Supplemental Digital Content is available in the text.
Abstract
Background.
Neutrophil-induced tissue damage contributes to the rejection in xenotransplantation. Therefore, suppressing neutrophil function could be effective in suppressing xenogeneic rejection. In a previous study, we demonstrated that the ectopic expression of human cluster of differentiation 31 (CD31) on porcine endothelial cells (PEC) significantly suppressed neutrophil-mediated cytotoxicity through the homophilic binding of CD31. Cluster of differentiation 177 (CD177) was recently reported to be a high-affinity heterophilic binding partner for CD31 on endothelial cells. Thus, we hypothesized that human CD177 on PEC might induce a stronger suppression in neutrophil-mediated cytotoxicity compared with CD31. In this study, the inhibitory function of human CD177 on PEC in neutrophil-mediated cytotoxicity was investigated.
Methods.
PEC were transfected with a cloning plasmid containing cDNA inserts that encoded for hCD177 and hCD31 genes. Neutrophil-induced cytotoxicity was evaluated by flow cytometry after coculturing with PEC or PEC/CD177 in the presence of phorbol 12-myristate 13-acetate. To elucidate the mechanisms responsible for hCD177-induced suppression, the phosphorylation of src homology region 2 domain containing phosphatase 1 was measured by immunoblot analysis.
Results.
Human CD177 on PEC induced a significant reduction in neutrophil-induced cytotoxicity. In addition, CD177 on PEC induced a significant increase in the phosphorylation of src homology region 2 domain-containing phosphatase 1 in neutrophils and suppressed NETosis.
Conclusions.
These findings suggest that human CD177 suppresses neutrophil-mediated cytotoxicity through the inhibition of NETosis.
INTRODUCTION
More severe innate immune responses are induced in xenotransplantation compared with allogeneic transplants. Previous reports suggest that neutrophils also contribute to cellular xenogeneic rejection.1-4 The inhibition of neutrophil xenorejection is necessary for suppressing rejection in xenotransplantation, and it has been reported that neutrophils induce tissue damage under xenogeneic conditions in both antibody-dependent and antibody-independent manners.1,2 At least 3 main mechanisms have been reported to explain tissue damage caused by activated neutrophils in transplantation, as follows: (i) Tissue damage is caused by the generation of reactive oxygen species (ROS), such as NADPH oxidase5-9; (ii) The release of digestive enzymes such as metalloproteinase-9 (MMP9) and neutrophil elastase (NE)10,11; (iii) In the response to various inflammatory stimuli, neutrophils induce a unique cell death process termed “NETosis.” They release a meshwork with serine proteases and antibacterial peptides, and this structure is named the nuclear extracellular traps (NETs). NETs from neutrophils cause an extensive endothelial cell damage in response to xenogeneic porcine antigen.12–14 In addition, in a previous study, we reported that cluster of differentiation 31 (CD31)15 suppressed complement-independent neutrophil-mediated cytotoxicity.
Cluster of differentiation 177 (CD177) is a 58–64-kDa glycosylphosphatidylinositol-anchored glycoprotein, and its expression is restricted to the cell surface of a subpopulation of neutrophils, with the average CD177-positive subpopulation ranging from 45% to 70%.16,17 CD177 was recently reported to be a high-affinity heterophilic binding partner for CD31 on endothelial cells.16,18 CD31 is ubiquitously expressed on various immune cells and induces the development of inhibitory signals by homophilic binding to CD31.19–23 The heterophilic interaction between CD177 and CD31 is approximately 15 times stronger than CD31 homophilic interactions.16,18 We also previously reported that the ectopic expression of human CD31 on porcine endothelial cells (PEC) induced a significant suppression of neutrophil-mediated xenogeneic cytotoxicity via the homophilic binding of CD31 to neutrophils.15 Based on the aforementioned collective findings, we hypothesized that the ectopic expression of CD177 on PEC might induce a stronger suppression against neutrophil-mediated cytotoxicity compared with CD31. In this study, the suppressive effect of hCD177 in neutrophil-mediated cytotoxicity was evaluated and compared with that of hCD31.
In this study, we present a novel strategy for inhibiting neutrophil-mediated xenogeneic cytotoxicity.
MATERIALS AND METHODS
Ethical Approval
The study design of this study was approved by the ethics review board of Osaka University (Approval No. 18395 [T1]).
Cells and Reagents
Porcine endothelial cells were obtained as described previously.24 DMEM supplemented with 10% fetal bovine serum was used for culturing of PEC and PEC transfectants. The SYTOX Green used in the study was obtained from Invitrogen (Carlsbad, CA).
The Construction of Plasmids
The cDNA of human CD177 (Gene Bank AB237911.1) was synthesized (Japan Integrated DNA Technologies, Tokyo, Japan) using a codon modification that is expressed at high levels in mammals. The DNA sequence was then confirmed by the ABI 310 automated sequencer (Applied Biosystems, Waltham, MA). In the next step, the construct was cloned into the pCXN2L expression vector. Plasmids were individually transformed into Escherichia coli DH5α for amplification.
Experiments Related to Transfection
Plasmids (5 µg) were transfected to PEC using the Lipofectamine LTX reagent (Invitrogen).
The transfected cells were then cultured for 24 h in complete medium. The hCD177-positive clone was selected with 0.4 mg/mL G418 (Invitrogen) and then separated by the limiting dilution method.
Flow Cytometry
To check the expression of CD177 and CD31 in each transfectant, PEC/CD177 and PEC/CD31 were stained with fluorescein isothiocyanate-coupled anti-human CD177 (clone: MEM-166, Bio Legend, San Diego, CA) and fluorescein isothiocyanate-coupled antihuman CD31 (clone: WM59, Bio Legend) were used. Flow cytometry was conducted using FACS Verse (BD Biosciences, San Diego, CA).
Neutrophil Isolation
Human-multinucleated granulocytes were separated from peripheral blood using Polymorphprep (Alere Technologies AS, Oslo, Norway). The polynuclear granulocyte suspensions were dissolved in ACK lysing buffer (0.83% NH4Cl, 10 mmol/L HEPES-NaOH, pH 7.4) and incubated at 37°C for 3 min to remove erythrocytes. The isolated cells were labeled with phycoerythrin (PE)-labeled anti-CD66b, and a purity in excess of 95% was confirmed by flow cytometry.
Cytotoxicity Assay
PEC, PEC/hCD177, and PEC/hCD31 were plated in flat bottom gelatin-coated 24-well dishes at a concentration of 6 × 104/well. After culturing for 24 h, neutrophils from peripheral blood were applied to each well at a concentration of 3 × 105/well in the medium of 200 nmol/L phorbol 12-myristate 13-acetate (PMA). After a 2-h incubation, cells were obtained and were fluorescently labeled with allophycocyanin-conjugated anti-CD11b antibody (Bio Legend), Annexin V (Bio Legend), and 7-AAD (MBL Life science, Nagoya, Japan), CD11b-negative cells are gated as the target cells. The % cytotoxicity was calculated as (% Annexin V+ 7-AAD+ cells in CD11b+ cells) + (% Annexin V+ 7-AAD– cells in CD11b+ cells) + (% Annexin V– 7-AAD+ cells in CD11b+ cells). For the blocking assay, neutrophils were treated with 200 μg/mL of antihuman CD31 (clone: WM59, Bio Legend) or isotype mouse IgG1 (Santa Cruz, Dallas, TX) for 2 h at room temperature before adding to each well.
The Detection of NETosis by SYTOX Green
PEC or PEC/hCD177 were plated in gelatin-coated 24-well dishes at a concentration of 4 × 104 cells/well. After 24 h of incubation, 4 × 105 neutrophils were mixed into individual wells. After 2-h stimulation with 50 nmol/L PMA, the cells were collected by gentle pipetting, followed by staining with 50 nmol/L SYTOX Green and PE-labeled anti-human CD66 antibody. % NETosis was calculated as (the number of CD66b+ SYTOX Green+ cells/the number of CD66b+ neutrophils) × 100 (%).
Extraction of Proteins
One hundred sixty thousand PEC, PEC/hCD177, and PEC/hCD31 were plated in 6-well dishes. After a 24-h incubation, 1.6 × 106 neutrophils were mixed into the individual wells. After a 30 min incubation in the presence of 200 nmol/L PMA, cells were collected by gently pipetting. The cell pellet was homogenized in homogenous solution of lysis buffer (10% SDS/62 mmol/L Tris pH 6.8). The samples were homogenized by ultrasonication for 30 s at the setting 3 using a handy sonic UR-20P sonicator (TOMY SEIKO Co, LTD, Tokyo, Japan) to extract proteins.
Western Blotting and SDS-PAGE
After the denaturation at 95°C, samples were loaded into 10% SDS-PAGE gel. The membranes were stained with anti-phosphorylated src homology region 2 domain containing phosphatase 1 (SHP-1) rabbit mAb (Cell Signaling Technology Japan, Tokyo, Japan) for 60 min at room temperature. The blots were then incubated with horseradish peroxidase–coupled antirabbit IgG. The signals were processed with ImmunoStar Zeta (WAKO, Osaka, Japan) and visualized on a LAS4000 (GE Healthcare, Little Chalfont, GB). Antibodies against human GAPDH (ProteinTech, Tokyo, Japan) were used as a loading control antibody.
Statistical Analysis
All results are indicated as mean ± SEM. Significant differences were detected by a paired 2-tailed t test using Microsoft Excel (Microsoft Japan, Tokyo, Japan).
RESULTS
Successful Transfection of Human CD177 Into Swine Endothelial Cells
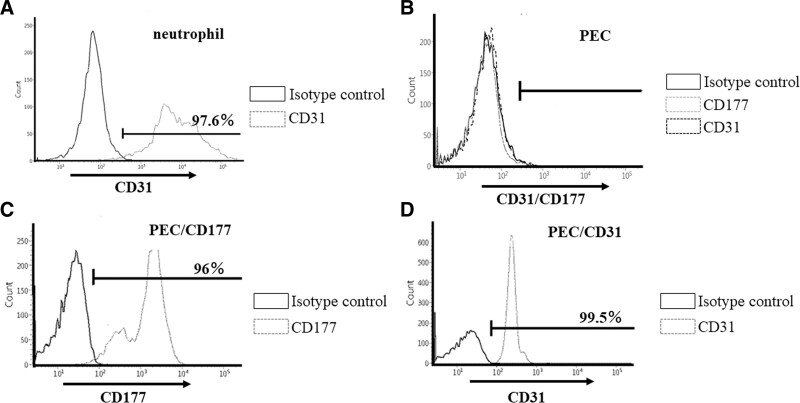
As an initial experiment, we investigated the expression of CD31, the receptor for CD177, by human neutrophils, and the findings indicated a significant expression of CD31 in these neutrophils (Figure 1A). To study the inhibitory effect of human CD177 against neutrophil-mediated xenogeneic rejection, we prepared a PEC transfectant (hCD177), and the efficiency of the transfection was confirmed by flow cytometry (Figure 1C and D).
FIGURE 1.
Successful transfection of human CD177 and CD31 in PEC. Expression of hCD31, the inhibitory receptor for CD177, in human neutrophils was confirmed by flow cytometry (A). To obtain PEC transfectants (PEC/hCD177 and PEC/hCD31), plasmids containing human CD177 and CD31 were transfected into PEC. The expression of human CD177 and CD31 in the obtained clones was evaluated by flow cytometry. The expression of human CD177 and CD31 in the obtained clones was evaluated by flow cytometry. More than 95% positive staining was observed in PEC/hCD177 (C) and PEC/hCD31 (D), and no expression was detected in naive PEC (B). CD177, cluster of differentiation 177; CD31, cluster of differentiation 31; PEC, porcine endothelial cells.
Human CD177 Inhibits Neutrophil-mediated Xenogeneic Cytotoxicity
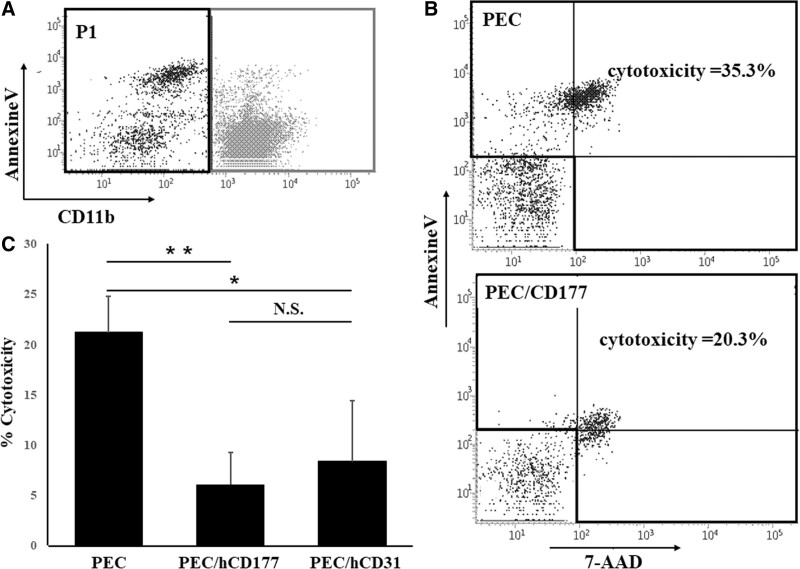
Using these transfectants, we examined the cytotoxic function mediated by peripheral blood-derived neutrophils. CD11b negative cells were gated as target cells (Figure 2A), and the cytotoxicity induced by neutrophils was quantitated by Annexin V and 7-AAD staining. The hCD177 and hCD31 on PEC significantly suppressed neutrophil-mediated cytotoxicity (Figure 2B). Furthermore, the significant inhibition of cytotoxicity by hCD177 was confirmed in neutrophils from different donors (Figure S1, SDC, http://links.lww.com/TXD/A340).
FIGURE 2.
hCD177-induced suppression of neutrophil-mediated cytotoxicity. Neutrophils were isolated, and the purity was checked by CD11b staining. PEC, PEC/hCD177, and PEC/hCD31 were plated at a concentration of 6 × 104 cells/well as target cells in a 24-well culture plate. After culturing for 24 h, PEC and PEC transfectants were cocultured with 3 × 105 neutrophils for 2 h in the presence of 200 nmol/L PMA, followed by triple-color staining with Annexin V-FITC, 7-AAD, and APC-conjugated anti-CD11b antibody. CD11b-negative cells were gated as the target cells (P1) (A). The % cytotoxicity was calculated as (% Annexin V+ 7-AAD+ cells) + (% Annexin V+ 7-AAD– cells) + (% Annexin V– 7-AAD+ cells) (B,C). The bars indicate the SEM, *P < 0.01, **P < 0.001, vs PEC. APC, allophycocyanin; FITC, fluorescein isothiocyanate; N.S., no significant difference, n = 7; PMA, phorbol 12-myristate 13-acetate; PEC, porcine endothelial cells.
Human CD177-induced Suppression in Neutrophil is Mediated by the Binding to CD31
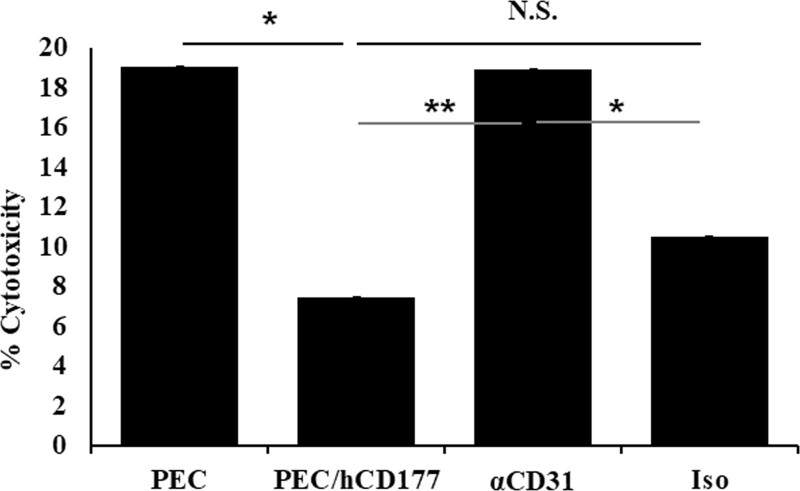
Next, we investigated the effect of anti-CD31 blocking antibody in hCD177-induced suppression. Although antihuman CD31 antibody does not affect neutrophil-induced PEC cytotoxicity (Figure S2, SDC, http://links.lww.com/TXD/A340), the neutrophils treated with anti-human CD31 caused significantly less inhibitory effect by hCD177 compared with untreated neutrophils (PEC/CD177) and neutrophils treated with isotype mouse IgG (Iso) (Figure 3), indicating that hCD177 suppresses neutrophil-mediated cytotoxicity via the heterophilic binding to hCD31.
FIGURE 3.
hCD177-induced suppression in neutrophil is mediated at least partially by the binding of CD177 to CD31. PEC and PEC/hCD177 were plated at a concentration of 6 × 104 cells/well as target cells in a 24 well gelatin-coated plate. After culturing for 24 h, 3 × 105 neutrophils were added to each well. Neutrophils were treated for 1 h with vehicle (PEC, PEC/hCD177) or 200 μg/mL of anti-human CD31 (α CD31) or isotype mouse IgG (Iso). Cells were cocultured for 2 h in the presence of 200 nmol/L PMA, followed by triple-color staining with Annexin V-FITC, 7-AAD, and APC-conjugated anti CD11b antibody. The % cytotoxicity was calculated as (% Annexin V+ 7-AAD+ cells) + (% Annexin V+ 7-AAD– cells) + (% Annexin V– 7-AAD+ cells). The bars indicate the SEM. *P < 0.05, **P < 0.005. APC, allophycocyanin; CD177, cluster of differentiation 177; CD31, cluster of differentiation 31; FITC, fluorescein isothiocyanate; N.S., no significant difference, n = 8; PEC, porcine endothelial cells; PMA, phorbol 12-myristate 13-acetate.
Human CD177 Suppresses NETosis
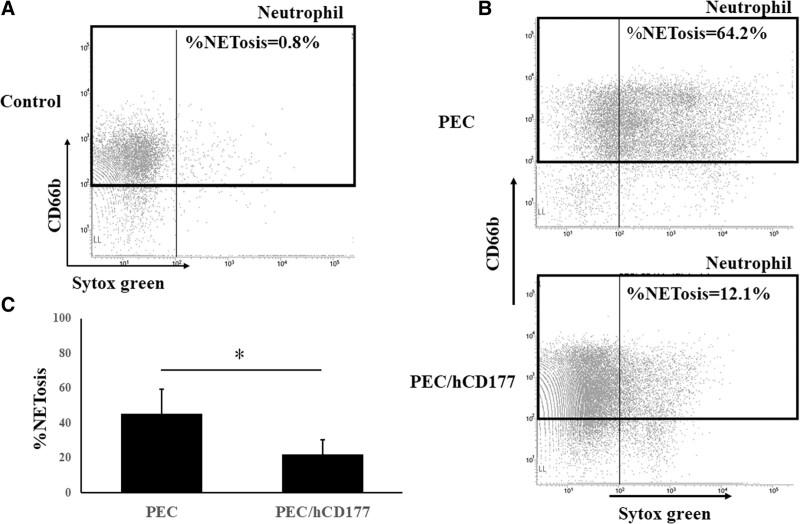
To assess the influence of human CD177 in NETosis, neutrophils were stained with SYTOX Green after a 2 h incubation with PEC or PEC/hCD177. Neutrophils that were incubated with PEC/hCD177 contained a lower percentage of SYTOX green+ cells compared with neutrophils cocultured with PEC. A significant reduction of % NETossis in cocultured neutrophils was induced by PEC/hCD177 (Figure 4A–C), indicating that human CD177 has the capacity to suppress the induction of NETossis in neutrophils.
FIGURE 4.
Suppression of NETosis by human CD177. PEC and PEC/hCD177 were cultured in 24 well gelatin-coated plates at a concentration of 1.6 × 105 cells/well. After 24 h culturing, PEC and PEC transfectants were cocultured with 3 × 105 neutrophils for 2 h in the presence of 50 nmol/L PMA. The cells were procured and stained with PE-labeled anti-CD66b and 50 nmol/L SYTOX green. Neutrophils were gated in CD66b positive, as shown in the black box. Representative data are demonstrated in (A, B), and a significantly decreased %NETosis was detected in the neutrophils that were cocultured with PEC/hCD177 (C). A significant decrease of SYTOX Green positive dead cells was detected in CD66b positive neutrophils that were cocultured with PEC/hCD177 compared with neutrophils with PEC. The bars indicate the SEM, *P < 0.05, vs PEC, n = 5. PE, phycoerythrin; PEC, porcine endothelial cells; PMA, phorbol 12-myristate 13-acetate.
Human CD177 Enhances the Phosphorylation of SHP-1 in Human Neutrophils
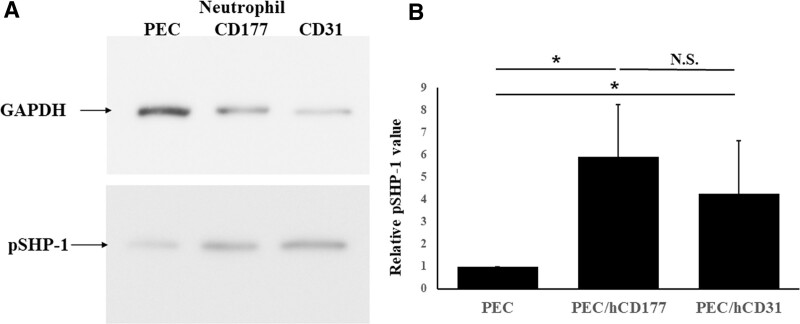
Because the inhibitory signals on granulocytes such as neutrophils indicate that the phosphorylation of ITIM causes the recruitment of SHP-1 to phosphorylated tyrosine residues within the motif, the degree of phosphorylation of SHP-1 was measured by immunoblotting. A significantly larger amount of phosphorylation of SHP-1 was detected in neutrophils that were cocultured with PEC/hCD177 and PEC/hCD31 compared with those that were cocultured with PEC (Figure 5A–B).
FIGURE 5.
SHP-1 phosphorylation induced by hCD177 and hCD31. PEC, PEC/hCD177, and PEC/hCD31 were seeded in gelatin-coated 6 well plates at a concentration of 1.6 × 105 cells/well and cocultured with neutrophils at a concentration of 1.6 × 106/well. Proteins were extracted from neutrophils after 30 min coculturing in the presence of 200 nmol/L PMA and loaded into 10% SDS-PAGE gel, followed by the transfer to PVDF membranes. The blot was stained with antiphosphorylated SHP-1 mAb and HRP-coupled secondary antibody. Representative data for 7 experiments are shown in (A). The extent of phosphorylation of SHP-1 by hCD177 and hCD31 was quantitatively evaluated using the Image J software program. All results of relative phosphorylated SHP-1 values are shown as the mean ± SEM (B). *P < 0.05. HRP, horseradish peroxidase; N.S., no significant difference, n = 7; PEC, porcine endothelial cells; PMA, phorbol 12-myristate 13-acetate; PVDF, polyvinylidene difluoride; SHP-1, src homology region 2 domain-containing phosphatase 1.
DISCUSSION
CD31 is a member of the Ig-ITIM family, and the homophilic ligation of CD31 leads to the phosphorylation of SHP-1 and SHP-2. CD177 has been reported to be activated on the surface of neutrophils after a severe bacterial infection or by stimulation, such as treatment with the granulocyte colony-stimulating factor.19 The binding ability of CD177 to CD31 is approximately 15 times stronger than the homophilic binding of CD31.5,18 Heterophilic interactions between hCD177 and CD31 play an important role in the inflammatory cascade in neutrophil-induced xenogeneic rejection. It is, therefore, possible that hCD177 on swine cells could bind to CD31 on human neutrophils, suggesting that this heterophilic ligation may exert a superior immunosuppressive effect in neutrophil-mediated rejection compared with hCD31 on swine cells. In this study, the neutrophil-mediated cytotoxicity was significantly suppressed by hCD177 on porcine cell. Furthermore, antihuman CD31 significantly reduced CD177-induced suppression in neutrophil-mediated cytotoxicity, indicating that the heterophilic binding of hCD177 to CD31 on neutrophil contributes to the suppressive effect of hCD177 in neutrophil-mediated cytotoxicity at least partially. The heterophilic binding of hCD177 to hCD31 on neutrophils was also reported to induce the phosphorylation of human CD31- and human CD177-dependent SHP-1, which was shown in a previous study to suppress NETosis.25–29
Although a better suppression by hCD177 was expected, the suppression of neutrophil-mediated cytotoxicity by human CD177 was on the same level with hCD31 (Figures 2 and 4). We assume that other mechanisms from NETosis, such as protease 3 (PR3), may also be associated with neutrophil-mediated cytotoxicity. It was recently reported that PR3 is expressed on CD177+ neutrophils.30 CD177 and PR3 are colonized on the neutrophil membrane, and PR3 is a ligand for CD177.31–33 The interaction between CD31 and CD177 may affect the anchoring of mPR3 to the neutrophil membrane.31 Therefore, we guessed that hCD31 on PEC must disturb the anchoring of mPR3 to CD177 on neutrophils and free PR3 released from neutrophils may influence the cytotoxicity. We also evaluated the release of PR3 from neutrophil by ELISA (Figure S3, SDC, http://links.lww.com/TXD/A340). However, hCD31 and hCD177 did not affect the PR3 release from neutrophils. Because PMA induced significant PR3 release from neutrophils, almost all PR3 may be released during NETosis regardless of hCD177 and hCD31 on porcine cells. These findings indicate that PR3 release from neutrophil may contribute to the neutrophil-mediated cytotoxicity but may have no relevance to the inhibitory effect of hCD31 and hCD177.
Sachs et al5 have reported the downregulation of CD31 expression in CD177-transfected U937 cells. In this study, porcine CD31 expression on PEC was not checked because antihuman CD31 antibody does not bind to porcine CD31. However, even if porcine CD31 expression is downregulated in PEC/hCD177, this may not affect the suppressive ability by hCD177 because of the incompatibility between human and porcine CD31.34 The expression of porcine CD31 expression should be investigated in the future study.
In a xenogeneic rejection situation, tissue damage is caused by neutrophils that are activated by ROS and NETs. In contrast, under stable conditions, ROS induce neutrophil apoptosis primarily through the action of cathepsin D, which promotes the activation of caspase 8.35–37 The end of inflammation is due to the efficient removal of neutrophils from the inflamed tissues by apoptosis of the neutrophils themselves.38 The apoptosis of neutrophils itself not only ends effector activity but also induces the generation of anti-inflammatory signals that are transmitted to other immune cells. Macrophages, which phagocytosed the apoptotic neutrophils, also induce anti-inflammatory signals in various immune cells. However, in this study, no significant differences in CD11b+ Annexin V+ apoptotic neutrophils between coculture with PEC/hCD31 and PEC/hCD177 were found (data not shown). A vivo study of the function of hCD177 is planned for the near future. In summary, our findings demonstrated that hCD177 inhibits neutrophil-mediated cytotoxicity by suppressing NETosis. Our findings in this study suggest that the generation of hCD177 transgenic pigs would be a very attractive strategy in terms of preventing xenotransplant rejection.
ACKNOWLEDGMENTS
The authors wish to thank Dr Milton S. Feather for his editing of the article.
Supplementary Material
Footnotes
Published online 23 July, 2021.
T.Y. participated in the performance of research, the writing of the article, and data analysis; A.M. participated in the performance of research, the writing of the article, data analysis, and study design. S.K., C.T., P.L., K.M., T.H., C.O., H.E., Y.T., and T.U. participated in data analysis. H.O. and S.M. participated in study design.
The authors declare no conflict of interest.
This work was supported by Grants-in Aid for Scientific Research, Japan (grant T19K090920).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.al-Mohanna FA, Collison KS, Allen SP, et al. Naive neutrophils and xenotransplantation. Lancet. 1996;348:1246. [DOI] [PubMed] [Google Scholar]
- 2.al-Mohanna F, Collison K, Parhar R, et al. Activation of naive xenogeneic but not allogeneic endothelial cells by human naive neutrophils: a potential occult barrier to xenotransplantation. Am J Pathol. 1997;151:111–120. [PMC free article] [PubMed] [Google Scholar]
- 3.Kim YT, Lee HJ, Lee SW, et al. Pre-treatment of porcine pulmonary xenograft with desmopressin: a novel strategy to attenuate platelet activation and systemic intravascular coagulation in an ex-vivo model of swine-to-human pulmonary xenotransplantation. Xenotransplantation. 2008;15:27–35. [DOI] [PubMed] [Google Scholar]
- 4.Harris DG, Quinn KJ, Dahi S, et al. Lung xenotransplantation: recent progress and current status. Xenotransplantation. 2014;21:496–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schofield ZV, Woodruff TM, Halai R, et al. Neutrophils–a key component of ischemia-reperfusion injury. Shock. 2013;40:463–470. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari RS, Andrade CF. Oxidative stress and lung ischemia-reperfusion injury. Oxid Med Cell Longev. 2015;2015:590987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loukogeorgakis SP, van den Berg MJ, Sofat R, et al. Role of NADPH oxidase in endothelial ischemia/reperfusion injury in humans. Circulation. 2010;121:2310–2316. [DOI] [PubMed] [Google Scholar]
- 8.Duilio C, Ambrosio G, Kuppusamy P, et al. Neutrophils are primary source of O2 radicals during reperfusion after prolonged myocardial ischemia. Am J Physiol Heart Circ Physiol. 2001;280:H2649–H2657. [DOI] [PubMed] [Google Scholar]
- 9.Kimura K, Shirabe K, Yoshizumi T, et al. Ischemia-reperfusion injury in fatty liver is mediated by activated NADPH oxidase 2 in rats. Transplantation. 2016;100:791–800. [DOI] [PubMed] [Google Scholar]
- 10.Uchida Y, Freitas MC, Zhao D, et al. The protective function of neutrophil elastase inhibitor in liver ischemia/reperfusion injury. Transplantation. 2010;89:1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hardison MT, Galin FS, Calderon CE, et al. An active role for matrix degradation in airway inflammation seen during lung transplantation allograft rejection. J Immunol. 2009;182:4423–4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu FC, Chuang YH, Tsai YF, et al. Role of neutrophil extracellular traps following injury. Shock. 2014;41:491–498. [DOI] [PubMed] [Google Scholar]
- 13.Sayah DM, Mallavia B, Liu F, et al. ; Lung Transplant Outcomes Group Investigators. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med. 2015;191:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang H, Tohme S, Al-Khafaji AB, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology. 2015;62:600–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HT, Maeda A, Sakai R, et al. Human CD31 on porcine cells suppress xenogeneic neutrophil-mediated cytotoxicity via the inhibition of NETosis. Xenotransplantation. 2018;25:e12396. [DOI] [PubMed] [Google Scholar]
- 16.Sachs UJ, Andrei-Selmer CL, Maniar A, et al. The neutrophil-specific antigen CD177 is a counter-receptor for platelet endothelial cell adhesion molecule-1 (CD31). J Biol Chem. 2007;282:23603–23612. [DOI] [PubMed] [Google Scholar]
- 17.Matsuo K, Lin A, Procter JL, et al. Variations in the expression of granulocyte antigen NB1. Transfusion. 2000;40:654–662. [DOI] [PubMed] [Google Scholar]
- 18.Newton JP, Buckley CD, Jones EY, et al. Residues on both faces of the first immunoglobulin fold contribute to homophilic binding sites of PECAM-1/CD31. J Biol Chem. 1997;272:20555–20563. [DOI] [PubMed] [Google Scholar]
- 19.Göhring K, Wolff J, Doppl W, et al. Neutrophil CD177 (NB1 gp, HNA-2a) expression is increased in severe bacterial infections and polycythaemia vera. Br J Haematol. 2004;126:252–254. [DOI] [PubMed] [Google Scholar]
- 20.Jackson DE, Kupcho KR, Newman PJ. Characterization of phosphotyrosine binding motifs in the cytoplasmic domain of platelet/endothelial cell adhesion molecule-1 (PECAM-1) that are required for the cellular association and activation of the protein-tyrosine phosphatase, SHP-2. J Biol Chem. 1997;272:24868–24875. [DOI] [PubMed] [Google Scholar]
- 21.Henshall TL, Jones KL, Wilkinson R, et al. Src homology 2 domain-containing protein-tyrosine phosphatases, SHP-1 and SHP-2, are required for platelet endothelial cell adhesion molecule-1/CD31-mediated inhibitory signaling. J Immunol. 2001;166:3098–3106. [DOI] [PubMed] [Google Scholar]
- 22.Hua CT, Gamble JR, Vadas MA, et al. Recruitment and activation of SHP-1 protein-tyrosine phosphatase by human platelet endothelial cell adhesion molecule-1 (PECAM-1). Identification of immunoreceptor tyrosine-based inhibitory motif-like binding motifs and substrates. J Biol Chem. 1998;273:28332–28340. [DOI] [PubMed] [Google Scholar]
- 23.Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010;87:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyagawa S, Shirakura R, Iwata K, et al. Effects of transfected complement regulatory proteins, MCP, DAF, and MCP/DAE hybrid, on complement-mediated swine endothelial cell lysis. Transplantation. 1994;58:834–840. [PubMed] [Google Scholar]
- 25.Van Avondt K, van der Linden M, Naccache PH, et al. Signal inhibitory receptor on Leukocytes-1 limits the formation of neutrophil extracellular traps, but preserves intracellular bacterial killing. J Immunol. 2016;196:3686–3694. [DOI] [PubMed] [Google Scholar]
- 26.Van Avondt K, Fritsch-Stork R, Derksen RH, et al. Ligation of signal inhibitory receptor on leukocytes-1 suppresses the release of neutrophil extracellular traps in systemic lupus erythematosus. PLoS One. 2013;8:e78459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steevels TA, van Avondt K, Westerlaken GH, et al. Signal inhibitory receptor on leukocytes-1 (SIRL-1) negatively regulates the oxidative burst in human phagocytes. Eur J Immunol. 2013;43:1297–1308. [DOI] [PubMed] [Google Scholar]
- 28.Secundino I, Lizcano A, Roupé KM, et al. Host and pathogen hyaluronan signal through human siglec-9 to suppress neutrophil activation. J Mol Med (Berl). 2016;94:219–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lizcano A, Secundino I, Döhrmann S, et al. Erythrocyte sialoglycoproteins engage Siglec-9 on neutrophils to suppress activation. Blood. 2017;129:3100–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauer S, Abdgawad M, Gunnarsson L, et al. Proteinase 3 and CD177 are expressed on the plasma membrane of the same subset of neutrophils. J Leukoc Biol. 2007;81:458–464. [DOI] [PubMed] [Google Scholar]
- 31.Deng H, Hu N, Wang C, et al. Interaction between CD177 and platelet endothelial cell adhesion molecule-1 downregulates membrane-bound proteinase-3 (PR3) expression on neutrophils and attenuates neutrophil activation induced by PR3-ANCA. Arthritis Res Ther. 2018;20:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Vietinghoff S, Tunnemann G, Eulenberg C, et al. NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils. Blood. 2007;109:4487–4493. [DOI] [PubMed] [Google Scholar]
- 33.Jerke U, Marino SF, Daumke O, et al. Characterization of the CD177 interaction with the ANCA antigen proteinase 3. Sci Rep. 2017;7:43328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasu K, Whyte A, Green SJ, et al. Alpha-galactosyl-mediated activation of porcine endothelial cells: studies on CD31 and VE-cadherin in adhesion and signaling. Transplantation. 1999;68:861–867. [DOI] [PubMed] [Google Scholar]
- 35.Yao W, Han X, Guan Y, et al. Neutrophil elastase inhibitors suppress oxidative stress in lung during liver transplantation. Oxid Med Cell Longev. 2019;2019:7323986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blomgran R, Zheng L, Stendahl O. Cathepsin-cleaved Bid promotes apoptosis in human neutrophils via oxidative stress-induced lysosomal membrane permeabilization. J Leukoc Biol. 2007;81:1213–1223. [DOI] [PubMed] [Google Scholar]
- 37.Conus S, Perozzo R, Reinheckel T, et al. Caspase-8 is activated by cathepsin D initiating neutrophil apoptosis during the resolution of inflammation. J Exp Med. 2008;205:685–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ortega-Gómez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO Mol Med. 2013;5:661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.