronal units. Collectively, the unexpected role for tanycytic networks as sensors of the peripheral energy state upstream of POMC neurons is very important for the evolving field of tanycyte science and represents a formidable example of the unlimited means of nature to find simple solutions to complex problems.
Acknowledgments
RHT is supported by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Action (H2020-MSCA-IF) NEUROPREG (grant agreement 891247) project. MC’s work on hypothalamic nutrient sensing and energy balance control is supported by the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement 725004) and the CERCA Programme/Generalitat de Catalunya. A portion of this work was carried out at the Esther Koplowitz Centre.
Version 1. 09/15/2021
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(18):e153279. https://doi.org/10.1172/JCI153279.
See the related article at Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons.
Contributor Information
Roberta Haddad-Tóvolli, Email: HADDAD@clinic.cat.
Marc Claret, Email: MCLARET@clinic.cat.
References
- 1.Myers MG, et al. Central nervous system regulation of organismal energy and glucose homeostasis. Nat Metab. 2021;3(6):737–750. doi: 10.1038/s42255-021-00408-5. [DOI] [PubMed] [Google Scholar]
- 2.García-Cáceres C, et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci. 2019;22(1):7–14. doi: 10.1038/s41593-018-0286-y. [DOI] [PubMed] [Google Scholar]
- 3.Lhomme T, et al. Tanycytic networks mediate energy balance by feeding lactate to glucose-insensitive POMC neurons. J Clin Invest. 2021;131(18):e140521. doi: 10.1172/JCI140521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Langlet F, et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 2013;17(4):607–617. doi: 10.1016/j.cmet.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balland E, et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19(2):293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc Natl Acad Sci U S A. 2013;110(4):1512–1517. doi: 10.1073/pnas.1212137110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frayling C, et al. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol. 2011;589(9):2275–2286. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36(2):91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortes-Campos C, et al. MCT2 expression and lactate influx in anorexigenic and orexigenic neurons of the arcuate nucleus. PLoS One. 2013;8(4):e62532. doi: 10.1371/journal.pone.0062532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortés-Campos C, et al. MCT expression and lactate influx/efflux in tanycytes involved in glia-neuron metabolic interaction. PLoS One. 2011;6(1):e16411. doi: 10.1371/journal.pone.0016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91(22):10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolborea M, et al. Hypothalamic tanycytes generate acute hyperphagia through activation of the arcuate neuronal network. Proc Natl Acad Sci U S A. 2020;117(25):14473–14481. doi: 10.1073/pnas.1919887117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rouach N, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322(5907):1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 14.Benninger RKP, Piston DW. Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics. Trends Endocrinol Metab. 2014;25(8):399–406. doi: 10.1016/j.tem.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston NR, et al. Beta cell hubs dictate pancreatic islet responses to glucose. Cell Metab. 2016;24(3):389–401. doi: 10.1016/j.cmet.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prevot V, et al. The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr Rev. 2018;39(3):333–368. doi: 10.1210/er.2017-00235. [DOI] [PubMed] [Google Scholar]
- 17.Elizondo-Vega RJ, et al. Nutrient sensing by hypothalamic tanycytes. Front Endocrinol (Lausanne) 2019;10:244. doi: 10.3389/fendo.2019.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cai D, Khor S. “Hypothalamic microinflammation” paradigm in aging and metabolic diseases. Cell Metab. 2019;30(1):19–35. doi: 10.1016/j.cmet.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 19.Ramalho AF, et al. Dietary fats promote functional and structural changes in the median eminence blood/spinal fluid interface-the protective role for BDNF. J Neuroinflammation. 2018;15(1):10. doi: 10.1186/s12974-017-1046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]