Abstract
Purpose
To investigate the prevalence of and risk factors for abdominal aortic aneurysm (AAA) in 65-year-old men in Oslo, Norway.
Materials and Methods
From May 2011, until September 2019, the annual population of 65-year-old men living in Oslo were invited to an ultrasonographic screening of the abdominal aorta. Candidates received a one-time invitation by mail, including a questionnaire on possible risk factors and comorbidities. Abdominal aortic outer-to-outer diameter and ankle-brachial index were measured by the screening team. Participants were allocated into three groups: non-, sub- and aneurysmal aorta. Written information on recommended follow-up regime was given to participants with an aortic diameter ≥25 mm. Univariate and multivariate analyses of potential risk factors were performed, in addition to descriptive analyses and significance testing.
Results
In total, 19,328 were invited, 13,215 men were screened, of which 12,822 accepted inclusion in the study. Aortic diameter was registered for 12,810 participants and 330 men had aortic diameter ≥30 mm, giving a prevalence of AAA of 2.6% (95% confidence interval (CI) 2.31–2.86). We identified 4 independent risk factors for AAA: smoking (OR = 3.64, 95% CI 2.90–4.58), hypertension (OR = 1.87, 95% CI 1.49–2.35), BMI >30 (OR = 1.02, 95% CI 1.00–1.03), and diabetes mellitus (OR = 0.52, 95% CI 0.35–0.79), the latter showing an inverse association with AAA growth. A subgroup of 862 men with aortic diameters 25–29 mm had a significantly higher prevalence of BMI >25, smoking and family history of AAA, compared to participants with aortic diameter <25 mm.
Conclusion
Among the participants in this study, the prevalence of abdominal aortic aneurysms was 2.6%. Participants with AAA more frequently reported cardiovascular risk factors, and less frequently diabetes mellitus.
Keywords: prevalence, sub-aneurysmal aorta, ultrasonography, men, smoking, diabetes
Plain Language Summary
This is a population-based screening study of the abdominal aorta in 65-year-old men in Oslo. Ultrasonographic screening of the abdominal aorta was offered to all 65-year-old men living in Oslo, Norway. In a population of 12,810 men, 330 cases of abdominal aortic aneurysms (AAA) were found, giving a prevalence of 2.6%.
Compared with studies from the 80s and 90s, this study revealed a lower prevalence of abdominal aortic aneurysms in 65-year-old men. This prevalence is nonetheless twice as high as that reported in recent studies (1.3–1.5%). Four risk factors for AAA were identified: smoking, hypertension, body-mass index (BMI) >30 and diabetes mellitus, the latter having an inverse effect on AAA growth. In addition, there was a significantly higher prevalence of peripheral artery disease and a history of stroke amongst participants with AAAs. Since the presence of AAA is a potential life-threatening condition, screening is an important tool in early detection of the disease.
Introduction
Abdominal aortic aneurysm is a potentially lethal condition, most prevalent in elderly men. Ruptured AAA (rAAA) has a high mortality rate. A nationwide analysis of rAAA in Portugal has shown that approximately 75% of patients with rAAA die before reaching hospital or during hospitalization.1 Budtz-Lilly et al have reported a peri-operative mortality for emergency surgery at 28.8%.2 Furthermore, the 30-day mortality ranges from around 30–60%.1,3–5 In contrast, mortality rates for elective AAA surgery are drastically lower, 30-day mortality range from 0.5% to 5.0%.6–9
Several large studies have shown that one-time ultrasound screening in men above 65 years reduces the incidence of sudden AAA ruptures and aneurysm-related mortality.9–15 Consequently, Sweden (2006) and the United Kingdom (2009) have implemented national screening programs for ultrasonographic detection of AAA.9,16 In the United States, one-time screening for AAA has been offered since 2007 to male members of Medicare between the ages of 65 to 75 years with any history of smoking.17
In Sweden, the prevalence of AAA in 65-year-old men is 1.5%.9 The national screening program in the United Kingdom has shown a AAA prevalence in ≥65-year-old men at 1.57%.16 A study from the United States found a prevalence of AAA in the population aged 50 to 84 years (both men and women) of 1.4%.18 This is in contrast to results from earlier studies from the 80s and 90s, which demonstrated a prevalence of 4.0–9.0%.19–22 Data from the Gloucestershire Aneurysm Screening Program also reveal a decline in AAA prevalence in 65-year-old men, from 5.0% in 1991 to 1.3% in 2015.23
Krohn et al examined 500 Norwegian men above 60 years in 1992, and found that 5.8% had small and 2.4% had large AAAs.24 In 2001, Singh et al found a AAA prevalence of 8.9% in 23–84-year-old men in the Tromsø study, in Norway.25,26 To our knowledge, there are no other studies on the prevalence of AAA in Norway, and there are no national or regional screening programs for AAA in Norway. The aim of the present study was to examine the current prevalence and risk factors for AAA in 65-year-old men in Oslo. We hypothesize that the prevalence may be lower than previously reported.
Materials and Methods
From May 2011, annually all 65-year-old men with permanent residence in Oslo were invited (one-time invitation by mail) to undergo an ultrasonography of the abdominal aorta. Names, dates of birth and contact information were extracted from the National Population Register. The invitation included a questionnaire on potential risk factors for and comorbidities with AAA, which was completed in collaboration with the screening team on the day of screening. The screening was performed in the outpatient clinic of the Department of Vascular Surgery at Oslo University Hospital.
The screening team consists of radiographers and nurses with special training in abdominal aorta ultrasonography. Their training was given by a consultant radiologist from the Department of Radiology and Interventional Radiology at Oslo University Hospital. The screening team has been consistent throughout the screening period. In the early phase, the consultant radiologist did sample tests to validate the ultrasonographic results. Ultrasonography of the abdominal aorta is a highly sensitive and specific modality for detection of AAAs.11,27,28
The screening team works at the Department of Vascular Surgery and the Department of Radiology and Interventional Radiology at Oslo University Hospital, and the costs were shared between these two departments. The screening project has no external funding.
The examinations were performed with a sector probe (Sonix SP; C5-2 probe, Ultrasonix). The aorta was examined in the axial plane with scans perpendicular to the longitudinal plane. The maximum aortic diameter was measured below the level of the renal arteries from the outer-to-outer (OTO) wall with electronic calipers, both in the transverse and anterior-posterior plane. The highest of these measurements was used. OTO is considered the most reliable diameter of the aorta.29 The sensitivity of the method was ensured consecutively through instrument calibration by an experienced radiologist. A biomedical scientist consecutively collected the data in a database (FileMaker Pro 14).
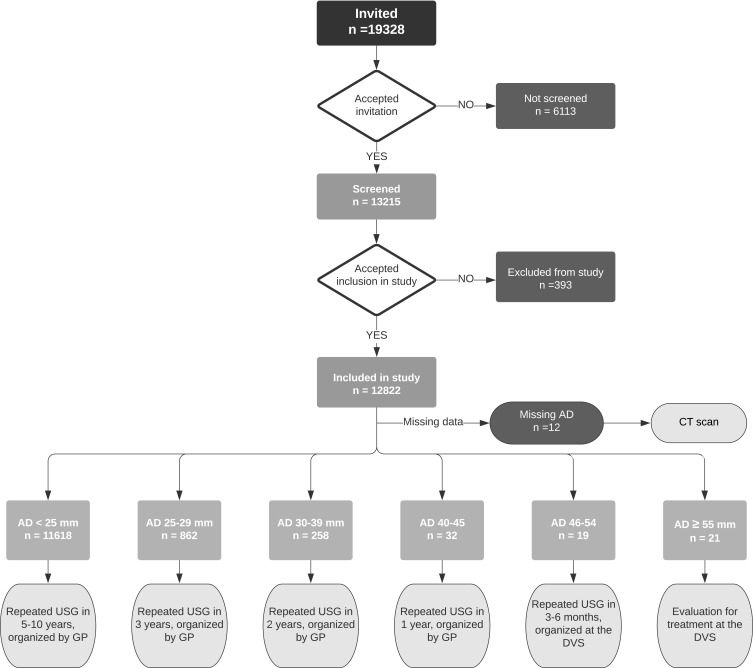
The internationally accepted definition of AAA was used; diameter of ≥30 mm.30 Written information on recommended follow-up regime was given to participants with an aortic diameter ≥25 mm. Follow-up until aortic diameter 45 mm is facilitated by the general practitioner. At aortic diameter ≥45 mm, participants are referred to the Department of Vascular Surgery for further follow-up and eventually treatment (Figure 1). In Norway, all citizens are entitled to a dedicated general practitioner, as part of the public healthcare system.
Figure 1.
Consort diagram. The diagram shows the allocation of the invited candidates, distribution of aortic diameters, and recommended follow-up regime depending on measured aortic diameter.
Abbreviations: AD, aortic diameter; USG, ultrasonography; GP, general practitioner; DVS, Department of Vascular Surgery.
The ankle- and brachial pressures were measured in both arms and both legs (both dorsalis pedis artery and posterior tibial artery) for each participant, by sphygmomanometry using a Doppler ultrasound transducer. The highest of the two systolic ankle pressures was taken as the numerator for each leg and the highest systolic brachial pressure as the denominator for the arm. An ankle-brachial index (ABI) <0.9 in either leg is considered abnormal and indicates peripheral artery disease (PAD).31
Statistical analyses were performed using SPSS (ver. 26). The program was used for descriptive analyses, categorical data including frequency values, tables and histograms for evaluation of normal distribution. Crosstabs were used to examine the distribution of variables, and Pearson’s chi square test was used for significance testing. Statistical significance was set at p-value ≤0.05.
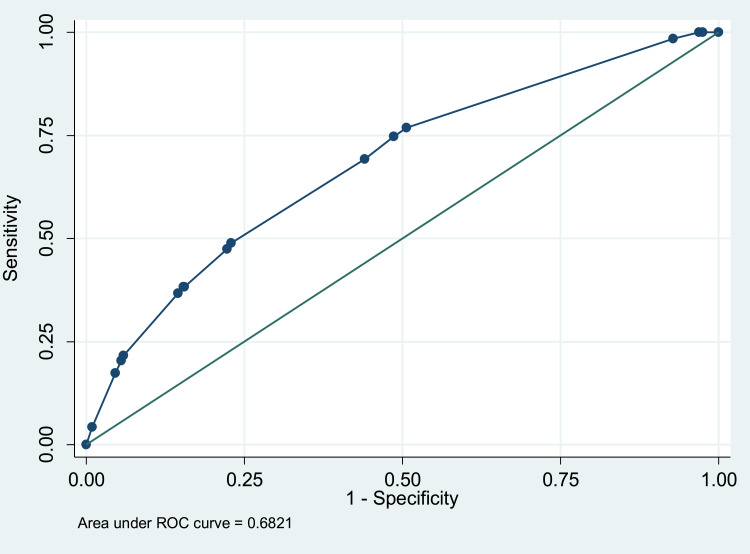
In the univariate analysis, contingency tables were used for the discrete variables and Student’s t-test for continuous variables. The logistic model was used in the multivariate analysis to pinpoint independent risk factors of AAA. The predictive accuracy of the model was evaluated by calibration and discrimination. Calibration, which measures the ability of the logistic model to assign the appropriate risk, was evaluated by the Hosmer and Lemeshow (H-L) goodness-of-fit test. The H-L measures the difference between expected and observed outcomes over deciles of risk. A statistically not significant H-L result (p-value > 0.05) suggests that the model predicts accurately on average. Discrimination, which measures the ability of the model to differentiate among those who have or do not have AAA, was evaluated by the analysis of the area under the receiver operating characteristic (ROC) curve. If the area under the curve is greater than 0.7 it can be concluded that the model has an acceptable discriminatory capability.32
The study was approved by the Regional Committees for Medical and Health Research Ethics (REC reference number 2009/866) and complies with the Declaration of Helsinki. Informed consents were obtained from all participants in writing. The study is registered at https://clinicaltrials.gov/show/NCT01248533.
Results
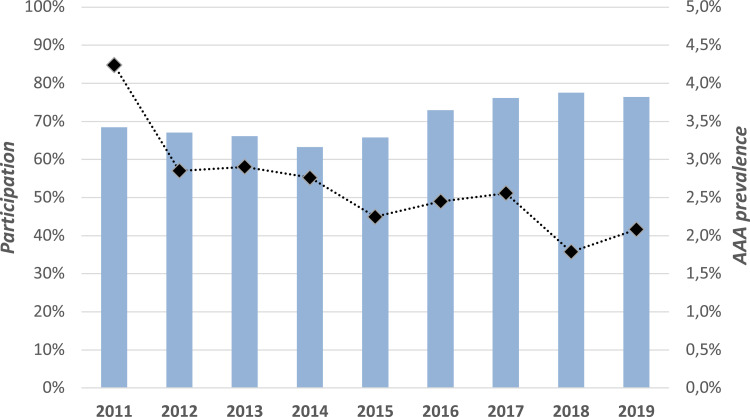
In total, 19,328 were invited, 13,215 men were screened, and 12,822 were included in the study. Of these, 12 participants were excluded due to missing data on aortic diameter, leaving 12,810 eligible for statistical analyses (Figure 1). For the 12 excluded participants, aortic diameter was not possible to obtain due to lack of ultrasonographic visibility for any reason (the participants were also examined by an experienced radiologist). These participants were further examined with a CT scan. The recorded aortic diameters for the participants were normally distributed. Annually, 1124–2784 men were invited (the lowest number represents the first year of screening, that is from May 2011), of which 63.3–77.6% accepted the invitation, and participation rate increased during the study period (Figure 2).
Figure 2.
Yearly participation (columns) and AAA point prevalence (diamonds).
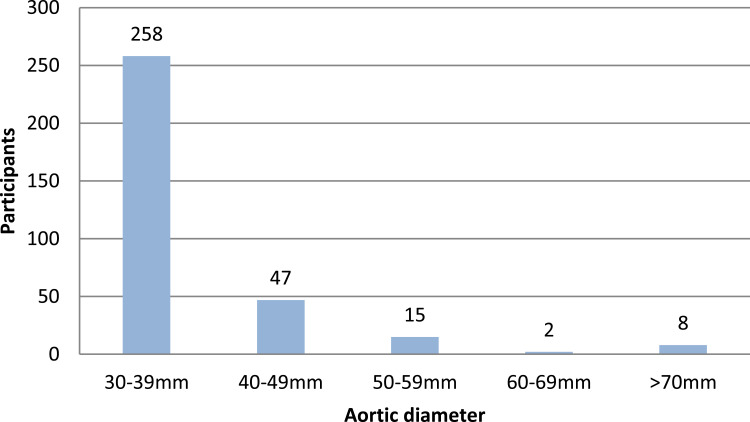
The mean aortic diameter in the studied cohort was 22.1 mm (SD 3.5 mm), and the AAA prevalence was 2.6% (95% CI 2.31–2.86). Figure 2 illustrates the annual prevalence of AAA during the study period. The distribution of aneurysm-size is shown in Figure 3.
Figure 3.
The distribution of aneurysms. The aneurysms are categorized by every 10th mm, n = 330.
Participants with AAA had significantly higher prevalence of BMI > 25, hypertension, intermittent claudication (IC), and ABI < 0.9, than participants with normal aortas (Table 1). More often, candidates with AAA reported a history of previous myocardial infarction (MI) or stroke, current smoking, and a family history of AAA. More participants with AAA were using statins and acetyl salicylic acid (ASA) than participants with normal aortic diameter. There was no statistical difference between participants with or without AAA on history of transient ischemic attack (TIA) or previous smoking. There was a higher, but not statistically significant, prevalence of diabetes mellitus amongst participants without aneurysms (Table 1).
Table 1.
Descriptive Data; Risk Factors and Comorbidities
| Variable | All Participants, n = 12,810 | Aortic Diameter ≥ 30 mm, n = 330 | Aortic Diameter < 30 mm, n = 12,480 | P-value* | Aortic Diameter 25–29 mm, n = 862 | P-value** |
|---|---|---|---|---|---|---|
| BMI > 25 | 65.5% | 75.2% | 65.3% | < 0.001 | 70.2% | 0.01 |
| BMI > 30 | 16.6% | 25.1% | 16.4% | < 0.001 | 20.8% | < 0.001 |
| ABI < 0.9 | 6.7% | 15.5% | 6.5% | < 0.001 | 8.2% | 0.017 |
| MI | 8.6% | 20.0% | 8.3% | < 0.001 | 8.6% | 0.377 |
| TIA | 2.9% | 4.5% | 2.9% | 0.063 | 3.4% | 0.218 |
| Stroke | 2.8% | 6.4% | 2.7% | < 0.001 | 3.6% | 0.056 |
| Hypertension | 40.2% | 53.0% | 39.9% | < 0.001 | 41.5% | 0.163 |
| Diabetes mellitus | 11.2% | 7.9% | 11.3% | 0.056 | 7.2% | < 0.001 |
| IC | 6.5% | 12.5% | 6.4% | < 0.001 | 7.7% | 0.061 |
| Current smoker | 16.8% | 39.7% | 16.2% | < 0.001 | 24.0% | < 0.001 |
| Past smoker | 43.6% | 45.2% | 43.6% | 0.306 | 43.6% | 0.506 |
| Family history of AAA | 8.3% | 17,3% | 8.1% | < 0.001 | 13.5% | < 0.001 |
| Statin | 33.1% | 48.8% | 32.7% | < 0.001 | 32.8% | 0.486 |
| ASA | 24.8% | 35.9% | 24.5% | < 0.001 | 25.9% | 0.175 |
Notes: Statistical significance was set at p-value ≤ 0.05, and significant p-values are presented in bold text. P-values are calculated for *Aortic diameter ≥ 30 mm versus aortic diameter < 30 mm, **Aortic diameter 25–29 mm versus aortic diameter < 25 mm.
Abbreviations: BMI, body mass index; ABI, ankle brachial index; MI, myocardial infarction; TIA, transitory ischemic attack; IC, intermittent claudication; ASA, acetylsalicylic acid.
Performing regression analyses, we found four independent risk factors for AAA: smoking, hypertension, obesity (BMI > 30) and diabetes mellitus. Smoking was the most important risk factor (Tables 2 and 3). The ROC curve is presented in Figure 4.
Table 2.
Univariate Analysis
| Discrete Variables | Number of Participants with AAA | Odds Ratio | 95% CI | P-value |
|---|---|---|---|---|
| Smoking | Yes/No | 3,40 | 2.71–4.26 | 0.0001 |
| Hypertension | Yes/No | 1,70 | 1.30–2.12 | 0.001 |
| Diabetes mellitus | Yes/No | 0,67 | 0.45–1.01 | 0.56 |
| Continuous Variables | Number of Participants | Mean | 95% CI | P-value |
| BMI | ||||
| AD ≥ 30 mm | 330 | 27.6 | ± 4.09 | 0.0001 |
| AD < 30 mm | 12,449 | 26.67 | ± 4.48 |
Notes: Risk factors univariate analysis. Abdominal aortic aneurysm; aortic diameter ≥ 30 mm.
Abbreviations: AD, aortic diameter; CI, confidence interval; BMI, body mass index.
Table 3.
Multivariate Analysis
| Variable | Number of Participants with AAA | Odds Ratio | 95% CI | P-value | |
|---|---|---|---|---|---|
| BMI > 30 | Yes | 330 | 1.02 | 1.00–1.03 | 0.0072 |
| No | 12,449 | ||||
| Diabetes mellitus | Yes | 329 | 0.52 | 0.35–0.79 | 0.0025 |
| No | 12,451 | ||||
| Hypertension | Yes | 330 | 1.87 | 1.49–2.35 | 0.0001 |
| No | 12,456 | ||||
| Smoking | Yes | 330 | 3.64 | 2.90–4.58 | 0.0001 |
| No | 12,479 | ||||
Notes: Multivariate analysis using the logistic model. Independent risk factors of abdominal aortic aneurysm; aortic diameter ≥ 30 mm.
Abbreviation: CI, confidence interval.
Figure 4.
ROC curve. Measures the ability of the regression model to differentiate among those who have or do not have AAA.
Abbreviation: ROC curve, receiver operating characteristic curve.
Sub-aneurysmal aortas (25–29 mm) were found in 6.7% of the participants. These participants more often presented with risk factors such as BMI > 25, stroke, current smoking and family history of AAA, but less often diabetes mellitus, when compared to the group with aortic diameter <25 mm (Table 1).
Discussion
This study revealed a AAA prevalence of 2.6% in the screened population of 65-year-old men living in Oslo. This is much lower than large screening studies from the 80s and 90s, and twice as high as recent findings in Sweden and the United Kingdom.9,16,19–23,33
The target population was invited one time only, achieving an annual participation rate up to 77.6%. This must be taken into consideration when discussing the findings. In large screening trials, the percentage of attendance varies from 63% to 80%.34 A single re-invitation could have increased the number of participants to approximately 80% of the invited, as seen in the Swedish screening program.9
Guidelines from European Society for Vascular Surgery (ESVS) and Society for Vascular Surgery (SVS) recommend treatment for AAA at a diameter ≥55 mm in men.28,31 In this study, 6.4% of the aneurysms were ≥55 mm at the time of screening, which shows that screening for AAA in men at the age of 65 years discovers individuals in need of treatment for a potentially lethal condition. These individuals amount to 0.2% of the screened population, and demonstrate a lower incidence than what was found in the four previous large screening studies (0.4–0.6%).35–37 Amongst the participants, 77.7% of the detected aneurysms were <40 mm, and this is consistent with previous data.27
Previous studies have examined the group of 65-year-old men with sub-aneurysmal aortas (25–29 mm) and found that these men are at risk of developing AAA within 5 years.23,38 Data from the screening program in Gloucestershire demonstrated that 28% of participants with sub-aneurysmal aortas develop AAA within 15 years.23 In our material, 6.4% had sub-aneurysmal aortas, and these participants presented some of the same risk factors as participants with AAA, such as BMI > 25, current smoking and a family history of AAA. These findings highlight a group that might benefit from follow-up ultrasonography.
Known risk factors for developing AAA include male sex, smoking, a family history of AAA and old age.18,23–25,39 Hypertension is another potential risk factor, which is also associated with an increased risk for AAA.18,26,31 We found four independent risk factors for AAA: smoking, hypertension, obesity, and diabetes mellitus. These were considered as potential etiological risk factors for AAA, while the other registrations represented the presence of concomitant cardiovascular disease.
In this material, there is a significantly higher number of smokers in the AAA group (compared to participants with aortic diameter < 30 mm), and in the sub-aneurysmal group (compared to participants with aortic diameter < 25 mm). No such difference was found among past smokers; however, the questionnaire did not differentiate on when the participants quit smoking. Other investigators have found that current smokers have a higher risk of developing AAA than past smokers.10,18,35 Smoking has also been associated with higher aneurysm growth rate and progression of sub-aneurysmal aortas to AAAs.9,36,37 In recent recommendations published by the US Preventive Services Task Force, it is concluded that, most likely there is little benefit in screening elderly men who have never smoked.40 In Norway, the prevalence of smokers has decreased during the last decades, according to Statistics Norway the number of daily smokers decreased from 42% to 9% during the period 1973–2020, and its effect on the future prevalence of AAA remains to be investigated.41
In this study, 53% of men with AAA reported to have hypertension, which is significantly higher than for men with aortic diameters <30 mm (39.9%). In a systematic review and meta-analysis from 2004, Cornuz et al found a weak positive association between hypertension and AAA, while a recent meta-analysis suggests that hypertension increases the risk of developing AAA by 66%.42,43 A review by Takagi et al showed no association between hypertension and AAA growth.44
Obesity was an independent, but weak risk factor for AAA in our study. A Swedish observational cohort study did not reveal an association between BMI and AAA in men and women aged 46–84 years, but they found an increased risk of developing AAA in individuals with abdominal adiposity.45 A systematic reviews from 2013 however, suggests a positive association between BMI and AAA.46
Diabetes mellitus was negatively associated with AAA. Recent meta-analyses suggest that in individuals with diabetes mellitus, the risk of developing AAA is reduced by 42%, and that the AAA growth in non-diabetic patients is slower compared to patients with diabetes.47,48 The biological pathways that are considered to limit AAA growth include effect on extracellular matrix, formation of glycation end-products and reduced inflammation. Medications, such as Metformin, used to treat diabetes mellitus may also limit the progression of AAA.49,50 Moreover, diabetes mellitus is an important determinant of mortality following surgical treatment of AAA.51 An ongoing prospective multicenter study in Norway aims to investigate the relationship between glycemic status and mortality in treatment of AAA (ABANDIA).
Concomitant existence of cardiovascular disease was not surprisingly predominant in the AAA group, as AAA shares a lot of similarities with atherosclerotic disease. In this study, a significantly higher prevalence of ABI < 0.9 in the AAA group was found (compared to participants with aortic diameter < 30 mm), but not in the sub-aneurysmal group (compared to participants with aortic diameter < 25 mm). ABI is highly specific (99%) and sensitive (94–97%) for the detection of high-grade stenoses in PAD.30 In the AAA group, 12.5% reported to experience claudication, whereas only 6.4% reported this in the group with normal aortic diameter, and this corresponded to the measured ABI. It is interesting that only 15.5% of the men with AAA had an ABI < 0.9, and the prevalence of ABI < 0.9 was surprisingly low (6.7%) among all 65-year-old men screened in Oslo. A meta-analysis from 2016 found a positive correlation between PAD and AAA, and a negative correlation between PAD and aneurysm growth.52 Lin et al recently published data on 6590 people with AAA, and found an increased risk of developing PAD in this cohort.53
Studies have shown a significant correlation between AAA and three-vessel coronary artery disease (CAD).18,54–56 The prevalence of AAA in patients with CAD has been reported to be higher than in the general population, and the severity of the disease seems to effect the prevalence.55,56 We find it interesting that the prevalence of MI is 20% in the AAA group in the present study, whereas it is 8.6% in the sub-aneurysmal group and 8.3% in the group with normal aortas.
Participants in the AAA group more often reported a history of stroke, whereas for TIA there was no significant difference. The risk factors and comorbidities were self-reported by the participants, and whether they were able to discriminate between stroke and TIA could influence these results. An association between a history of stroke and AAA in women was found by Chabok et al.57 Other investigators have also found a higher prevalence of AAA in patients with stroke or TIA.58,59
A systematic review by Fleming et al shows an increased risk of developing AAA in individuals with a family history of AAA.34 In our material, both in the AAA group and in the sub-aneurysmal group, the prevalence of a family history of AAA was significantly higher than that found in the group with aortic diameter < 30 mm and <25 mm, respectively. Unfortunately, not enough data was obtained from the participants to analyze this as a potential risk factor with regression analyses.
As participation ranged from 63.3% to 77.6% annually, the results must be interpreted with caution. We have no data on the remaining population of the invited 65-year-old men, and it would be of interest to study this group and their risk factors in the future. In addition, there are regional differences in health behavior in Norway, and the healthiest part of the population lives in the capital (Statistics Norway, survey on health 2015). Whether there are regional differences in the AAA prevalence in Norway remains to be studied.
For future similar studies, our recommendation would be to translate invitation letters to different languages addressing the increasing heterogeneous population in our society today. Moreover, a second invitation would increase participation rate. To improve the effect of screening, one could consider to include a follow-up program for all participants with AAA. This requires, however, extended resources.
Conclusions
Screening for AAA in 65-year-old men in Oslo revealed a prevalence of 2.6%. In the AAA group, we found a higher prevalence of risk factors and comorbidities, such as smoking, overweight and cardiovascular disease. We found four independent risk factors for AAA: smoking, hypertension, obesity and diabetes mellitus, the last having an inverse effect. The data also suggest that people with sub-aneurysmal aortas are at risk of developing AAA. This highlights the potential health benefit of offering follow-up ultrasonographies to individuals in this group. Furthermore, the data indicate that individuals with certain risk factor profiles more often develop AAAs, which may give a rationale for selective, risk factor-based screening and follow-up.
Acknowledgments
Professor Jorgen Jorgensen (1947–2017) is gratefully acknowledged for initiating the aorta screening project in Oslo in May 2011. We are grateful to Dr. Oyvind Risum for valuable help with regression analyses. We also thank the screening team for their efforts in inviting and examining the participants, and collecting the data.
Data Sharing Statement
For legal reasons, the raw data from this study cannot be shared publicly.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dias-Neto M, Castro-Ferreira R, Mani K, Freitas A, Leite-Moreira A, Sampaio SM. Nationwide analysis of ruptured abdominal aortic aneurysm in Portugal (2000–2015). Eur J Vasc Endovasc Surg. 2020;60(1):27–35. doi: 10.1016/j.ejvs.2020.02.024 [DOI] [PubMed] [Google Scholar]
- 2.Budtz-Lilly J, Björck M, Venermo M, et al. Editor’s choice - The impact of centralisation and endovascular aneurysm repair on treatment of ruptured abdominal aortic aneurysms based on international registries. Eur J Vasc Endovasc Surg. 2018;56(2):181–188. doi: 10.1016/j.ejvs.2018.01.014 [DOI] [PubMed] [Google Scholar]
- 3.Powell JT, Sweeting MJ, Thompson MM, et al. Endovascular or open repair strategy for ruptured abdominal aortic aneurysm: 30 day outcomes from IMPROVE randomised trial. BMJ. 2014;348(1):f7661. doi: 10.1136/bmj.f7661 [DOI] [PubMed] [Google Scholar]
- 4.Briggs CS, Sibille JA, Yammine H, et al. Short-term and midterm survival of ruptured abdominal aortic aneurysms in the contemporary endovascular era. J Vasc Surg. 2018;68(2):408e1–414e1. doi: 10.1016/j.jvs.2017.12.037 [DOI] [PubMed] [Google Scholar]
- 5.Roosendaal LC, Kramer GM, Wiersema AM, Wisselink W, Jongkind V. Outcome of ruptured abdominal aortic aneurysm repair in octogenarians: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2020;59(1):16–22. doi: 10.1016/j.ejvs.2019.07.014 [DOI] [PubMed] [Google Scholar]
- 6.Ammar AD. Mortality for open abdominal aortic aneurysm repair before and after Endovascular Aortic Repair (EVAR). Am Surg. 2019;85(12):1341–1344. doi: 10.1177/000313481908501226 [DOI] [PubMed] [Google Scholar]
- 7.Qadura M, Pervaiz F, Harlock JA, et al. Mortality and reintervention following elective abdominal aortic aneurysm repair. J Vasc Surg. 2013;57(6):1676–1683, 1683.e1. doi: 10.1016/j.jvs.2013.02.013 [DOI] [PubMed] [Google Scholar]
- 8.Beck AW, Goodney PP, Nolan BW, Likosky DS, Eldrup-Jorgensen J, Cronenwett JL. Predicting 1-year mortality after elective abdominal aortic aneurysm repair. J Vasc Surg. 2009;49(4):838–843;discussion 843–844. doi: 10.1016/j.jvs.2008.10.067 [DOI] [PubMed] [Google Scholar]
- 9.Wanhainen A, Hultgren R, Linné A, et al. Outcome of the Swedish nationwide abdominal aortic aneurysm screening program. Circulation. 2016;134(16):1141–1148. doi: 10.1161/CIRCULATIONAHA.116.022305 [DOI] [PubMed] [Google Scholar]
- 10.Cosford PA, Leng GC. Screening for abdominal aortic aneurysm. Cochrane Database Syst Rev. 2007;(2):CD002945. doi: 10.1002/14651858.CD002945.pub2 [DOI] [PubMed] [Google Scholar]
- 11.Guirguis-Blake JM, Beil TL, Senger CA, Coppola EL. Primary care screening for abdominal aortic aneurysm: updated evidence report and systematic review for the US preventive services task force. JAMA. 2019;322(22):2219–2238. doi: 10.1001/jama.2019.17021 [DOI] [PubMed] [Google Scholar]
- 12.Ali MU, Fitzpatrick-Lewis D, Miller J, et al. Screening for abdominal aortic aneurysm in asymptomatic adults. J Vasc Surg. 2016;64(6):1855–1868. doi: 10.1016/j.jvs.2016.05.101 [DOI] [PubMed] [Google Scholar]
- 13.Ali MU, Fitzpatrick-Lewis D, Kenny M, Miller J, Raina P, Sherifali D. A systematic review of short-term vs long-term effectiveness of one-time abdominal aortic aneurysm screening in men with ultrasound. J Vasc Surg. 2018;68(2):612–623. doi: 10.1016/j.jvs.2018.03.411 [DOI] [PubMed] [Google Scholar]
- 14.Takagi H, Goto S, Matsui M, Manabe H, Umemoto T. A further meta-analysis of population-based screening for abdominal aortic aneurysm. J Vasc Surg. 2010;52(4):1103–1108. doi: 10.1016/j.jvs.2010.02.283 [DOI] [PubMed] [Google Scholar]
- 15.Thompson SG, Ashton HA, Gao L, Buxton MJ, Scott RAP. Final follow-up of the Multicentre Aneurysm Screening Study (MASS) randomized trial of abdominal aortic aneurysm screening. Br J Surg. 2012;99(12):1649–1656. doi: 10.1002/bjs.8897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis M, Harris M, Earnshaw JJ. Implementation of the national health service abdominal aortic aneurysm screening program in England. J Vasc Surg. 2013;57(5):1440–1445. doi: 10.1016/j.jvs.2012.10.114 [DOI] [PubMed] [Google Scholar]
- 17.Chun KC, Dolan KJ, Smothers HC, et al. The 10-year outcomes of a regional abdominal aortic aneurysm screening program. J Vasc Surg. 2019;70(4):1123–1129. doi: 10.1016/j.jvs.2019.01.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent KC, Zwolak RM, Egorova NN, et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52(3):539–548. doi: 10.1016/j.jvs.2010.05.090 [DOI] [PubMed] [Google Scholar]
- 19.Lederle FA, Johnson GR, Wilson SE, et al. Prevalence and associations of abdominal aortic aneurysm detected through screening. Aneurysm Detection and Management (ADAM) veterans affairs cooperative study group. Ann Intern Med. 1997;126(6):441–449. doi: 10.7326/0003-4819-126-6-199703150-00004 [DOI] [PubMed] [Google Scholar]
- 20.Newman AB, Arnold AM, Burke GL, O’Leary DH, Manolio TA. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Ann Intern Med. 2001;134(3):182–190. doi: 10.7326/0003-4819-134-3-200102060-00008 [DOI] [PubMed] [Google Scholar]
- 21.Collin J, Araujo L, Walton J, Lindsell D. Oxford screening programme for abdominal aortic aneurysm in men aged 65 to 74 years. Lancet Lond Engl. 1988;2(8611):613–615. doi: 10.1016/s0140-6736(88)90649-6 [DOI] [PubMed] [Google Scholar]
- 22.Chichester Aneurysm Screening Group, Viborg Aneurysm Screening Study, Western Australian Abdominal Aortic Aneurysm Program. A comparative study of the prevalence of abdominal aortic aneurysms in the United Kingdom, Denmark, and Australia. J Med Screen. 2001;8(1):46–50. doi: 10.1136/jms.8.1.46. [DOI] [PubMed] [Google Scholar]
- 23.Oliver-Williams C, Sweeting MJ, Turton G, et al. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br J Surg. 2018;105(1):68–74. doi: 10.1002/bjs.10715 [DOI] [PubMed] [Google Scholar]
- 24.Krohn CD, Kullmann G, Kvernebo K, Rosén L, Kroese A. Ultrasonographic screening for abdominal aortic aneurysm. Eur J Surg Acta Chir. 1992;158(10):527–530. [PubMed] [Google Scholar]
- 25.Singh K, Bønaa KH, Jacobsen BK, Bjørk L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: the Tromsø Study. Am J Epidemiol. 2001;154(3):236–244. doi: 10.1093/aje/154.3.236 [DOI] [PubMed] [Google Scholar]
- 26.Forsdahl SH, Singh K, Solberg S, Jacobsen BK. Risk factors for abdominal aortic aneurysms: a 7-year prospective study: the Tromsø Study, 1994–2001. Circulation. 2009;119(16):2202–2208. doi: 10.1161/CIRCULATIONAHA.108.817619 [DOI] [PubMed] [Google Scholar]
- 27.Guirguis-Blake JM, Beil TL, Senger CA, Whitlock EP. Ultrasonography screening for abdominal aortic aneurysms: a systematic evidence review for the U.S. preventive services task force. Ann Intern Med. 2014;160(5):321–329. doi: 10.7326/M13-1844 [DOI] [PubMed] [Google Scholar]
- 28.Chaikof EL, Dalman RL, Eskandari MK, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. 2018;67(1):2e2–77.e2. doi: 10.1016/j.jvs.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 29.Watson JDB, Gifford SM, Bandyk DF. Aortic aneurysm screening using duplex ultrasound: choosing wisely who to examine. Semin Vasc Surg. 2020;33(3–4):54–59. doi: 10.1053/j.semvascsurg.2020.05.002 [DOI] [PubMed] [Google Scholar]
- 30.Wanhainen A, Verzini F, Van Herzeele I, et al. Editor’s choice - European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93. doi: 10.1016/j.ejvs.2018.09.020 [DOI] [PubMed] [Google Scholar]
- 31.Aboyans V, Ricco J-B, Bartelink M-LE-L, et al. Editor’s choice - 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2018;55(3):305–368. doi: 10.1016/j.ejvs.2017.07.018 [DOI] [PubMed] [Google Scholar]
- 32.Kleinbaum DG, Kupper LL,Morgenstern H. Epidemiologic Research: Principles and Quantitative Methods. John Wiley and Sons. 1982 [Google Scholar]
- 33.Scott RA, Ashton HA, Kay DN. Abdominal aortic aneurysm in 4237 screened patients: prevalence, development and management over 6 years. Br J Surg. 1991;78(9):1122–1125. doi: 10.1002/bjs.1800780929 [DOI] [PubMed] [Google Scholar]
- 34.Fleming C, Whitlock EP, Beil TL, Lederle FA. Screening for abdominal aortic aneurysm: a best-evidence systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2005;142(3):203–211. doi: 10.7326/0003-4819-142-3-200502010-00012 [DOI] [PubMed] [Google Scholar]
- 35.Ashton HA, Buxton MJ, Day NE, et al. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet Lond Engl. 2002;360(9345):1531–1539. doi: 10.1016/s0140-6736(02)11522-4 [DOI] [PubMed] [Google Scholar]
- 36.Lindholt JS, Juul S, Fasting H, Henneberg EW. Screening for abdominal aortic aneurysms: single centre randomised controlled trial. BMJ. 2005;330(7494):750. doi: 10.1136/bmj.38369.620162.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004;329(7477):1259. doi: 10.1136/bmj.38272.478438.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Svensjö S, Björck M, Wanhainen A. Editor’s choice: five-year outcomes in men screened for abdominal aortic aneurysm at 65 years of age: a population-based cohort study. Eur J Vasc Endovasc Surg. 2014;47(1):37–44. doi: 10.1016/j.ejvs.2013.10.007 [DOI] [PubMed] [Google Scholar]
- 39.Benson RA, Poole R, Murray S, Moxey P, Loftus IM. Screening results from a large United Kingdom abdominal aortic aneurysm screening center in the context of optimizing United Kingdom national Abdominal Aortic Aneurysm Screening Programme protocols. J Vasc Surg. 2016;63(2):301–304. doi: 10.1016/j.jvs.2015.08.091 [DOI] [PubMed] [Google Scholar]
- 40.Owens DK, Davidson KW, Krist AH, et al. Screening for abdominal aortic aneurysm: US preventive services task force recommendation statement. JAMA. 2019;322(22):2211–2218. doi: 10.1001/jama.2019.18928 [DOI] [PubMed] [Google Scholar]
- 41.Percentage daily smokers and occasional smokers, by sex and age (per cent) 1973–2020. Statistics Norway; October6, 2021. Available from:https://www.ssb.no/en/statbank/table/05307/. Accessed August11, 2021.
- 42.Cornuz J, Sidoti Pinto C, Tevaearai H, Egger M. Risk factors for asymptomatic abdominal aortic aneurysm: systematic review and meta-analysis of population-based screening studies. Eur J Public Health. 2004;14(4):343–349. doi: 10.1093/eurpub/14.4.343 [DOI] [PubMed] [Google Scholar]
- 43.Kobeissi E, Hibino M, Pan H, Aune D. Blood pressure, hypertension and the risk of abdominal aortic aneurysms: a systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2019;34(6):547–555. doi: 10.1007/s10654-019-00510-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takagi H, Umemoto T. Association of hypertension with abdominal aortic aneurysm expansion. Ann Vasc Surg. 2017;39:74–89. doi: 10.1016/j.avsg.2016.04.019 [DOI] [PubMed] [Google Scholar]
- 45.Stackelberg O, Björck M, Sadr-Azodi O, Larsson SC, Orsini N, Wolk A. Obesity and abdominal aortic aneurysm. Br J Surg. 2013;100(3):360–366. doi: 10.1002/bjs.8983 [DOI] [PubMed] [Google Scholar]
- 46.Cronin O, Walker PJ, Golledge J. The association of obesity with abdominal aortic aneurysm presence and growth. Atherosclerosis. 2013;226(2):321–327. doi: 10.1016/j.atherosclerosis.2012.10.041 [DOI] [PubMed] [Google Scholar]
- 47.Aune D, Schlesinger S, Norat T, Riboli E. Diabetes mellitus and the risk of abdominal aortic aneurysm: a systematic review and meta-analysis of prospective studies. J Diabetes Complications. 2018;32(12):1169–1174. doi: 10.1016/j.jdiacomp.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 48.Takagi H, Umemoto T. Diabetes and abdominal aortic aneurysm growth. Angiology. 2016;67(6):513–525. doi: 10.1177/0003319715602414 [DOI] [PubMed] [Google Scholar]
- 49.Dattani N, Sayers RD, Bown MJ. Diabetes mellitus and abdominal aortic aneurysms: a review of the mechanisms underlying the negative relationship. Diab Vasc Dis Res. 2018;15(5):367–374. doi: 10.1177/1479164118780799 [DOI] [PubMed] [Google Scholar]
- 50.Golledge J, Moxon J, Pinchbeck J, et al. Association between metformin prescription and growth rates of abdominal aortic aneurysms. Br J Surg. 2017;104(11):1486–1493. doi: 10.1002/bjs.10587 [DOI] [PubMed] [Google Scholar]
- 51.Hjellestad ID, Søfteland E, Nilsen RM, Husebye ES, Jonung T. Abdominal aortic aneurysms–glycaemic status and mortality. J Diabetes Complications. 2016;30(3):438–443. doi: 10.1016/j.jdiacomp.2015.12.015 [DOI] [PubMed] [Google Scholar]
- 52.Takagi H, Umemoto T. Association of peripheral artery disease with abdominal aortic aneurysm growth. J Vasc Surg. 2016;64(2):506–513. doi: 10.1016/j.jvs.2016.01.059 [DOI] [PubMed] [Google Scholar]
- 53.Lin Y-T, Chen H-J, Chen P-C, Sung F-C. Increased risk of peripheral arterial disease in patients with abdominal aortic aneurysm: a retrospective cohort study (version 5). Angiology. 2019;70(1):41–46. doi: 10.1177/0003319718757615 [DOI] [PubMed] [Google Scholar]
- 54.Koshty A, Bork M, Böning A, Gündüz D, Pleger SP. Coronary artery disease as a relevant risk factor in screening of abdominal aortic ectasia and aneurysm. Thorac Cardiovasc Surg. 2021;69(1):57–62. doi: 10.1055/s-0038-1676336 [DOI] [PubMed] [Google Scholar]
- 55.Reutersberg B, Salvermoser M, Haller B, et al. Screening cardiovascular patients for aortic aneurysms (SCAN) - high prevalence of abdominal aortic aneurysms in coronary heart disease patients requiring intervention. VASA Z Gefasskrankheiten. 2020;49(5):375–381. doi: 10.1024/0301-1526/a000881 [DOI] [PubMed] [Google Scholar]
- 56.Hernesniemi JA, Vänni V, Hakala T. The prevalence of abdominal aortic aneurysm is consistently high among patients with coronary artery disease. J Vasc Surg. 2015;62(1):232–240.e3. doi: 10.1016/j.jvs.2015.02.037 [DOI] [PubMed] [Google Scholar]
- 57.Chabok M, Nicolaides A, Aslam M, et al. Risk factors associated with increased prevalence of abdominal aortic aneurysm in women. Br J Surg. 2016;103(9):1132–1138. doi: 10.1002/bjs.10179 [DOI] [PubMed] [Google Scholar]
- 58.van Lindert NHA, Bienfait HP, Gratama JWC, et al. Screening for aneurysm of the abdominal aorta: prevalence in patients with stroke or TIA. Eur J Neurol. 2009;16(5):602–607. doi: 10.1111/j.1468-1331.2009.02550.x [DOI] [PubMed] [Google Scholar]
- 59.Vänni V, Turtiainen J, Kaustio U, Toivanen J, Rusanen M, Hernesniemi J. Prospective ultrasound screening of men with cerebrovascular disease for abdominal aortic aneurysms. Scand J Surg. 2020. doi: 10.1177/1457496920917269 [DOI] [PubMed] [Google Scholar]