Abstract
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant disease that causes myocardial remodeling. Physical exercise (PE) is a therapeutic resource used in Supervised Cardiac Rehabilitation (SCR) to improve Quality of Life (QL), reducing cardiovascular morbidity and mortality. Therefore, the aim of this study is to report how SCR using a personalized exercise prescription, promoted Reverse Myocardial Remodeling (RMR), improved functionality and QL of a patient with HCM. This is a case report of a 43-year-old sedentary female patient with a Body Mass Index (BMI) of 24.7 kg/m2. The patient was diagnosed with Septal Type Asymmetric HCM. Heart Failure (HF) grade III / IV, according to the New York Heart Association (NYHA), was initially treated with 40mg of Propranolol Hydrochloride twice a day, and presented with excessive fatigue, and angina. The echocardiogram showed a final diastolic volume (FDV) of 130 ml, a final systolic volume (FSV) of 44 ml, a left ventricular mass (LVM) of 236 g, interventricular septum thickness of 14 mm, left ventricular posterior wall (LVPW) thickness of 9 mm, left atrium diameter 46 mm, left ventricular end diastolic diameter of 52mm, septum/left ventricular wall ratio of 1.55 mm, and ejection fraction (EF) of 66% (Teicholz). It was obtained as a result of decreased FDV 130 vs. 102ml, decreased FSV 44 vs. 32 ml, decreased LVM 236 vs. 201 g, increased EF 66 vs. 69%, 26% improvement in QL, and 50% reduction in the dosage of Propranolol Hydrochloride. These results suggest that a personalized SCR program is an adjuvant treatment capable of promoting RMR and improving QL and functionality in a patient with HCM.
Keywords: Ventricular remodeling, cardiac rehabilitation, heart failure, quality of life
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is an autosomal dominant disease that causes myocardial remodeling (concentric hypertrophy) (8). It expresses an intracellular anomaly, affecting the spatial organization of myofilaments, and favors the development of arrhythmias, such as supraventricular and non-sustained ventricular tachycardia/or sustained, which confers an increased risk of sudden death (3, 8).
HCM presents with diastolic ventricular dysfunction, preservation of systolic function, and normal ejection fraction (EF), assessed by Echocardiogram (ECO) (3). Clinically, a decrease in functional capacity is observed, which directly compromises the activities of daily living (ADL) leading the individual to a progressive decline in quality of life (QL) (12). Physical exercise (PE) is able to promote reverse myocardial remodeling (RMR) in hearts with concentric hypertrophy and/or dilatation, as well as being a therapeutic resource used in Cardiac Rehabilitation (CR) to improve QL and reducing cardiovascular morbidity and mortality (13).
Studies on PE in HCM showed improvement in functional capacity and QL, however, they did not investigate RMR (4, 11). Thus, this study aims to report as a Supervised Cardiac Rehabilitation (SCR) work, using a prescription of personalized exercise, promoted RMR, improved functionality and QL of a patient with HCM.
METHODS
Participants
This is a case report of a sedentary female patient, 43 years of age, and a Body Mass Index (BMI) 24.7 kg/m2. At 16 years old, she was diagnosed with hypertrophic asymmetric cardiomyopathy of septal predominance, through screening tests (electrocardiogram and ECO). These tests were repeated annually, due to the genetic load of maternal origin. However, the symptoms did not appear until the age of 16, accentuating at the age of 17. Since then, annual examinations have been carried out in conjunction with family research, in which a genetic load of maternal origin has been discovered. As of 2010, the ECO was always performed with the same echocardiographer.
The patient was referred to the Cardiovascular Rehabilitation service at the Clinical School of the Adventist College of Bahia, Cachoeira, Brazil (BA), in February 2019. For the treatment of heart failure (HF) due to HCM, she used propranolol hydrochloride (40 mg) twice a day. She presented with cardiovascular complications; among them, a picture of non-sustained ventricular and supraventricular tachycardia determined through the 24-hour ECG-Holter exam, HF grade III/IV according to the New York Heart Association (NYHA), excessive fatigue, and angina on habitual efforts.
During the physical therapy evaluation, the patient answered the Minnesota Living with Heart Failure Questionnaire (MLHFQ), with a score ranging from zero to 105, obtaining a score of 64. She reported discomfort during sleep, a feeling of suffocation (characteristic of paroxysmal nocturnal dyspnea), and felt it was necessary to support stand on the pillows at a 45° angle, while remaining supine to get to sleep. She also reported great difficulty in performing activities of daily living, such as bathing, walking, sweeping the house, hanging clothes, getting dressed, going up and down stairs, and picking up objects in high or very low places due to dyspnea during small efforts, as well recurrent syncope.
In the Doppler echocardiogram exam performed on April 24, 2018, the following descriptive variables were verified: final diastolic volume: 130 ml, final systolic volume: 44 ml, left ventricular mass: 236 g, thickness of interventricular septum: 14 mm, thickness of left ventricular posterior wall (LVPW): 9 mm, left atrium diameter: 46 mm, left ventricular end diastolic diameter: 52 mm, left ventricular septum/wall ratio: 1.55 mm, and an ejection fraction of 66% (Teicholz). The report attested moderate left ventricular diastolic dysfunction (Type II), increased left ventricular filling pressure, moderate left atrial dilation (Type II), mild degree mitral valve insufficiency, degree aortic valve insufficiency discreet, and concentric left ventricular hypertrophy and dilation.
The ECG-Holter, performed on December 4, 2017, demonstrated the presence of ventricular arrhythmias (five of which were isolated and one episode in pairs) and supraventricular arrhythmias (115 were isolated, nineteen paired, and three non-sustained tachycardias). According to the Brazilian Guidelines for Implantable Electronic Cardiac Devices (7), the recommendations for implantation of an implantable cardiodefibrillator in patients with HCM are divided into classes: Class I - Patients with HCM who have presented ventricular tachycardia Sustained Ventricular Fibrillation of non-reversible cause and expectation life span of at least one year, Class II - Patients with HCM who have one or more major risk factors for sudden cardiac death and life expectancy of at least one year, Class III - HCM patients without risk factors. After the patient underwent an evaluation with the arrhythmologist, she was not indicated for the implantation of the implantable cardiodefibrillator. In view of the physiotherapeutic evaluation performed and the patient's clinical condition, the objectives of the SCR were: Increased functional capacity, improved ADL, without the patient having dyspnea or syncope and consequently improving QL.
This case report was approved by the Research Ethics Committee of Adventist College of Bahia, Cachoeira, BA, Brazil under CAAE n° 39825620.2.0000.0042, in accordance with the Declaration of Helsinki. This research was carried out fully in accordance with the ethical standards of the International Journal of Exercise Science (9).
Protocol
The individualized SCR protocol started on February 12, 2019 and lasted for five months, with a frequency of two weekly sessions. The sessions consisted of inspiratory muscle training (IMT), neuromuscular exercises, handgrip exercises, and cyclic exercises, performed under electrocardiographic monitoring (ECAFIX multi-parameter cardiac monitor, Active® model, São Paulo, SP, Brazil).
Initially, two subjective effort perception scales (SEPS) were presented and anchored to determine the intensity of the exercises. The Borg scale applied in cyclic exercises and the SEPS of Omni used to determine the effort of the active musculature in neuromuscular exercises. In the first month, an adaptive training was elaborated, after that period the patient underwent a reassessment, and the SCR protocol was updated for a conditioning training (Table 1). It is worth noting that Borg was applied in limiting neuromuscular exercises, so that a cardiorespiratory effort > 9 was not exceeded in the first month, and > 13 in the following months. However, the intensity of neuromuscular exercise was determined by Omni.
Table 1.
Exercise protocol for February (adaptive training) and March to June (conditioning training).
| Month/Year | Employed Exercise | Volume | Duration | Interval | Intensity |
|---|---|---|---|---|---|
| February/2019 | Inspiratory Muscle Training | 3 series of 10 inspirations 2 times weekly |
- | 1 minute recovery between sets | Light Borg from 7 to 9* |
| Neuromuscular Exercise | 4 monoarticular exercises¥ 2 sets of 10 reps 2 times weekly |
- | 1 minute recovery between sets | Light Omni from 1 to 3# Borg from 7 to 9* |
|
| Treadmill | 2 sets 2 times weekly |
30 seconds | 1 minute recovery between sets | Light Borg from 7 to 9*, reaching an average speed of 0.8 km/h |
|
| March to June/2019 | Handgrip | 5 series at 10% of the load obtained in dynamometry 2 times weekly |
1 minute of continuous handgrip | 1 minute recovery between sets | Moderate Omni from 3 to 5# Borg from 11 to 13* |
| Neuromuscular Exercise | 4 monoarticular exercises¥ 2 sets of 12 reps 2 times weekly |
- | 2 minutes of recovery between sets | Moderate Omni from 3 to 5# Borg from 11 to 13* |
|
| Treadmill | 5 sets 2 times weekly |
1 minute | 2 minutes of recovery between sets | Moderate Borg from 11 to 13* Heating speed on average 1.1km/h Conditioning speed on average at 2.0km/h |
Elbow flexion, elbow extension, knee flexion and knee extension,
Borg used for the patient to achieve the proposed muscle effort, without exceeding a cardiorespiratory effort > 9 in the adaptation training and >13 in the conditioning training,
Subjective Effort Scale referred to active muscles.
The IMT load was prescribed according to Oliveira et al. (10), which is based on the glycemic threshold through the maximum incremental test of resistance of the inspiratory muscles. The resistance of inspiratory muscles was assessed using POWERbreathe® K5, connected to the computer using the BreatheLink® software. This test is characterized as non-continuous incremental and consists of up to ten stages with an interval of two minutes between them. The test starts with 10% of the maximum value, increases 10% at each level, with blood glucose, Borg, blood pressure and heart rate collections at the end of each level. As the equipment only imposes the load determined on the fourth inspiration, 19 incursions were made at each level, with a five-second breathing cycle guided by a beep of the device. The test was interrupted on the load in which the volunteer was unable to win it or expressed an inability to continue the test, calling this the point of exhaustion.
The glycemic threshold was detected by means of blood collections through puncture in one of the digital pulps. After asepsis with alcohol (70%) using lancets and disposable procedure gloves, blood glucose values were obtained by applying the blood on a test tape attached to the glucose monitor G-TECH free® (expressed in mg/dL) that obtained the results immediately after contact with the blood in the lancet. The glycemic threshold was determined by visual inspection, as the lowest value of the glycemic curve built in the test. At the point where the glycemic threshold was found, the Borg of the obtained load was anchored. This allowed us to evolve the load in the other months by adjusting it using the Borg. During the first month, we opted for the IMT to be performed during the service session at the school clinic, aiming at adapting the patient to the device. Subsequently, the IMT was allowed to be done at home three times a week on days when it did not attend the SCR.
To determine the handgrip load, we used the WCT Fitness® HandGrip dynamometer and followed the dynamometer protocol of the American Society of Hand Therapists (2). Therefore, the patient was positioned in sedestation in a chair with a straight back and without support for the arms, with arms adducted and neutrally rotated shoulders, elbow flexed at 90° and forearm in neutral position. After being in the oriented position, the patient was asked to squeeze the dynamometer as tightly as possible, making three attempts on each hand with a one-minute rest between attempts. At the end, we considered 10% of the maximum strength obtained from the non-dominant hand.
RESULTS
Table 2 represents the comparison of the Doppler echocardiogram variables and the percentage of improvement before and after the patient joined an SCR program. It is worth mentioning that both exams were performed by the same echocardiographer and the patient’s weight and height, respectively, were 72 kg and 167 cm in both exams.
Table 2.
Echocardiographic measurements comparing pre and post SCR values.
| Variables | Pre SCR 12/02/2019 | Post SCR 07/05/2019 | Reference values* | Evolution percentage |
|---|---|---|---|---|
| Left Atrium diameter (mm) | 46 | 44 | 40 | 4% |
| Final Diastolic Volume (ml) | 130 | 102 | 46 – 106 | 22% |
| Final stroke volume (ml) | 44 | 32 | 14 – 42 | 27% |
| Diastolic thickness of LVPW (mm) | 9 | 9 | 7 – 10 | - |
| Diastolic Thickness of the Septum (mm) | 14 | 14 | 7 – 10 | - |
| Left Ventricular Mass (g) | 236 | 201 | 67 – 162 | 15% |
| Mass of the LV/ Body Surface (g/m2) | 131 | 111 | 43 – 95 | 15% |
| Ejection Fraction (%) # | 66 | 69 | 54 – 74 | 5% |
Reference values of the American Society of Echocardiography and the European Cardiovascular Imaging Association (6),
Ejection Fraction (Simpson),
LVPW: Left Ventricular Posterior Wall, LV: Left Ventricle.
From the results obtained in Table 2, we can highlight a 15% decrease in left ventricular mass, a 27% decrease in final stroke volume, a 22% decrease in final diastolic volume, and a 4% reduction in the diameter of the Left Atrium (LA), which reflects a decrease in dilation and cardiac functional improvement.
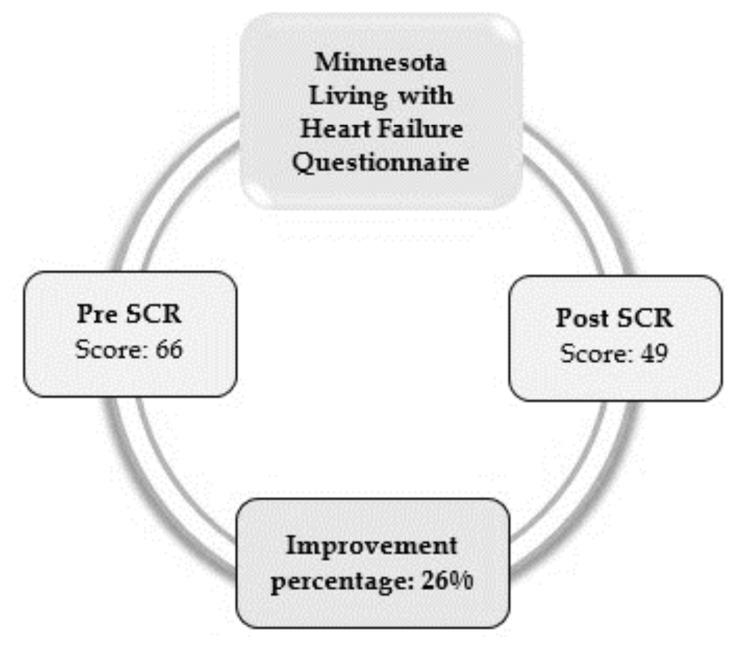
Figure 1 represents the MLHFQ score (an important tool to assess QL in individuals with HF), which was applied before and after the SCR. After the SCR, there was an improvement in the physical and emotional aspects, such as: easier walking, carrying out domestic activities, and decreased concern.
Figure 1.
Score obtained in the QL test before and after the SCR.
The SCR program also made it possible to reduce the dosage of the drug, propranolol hydrochloride, in half. In the last month of SCR, a patient started to show bradycardia and hypotension during and after exercise. In the face of these events, the same was referred to the cardiologist who reduced the dosage from 40 mg 2x a day to 20 mg 2x a day
DISCUSSION
The results of this case report suggest that a SCR program is a cost-effective adjunctive treatment in HCM, capable of promoting RMR and improving functionality and QL. It is well established in the literature that physical exercise is capable of inducing RMR, both for dilation and concentric hypertrophy (5). Although we have not seen regression of concentric hypertrophy, we have seen reversal of dilation. This is observed in Table 2, in which there is a substantial reduction in the final diastolic and systolic volumes, in the left atrium diameter, and in the indexed and non-indexed left ventricular mass. These improvements, both numerically and percentage-wise, are reinforced by the ECO-dependent evaluation character. The fact that he was the same evaluator before and after the program, and the fact that he has followed the patient for ten consecutive years, reinforces the reliability of echocardiographic results. Furthermore, it should also be noted that not only did the left ventricular systolic and diastolic volumes regress, but also that they adjusted now to the are within normal limits values of these variables.
The increased volume of the LA is an important predictor of negative cardiovascular outcomes (3, 6). The regression of LA dilation (as seen in Table 2), even an improvement of 4%, for a patient with HCM is extremely significant. The decrease in LA is reflected in a reduction in the risk of mortality and hospitalization, incidence of atrial fibrillation and stroke, as well as in an increase in life expectancy (4).
The mechanisms by which exercise triggers RMR have been described in the literature for some time. We highlight here the effect of aerobic intensity cyclic exercise on microRNAs, used in the patient's program. Experimental studies with rats demonstrate that physical exercise induces the action of microRNAs 21, 132, 155, 146b, 208b, 212 and 214, which are indicated to induce RMR and improve cardiac function (14). Although, in this case, cardiac function has undergone a slight quantitative improvement (ejection fraction increasing by 5%), it was significant in the patient's clinical context. The significant improvement in QL assessed by the Minnesota questionnaire, points this out. The evolution of performing ADL as performing domestic activities were the points most highlighted by the patient after the SCR program. We cannot forget that other factors, such as the decrease in sympathetic hyperactivity (common in these patients), improvement in the activity of the renin-angiotensin system, and adaptations in the peripheral muscles, may have had as strong an impact on QL as the central improvements (4, 12).
In HCM cases, some patients may have syncope. At the beginning of treatment, this was one of the main complaints of the patient, along with dyspnea on light and moderate exertion, possibly caused by a myocardial hypoperfusion and increased oxygen demand characteristic of HCM (1). After the five months of SCR, the patient reported that she was no longer presenting with episodes of syncope, a condition that contributed to the improvement of her emotional perception and, consequently, in QL.
Another point to be highlighted in this case is the work of the multidisciplinary team. The benefits mentioned above were due to a personalized physical exercise prescription, individualized according to the patient's clinical and biological characteristics. For that, the moments of reevaluation and periodization of training carried out by the multidisciplinary team, composed of doctors (assistant cardiologist and arrhythmologist) and physiotherapists (specialists in Cardiovascular Rehabilitation) made all the difference in the relevance of the results obtained.
Finally, we point out that, as this is a case report, the results presented here must be analyzed with caution and impartiality. Despite this, this case report advances something new, the possibility of exercise causing reversal of dilation in HCM, and is something that in our knowledge has not yet been reported in the literature. We know that a case report is only based on the hierarchical scale of scientific evidence. However, it is able to shed light on fields that are still little explored in science, the main contribution of this work. In view of the above, this study suggests that an individualized and personalized SCR program is an adjuvant treatment capable of promoting RMR and improving the QL and functionality of patients with HCM.
REFERENCES
- 1.Cao Y, Zhang PY. Review of recent advances in the management of hypertrophic cardiomyopathy. Eur Rev Med Pharmacol Sci. 2017;21(22):5207–5210. doi: 10.26355/eurrev_201711_13841. [DOI] [PubMed] [Google Scholar]
- 2.Fess EE. Clinical assessment recommendations. 2nd Ed. Chicago: American Society of Hand Therapists; 1992. Grip strength. [Google Scholar]
- 3.Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines developed in collaboration with the American Ass. J Am Coll Cardiol. 2011;58(25):212–260. doi: 10.1016/j.jacc.2011.10.825. [DOI] [PubMed] [Google Scholar]
- 4.Klempfner R, Kamerman T, Schwammenthal E, Nahshon A, Hay I, Goldenberg I, et al. Efficacy of exercise training in symptomatic patients with hypertrophic cardiomyopathy: results of a structured exercise training program in a cardiac rehabilitation center. Eur J Prev Cardiol. 2015;22(1):13–19. doi: 10.1177/2047487313501277. [DOI] [PubMed] [Google Scholar]
- 5.Konhilas JP, Watson PA, Maass A, Boucek DM, Horn T, Stauffer BL, et al. Exercise can prevent and reverse the severity of hypertrophic cardiomyopathy. Circ Res. 2006;98(4):540–548. doi: 10.1161/01.RES.0000205766.97556.00. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for quantification of cardiac chambers by echocardiography in adults: An update from the American society of echocardiography and the European cardiovascular imaging association citation for original document: Recommendations for cardiac. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Martinelli Filho M, Zimerman LI, Lorga AM, Vasconcelos JTMRAJ. Guidelines for implantable electronic cardiac devices of the Brazilian society of cardiology. Arq Bras Cardiol. 2007;89(6):210–238. [Google Scholar]
- 8.Mattos BP, Torres MAR, Freitas VC, Scolari FL, Loreto MS. Ventricular arrhythmias and left ventricular hypertrophy in hypertrophic cardiomyopathy. Arq Bras Cardiol. 2013;5(100):452–459. doi: 10.5935/abc.20130078. [DOI] [PubMed] [Google Scholar]
- 9.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira FTO, Petto J, Esquivel MS, Dias CMCC, Oliveira ACS, Aras R. Comparison of the strength and resistance of inspirational muscles between assets and sedentary. J Phys Res. 2018;8(2):223–229. [Google Scholar]
- 11.Reineck E, Rolston B, Bragg-Gresham JL, Salberg L, Baty L, Kumar S, et al. Physical activity and other health behaviors in adults with hypertrophic cardiomyopathy. Am J Cardiol. 2013;111(7):1034–1039. doi: 10.1016/j.amjcard.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Saberi S, Wheeler M, Bragg-Gresham J, Hornsby W, Agarwal PP, Attili A, et al. Effect of moderate-intensity exercise training on peak oxygen consumption in patients with hypertrophic cardiomyopathy: A randomized clinical trial. JAMA. 2017;317(13):1349–1357. doi: 10.1001/jama.2017.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saberi S, Day SM. Exercise and hypertrophic cardiomyopathy: Time for a change of heart. Circulation. 2018;137(5):419–421. doi: 10.1161/CIRCULATIONAHA.117.029989. [DOI] [PubMed] [Google Scholar]
- 14.Souza RWA, Fernandez GJ, Cunha JPQ, Piedade WP, Soares LC, Souza PAT, et al. Regulation of cardiac microRNAs induced by aerobic exercise training during heart failure. Am J Physiol Heart Circ Physiol. 2015;309(10):1629–1641. doi: 10.1152/ajpheart.00941.2014. [DOI] [PubMed] [Google Scholar]