Abstract
As the number of individuals vaccinated against SARS-CoV-2 rises worldwide, population-level data regarding the vaccines' ability to reduce infection are being generated. Randomised trials have shown that these vaccines dramatically reduce symptomatic COVID-19; however, less is known about their effects on transmission between individuals. The natural course of infection with SARS-CoV-2 involves infection of the respiratory epithelia and replication within the mucosa to sufficient viral titres for transmission via aerosol particles and droplets. Here we discuss the available data on the existing, approved SARS-CoV-2 vaccines' capacity to reduce transmissibility by reducing primary infection, viral replication, capacity for transmission, and symptomaticity. The potential for mucosal-targeted SARS-CoV-2 vaccine strategies to more effectively limit transmission than intramuscular vaccines is considered with regard to known immunological mechanisms. Finally, we enumerate the population-level effects of approved vaccines on transmission through observational studies following clinical trials and vaccine distribution in real-world settings.
Introduction
The COVID-19 pandemic, a Public Health Emergency of International Concern, is caused by widespread infection with SARS-CoV-2 and development of an infectious respiratory tract illness.1, 2, 3, 4 As of Sept 10, 2021, 223 022 538 cases of COVID-19 and 4 602 882 deaths had been confirmed by the WHO Coronavirus Dashboard worldwide, although these figures are dramatic underestimates. In December, 2020, two mRNA vaccine candidates received emergency use authorisations from the US Food and Drug Administration: Pfizer-BioNTech vaccine candidate BNT162b2 and the Moderna candidate mRNA-1273.5, 6 Around the same time, AstraZeneca and Oxford University announced positive interim results for their viral vector vaccine ChAdOx1.7 Since then, a number of vaccines against COVID-19 have been approved around the world, culminating in over 3 billion doses given to date. Although many vaccine trials have shown a significant capacity to prevent symptomatic COVID-19, their ability to limit viral transmission between individuals is less well understood. Evaluating how well SARS-CoV-2 vaccines can reduce transmission has major epidemiological, social, and policy implications, as inefficient transmission reduction by vaccines would hinder efforts to reach herd immunity.
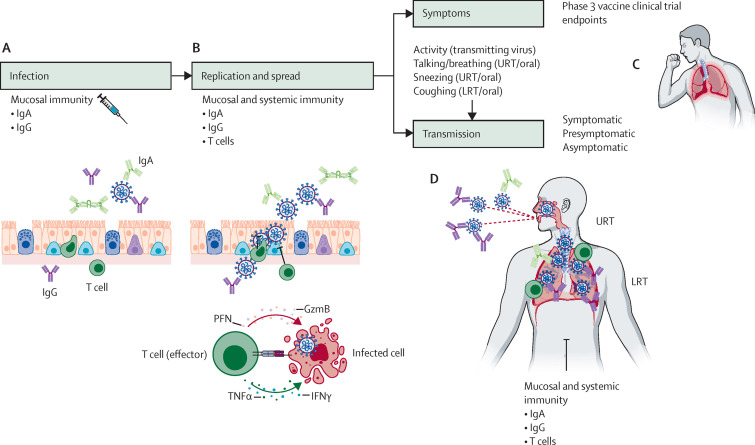
By revisiting the basic immunological mechanisms underlying transmission, we delineate how vaccines can reduce the infectious capacity of a vaccinated individual. We dissect these mechanisms and assess how well the currently available COVID-19 vaccines elicit these responses. We focus on four relevant features by which vaccine-induced immunity can reduce transmission: infection, viral replication, threshold for host-to-host spread, and degree of symptomaticity. We define the infection stage as when the virus enters target cells at the site of exposure, and the replication phase as when SARS-CoV-2 proliferates within the infected cells. If the virus replicates to high enough levels within its host, then host-to-host transmission might occur. Vaccines can further reduce the degree of transmissibility by lowering symptomaticity (eg, coughing and sneezing). Finally, vaccines can induce immune responses that reduce the infectivity of the emitted virus. Vaccines can theoretically suppress SARS-CoV-2 at all these stages to prevent transmission (figure ).
Figure.
Parenteral vaccine-mediated immunity and possible mechanisms of transmission reduction
(A) Intramuscular immunisation with currently approved COVID-19 vaccines elicits systemic IgG and IgA responses, and, in some cases, dimeric IgA that can be transported across the mucosal epithelia. Some of the serum antibodies are transported or spill over into the respiratory mucosa as serum exudate to prevent viral entry into host airway epithelial cells. (B) Once the virus manages to infect the host cells, intrahost replication and spread can be prevented by IgG and IgA antibodies as well as T cells specific to the virus. (C) If the vaccines reduce symptoms such as coughing and sneezing, the emission load from vaccinated individuals will be reduced, leading to less effective transmission. (D) Even if the virus manages to replicate within the respiratory mucosa, vaccine-induced immune responses will reduce transmittable viral load within the URT and LRT. In addition, antibodies might coat the emitted virus to render the virus less infectious in the recipient host, preventing interhost transmission. URT=upper respiratory tract. LRT=lower respiratory tract.
We discuss the immunological mechanisms that can contribute to vaccine-mediated reductions in transmission of SARS-CoV-2 (reduction of infection, viral replication, capacity for host-to-host spread, and symptomaticity) and present the extant evidence for whether the existing vaccines elicit such responses. We highlight existing studies showing the effect of vaccination on asymptomatic infection, which is a proxy for transmission reduction. We also discuss vaccination strategies that are designed to fortify transmission blockade and might be used in the future.
Mechanisms limiting SARS-CoV-2 infection and replication during natural infection
Columnar epithelial cells lining the upper respiratory tract are protected by a layer of glycoprotein-rich mucin. This mucus layer poses a physical and chemical barrier to infection by entrapping viral particles that are then swept up the upper respiratory tract via mucociliary clearance. In its natural course, SARS-CoV-2 bypasses this mucosal barrier and directly infects the epithelial cells via spike protein interaction with host angiotensin-converting enzyme 2 (ACE2). Primary infection results in engagement of innate immune responses, including production of antiviral interferon and cytokines. If this innate defence layer is insufficient to control viral replication, local dendritic cells initiate adaptive immune responses by taking up SARS-CoV-2 antigens, sensing viral pathogen-associated molecular patterns, migrating to the draining lymph nodes, and activating differentiation of naive T and B cells. Once differentiated, T cells specific to SARS-CoV-2 organise an adaptive immune response by clearing infected cells and helping B cells generate class-switched virus-specific IgG and IgA responses.8, 9, 10
During primary SARS-CoV-2 infection, immunological memory is generated through the production of virus-specific follicular helper T cells, memory B cells, and circulating memory T cells, including effector memory, central memory, and terminally differentiated effector T cells, which have been shown to persist for at least 8 months after symptom onset in SARS-CoV-2-infected individuals.11 These long-term memory cell populations are necessary to mount a rapid response to reinfection, primarily by serving as a source of virus-specific lymphocytes capable of both immediate response and giving rise to a higher-affinity antibody response. Plasma cells seeding the mucosal lamina propria secrete IgA dimers, which are transported across the epithelial layers into the mucosa by way of polymeric immunoglobulin receptors and function as neutralising antibodies. Secreted dimeric IgA is on average 15 times more potent than serum monomeric IgA at neutralising the spike protein.12 Circulating IgG is also transported into the mucosal lumen via neonatal Fc receptors or as serum exudate. Generation of these robust neutralising antibodies at the mucosa is a potent effector mechanism capable of providing sterilising immunity against reinfection.8
Even if neutralising antibodies alone are insufficient, reinfection with SARS-CoV-2 can be limited by non-neutralising antibodies and T cells.13 Non-neutralising antibodies activate immune pathways such as complement-dependent and antibody-dependent cellular cytotoxicity and phagocytosis to clear viral particles and infected host cells, and local and systemic memory T cells proliferate, recognise class I MHC-presented antigens from infected cells, and secrete cytokines such as IFNγ to induce antiviral responses. Consequently, subsequent infections are cleared quickly, often with minimal symptoms.
Mechanisms limiting SARS-CoV-2 infection and replication induced by vaccination
Effect of SARS-CoV-2 vaccination on infection
When droplets or aerosols containing infectious SARS-CoV-2 land in the respiratory mucosa of a susceptible individual, spike protein binds ACE2 on host airway epithelial cells, mediating viral entry and host infection. Thus, blocking the spike protein's capacity to interact with ACE2 at the mucosal surface obstructs viral entry into cells,14 preventing infection without the need to invest in a substantial acute immunological response (figure A).
Vaccination is an effective strategy to elicit neutralising antibodies that can limit infection, replication, and potentially transmission. Depending on the vaccine, IgA and IgG generated locally or systemically can be transported to the mucosal surface, where they mechanically hinder spike attachment to ACE2 and initiate clearance of viral particles. If infection of host cells is prevented, sterilising immunity is achieved. In viral challenge experiments, sterilising immunity is inferred if viral load is undetectable in the days following the challenge.
All SARS-CoV-2 vaccines currently approved for use worldwide are injected intramuscularly. Although these vaccines are expected to induce neutralising antibodies in circulation, neutralising antibody generation local to mucosal surfaces lining the epithelia of the respiratory tract or transcytosis of circulating neutralising antibodies to mucosal surfaces might be limited. Indeed, another adenovirus-vectored vaccine, the Ad26.COV2.S vaccine from Johnson & Johnson–Janssen, did not induce IgA or IgG neutralising antibodies in the saliva.15 Nevertheless, after injection of REGN-COV2 neutralising antibody cocktail produced by Regeneron or vaccination with mRNA-1273 in rhesus macaques, no virus was detected after challenge (1 × 105 pfu for REGN-COV2; 8 × 105 pfu for mRNA-1273) in nasal swab nor bronchoalveolar lavage.16, 17 Additionally, IgA and IgG neutralising antibodies, including highly effective secreted dimeric IgA, have been observed in the saliva of patients vaccinated with BNT162b2 or mRNA-1273.15, 18, 19, 20 These studies show variable capacity among the parenteral vaccines to induce sterilising immunity at the mucosa, with ChAdOx1 vaccine being potentially less effective than the mRNA vaccines. Factors potentially influencing whether these vaccines induce mucosal sterilising immunity include the mechanism of the vaccine and the quantity and quality of circulating neutralising antibodies generated. Indeed, mucosal vaccines might provide more robust protection than parenteral vaccines against infection. The degree to which mucosal antibodies elicited by vaccines can prevent infection still needs to be resolved.
Effect of SARS-CoV-2 vaccination on intrahost viral replication
If neutralising antibody concentrations are insufficient to elicit sterilising immunity, SARS-CoV-2 can infect mucosal epithelial cells and proliferate within a susceptible host. We define replication as generation of multiple copies of SARS-CoV-2 within cells above the initial exposure amount. Vaccine-induced immunity, including neutralising antibodies, non-neutralising antibodies, and memory T cells, limits SARS-CoV-2 replication, thereby reducing production of transmissible virus (figure B). In participants immunised with two doses of BNT162b1, mean serum concentrations of IgG, markers of CD8 T-cell activity, and markers of T-helper 1-mediated CD4 T-cell activity increased,21 with similar results in patients administered two doses of mRNA-1273.22, 23
BNT162b2 immunisation reduces SARS-CoV-2 viral load in patients infected at least 12 days after receiving the first dose of vaccine,24 and ChAdOx1 immunisation reduces oropharyngeal and nasopharyngeal viral load in patients subsequently infected with SARS-CoV-2.25 These data suggest that currently approved vaccines reduce viral load within infected individuals by limiting viral replication within the mucosa.
Effect of SARS-CoV-2 vaccination on host-to-host transmission
If immunity fails to block infection and replication, SARS-CoV-2 viral load will increase and the virus will potentially spread to other individuals. Although the viral replication stage concerns the intrahost proliferative capacity of SARS-CoV-2, this transmission stage describes the capacity of interhost spread, which involves the extent and infectivity of viral emission by an infected host. Immune responses that can potentially reduce host-to-host spread of the virus include those that reduce the number of infectious viral particles emitted by the immunised infected person, the infectivity of the emitted viral particles, or both.
Let us consider the relevant sources of virus that can be transmitted to another individual depending on the mode of spreading. The source of transmitted virus can be inside the lower respiratory tract (via coughing), the upper respiratory tract (via sneezing), or the oral cavity (via speaking, breathing by mouth, coughing, and sneezing; figure C). In individuals diagnosed with COVID-19, nasopharyngeal viral load of SARS-CoV-2 is a strong direct correlate for human-to-human transmission, with viral load peaking shortly after initial infection or symptom onset.26 If the vaccine-induced immunity reduces effective viral load in these respective organs, the number of infectious particles will be lowered, and transmission will also be reduced. Ample evidence exists that vaccines reduce viral load in the lower and upper respiratory tracts from non-human primate studies.17, 27, 28, 29, 30 In addition, vaccinated individuals with a breakthrough infection are less infectious than unvaccinated individuals.31 The particles emitted by these individuals might be less infectious through induction of antibodies that can coat the virus, which, through steric hindrance or complement fixation, can prevent virions from infecting susceptible hosts. The extent of vaccine-induced transmission blockade can be studied through contact-tracing studies comparing exposed, vaccinated with exposed, unvaccinated individuals.
Effect of SARS-CoV-2 vaccination on symptomatic transmission
Successful SARS-CoV-2 infection and replication prompt a widespread host immunological response, which leads to the symptoms characteristic of COVID-19, including cough, fever, and shortness of breath.32 Symptoms can directly impact the dissemination of transmissible virus from the infected individual (figure D).
The most striking outcome of SARS-CoV-2 vaccine trials was their effectiveness at preventing symptomatic infection and severe-to-lethal COVID-19. Nevertheless, the impact of reduced disease severity among vaccinated individuals on the risk of causing secondary infections has, to our knowledge, not been systematically investigated. The difference in transmissibility between symptomatic and asymptomatic SARS-CoV-2 infection can, however, provide a hint. Although asymptomatic individuals do not necessarily have a lower viral peak load than symptomatic individuals,33, 34 the reduction among asymptomatic individuals of prolific aerosol-generating symptoms such as coughing could reduce the possibility of transmission. Indeed, an index case who is asymptomatic infects fewer contacts, and the contacts that are infected are more likely to be asymptomatic themselves.35 Additionally, compared with asymptomatic individuals, symptomatic individuals appear to have higher viral loads for longer and are thereby infectious for longer.33, 34 As vaccination strongly decreases the incidence of symptomatic infection, the fraction of time in which the vaccinated individual is infectious, even if a similar peak viral load is reached, might be decreased.36
In the real world, individuals with symptomatic infection will be more likely than those with asymptomatic infection to self-isolate, which might lower the degree of transmission from them, despite the increased transmissibility potential of these individuals. We do not explore the effect of this epidemiological reality in this review.
Here, we propose that current vaccines targeting SARS-CoV-2 operate through four mechanisms: (1) reduction of initial infection; (2) reduction of viral replication in infected individuals; (3) reduction of viral particles emitted by infected individuals and, in those that are emitted, a potentially diminished infectivity; and (4) reduction of symptoms, further blunting viral expulsion from any given infected individual. The cumulative effect of these four steps might contribute substantially to the reduction of viral transmission on a population level.
Real-world evidence for the efficacy and effectiveness of vaccines against infection and transmissionA variety of study designs have measured the direct effect of COVID-19 vaccines against PCR-positive infection or seropositivity, which are proxies for assessing transmissibility reduction. We summarise the available evidence for vaccine-mediated reduction in transmissibility in the appendix (pp 2–16).25, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60 The studies can be divided into randomised clinical trials and observational studies. The randomised clinical trials derive from the original vaccine trials, in which infection and seropositivity were not primary endpoints. Of the four trials that performed asymptomatic testing, all vaccines showed some effect against infection (appendix pp 2–3). The observational studies encompass a variety of designs formulated to assess the effectiveness of vaccination against infection in real-world settings; the studies reported show the tremendous effect of vaccination against SARS-CoV-2 positivity (clustering around 90% effectiveness), yet most of these studies are composed of individuals who have received the mRNA vaccines, especially the Pfizer-BioNTech BNT162b2 vaccine. These real-world data show the striking ability of the mRNA vaccines to prevent SARS-CoV-2 infection altogether (appendix pp 3–12).40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60 Some epidemiological evidence is emerging that the infectiousness of vaccinated individuals testing positive is reduced, with both the Pfizer-BioNTech and the Oxford-AstraZeneca vaccines providing moderate transmission prevention after one dose,54, 60 perhaps by reducing viral load and the infectious period.60 Similar data for other vaccine platforms were not available for analysis at the time of writing. Throughout the summer of 2021, we have witnessed an apparent decline in the effectiveness of the vaccines against both asymptomatic and symptomatic infection, possibly due to the combination of proliferation of the delta (B.1.612.2) variant (first isolated in December, 2020), waning immunity of the vaccine, and high community prevalence. Nevertheless, vaccines, especially mRNA-1273, maintain a relatively high level of protection against infection (appendix pp 13–16).
Future approaches in transmission-blocking vaccines
Parenteral, intramuscular SARS-CoV-2 vaccines are optimised to reduce disease, not to generate mucosal immunity. High concentrations of IgA and IgG that enter from systemic circulation to the mucosa after vaccination might not be directly related to their ability to prevent SARS-CoV-2 infection and replication at the site of infection.61 Preventing viral infection and replication in the mucosa halts the transmissibility potential of a host. Local neutralising antibodies against SARS-CoV-2 can be elicited with mucosal vaccines. Vaccination routes targeting mucosa can also stimulate neutralising antibody production at other, distal mucosal sites, in addition to providing similar systemic immunity conferred by traditional systemic, intramuscular vaccines.62
As SARS-CoV-2 primarily spreads by respiratory droplets and aerosols, viral load within the oropharynx is a key determinant of transmission risk. Vaccination approaches that specifically target the mucosa can elicit local neutralising IgA antibodies to reduce both the viral load to below the transmissibility threshold and symptomaticity. Mucosal vaccines could generate a more robust plasma cell response through generation of dimeric IgA, which is transported into the mucosal lumen, and produces a more potent response in comparison with its monomeric counterpart.12
A variety of intranasal vaccines against SARS-CoV-2 are in development and hold promise as next-generation vaccines. The candidates include the Altimmune AdCOVID intranasal vaccine, administered as a single dose and shown to stimulate neutralising IgG, mucosal IgA, and T-cell-based immune responses (NCT04679909, NCT04442230). Another approach is an intranasal version of the ChAdOx1 vaccine, which has also shown an ability to induce robust IgA neutralising antibodies and cell-mediated immunity superior to that shown in the intramuscular form of the vaccine in mice.63 A handful of other vaccines targeting the oropharynx are in various stages of development by collaborating entities, such as COVI-VAC (a collaboration of Codagenix and the Serum Institute of India; NCT04619628). Since SARS-CoV-2 can infect the oral epithelial cells,64 generating antibody and cellular immunity within the oral mucosa could effectively block viral transmission at the source.
Since SARS-CoV-2 can affect the gastrointestinal system,65 targeted vaccination to the enteric mucosa against enteric SARS-CoV-2 would decrease the burden of gastrointestinal symptoms and stimulate protective mucosal immunity distally, including within the oropharynx. Vaxart is developing a single-dose, orally administered, adenovirus 5-vectored SARS-CoV-2 vaccine, VXA-CoV2-1, which phase 1 trials (NCT04563702) have found can elicit strong IgA and CD8 T-cell responses in participants' nasal mucosa.66 Oral vaccines targeting three components of SARS-CoV-2 (the spike, membrane, and envelope proteins) have been proposed or are in varying stages of development.67, 68
As use of mucosal vaccines for prevention of SARS-CoV-2 infection is limited, we can only speculate on their effectiveness in real-world settings. The intramuscular ChAdOx1 vaccine limited infection in rhesus macaques,27 whereas the intranasal ChAdOx1 formulation was able to induce sterilising immunity,63 suggesting that properly designed mucosal vaccines might be more effective at altogether preventing infection. The duration of mucosal vaccine-induced immunity is unclear. However, as many of the currently approved parenteral vaccines have been found to be durable, a vaccination schedule mixing mucosal with parenteral vaccines could provide both robust and lasting immunity. One such approach includes prime and pull, where a current parenteral vaccine can be used to elicit circulating T and B effector cells, which can be pulled into the mucosa using a second step involving chemokines or other stimulants.69
Conclusion
With an estimated 40% of the world's population at least partially vaccinated, efforts to increase vaccine uptake to levels commensurate with a decrease in SARS-CoV-2 infection rates and community-level herd immunity continue.70 As vaccine uptake increases globally, we are beginning to see suppression of person-to-person SARS-CoV-2 transmission. By leveraging various arms of the adaptive immune response in both mucosal and systemic environments, several of the available vaccines appear to limit infection and viral replication, often lowering viral load beneath the threshold for transmission and preventing symptoms. Although many studies show significant efficacy in preventing transmission for some vaccines, such as the Pfizer-BioNTech, Moderna, and Johnson & Johnson vaccines, scant data exist for other approved vaccines, such as the Sinopharm vaccine and Sputnik V.71, 72 Although these vaccines might have some effect on transmission, the magnitude to which they prevent transmission will considerably affect the course of outbreaks in countries using these vaccines. The most pressing unanswered questions include the extent to which emerging variants can evade existing immunity and whether immunity wanes over time. The emergence of the delta variant tests our waning vaccination-derived immunity and previous estimates of vaccine effectiveness. Fortunately, boosters appear to restore protection against infection.73 Risk of infection after a vaccine can be modulated by patient characteristics (eg, age), circumstances (eg, time since vaccination), and virus characteristics (eg, lineage).74 For this reason, comprehensive surveillance of viral genotypes, clinical presentation, case history, and demographics of individuals infected with SARS-CoV-2 after vaccination is essential. Although no variant of concern has completely evaded vaccine-derived immunity, some vaccines have compelling in-vitro evidence for decreased efficacy against certain variants, especially variants with the E484K mutation.75 Continued careful follow-up in the laboratory, clinical settings, and epidemiological studies over the coming months will illuminate the ultimate capacity of these vaccines to prevent transmission and will identify the need for updated vaccines.
This online publication has been corrected. The corrected version first appeared at thelancet.com/infection on October 19, 2021
Declaration of interests
AI is an investigator of the Howard Hughes Medical Institute; serves as a paid consultant to 4BIO; and is a co-founder of RIGImmune. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
The authors received no funding for the preparation of this Personal View. BioRender was used to create the figure for this paper. The views expressed are those of the authors and do not represent the views of any affiliated institutions.
Contributors
CCK, HTL, DM, CNV, and AI conceptualised this Personal View. CCK, HTL, DM, and CNV wrote the manuscript. DV and CNV prepared the appendix and AI and HTL prepared the figure. All authors contributed to the revision and discussions of the scope of this Personal View and approved the final manuscript.
Supplementary Material
References
- 1.WHO Listings of WHO's response to COVID-19. https://www.who.int/news/item/29-06-2020-covidtimeline
- 2.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922–1924. doi: 10.15585/mmwr.mm6950e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices' interim recommendation for use of Moderna COVID-19 vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653–1656. doi: 10.15585/mmwr.mm695152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasaki A. Exploiting mucosal immunity for antiviral vaccines. Annu Rev Immunol. 2016;34:575–608. doi: 10.1146/annurev-immunol-032414-112315. [DOI] [PubMed] [Google Scholar]
- 9.Ma H, Zeng W, He H, et al. Serum IgA, IgM, and IgG responses in COVID-19. Cell Mol Immunol. 2020;17:773–775. doi: 10.1038/s41423-020-0474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 11.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371 doi: 10.1126/science.abf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Lorenzi JCC, Muecksch F, et al. Enhanced SARS-CoV-2 neutralization by dimeric IgA. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abf1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu LL, Suscovich TJ, Fortune SM, Alter G. Beyond binding: antibody effector functions in infectious diseases. Nat Rev Immunol. 2018;18:46–61. doi: 10.1038/nri.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11:1–6. doi: 10.1038/s41467-020-16256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahass GR, Salomon-Shulman RE, Blacker G, et al. Intramuscular SARS-CoV-2 vaccines elicit varying degrees of plasma and salivary antibody responses as compared to natural infection. medRxiv. 2021 doi: 10.1101/2021.08.22.21262168. published online Aug 30. (preprint). [DOI] [Google Scholar]
- 16.Baum A, Ajithdoss D, Copin R, et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. 2020;370:1110–1115. doi: 10.1126/science.abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbett KS, Flynn B, Foulds KE, et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ketas TJ, Chaturbhuj D, Portillo VMC, et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. Pathog Immun. 2021;6:116–134. doi: 10.20411/pai.v6i1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mades A, Chellamuthu P, Lopez L, et al. Detection of persistent SARS-CoV-2 IgG antibodies in oral mucosal fluid and upper respiratory tract specimens following COVID-19 mRNA vaccination. medRxiv. 2021 doi: 10.1101/2021.05.06.21256403. published online May 7. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohamed SS, Chao GY, Isho B, et al. A mucosal antibody response is induced by intra-muscular SARS-CoV-2 mRNA vaccination. medRxiv. 2021 doi: 10.1101/2021.08.01.21261297. published online Aug 4. (preprint). [DOI] [Google Scholar]
- 21.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 22.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine-Tiefenbrun M, Yelin I, Katz R, et al. Initial report of decreased SARS-CoV-2 viral load after inoculation with the BNT162b2 vaccine. Nat Med. 2021;27:790–792. doi: 10.1038/s41591-021-01316-7. [DOI] [PubMed] [Google Scholar]
- 25.Emary KRW, Golubchik T, Aley PK, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B.1.1.7): an exploratory analysis of a randomised controlled trial. Lancet. 2021;397:1351–1362. doi: 10.1016/S0140-6736(21)00628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawasuji H, Takegoshi Y, Kaneda M, et al. Transmissibility of COVID-19 depends on the viral load around onset in adult and symptomatic patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0243597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Doremalen N, Lambe T, Spencer A, et al. ChAdOx1 nCoV-19 vaccine prevents SARS-CoV-2 pneumonia in rhesus macaques. Nature. 2020;586:578–582. doi: 10.1038/s41586-020-2608-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science. 2020;369:806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel AB, Kanevsky I, Che Y, et al. BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 30.Brouwer PJM, Brinkkemper M, Maisonnasse P, et al. Two-component spike nanoparticle vaccine protects macaques from SARS-CoV-2 infection. Cell. 2021;184:1188–1200. doi: 10.1016/j.cell.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shamier MC, Tostmann A, Bogers S, et al. Virological characteristics of SARS-CoV-2 vaccine breakthrough infections in health care workers. medRxiv. 2021 doi: 10.1101/2021.08.20.21262158. published online Aug 21. (preprint). [DOI] [Google Scholar]
- 32.Dantzer R, Bluthé RM, Layé S, Bret-Dibat JL, Parnet P, Kelley KW. Cytokines and sickness behavior. Ann N Y Acad Sci. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- 33.Gonçalves A, Maisonnasse P, Donati F, et al. SARS-CoV-2 viral dynamics in non-human primates. PLoS Comput Biol. 2021;17 doi: 10.1371/journal.pcbi.1008785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ge Y, Martinez L, Sun S, et al. COVID-19 transmission dynamics among close contacts of index patients with COVID-19: a population-based cohort study in Zhejiang province, China. JAMA Intern Med. 2021 doi: 10.1001/jamainternmed.2021.4686. published online Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chia PY, Ong SW, Chiew CJ, et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine-breakthrough infections: a multi-center cohort study. medRxiv. 2021 doi: 10.1101/2021.07.28.21261295. published online July 31. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.US Food and Drug Administration Briefing document FDA. Moderna COVID-19 vaccine. 2020. https://www.fda.gov/media/144453/download
- 38.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.US Food and Drug Administration Briefing document FDA. Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. 2021. https://www.fda.gov/media/146217/download
- 40.Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hall VJ, Foulkes S, Saei A, et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tande AJ, Pollock BD, Shah ND, et al. Impact of the COVID-19 vaccine on asymptomatic infection among patients undergoing pre-procedural COVID-19 molecular screening. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab229. published online March 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pawlowski C, Lenehan P, Puranik A, et al. FDA authorized mRNA COVID-19 vaccines are effective per real-world evidence synthesized across a multistate health system. Med (NY) 2021;2:979. doi: 10.1016/j.medj.2021.06.007. 92.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones NK, Rivett L, Seaman S, et al. Single-dose BNT162b2 vaccine protects against asymptomatic SARS-CoV-2 infection. eLife. 2021;10 doi: 10.7554/eLife.68808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daniel W, Nivet M, Warner J, Podolsky DK. Early evidence of the effect of SARS-CoV-2 vaccine at one medical center. N Engl J Med. 2021;384:1962–1963. doi: 10.1056/NEJMc2102153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keehner J, Horton LE, Pfeffer MA, et al. SARS-CoV-2 infection after vaccination in health care workers in California. N Engl J Med. 2021;384:1774–1775. doi: 10.1056/NEJMc2101927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benenson S, Oster Y, Cohen MJ, Nir-Paz R. BNT162b2 mRNA Covid-19 vaccine effectiveness among health care workers. N Engl J Med. 2021;384:1775–1777. doi: 10.1056/NEJMc2101951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pritchard E, Matthews PC, Stoesser N, et al. Impact of vaccination on new SARS-CoV-2 infections in the UK. medRxiv. 2021 doi: 10.1101/2021.04.22.21255913. published online June 9. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.White EM, Yang X, Blackman C, Feifer RA, Gravenstein S, Mor V. Incident SARS-CoV-2 infection among mRNA-vaccinated and unvaccinated nursing home residents. N Engl J Med. 2021;385:474–476. doi: 10.1056/NEJMc2104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Angel Y, Spitzer A, Henig O, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA. 2021;325:2457–2465. doi: 10.1001/jama.2021.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thompson MG, Burgess JL, Naleway AL, et al. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385:320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harris RJ, Hall JA, Zaidi A, Andrews NJ, Dunbar JK, Dabrera G. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med. 2021;385:759–760. doi: 10.1056/NEJMc2107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Swift MD, Breeher LE, Tande AJ, et al. Effectiveness of mRNA COVID-19 vaccines against SARS-CoV-2 infection in a cohort of healthcare personnel. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab361. published online April 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang L, Hijano DR, Gaur AH, et al. Asymptomatic and symptomatic SARS-CoV-2 infections after BNT162b2 vaccination in a routinely screened workforce. JAMA. 2021;325:2500–2502. doi: 10.1001/jama.2021.6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavanaugh AM, Fortier S, Lewis P, et al. COVID-19 outbreak associated with a SARS-CoV-2 R.1 lineage variant in a skilled nursing facility after vaccination program—Kentucky, March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:639–643. doi: 10.15585/mmwr.mm7017e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zacay G, Shasha D, Bareket R, et al. BNT162b2 vaccine effectiveness in preventing asymptomatic infection with SARS-CoV-2 virus: a nationwide historical cohort study. Open Forum Infect Dis. 2021;8 doi: 10.1093/ofid/ofab262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fabiani M, Ramigni M, Gobbetto V, Mateo-Urdiales A, Pezzotti P, Piovesan C. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS-CoV-2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.17.2100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prunas O, Warren JL, Crawford FW, et al. Vaccination with BNT162b2 reduces transmission of SARS-CoV-2 to household contacts in Israel. medRxiv. 2021 doi: 10.1101/2021.07.13.21260393. published online July 16. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bleier BS, Ramanathan M, Jr, Lane AP. COVID-19 vaccines may not prevent nasal SARS-CoV-2 infection and asymptomatic transmission. Otolaryngol Head Neck Surg. 2021;164:305–307. doi: 10.1177/0194599820982633. [DOI] [PubMed] [Google Scholar]
- 62.Yusuf H, Kett V. Current prospects and future challenges for nasal vaccine delivery. Hum Vaccin Immunother. 2017;13:34–45. doi: 10.1080/21645515.2016.1239668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassan AO, Kafai NM, Dmitriev IP, et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell. 2020;183:169. doi: 10.1016/j.cell.2020.08.026. 84.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang N, Pérez P, Kato T, et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat Med. 2021;27:892–903. doi: 10.1038/s41591-021-01296-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo M, Tao W, Flavell RA, Zhu S. Potential intestinal infection and faecal–oral transmission of SARS-CoV-2. Nat Rev Gastroenterol Hepatol. 2021;18:269–283. doi: 10.1038/s41575-021-00416-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaxart Investor Relations Vaxart announces positive preliminary data from phase 1 clinical trial evaluating its oral COVID-19 tablet vaccine candidate. 2021. https://investors.vaxart.com/news-releases/news-release-details/vaxart-announces-positive-preliminary-data-phase-1-clinical
- 67.Oravax Press releases: Oramed forms a joint venture, Oravax Medical Inc., for the development of novel oral COVID-19 vaccines. 2021. https://www.ora-vax.com/press-releases/oramed-provides-update-on-oravax-oral-vaccine-maker-gets-irb-approval-for-clinical-trial.php
- 68.Arora K, Rastogi R, Arora NM, et al. Multi-antigenic virus-like particle of SARS-CoV-2 produced in Saccharomyces cerevisiae as a vaccine candidate. bioRxiv. 2020 doi: 10.1101/2020.05.18.099234. published online May 19. (preprint). [DOI] [Google Scholar]
- 69.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ritchie H, Ortiz-Ospina E, Beltekian D, et al. Statistics and research: coronavirus pandemic (COVID-19) 2020. https://ourworldindata.org/coronavirus
- 71.Al Kaabi N, Zhang Y, Xia S, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45. doi: 10.1001/jama.2021.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Logunov DY, Dolzhikova IV, Shcheblyakov DV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime–boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet. 2021;397:671–681. doi: 10.1016/S0140-6736(21)00234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bar-On YM, Goldberg Y, Mandel M, et al. BNT162b2 vaccine booster dose protection: a nationwide study from Israel. medRxiv. 2021 doi: 10.1101/2021.08.27.21262679. published online Aug 31. (preprint). [DOI] [Google Scholar]
- 74.Centers for Disease Control and Prevention National Center for Immunization and Respiratory Diseases. Public health investigations of COVID-19 vaccine breakthrough cases: case investigation protocol. 2021. https://www.cdc.gov/vaccines/covid-19/downloads/COVID-vaccine-breakthrough-case-investigations-Protocol.pdf
- 75.Jangra S, Ye C, Rathnasinghe R, et al. The E484K mutation in the SARS-CoV-2 spike protein reduces but does not abolish neutralizing activity of human convalescent and post-vaccination sera. medRxiv. 2021 doi: 10.1101/2021.01.26.21250543. published online Jan 29. (preprint). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.