Abstract
Resistance exercise has been shown to induce an acute hormonal response. The purpose of this study was to examine upper-body adaptations and the endocrine response to strength training in men and women when subjected to two different types of lower-body resistance training protocols. Nine males and eight females were assigned to either a Heavy Load (HL) (N = 10) or Mixed Load (ML) (N = 7) training routine three times per week for ten weeks. The HL-group executed low-volume, high-load resistance exercise for both lower and upper-body (4–6 reps at 80–90% of one repetition maximum (1-RM), three-minute inter-set rest). The ML-group performed the HL-protocol for the upper-body, but a high-volume, moderate-load protocol for the lower body (10–15 reps at 60–70% of 1-RM, one-minute inter-set rest). Volume load, 1-RM strength and hormonal measurements were analyzed by repeated-measures linear mixed models. Both groups increased their 1-RM in all assessments (p < 0.01) with no significant difference between groups at any time. Growth hormone (GH), testosterone and bioavailable testosterone (T/SHBG) increased in both groups during a standardized exercise session (p < 0.01) with ML having a greater increase in GH. The notion that acute elevations in anabolic hormones is important for muscle strength adaptation cannot be supported by the present study.
Keywords: Growth hormone, testosterone, resistance training, exercise physiology, anabolism
INTRODUCTION
An acute bout of resistance exercise often results in a short-lasting increase in putative anabolic hormones such as growth hormone (GH), insulin-growth factor-1 (IGF-1), and testosterone (25, 36, 46). Several strength training methods aimed to maximize the acute anabolic response have been developed, including heavy loads (60–80% 1RM), multiple exercises, high training volume, and short rest periods (30 – 90s) (7, 21, 22, 30). Studies involving blood flow restricted (BFR) training support the view that high metabolic stress significantly increases hormonal levels (27, 29, 37). Observed positive relationships between anabolic hormones and hypertrophy-type training may postulate a mechanism for muscle hypertrophy (14, 37), like testosterone, GH and IGF-1 have been shown to mediate anabolic signaling (12, 37). The importance of circulating hormones has recently been challenged, as it has been suggested that muscle adaptation is solely reliant on intrinsically located signaling, such as the mammalian target of rapamycin (mTOR), phosphorylation of p70S6K and down-stream translation-related targets (13, 43). Anabolic signaling was unaffected by post-exercise hormonal elevations in one study (45), and others have shown that mTOR-mediated increases in muscle protein synthesis are evident (13). Thus, mTOR-independent mechanisms may play a role in promoting prolonged muscle protein synthesis and muscle hypertrophy.
Circulating GH stimulates the synthesis and secretion of IGF-1 from the liver and other tissues. In turn, IGF-1 stimulates cell growth and differentiation in multiple target tissues via IGF receptors (3). Amongst other mechanisms, GH affects collagen synthesis, increasing lean body mass, whole-body protein synthesis and alters lipolysis (3, 46). While GH is anabolic during childhood and puberty (1), its relevance for muscle tissue growth in adults is debated (43, 46). Yet, the importance of GH as an anabolic hormone is claimed by some (2, 15, 23, 24, 29, 36). Synergistic effects of GH on testosterone-mediated protein synthesis (42), and facilitated effects on muscle repair and remodeling is suggested (37).
As training, even with heavy loads, increases circulating testosterone for 15 – 30 minutes (23), some argue that upper-body training should be performed in this time-window, when prior lower-body training may exert an effect on distal muscle groups (36). Others argue that training effects are optimal when muscle protein synthesis for the targeted muscle is increased, that is, once training of the upper-body has been performed (35). Several studies have reported potentiating effects (greater gains in strength and/or hypertrophy) of lower-body training prior to upper-body strength and hypertrophy training (2, 15, 27, 29). Ronnestad, Nygaard, and Raastad (36) reported greater 1-RM and cross-sectional area (CSA) increases in the bicep brachii muscle for the group when leg training is performed before arm training. Meanwhile, some studies report no enhancing effect with exposure of loaded muscles to exercise-induced elevations in anabolic hormones (44, 45).
Training protocols designed to manipulate circulating endogenous GH and testosterone via leg exercises using high-volume training with short inter-set rest can induce a distal transfer effect (2, 23, 27, 29). Although, the relevance of short inter-set rest has more recently become challenged (39). Such regimens are suggested to result in superior strength training adaptions and greater strength gains, with or without an additive increase in muscle mass. Importantly, by using a within-person unilateral design, inter-individual differences in potential for hypertrophy and strength gains are minimized. However, the control arm (i.e., without added leg training) could be subject to cross-education, i.e., an enhanced performance due to neural factors, potentially blunting effects of elevated hormonal levels (16, 18, 20, 26, 28). Therefore, bilateral training and multiple-group designs examining the effect of lower-body resistance training on upper-body strength performances are of interest. To our knowledge, only three studies have used this design, including young males only, spanning six (2), seven (29), and nine (15) weeks, with hormonal levels measured in one study only (15). As described by Schoenfeld (37), inconsistent findings require further study.
The purpose of this study was to examine upper-body adaptation to resistance training and acute endocrine responses in men and women, subjected to low volume upper-body resistance training with heavy load and lower-body resistance training with either low volume with heavy load or high volume with moderate load.
METHODS
Participants
Twenty-six healthy individuals (thirteen females), aged 18 to 45 years, free of musculoskeletal pain or injuries, and any chronic disease, were recruited in Umeå, Sweden, via posters and social media. The large age range of participants was due to participation in another study, not described here. Other resistance training activities were prohibited during the study, which focused on recreational athletes with varying current training status (0 – 3 resistance exercise session/week). After receiving verbal and written information about the study, all participants signed a consent form. The Regional Ethical Review Board of Umeå (#2017-121-31M) granted approval of the study which was designed in accordance with the seventh Declaration of Helsinki (2013). Further, this research was carried out fully in accordance with the ethical standards of the International Journal of Exercise Science (32) and with α = 0.05, β = 0.8, and effect size of 1.2 (10) and a required sample size per group of ten.
All females were pre-menopausal. Subjects were matched based on anthropometrics and one-repetition max (1-RM) strength into either Heavy Load (HL) group or Mixed Load (ML) group. Nine participants dropped out during the study intervention due to non-intervention related injuries (two), illness (five; influenza, stomach flu, and the common cold) and personal reasons (two). Hence, 17 participants completed the entire study, while 19 completed the mid tests and hormonal measurements week six (Table 1). At baseline, no variable was statistical different between groups (Table 1).
Table 1.
Participants’ baseline anthropometric and strength assessments.
| All | HL | ML | p | |
|---|---|---|---|---|
| N | 17 | 10 | 7 | |
| Males | 9 | 4 | 5 | |
| Females | 8 | 6 | 2 | |
| Age (years) | 30.9 ± 7.6 | 29.3 ± 7.2 | 33.1 ± 8.1 | 0.319 |
| Height (cm) | 172 ± 8.2 | 170.2 ± 10.1 | 173.7 ± 3.9 | 0.399 |
| Weight (kg) | 70.9 ± 11.4 | 68.1 ± 12.9 | 74.9 ± 8.0 | 0.239 |
| BMI (kg/m2) | 23.9 ± 2.7 | 23.4 ± 4.0 | 24.8 ± 1.7 | 0.308 |
| 1-RM BP (kg) | 59.1 ± 28.2 | 56.2 ± 33.5 | 63.4 ± 20.0 | 0.713 |
| 1-RM BP/kg bw | 0.81 ± 0.30 | 0.79 ± 0.34 | 0.84 ± 0.26 | 0.713 |
| 1-RM LP (kg) | 262.1 ± 88.6 | 253.0 ± 102.8 | 275.1 ± 69.0 | 0.628 |
| 1-RM LP/kg bw | 3.62 ± 0.83 | 3.60 ± 0.88 | 3.66 ± 0.82 | 0.876 |
| 1-RM LPD (kg) | 53.5 ± 21.9 | 49 ± 24.5 | 60 ± 17.1 | 0.323 |
| 1-RM LPD/kg bw | 0.73 ± 0.23 | 0.69 ± 0.23 | 0.79 ± 0.22 | 0.343 |
HL = Heavy Load, ML = Mixed Load, 1-RM = one-repetition maximum BP = Bench Press, LP = Leg Press, LPD Lat-Pull Down. Values are Mean ± SD.
Protocol
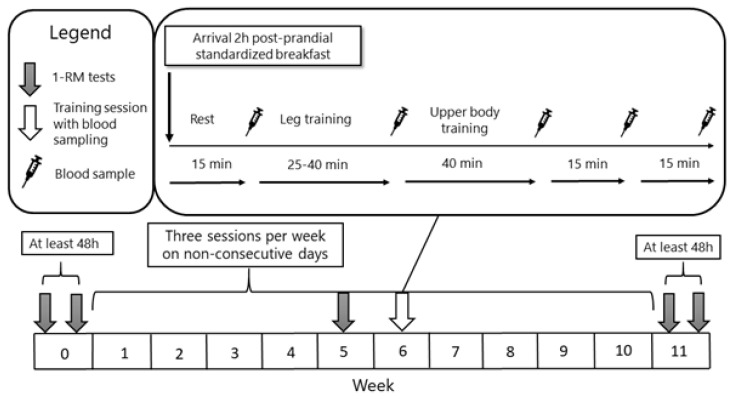
Participants performed three resistance training sessions per week on non-consecutive days for ten weeks (Figure 1). Compliance was defined as attending 85% of the training sessions. One-repetition max tests were conducted before (Pre), during the 5th week (Mid), and after the training intervention (Post).
Figure 1.
Schematic picture over the study timeline. Participants trained a total of 30 times over a ten-week period, with 1-RM test before, during and after the intervention. Blood sampling for hormonal measurements took place at five time points during a standardized training session in the middle of the intervention period.
Maximal dynamic strength was assessed by 1-RM tests in bench press (BP), lat pull-down (LPD), and leg press (LP). Participants attended the laboratory having refrained from any other physical activities for at least 48 hours before testing. Participants completed their 1-RM testing alone with the test leader. Most, but not all participants, were familiar with the performance assessments. To minimize the influence of day-to-day variations at testing, the procedure was repeated within three to six days, with the same examiner. Intra-class correlation coefficients were 0.99 for the 1RM BP (Standard error of mean [SEM]: 0.50 kg; Mean Difference [MD]: 1.58 kg), 0.98 for the 1RM LP (SEM: 2.89 kg; MD: 14.06 kg) and 0.99 for the 1 RM LPD (SEM: 0.47 kg; MD: 0.74 kg). The best results from the two testing occasions were chosen for analysis.
Test procedures were performed following the NSCA guidelines (40). Initially, participants performed a five-minute moderate cardiovascular warm-up at a bike ergometer at self-selected intensity. Prior 1-RM assessment, a specific warm-up of the given exercise was performed (5–10, 3–5, and 2–3 repetitions at 40–60%, 70–80% and 80–90% of estimated 1-RM, respectively). Rest intervals between warm-up sets were one to two minutes. Following the warm-up and rest of three min, participants performed single repetitions of increasing load for 1-RM determination. Three to four minutes of rest were provided between each attempt.
Bench press testing was performed on a flat bench in the standard supine position with five-point body contact (back of the head, upper back/shoulders, lower back/buttocks, both feet). High-quality barbells and weight plates were used (Eleiko Sport, Halmstad, Sweden). With a pronated grip, participants lowered the bar to mid-chest and then pressed the bar until fully extended arms. Participants were required to pause briefly at the bottom (stop momentum) of the lowering phase and wait for a signal before starting the concentric phase. Leg press testing was performed in a plate-loaded linear hip-sled machine (Hammer strength, Brunswick Co., Lake Forest, IL, USA). Participants lowered the platform until thighs were parallel to the foot platform and after a given signal, fully extended their hips and knees. Total loading was determined with the weight of the loaded plates, plus the weight of the sled. Lat pull-down was conducted in a weight magazine machine (Life fitness, Brunswick Co., Lake Forest, IL, USA) with a straight bar. With a pronated grip slightly wider than shoulder-width, participants lowered the bar until contact with the upper chest area. Proper form without extensive movement of the torso was required. Verbal encouragement was provided during all 1-RM attempts.
The resistance training protocol consisted of three sets of seven exercises in the following order: leg press, leg extension, leg curl, bench press, seated dumbbell shoulder press, seated low row, and wide grip lat pull-downs. Both groups performed the same training protocol for the upper-body, focusing on strength development: Three sets per exercise, 4–6 reps at 80–90% of 1-RM, with an inter-set rest of three minutes. For lower body exercises, HL performed the same protocol, whereas ML underwent a protocol designed for eliciting muscle hypertrophy and/or enhanced endocrine response: Three sets per exercise, 10–15 reps at 60–70% of 1-RM with an inter-set rest of 60 seconds. While a repetition range of 6–12 could be argued optimal for hypertrophy, the higher repetition range was used to have a clear difference between the two protocols. Only the lower-body training regimen was altered to enable potential differences in endocrine response, while keeping the upper-body training regimen equal.
The third set of each exercise was performed to the point of voluntary concentric muscular failure. That is, an inability to perform another repetition with proper form. For set one and two, participants were instructed to perform a near max exertion, stopping 1–2 reps short from concentric failure. For this, participants were provided descriptions regarding “reps in reserve”. Repetitions were performed in a controlled manner with a concentric phase of one second and an eccentric action of approximately two seconds. Training load was adjusted successively (e.g., increased loading for a given exercise when failure was not met in third set) to ensure adequate training intensity and for achieving failure in the targeted repetition range. Participants recorded all workouts in a logbook, supervised by the investigators which provided continuous feedback regarding training loads. After an initial introduction to the training program, one-third of the training sessions were supervised by one of the investigators.
To facilitate recovery and to standardize post-training nutrition, participants were provided with a liquid dietary supplement, containing 30g whole milk protein and 10g of carbohydrates (Lean Recovery, Norrmejerier, Umeå, Sweden), consumed within one hour after each exercise session.
The acute hormonal response was analyzed during one of the three training session (i.e., session 16, 17, or 18) in the sixth week of the intervention (Figure 1). While more sessions with blood samples was warranted, the timing at six weeks was chosen to, in untrained subjects, ensure adequate experience with the training protocol and to minimize early-phase endocrine adaptations, resulting in more generalizable results (25). Participants avoided physical exertion and alcohol for 48 hours, as well as caffeine for a minimum of six hours, before attending the laboratory. Participants arrived at the laboratory two hours postprandial, after consuming a standardized meal (7 kcal/kg bodyweight, 65E% carbohydrates, 16E% proteins, 19E% fat) consisting of either oatmeal with milk, or cereal with milk. During the training session, participants could drink water ad libitum, however, limited to 300 mL to avoid overhydration.
Five venous blood samples were obtained during the training session (Figure 1): resting levels after 15 minutes of rest (Pre), immediately after the leg exercises (Intra), three minutes after the full training protocol (Post), and subsequent samples at 15- and 30-minutes post-exercise. To minimize invasive sampling, a peripheral venous catheter (Venflon Pro Safety, Becton,
Dickinson and Co. Plymouth, UK) was inserted post-exercise for the remaining three samples. Diurnal variation of testosterone levels has been well documented with a peak between 05:30 and 08:00 (AM) (5). While this can influence the acute endocrine response to exercise, it is known that testosterone can increase in both morning and evening sessions with resistance training, possibly with a higher and more anabolic testosterone to cortisol ratio in the evening (4). In this study, blood samples were taken within the time interval from 08:00 to 11:00 (AM) to limit the influence of diurnal variations between participants.
Blood samples were drawn from an antecubital vein, directly collected into a 5 ml serum separator tube (SST II Advance, Becton, Dickinson, and Co. Plymouth, UK) and a 3 ml lithium-heparinized plasma tube (LH PST II, Becton, Dickinson, and Co. Plymouth, UK). Tubes were stored for 30 minutes in room temperature before being centrifuged for 10 minutes at 3900 rpm in 4°C (Allegra X-22R, Beckman and Coulter, Brea, CA, USA). Testosterone, sex-hormone-binding hormone (SHBG) and GH were analyzed using standard immunoassay procedures at the Clinical Chemistry Laboratory, Umeå University Hospital. Coefficient of variation is 6% for testosterone, 4–5% for SHBG (at 20 and 30 nmol/L, respectively) and 6–8% for GH (3–7 ug/L). The ratio between testosterone and SHBG (T/SHBG) is seen as the bioavailable testosterone and influences the magnitude of free testosterone, available for interaction with androgen receptors (24).
17 participants completed the study, while 19 participated past the blood sampling for hormonal analysis. Thus, for strength assessments, N = 10 for HL and N = 7 for ML. For hormonal measurements, N = 10 for HL and N = 9 for ML. Data points under the limit of detection (< 0.06 μg/L for GH, < 0.2 nmol/L for testosterone) were treated as missing data, as data cannot be used for statistical calculation (number below detection limits are neither 0 nor 0.06/0.2). Area under the curve (AUC) was calculated for hormonal measurements by integrating the function for the quartic polynomial curve using the equation for a definite integral (see equations). This provided results similar to the trapezoidal rule approximation, as tested by the authors. Since the training protocols differ in time, where the leg training for HL is 15 minutes longer, AUC is expressed as time adjusted AUC (area units per minute). AUC1 includes measurements from rest to post-training (i.e., the training session), AUC2 includes the Post+15 and Post+30 measures as well. Volume load, calculated from the third training session each week, was defined as (weight × repetitions × sets) for each exercise, and summed, resulting in volume load for that session. Since adherence was not 100%, volume load was not calculated for every training session.
Statistical Analysis
Pre to Mid- and Post-intervention 1-RM strength, hormonal measurements and volume load were analyzed using repeated measures three-way linear mixed models (LMM). Group, Time (i.e., Pre, Mid and Post for 1-RM data; Pre, Intra, Post, Post+15 and Post+15 for hormonal data; Week 1–10 for volume load data) and Sex was set as fixed effects and Subject as random effect. While effects of Group×Sex and Group×Time×Sex are executed in the statistical models, they were not analyzed due to low sample size and low statistical power, as an effect of separation by group and sex (which results in N = 2–6 for Group×Sex effects). Models are generated using restricted maximum likelihood (REML). Fixed effects are reported in the results while a complete model report will be found in supplementary materials.
Tukey’s honestly significant different (HSD) post hoc test was used to identify pairwise differences when a significant main or interaction effect was revealed. For 1-RM and hormonal changes, post hoc tests was executed on least square means, controlling for covariates and missing data in the model (41). Baseline measurements, AUC, total training load and single-level effects (Group and Sex) were compared using two-tailed independent t-tests. Effect sizes for pairwise measurements were calculated using Cohen’s d and interpreted as small (> 0.2), medium (> 0.5), and large (> 0.8) (6). Intra-class correlation estimates were calculated using a two-way random-effects model. The level of significance was set at α = 0.05. All statistical analyses were performed using a statistical software package (JMP 14.1, SAS Institute Inc. Cary, NC, USA). As data was not skewed, all descriptive data is expressed as mean ± SD.
RESULTS
Linear mixed model data for 1-RM strength, hormonal response and volume load, as well as descriptive data for 1-RM strength and hormonal response, are presented in detail in supplementary material 1. Additional statistical details for LMMs, including least square mean plots and least square post-hoc test details, are found in supplied HTML-files 1 to 7.
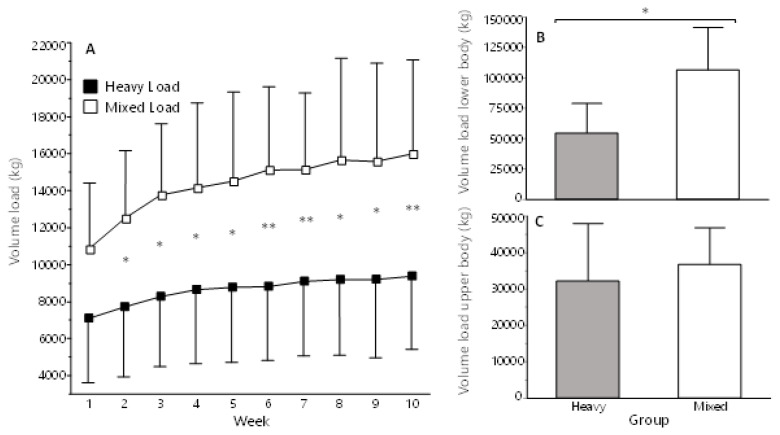
The ML group completed 90% (27 ± 2) of all training sessions, and the HL 94% (28 ± 2). For volume load, there was a main effect of Group, Time, Sex and Group×Time (p < 0.05 for all). Volume load was significantly higher in ML (p < 0.05) than HL at week two and subsequent weeks (Supplementary Materials 1, Table 1, and HTML-files 1 and 2). For total volume load, ML had significantly higher total- and lower body volume (p = 0.022; Cohen’s d = 1.36 [95% CI: 0.26–2.42] and p = 0.008; d = 1.25 [0.42–2.10], respectively), but not upper-body volume (p = 0.514; d = 0.11 [−0.27–0.43]).
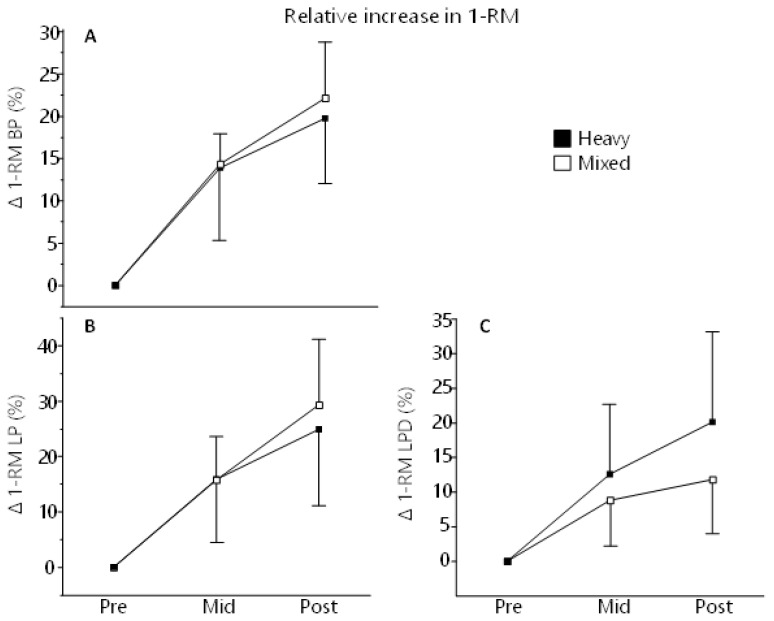
Both groups increased their strength significantly to from Pre to Mid (p < 0.05) and Post (p < 0.01) measurements. Descriptive statistics for 1-RM strength are found in Table 2. Delta values for 1-RM measurements are depicted in Figure 3.
Figure 2.
A) Volume load each week for both groups. B) Total lower body volume load for the whole intervention C) Total upper body volume load for the whole intervention. Volume load is calculated from the third training session each week and is defined as weight × repetitions × sets and is summed for all exercises. * = Significant difference, p ≤ 0.05. ** = Significant difference, p ≤ 0.01.
Table 2.
Strength characteristics at baseline (Pre), at week five (Mid) and after 10 weeks (Post).
| Heavy | Mixed | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Measure | Pre | Mid | Post | Pre | Mid | Post |
| 1-RM BP | 56.2±33.5 | 60.5±37.5** | 66.7±38.3*** | 63.4±20.0 | 72.1±21.6*** | 76.6±21.6*** |
| 1-RM LP | 253.0±102.8 | 273.7±128.1* | 310.5±119.0*** | 275.1±69.0 | 319.7 ± 85.7* | 351.6±77.0*** |
| 1-RM LPD | 49.0±24.5 | 52.5±26.0** | 57.0±24.4*** | 60.0±17.1 | 65.0±18.1* | 66.1±16.2** |
Data (kg) are presented as mean ± SD. BP = bench press; LP = leg press; LPD = lat pull-down.
Significant different from Pre, p ≤ 0.05.
Significant different from Pre, p ≤ 0.01.
Significant different from Pre, p ≤ 0.001.
Figure 3.
The relative increase in 1-RM BP (A), LP (B) and LPD (C) expressed as delta values (%) from baseline to MID (week 5) and POST (after 10 weeks of training). There was no difference between groups at any time point.
In all strength assessments, there was a main effect of Time and Sex (p < 0.0001) but not for Group or Group×Time. In BP, but not LP or LPD, there was an effect of Sex×Time (p = 0.0011) (Supplementary materials 1, Table 2, and HTML-file 3). For relative change in 1-RM, there was a main effect of Time in all assessments (p < 0.0001), but not for Group, Sex or Group×Time. In LPD, but not BP or LP, there was an effect for Sex×Time (p = 0.0117) where women increased more in strength then men from Pre to Post (24% vs 10%, p < 0.05) (Supplementary Materials 1, Table 3, and HTML-file 4).
Change in blood hormone levels from initial concentrations were not significantly different between men and women (Sex×Time effect), thus when investigating changes (delta-values) men and women are pooled in the same analysis (Group, Group×Time). Delta values for hormonal response during workout are depicted in Figure 3. Descriptive statistics for hormonal responses are depicted in Table 3. To illustrate sex differences, descriptive data are shown by sex in addition to group.
Table 3.
Exercise-induced hormonal response. Descriptive data by group and sex.
| Group | Time | Testosterone (nmol/L) | T/SHBG (ratio) | GH (ug/L) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||
| Heavy | Pre | 9.08 | 10.22 | 0.19 | 0.22 | 0.61 | 0.92 |
| Heavy | Mid | 11.91 | 13.02 | 0.24 | 0.27 | 5.76 | 7.23 |
| Heavy | Post | 11.79 | 13.28 | 0.23 | 0.26 | 4.97 | 6.65 |
| Heavy | Post-15 | 11.34 | 12.77 | 0.24 | 0.27 | 3.41 | 4.57 |
| Heavy | Post-30 | 11.48 | 12.64 | 0.24 | 0.26 | 1.97 | 2.47 |
| Mixed | Pre | 12.84 | 10.92 | 0.19 | 0.16 | 0.46 | 0.53 |
| Mixed | Mid | 12.98 | 12.33 | 0.21 | 0.17 | 8.00† | 7.22 |
| Mixed | Post | 12.67 | 11.8 | 0.21 | 0.18 | 4.10 | 4.42 |
| Mixed | Post-15 | 12.22 | 11.57 | 0.22 | 0.18 | 2.06 | 2.25 |
| Mixed | Post-30 | 11.53 | 10.90 | 0.21 | 0.17 | 1.08 | 1.19 |
| Female | Pre | 0.96* | 0.29 | 0.02* | 0.01 | 0.70 | 0.76 |
| Female | Mid | 1.00* | 0.44 | 0.02* | 0.02 | 10.34* | 7.17 |
| Female | Post | 1.01* | 0.41 | 0.02* | 0.02 | 6.74 | 6.32 |
| Female | Post-15 | 1.01* | 0.42 | 0.02* | 0.02 | 4.26 | 4.25 |
| Female | Post-30 | 1.01* | 0.44 | 0.02* | 0.02 | 2.30 | 2.36 |
| Male | Pre | 19.63 | 5.64 | 0.38 | 0.11 | 0.07 | 0.01 |
| Male | Mid | 23.89 | 5.21 | 0.43 | 0.11 | 3.41 | 5.37 |
| Male | Post | 23.44 | 5.73 | 0.43 | 0.09 | 2.33 | 3.64 |
| Male | Post-15 | 22.56 | 6.00 | 0.44 | 0.11 | 1.21 | 1.91 |
| Male | Post-30 | 22.00 | 5.55 | 0.43 | 0.10 | 0.76 | 1.03 |
Descriptive statistics of hormones measured before, during and after a standardized exercise session.
Significant different between sexes, p ≤ 0.05.
Significant difference from ”Pre”, p ≤ 0.05.
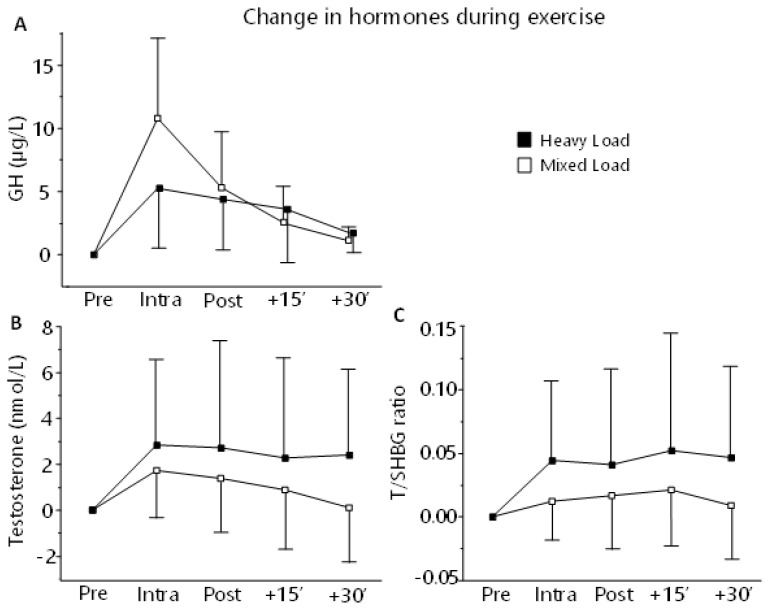
For GH response, there was a main effect of Time (p < 0.01) where GH concentration was significantly elevated from baseline at Intra (p < 0.05) which also was higher than Post+15 and Post+30 (p < 0.05). There was an effect of Sex (p < 0.01), with females having a higher concentration of GH in general. There was no effect for Group or any interaction. When normalized to relative changes, there was an effect of Group, Time, Sex and Time×Group (p < 0.01). For Time×Group effect, ML had a greater increase in GH at Intra and Post than HL (p < 0.05), and for Sex effect, males show higher relative increase in GH (p< 0.0001) (Supplementary Materials 1, Table 4 and 5, and HTML-file 5–6).
There was no difference between groups in AUC for GH (p = 0.693, d = 0.18 [−0.73, 1.08] for AUC1, p = 0.506, d = 0.31 [−0.61, 1.20] for AUC2.
For testosterone, there was an effect of Sex (p < 0.0001) with males having a higher concentration than females. There was no effect of Time, Group, or any interaction. When normalized to relative changes, there was an effect of Time (p = 0.04), but not for Group or any interaction (Supplementary Materials 1, Table 4 and 5, HTML-file 5 and 7).
For T/SHBG, there was an effect of Sex (p < 0.0001) and Group, with males and HL-group showing a higher T/SHBG ratio then females and ML-group respectively. When normalized to relative changes, there was an effect of Sex with females having a higher increase overall (p < 0.003), but no Sex×Time effect. There was also effects of Time and Group (p < 0.001) but not Time×Group (Supplementary Materials 1, Table 4 and 5, HTML-file 5 and 7).
There was no difference between groups in AUC for testosterone or T/SHBG response (Testosterone: p = 0.675, d = 0.46 [−0.71, 1.10] for AUC1, p = 0.502, d = 0.46 [−1.10, 0.71] for AUC2, T/SHBG: p = 0.976, d = 0.00 [−0.95, 0.95] for AUC1, p = 0.823, d = 0.09 [−0.95, 0.95] for AUC2).
Figure 4.
Acute changes in GH (A), Testosterone (B) and T/SHBG (C) measured during a standardized training session at week six during the intervention. Blood sampling took place two hours post-prandial, after 15 minutes seated rest (Pre), and then after leg exercises (Intra), after completed workout (Post) and then after 15 (+15′) and 30 (30′) minutes of rest.
DISCUSSION
The main finding of this study was that a ten-week resistance-training program using a combination of hypertrophy-type and maximal strength training for lower- and upper-body exercises (ML), respectively, did not induce different changes in dynamic 1-RM (upper-body) strength compared to a maximal strength-only training program (HL). Both groups increased their 1-RM in a similar manner. In response to one bout of exercise, both training regimens resulted in elevations of testosterone, T/SHBG and GH with an Intra- and Post-exercise difference between groups in relative change in GH. Females had lower levels of testosterone and T/SHBG than men, but showed a greater relative increase in T/SHBG (main effect of Sex). Meanwhile, while females had higher concentrations of GH and a greater absolute change, men showed a greater relative increase in GH. As expected, ML had a greater volume load than HL, due to the lower load in the lower-body exercises. While this type of training lead to a greater increase in GH, it did not potentiate 1-RM strength in the upper body.
The similar elevation of testosterone and T/SHBG between groups, is in contrast to studies utilizing a within-person design, where one arm is trained unilaterally at one session and the contralateral arm is trained in combination with the lower extremities at another session (15, 36, 44, 45). This is possibly due to the small discrepancy between the training protocols in the present study (same exercises and muscle engagement, with different loadings). The inclusion of both sexes increases within-group variance and decreases (females) absolute changes in hormonal measurements on a group level. However, it increases power for detecting associations between hormonal responses and strength adaptation and obviously, any effects of sex in the statistical models. Participants in the age of 35 – 45 years old displayed, as expected, a muted hormonal response to exercise, making the overall effect less obvious compared to other studies. Therefore, it is reasonable that the magnitude of between-group difference in hormonal elevations in this study is small, which complicates any conclusiveness.
One study reported greater relative increase in GH and isometric arm strength, in a group with leg training in addition to a one-arm protocol only (15). However, there were no group differences in dynamic 1-RM strength increase or exercise-induced elevations in testosterone. As noted by the authors, the low sample size, initial strength differences and large variability in data set do not allow for conclusiveness of the results. In studies by West et. al., utilizing a unilateral within-subject design with leg training and arm training for one arm, and arm training only, transient exercise-induced elevations in testosterone and GH coincided with neither elevated muscle protein synthesis and anabolic signaling (45), nor with muscle hypertrophy and muscle strength in of the elbow flexors (44). Fundamentally, as mentioned by the authors, a cross-education effect cannot be neglected, with possible blunting of an effect of transient elevations in anabolic hormones.
As mentioned, some argue that elevations in GH can be a cause for greater muscle protein synthesis and strength adaptations (15, 29, 36), while some argue it does not (43–45). In this study, both lower-body-training regimens gave rise to GH increase while ML increased it greater than HL. Yet, exercise-induced increase in GH did not potentiate strength gains, neither alone or in a multivariate analysis combined with testosterone and T/SHBG (not presented). Few studies have studied the response of GH in response of exercise between men and women. Our finding with higher baseline and greater absolute change in GH concentration, are somewhat in line with previous research and is not surprising, given the significant increase of GH basal levels in women during late follicular phase (11). The magnitude of these differences (approximately threefold higher in women Intra and Post-workout), however, are more substantial in this study, compared to others (11, 47). With the low samples size and great inter-individual variability, this could simply be due to randomness.
Circulating testosterone in adults explains most sex differences in strength performance (17). Therefore, it is not surprising that Sex had an effect on 1-RM strength with males showing superior performance. Interestingly, women showed similar, or even greater (LPD, Sex×Time) relative strength gain. The 1-RM strength gain in this study (~22% for BP, 27% for LP, and 17% for LPD) is in line with similar studies examining dynamic 1-RM strength (38).
Studies investigating the effect of exercise-induced elevations of endogenous hormones have reported equivocal effects on potentiating effects on muscle hypertrophy (2, 27, 36, 43). While some reported greater 1-RM and CSA increase in the bicep bracchi for the group with added leg training before arm training (36), some methodological considerations question its conclusiveness as no Time×Group interaction was analyzed (35). Madarame, Neya, Ochi, Nakazato, Sato, and Ishii (27) reported a greater relative CSA increase in the group with leg training using BFR, compared to leg training without BFR, both at an intensity of 30% 1-RM. While the study utilized an analysis of variance design, no Time×Group interaction was presented. Blood-flow restricted training induced greater noradrenaline elevation, but no differences were seen for GH and testosterone. Nevertheless, the authors conclude that circulating factors were involved in the remote effect of exercise on muscular size. Unfortunately, the present study did not include measurements of skeletal muscle hypertrophy. Given the results of similar 1-RM increase and the minor difference in hormonal change, we do not anticipate a greater increase in muscle size with ML compared to HL.
A key strength of the present study was the multiple-group design which omits potential inference of the cross-transfer effect. Another strength was the intervention period lasting ten-weeks, rather than six to eight, commonly applied in similar studies (2, 29). Limitations were the lack of fat-free mass and skeletal muscle hypertrophy measurements. Dropouts lowered the sample size from 26 to 17, leading to small sample size for between-group and sex comparisons, unfortunately. Subsequently, the sample size became smaller than desired to achieve adequate statistical power. Another limitation is the lack of additional data points on hormonal response, such as pre- and post-training data. While the comparison is between groups for rate of change, more sample sessions would have been advantageous to shed more on the endocrine responses. Data variability and a heterogeneous sample population (such as the age range) reduced external validity, statistical power, and effect size. For higher degree of standardization, more familiarization of the exercises and exercising to exhaustion would have been advantageous, especially regarding the beginners. Consequently, results must be interpreted conservatively. Hormone analyses in women is influenced by the menstrual cycle, as levels of several hormones, including GH and testosterone fluctuate over a month in women (48), with testosterone peaking in the mid-cycle. Basal levels of GH can be influenced by oral contraceptives and could thus influence the results, such as the effect of Sex with females having higher GH (9). Yet, the acute effect to exercise in testosterone levels as studied here, do not vary with the menstrual cycle or the use of oral contraceptives (8). However, not controlling for menstrual cycle or contraceptives in females add some uncertainty to the results related to hormonal change.
The present study, as well as most studies in the context of resistance exercise and endocrine responses, measures testosterone in serum or plasma. Possibly, investigating more potent intracellular derivate, such as dihydrotestosterone and the actual myocytic androgen action via various receptors, could be more relevant. Also, it is suggested that androgen receptor content, but not systemic hormones, is associated with resistance training-induced skeletal muscle hypertrophy (31). At least, androgen receptors are mediators of testosterone effects and contributes to complexity of myogenic pathways (19). Further, it has recently come to notice that androgen receptors in males compared to females are differentially phosphorylated at some, but not all sites, which could have implications on the adaptations to resistance training (33), making the concentration of anabolic hormones in blood only a small component of factors contributing to skeletal muscle adaptation.
As proposed by some, regulators (mTOR) and signaling proteins, such as p70S6K and acute elevations of muscle protein synthesis, can better reflect of the potential for resistance training-induced hypertrophy and strength (43). Further, studies examining changes to the muscle proteome and associations between protein expression and adaptations are expanding our knowledge concerning molecular mechanisms involved in skeletal muscle response to resistance training, however, there are still several gaps to fill as described more in-depth by others (34).
It is concluded that both groups increased their 1-RM in all assessments with no difference between groups. The different training protocols affected acute changes in GH, testosterone and T/SHBG levels where hypertrophy-type leg training induced a greater relative increase in GH compared to a maximal strength-type training regime, during the selected session with hormone measurements. The greater relative intra-workout increases in GH did not correspond to greater upper-body adaptations. Men and women had a similar increase in relative 1-RM. The notion that acute elevations in anabolic hormones is relevant for muscle strength adaptation in recreational athletes cannot be supported by the present study.
Equations:
Supplementary Information
REFERENCES
- 1.Albertsson-Wikland K, Kristrom B, Lundberg E, Aronson AS, Gustafsson J, Hagenas L, Ivarsson SA, Jonsson B, Ritzen M, Tuvemo T, Westgren U, Westphal O, Aman J. Growth hormone dose-dependent pubertal growth: A randomized trial in short children with low growth hormone secretion. Horm Res Paediatr. 2014;82(3):158–170. doi: 10.1159/000363106. [DOI] [PubMed] [Google Scholar]
- 2.Bartolomei S, Hoffman JR, Stout JR, Merni F. Effect of lower-body resistance training on upper-body strength adaptation in trained men. J Strength Cond Res. 2018;32(1):13–18. doi: 10.1519/JSC.0000000000001639. [DOI] [PubMed] [Google Scholar]
- 3.Bergan-Roller HE, Sheridan MA. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. Gen Comp Endocrinol. 2018;258:119–133. doi: 10.1016/j.ygcen.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Bird SP, Tarpenning KM. Influence of circadian time structure on acute hormonal responses to a single bout of heavy-resistance exercise in weight-trained men. Chronobiol Int. 2004;21(1):131–146. doi: 10.1081/cbi-120027987. [DOI] [PubMed] [Google Scholar]
- 5.Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94(3):907–913. doi: 10.1210/jc.2008-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen J. Statistical power analysis for the behavioral sciences. London, UK: Routledge; 1988. [Google Scholar]
- 7.Crewther B, Cronin J, Keogh J, Cook C. The salivary testosterone and cortisol response to three loading schemes. J Strength Cond Res. 2008;22(1):250–255. doi: 10.1519/JSC.0b013e31815f5f91. [DOI] [PubMed] [Google Scholar]
- 8.Enea C, Boisseau N, Ottavy M, Mulliez J, Millet C, Ingrand I, Diaz V, Dugue B. Effects of menstrual cycle, oral contraception, and training on exercise-induced changes in circulating dhea-sulphate and testosterone in young women. Eur J Appl Physiol. 2009;106(3):365–373. doi: 10.1007/s00421-009-1017-6. [DOI] [PubMed] [Google Scholar]
- 9.Faria AC, Bekenstein LW, Booth RA, Jr, Vaccaro VA, Asplin CM, Veldhuis JD, Thorner MO, Evans WS. Pulsatile growth hormone release in normal women during the menstrual cycle. Clin Endocrinol (Oxf) 1992;36(6):591–596. doi: 10.1111/j.1365-2265.1992.tb02270.x. [DOI] [PubMed] [Google Scholar]
- 10.Flanagan EP. The effect size statistic—applications for the strength and conditioning coach. Strength Cond J. 2013;35(5):37–40. [Google Scholar]
- 11.Giannoulis MG, Boroujerdi MA, Powrie J, Dall R, Napoli R, Ehrnborg C, Pentecost C, Cittadini A, Jørgensen JOL, Sonksen PH. Gender differences in growth hormone response to exercise before and after rhgh administration and the effect of rhgh on the hormone profile of fit normal adults. Clin Endocrinol (Oxf) 2005;62(3):315–322. doi: 10.1111/j.1365-2265.2005.02216.x. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez AM, Hoffman JR, Stout JR, Fukuda DH, Willoughby DS. Intramuscular anabolic signaling and endocrine response following resistance exercise: Implications for muscle hypertrophy. Sports Med. 2016;46(5):671–685. doi: 10.1007/s40279-015-0450-4. [DOI] [PubMed] [Google Scholar]
- 13.Goodman CA. The role of mTORC1 in mechanically-induced increases in translation and skeletal muscle mass. J Appl Physiol. 1985;2019;127(2):581–590. doi: 10.1152/japplphysiol.01011.2018. [DOI] [PubMed] [Google Scholar]
- 14.Goto K, Ishii N, Kizuka T, Takamatsu K. The impact of metabolic stress on hormonal responses and muscular adaptations. Med Sci Sports Exerc. 2005;37(6):955–963. [PubMed] [Google Scholar]
- 15.Hansen S, Kvorning T, Kjaer M, Sjogaard G. The effect of short-term strength training on human skeletal muscle: The importance of physiologically elevated hormone levels. Scand J Med Sci Sports. 2001;11(6):347–354. doi: 10.1034/j.1600-0838.2001.110606.x. [DOI] [PubMed] [Google Scholar]
- 16.Hendy AM, Spittle M, Kidgell DJ. Cross education and immobilisation: Mechanisms and implications for injury rehabilitation. J Sci Med Sport. 2012;15(2):94–101. doi: 10.1016/j.jsams.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Hirschberg AL, Elings Knutsson J, Helge T, Godhe M, Ekblom M, Bermon S, Ekblom B. Effects of moderately increased testosterone concentration on physical performance in young women: A double blind, randomised, placebo controlled study. Br J Sports Med. 2019;54(10):599–604. doi: 10.1136/bjsports-2018-100525. [DOI] [PubMed] [Google Scholar]
- 18.Hortobagyi T, Lambert NJ, Hill JP. Greater cross education following training with muscle lengthening than shortening. Med Sci Sports Exerc. 1997;29(1):107–112. doi: 10.1097/00005768-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Kadi F. Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol. 2008;154(3):522–528. doi: 10.1038/bjp.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamen G. Neural issues in the control of muscular strength. Res Q Exerc Sport. 2004;75(1):3–8. doi: 10.1080/02701367.2004.10609127. [DOI] [PubMed] [Google Scholar]
- 21.Kraemer WJ American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer WJ, Ratamess NA. Fundamentals of resistance training: Progression and exercise prescription. Med Sci Sports Exerc. 2004;36(4):674–688. doi: 10.1249/01.mss.0000121945.36635.61. [DOI] [PubMed] [Google Scholar]
- 23.Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35(4):339–361. doi: 10.2165/00007256-200535040-00004. [DOI] [PubMed] [Google Scholar]
- 24.Kraemer WJ, Ratamess NA, Nindl BC. Recovery responses of testosterone, growth hormone, and igf-1 after resistance exercise. J Appl Physiol. 1985;2017;122(3):549–558. doi: 10.1152/japplphysiol.00599.2016. [DOI] [PubMed] [Google Scholar]
- 25.Kraemer WJ, Staron RS, Hagerman FC, Hikida RS, Fry AC, Gordon SE, Nindl BC, Gothshalk LA, Volek JS, Marx JO, Newton RU, Hakkinen K. The effects of short-term resistance training on endocrine function in men and women. Eur J Appl Physiol Occup Physiol. 1998;78(1):69–76. doi: 10.1007/s004210050389. [DOI] [PubMed] [Google Scholar]
- 26.Lee M, Carroll TJ. Cross education: Possible mechanisms for the contralateral effects of unilateral resistance training. Sports Med. 2007;37(1):1–14. doi: 10.2165/00007256-200737010-00001. [DOI] [PubMed] [Google Scholar]
- 27.Madarame H, Neya M, Ochi E, Nakazato K, Sato Y, Ishii N. Cross-transfer effects of resistance training with blood flow restriction. Med Sci Sports Exerc. 2008;40(2):258–263. doi: 10.1249/mss.0b013e31815c6d7e. [DOI] [PubMed] [Google Scholar]
- 28.Manca A, Dragone D, Dvir Z, Deriu F. Cross-education of muscular strength following unilateral resistance training: A meta-analysis. 2017;117(11):2335–2354. doi: 10.1007/s00421-017-3720-z. [DOI] [PubMed] [Google Scholar]
- 29.May AK, Russell AP, Warmington SA. Lower body blood flow restriction training may induce remote muscle strength adaptations in an active unrestricted arm. Eur J Appl Physiol. 2018;118(3):617–627. doi: 10.1007/s00421-018-3806-2. [DOI] [PubMed] [Google Scholar]
- 30.McCaulley GO, McBride JM, Cormie P, Hudson MB, Nuzzo JL, Quindry JC, Travis Triplett N. Acute hormonal and neuromuscular responses to hypertrophy, strength, and power type resistance exercise. Eur J Appl Physiol. 2009;105(5):695–704. doi: 10.1007/s00421-008-0951-z. [DOI] [PubMed] [Google Scholar]
- 31.Morton RW, Sato K, Gallaugher MPB, Oikawa SY, McNicholas PD, Fujita S, Phillips SM. Muscle androgen receptor content but not systemic hormones is associated with resistance training-induced skeletal muscle hypertrophy in healthy, young men. Front Physiol. 2018;9:1373. doi: 10.3389/fphys.2018.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navalta JW, Stone WJ, Lyons TS. Ethical issues relating to scientific discovery in exercise science. Int J Exerc Sci. 2019;12(1):1–8. doi: 10.70252/EYCD6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicoll JX, Fry AC, Mosier EM. Sex-based differences in resting mapk, androgen, and glucocorticoid receptor phosphorylation in human skeletal muscle. Steroids. 2019;141:23–29. doi: 10.1016/j.steroids.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Petriz BA, Gomes CP, Almeida JA, de Oliveira GP, Jr, Ribeiro FM, Pereira RW, Franco OL. The effects of acute and chronic exercise on skeletal muscle proteome. J Cell Physiol. 2017;232(2):257–269. doi: 10.1002/jcp.25477. [DOI] [PubMed] [Google Scholar]
- 35.Phillips SM. Strength and hypertrophy with resistance training: Chasing a hormonal ghost. Eur J Appl Physiol. 2012;112(5):1981–1983. doi: 10.1007/s00421-011-2148-0. author reply 1985-1987. [DOI] [PubMed] [Google Scholar]
- 36.Ronnestad BR, Nygaard H, Raastad T. Physiological elevation of endogenous hormones results in superior strength training adaptation. Eur J Appl Physiol. 2011;111(9):2249–2259. doi: 10.1007/s00421-011-1860-0. [DOI] [PubMed] [Google Scholar]
- 37.Schoenfeld BJ. Postexercise hypertrophic adaptations: A reexamination of the hormone hypothesis and its applicability to resistance training program design. J Strength Cond Res. 2013;27(6):1720–1730. doi: 10.1519/JSC.0b013e31828ddd53. [DOI] [PubMed] [Google Scholar]
- 38.Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW. Strength and hypertrophy adaptations between low- vs. high-load resistance training: A systematic review and meta-analysis. J Strength Cond Res. 2017;31(12):3508–3523. doi: 10.1519/JSC.0000000000002200. [DOI] [PubMed] [Google Scholar]
- 39.Schoenfeld BJ, Pope ZK, Benik FM, Hester GM, Sellers J, Nooner JL, Schnaiter JA, Bond-Williams KE, Carter AS, Ross CL, Just BL, Henselmans M, Krieger JW. Longer interset rest periods enhance muscle strength and hypertrophy in resistance-trained men. J Strength Cond Res. 2016;30(7):1805–1812. doi: 10.1519/JSC.0000000000001272. [DOI] [PubMed] [Google Scholar]
- 40.Baechle THRWE. Essentials of strength training and conditioning. 3rd ed. Champaign, IL: Human Kinetics; 2008. [Google Scholar]
- 41.Verbeke GMG. Linear mixed models in practice Lecture notes in statistics. New York, NY: Springer; 1997. Linear mixed models for longitudinal data. [Google Scholar]
- 42.Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: The up-stream regulatory elements. Sports Med. 2010;40(12):1037–1053. doi: 10.2165/11536910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 43.West DW, Burd NA, Staples AW, Phillips SM. Human exercise-mediated skeletal muscle hypertrophy is an intrinsic process. Int J Biochem Cell Biol. 2010;42(9):1371–1375. doi: 10.1016/j.biocel.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 44.West DW, Burd NA, Tang JE, Moore DR, Staples AW, Holwerda AM, Baker SK, Phillips SM. Elevations in ostensibly anabolic hormones with resistance exercise enhance neither training-induced muscle hypertrophy nor strength of the elbow flexors. J Appl Physiol. 1985;2010;108(1):60–67. doi: 10.1152/japplphysiol.01147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, De Lisio M, Tang JE, Parise G, Rennie MJ, Baker SK, Phillips SM. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol. 2009;587(Pt 21):5239–5247. doi: 10.1113/jphysiol.2009.177220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.West DWD, Phillips SM. Anabolic processes in human skeletal muscle: Restoring the identities of growth hormone and testosterone. Phys Sportsmed. 2010;38(3):97–104. doi: 10.3810/psm.2010.10.1814. [DOI] [PubMed] [Google Scholar]
- 47.Wideman L, Weltman JY, Shah N, Story S, Veldhuis JD, Weltman A. Effects of gender on exercise-induced growth hormone release. J Appl Physiol. 1985;1999;87(3):1154–1162. doi: 10.1152/jappl.1999.87.3.1154. [DOI] [PubMed] [Google Scholar]
- 48.Wikstrom-Frisen L, Boraxbekk CJ, Henriksson-Larsen K. Effects on power, strength, and lean body mass of menstrual/oral contraceptive cycle based resistance training. J Sports Med Phys Fitness. 2017;57(1–2):43–52. doi: 10.23736/S0022-4707.16.05848-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.