Abstract
Purpose
Tracheostomy is an aerosol-generating procedure, thus performing it during the COVID-19 pandemic arises considerations such as the most appropriate timing and the patients to whom it is suitable. Medical teams lack sufficient data to assist determining whether or not to conduct tracheostomy, its short- and long-term implications are not fully understood. This study aims to shed light on the critically ill COVID-19 patients that require tracheostomy, and to investigate its value.
Methods
A retrospective multicentral case-control study of 157 hospitalized critically ill COVID-19 patients, among whom 30 patients went through tracheostomy and consisted of our study group.
Results
The mean age was similar between study and control groups (68.9 ± 12.7 years vs 70.5 ± 15.8 years, p = 0.57), as well as comorbidity prevalence (56.7% vs 67.7%, p = 0.25). Patients in the study group were hospitalized for longer duration until defined critically ill (5 ± 4.3 vs 3 ± 3.9 days; p = 0.01), until admitted to the intensive care unit (6 ± 6.6 vs 2.5 ± 3.7 days respectively; p = 0.005), and until discharged (24 ± 9.7 vs 10.7 ± 9.1 days, p < 0.001). Mortality rate was lower in the study group (30% vs 59.8%, p = 0.003). Kaplan Meier survival analysis revealed a statistically significant difference in survival time between groups (Log rank chi-sq = 20.91, p < 0.001) with mean survival time of 41 ± 3.1 days vs 21 ± 2.2 days. Survival was significantly longer in the study group (OR = 0.37, p = 0.004).
Conclusion
Tracheostomy allows for more prolonged survival for gradually deteriorating critically ill COVID-19 patients. This should be integrated into the medical teams' considerations when debating whether or not to conduct tracheostomy.
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; BP, blood pressure; COVID-19, Coronavirus disease 2019; CPAP, continuous positive airway pressure; COPD, chronic obstructive pulmonary disease; ICU, intensive care unit; PEEP, positive end-expiratory pressure; RT-PCR, reverse transcriptase–polymerase chain reaction; SARS-Cov-2, severe acute respiratory syndrome due to coronavirus 2; TPN, total parenteral nutrition
Keywords: Tracheostomy, COVID19, SARS-Cov-2, Mechanical ventilation
1. Introduction
By December 2019, the pandemic of Coronavirus disease 2019 (COVID-19) emerged in China and rapidly spread overseas. The symptoms of COVID-19 patients resemble viral pneumonia, 19% of patients are classified as severe-critical [1], 10–15% of patients require invasive mechanical ventilation due to acute respiratory distress [2], [3], [4], [5], [6]. Tracheostomy reduces the length of mechanical ventilation in COVID-19 negative patients and is usually recommended 7–10 days after the beginning of invasive ventilation [7], [8]. Current reports show that the mean ventilation period of COVID-19 patients is 10–14 days, therefore tracheostomy may be of value [2], [4], [5], [6].
Performing tracheostomy during COVID-19 pandemic arises several considerations, such as the most appropriate timing for performing the procedure, its short and long term advantages, and the proper tracheostomy technique [9]. Moreover, given the high infectivity of COVID-19, mainly during aerosol-generating procedures, healthcare teams attempt to perform tracheostomy only on certain patient populations and in specific settings [9], [10], [11], [12].
Patient selection is based on ventilation parameters, length of intubation, risks and benefits for the individualized patient and risks of the health-care workers [12], [13], [14], [15].
Current literature sparse sufficient data to assist clinicians decision making in the era of tracheostomy for COVID-19 patients. The optimal timing for tracheostomy is being under debate and was suggested between 7 and over 14 days of intubation, various aerosol depleting techniques had been suggested, and the long term advantages of tracheostomy in COVID-19 patients are yet to be discovered [6], [9], [10], [11], [16], [17].
This multicentral study aims to shed light on the critically ill COVID-19 patients that required tracheostomy, and to compare outcomes with COVID-19 patients who did not go through tracheostomy, in order to investigate the value of tracheostomy on COVID-19 patients.
2. Materials and methods
This was a retrospective case-control study of critically ill patients with laboratory-confirmed severe acute respiratory syndrome due to coronavirus 2 (SARS-Cov-2) infection, among 14 participating Israeli hospitals between March 5th and May 25th, 2020. During the COVID-19 pandemic, The Israel Ministry of Health, Jerusalem, Israel, waived the need for individual institutional ethics board and informed consent from COVID-19 patients in retrospective studies in light of the urgent need to collect data.
SARS-Cov-2 infection was defined as a positive result of real-time reverse transcriptase–polymerase chain reaction (RT-PCR) assay of nasal and pharyngeal swabs. Only laboratory-confirmed cases were included in the analysis. We extracted patients' and treatment data from electronic medical records, and recorded it on an online questionnaire-based electronic worksheet (SurveyMonkey) accessible online to registry associates.
Patients were defined critically ill by one or more of the following conditions: acute respiratory distress syndrome (ARDS; 200 mmHg < PaO2/FiO2a ≤ 300 mmHg (with positive end-expiratory pressure (PEEP) or continuous positive airway pressure (CPAP) ≥ 5 cmH2O, or non-ventilated; if PaO2 is not available, SpO2/FiO2 ≤ 315 suggests ARDS), sepsis (life-threatening organ dysfunction caused by a dysregulated host response to suspected or proven infection; sepsis-related SOFA score of ≥2 points) or acute organ dysfunction respiratory (hypoxemia defined by low PaO2/FiO2); coagulation (low platelets); liver (high bilirubin); cardiovascular (hypotension); central nervous system (low level of consciousness defined by Glasgow Coma Scale); and renal (low urine output or high creatinine).
We obtained data regarding demographics, medical comorbidities, medication history, body mass index (BMI), vital signs, chest X-ray and labs on admission to the intensive care unit (ICU), anti-COVID19 pharmacological therapy, methods of nutrition, time from admission to tracheostomy, ICU length of stay, complications and outcome (death, discharge, transferred to a lower level of care) as of May 25th, 2020.
2.1. Statistical analysis
Data were recorded on an Excel spreadsheet. Data analysis was performed using SPSS version 23 statistical software. Descriptive statistics are presented using prevalence and percentage values for categorical variables, averages and standard deviations for continuous variables. Statistical analysis was performed using a significance level of p = 0.05.
In order to compare the demographic characteristics, co-morbidities, vital signs upon admission and ICU interventions between the two groups, t-tests or the Mann-Whitney test, in the case of non-normally distributed data were performed for continuous data and chi-square or Fisher's exact test were performed for the categorical data. Kaplan Meier survival analysis was performed to assess mortality between the two groups. In order to adjust mortality for group differences, Stepwise Cox regression analysis was performed forcing gender into the model and using significant vital sign differences, time until defined critically ill and ICU-interventions as potential covariates. Stepwise logistic regression was performed to assess the ARDS secondary outcome. Using ROC analysis and Youden index we investigated a potential cut-off values for differentiating factors as presented by Fluss et al. [18].
3. Results
As of May 25th, 2020, Israel had 16,734 confirmed COVID-19 cases, among whom 544 patients were defined critically ill [19]. The participating hospitals treated 157 critically ill patients, and conducted 108 mechanical ventilations. Thirty patients required tracheostomy and consisted of our study group, while 127 patients did not go through tracheostomy and consisted of our control group.
Male gender was more prevalent in the study group (86.7% vs 64.6%, p = 0.027), with a mean age of 68.9 ± 12.7 years in the study group compared with 70.5 ± 15.8 years in the control group (p = 0.57). The study group contained a higher prevalence of patients born in western countries (Western Europe, North America and Israel) compared with the control group (70.8% vs 44.9% respectively, p = 0.024).
There was no significant variance in comorbidity prevalence between groups (56.7% study vs 67.7% control, p = 0.25). Both study and control groups did not have high prevalence of smokers, past smokers, or patients with Chronic Obstructive Pulmonary Disease (COPD) (0% vs 1.6% smokers, p > 0.99; 10% vs 6.3% past smokers, p = 0.44; 0% vs 9.4% patients with COPD, p = 0.13 respectively), Table 1 presents all the specific investigated comorbidities. Table 2 presents the patient's medication history.
Table 1.
Comorbidities of the study and control groups.
| Tracheostomy no. (%) | Non tracheostomy no. (%) | p | |
|---|---|---|---|
| Smoker | 0 (0) | 2 (1.6) | >0.99 |
| Past smoker | 3 (10) | 8 (6.3) | 0.44 |
| COPD | 0 (0) | 13 (9.4) | 0.13 |
| Hypertension | 16 (53.3) | 72 (56.7) | 0.74 |
| Diabetes | 10 (33.3) | 53 (41.7) | 0.40 |
| IHD | 10 (33.3) | 24 (18.9) | 0.08 |
| CHF | 2 (6.7) | 16 (12.6) | 0.53 |
| Active malignancy | 0 (0) | 8 (6.3) | 0.36 |
| Past malignancy | 3 (10) | 10 (7.9) | 0.72 |
| Chronic renal failure | 3 (10) | 21 (16.5) | 0.57 |
| Dementia | 2 (6.7) | 21 (16.5) | 0.25 |
| Immunodefficiency | 1 (3.3) | 5 (3.9) | >0.99 |
| Chronic liver disease (cirhossis, hepatitis, hepatic transplantation) | 0 (0) | 1 (1.6) | >0.99 |
| Dialysis | 0 (0) | 1 (0.8) | >0.99 |
| Overweight (BMI > 25) | 11 (73.3) | 26 (52) | 0.23 |
Abbreviations: COPD- chronic obstructive pulmonary disease; IHD- ischemic heart disease; CHF- congestive heart failure; BMI- body mass index.
Table 2.
Medications of the study and control groups.
| Tracheostomy no. (%) | Non tracheostomy no. (%) | p | |
|---|---|---|---|
| Aspirin | 11 (36.7) | 38 (30%) | 0.47 |
| Beta blockers | 11(36.7) | 39 (30.7) | 0.53 |
| Calcium channel blocker | 7 (23.3) | 18 (14.2) | 0.22 |
| ACE inhibitors | 5 (16.7) | 33 (26) | 0.28 |
| Diuretics | 2 (6.7) | 24 (18.9) | 0.17 |
| Steroids | 0 (0) | 8 (6.3) | 0.36 |
| Plavix | 4 (13.3) | 9 (7.1) | 0.28 |
| Coumadin | 0 (0) | 2 (1.6) | >0.99 |
| Inhalers | 1 (3.3) | 9 (7.1) | 0.69 |
| Immunosuppresors | 1 (3.3) | 2 (1.6) | 0.47 |
Abbreviations: ACE- angiotensin converting enzyme.
3.1. Hospitalization
Patients in the study group were hospitalized for longer duration until defined critically ill, compared with the control group (5 ± 4.3 days vs 3 ± 3.9 days respectively; p = 0.01) and until admitted to the intensive care unit (ICU) (6 ± 6.6 days vs 2.5 ± 3.7 days respectively; p = 0.005). Vital signs of the patients among entry to the ICU did not significantly differ between groups as presented in Table 3 . The only exception was diastolic blood pressure (BP), which was lower in the study group. The most prevalent finding on chest x-ray was pulmonary consolidation, with similar prevalence in the study and control group (96.7% vs 90.2% respectively, p = 0.26).
Table 3.
Vital signs upon admission to the ICU of the study and control groups.
| Tracheostomy Mean ± SD | Non tracheostomy Mean ± SD | p | |
|---|---|---|---|
| Heart rate (beats per minute) | 90 ± 17.5 | 88.8 ± 22.5 | 0.75 |
| Temperature (degrees Celsius) | 37.8 ± 0.99 | 37.5 ± 2.2 | 0.51 |
| Saturation | 88.5 ± 10.5 | 89.7 ± 11.3 | 0.6 |
| Respiratory rate (per minute) | 28.4 ± 9.1 | 23 ± 10.3 | 0.08 |
| Systolic BP | 135.4 ± 19.6 | 133.1 ± 25 | 0.66 |
| Diastolic BP | 64.9 ± 17.6 | 72.7 ± 13.6 | 0.01 |
Abbreviations- ICU- intensive care unit; BP- blood pressure.
3.2. Treatment
Table 4 presents the ICU interventions given to the patients. A higher rate of patients in the study group were treated with Anti IL6 (20% vs 3.9% respectively, p = 0.002), corticosteroids (36.7% vs 19.7% respectively, p = 0.047), vasopressors (76.7% vs 44.1% respectively, p < 0.001), Remdesivir (10% vs 1.6% respectively, p = 0.048) and Tocilizumab (26.7% vs 8.7% respectively, p = 0.007). The use of noninvasive ventilation prior to intubation-bilevel positive airway pressure (BiPAP) or continuous positive airway pressure (CPAP) was more prevalent among the study group (53.3% vs 23.6% respectively, p < 0.001), as well as feeding through nasogastric tube (73.3% vs 44.9% respectively, p = 0.005) and total parenteral nutrition (TPN) (30% vs 6.3% respectively, p < 0.001). The median time to from ICU admission to performing tracheostomy was 13 days (range, 5–32 days).
Table 4.
ICU interventions given to the study and control groups.
| Tracheostomy no. (%) | Non tracheostomy no. (%) | p | |
|---|---|---|---|
| Antibiotics | 28 (93.3) | 104 (81.9) | 0.17 |
| Antifibrinolytics | 2 (6.7) | 2 (1.6) | 0.17 |
| Anti-IL6 | 6 (20) | 5 (3.9) | 0.002 |
| Chloroquine | 9 (30) | 27 (21.3) | 0.31 |
| Corticosteroids | 11 (36.7) | 25 (19.7) | 0.047 |
| CPAP/BiPAP | 16 (53.3) | 30 (23.6) | 0.001 |
| ECMO | 0 (0) | 5 (3.9) | 0.58 |
| Hydroxychloroquine | 18 (60) | 73 (57.5) | 0.8 |
| Interferon beta | 1 (3.3) | 0 (0) | 0.19 |
| IVIG | 1 (3.3) | 6 (4.7) | >0.99 |
| Lopinavir/ritonavir | 5 (16.7) | 17 (13.4) | 0.64 |
| Nasogastric tube | 22 (73.3) | 57 (44.9) | 0.005 |
| Oxygen support before intubation | 27 (90) | 100 (78.7) | 0.2 |
| Remdesivir | 3 (10) | 2 (1.6) | 0.048 |
| Renal replacement therapy | 5 (16.7) | 9 (7.1) | 0.1 |
| Rezolsta | 3 (10) | 4 (3.1) | 0.13 |
| Tocilizumab | 8 (26.7) | 11 (8.7) | 0.007 |
| TPN | 9 (30) | 8 (6.3) | <0.001 |
| Vasopressors | 23 (76.7) | 56 (44.1) | 0.001 |
Abbreviations: ICU- intensive care unit; IL6- interleukin 6; IVIG- intravenous immunoglobulins; CPAP- continuous positive airway pressure; BiPAP- bilevel positive airway pressure; TPN- total parenteral nutrition; ECMO- extracorporeal membrane oxygenation;
3.3. Prognosis
As for complications, both ARDS and secondary infections were more prevalent in the study group (63.3% vs 40.2%, p = 0.02; 40% vs 15%, p = 0.002, respectively). All complications are summarized in Table 5 . The total duration of hospitalization was significantly longer in the study group (30.2 ± 11.7 days vs 13.2 ± 9.2 days respectively, p < 0.001), as well as the total duration of stay in the ICU (24 ± 9.7 days vs 10.7 ± 9.1 days respectively, p < 0.001). However, time to ventilation did not significantly differ between the study and control group (4.5 ± 3.2 days vs 5.7 ± 7.91 days respectively, p = 0.31).
Table 5.
Complications of the study and control groups during hospitalization.
| Tracheostomy n (%) | Non tracheostomy n (%) | p | |
|---|---|---|---|
| ARDS | 19 (63.3) | 51 (40.2) | 0.02 |
| Sepsis | 13 (43.3) | 45 (35.4) | 0.42 |
| Acute organ dysfunction | 19 (63.3) | 90 (70.9) | 0.42 |
| Respiratory failure | 26 (86.7) | 90 (70.9) | 0.11 |
| Heart failure | 1 (3.3) | 10 (7.9) | 0.69 |
| Septic shock | 5 (16.7) | 26 (20.5) | 0.64 |
| Coagulopathy | 2 (6.7) | 4 (3.1) | 0.32 |
| Acute kidney injury | 9 (30) | 30 (23.6) | 0.47 |
| Secondary infection | 12 (40) | 19 (15) | 0.002 |
| Death | 9 (30) | 76 (59.8) | 0.003 |
Abbreviations: ARDS- acute respiratory distress syndrome;
As for the end of the follow-up period, nine patients (30%) of the study group passed away, compared with 76 patients (59.8%) in the control group (p = 0.003). When investigating these patients, the dead patients in the study group were ventilated after a shorter duration from admission (3.6 ± 3.2 days vs 5.9 ± 8.6 days respectively, p = 0.45) and for a longer duration (22.7 ± 11.4 days vs 4.9 ± 6.6 days respectively, p = 0.002).
Among the 21 patients who went through tracheostomy and were alive at the end of the follow-up period, four patients (19.05%) were decannulated.
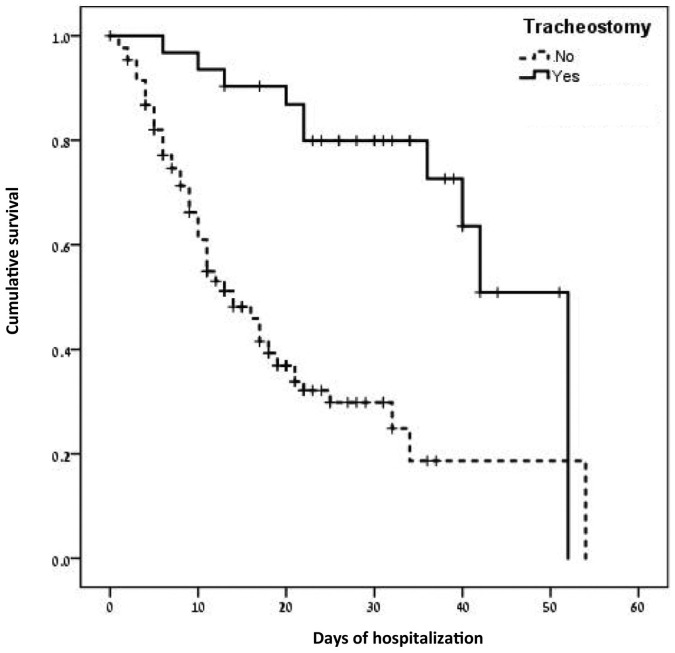
Kaplan Meier survival analysis revealed a statistically significant difference in survival time between the two groups (Log rank chi-sq = 20.91, p < 0.001) with mean survival time of 41 ± 3.1 days in the tracheostomy group and 21 ± 2.2 days in the control group (Fig. 1 ). Cox regression analysis using all significant variables as covariates (tracheostomy, gender, diastolic BP, time until defined critically ill, anti IL6, corticosteroids, Remdesivir, Tocilizumab, vasopressors, CPAP/BiPAP, nasogastric tube, TPN) and stepwise regression approach after adjustment for gender revealed a statistically significant difference in survival (mortality) between the tracheostomy and control groups (OR = 0.37, HR 0.13–0.96, p = 0.004), Table 6 .
Fig. 1.
Kaplan Meier overall survival analysis of the study and control groups.
Table 6.
Multivariate analysis of overall survival of the study cohort.
| All variables | OR | HR (95% CI) | p value |
|---|---|---|---|
| Tracheostomy | 0.35 | 0.13–0.96 | 0.04 |
| Gender | 2.91 | 1.67–5.05 | <0.001 |
| Diastolic BP | 0.99 | 0.97–1.01 | 0.38 |
| Time until defined critically ill | 0.94 | 0.87–1.01 | 0.08 |
| Anti- IL6 | 0.64 | 0.17–2.49 | 0.53 |
| Corticosteroids | 0.92 | 0.48–1.77 | 0.81 |
| CPAP/BiPAP | 0.29 | 0.15–0.59 | 0.001 |
| Nasogastric tube | 1.03 | 0.6–1.78 | 0.92 |
| Remdesivir | 0.82 | 0.09–7.32 | 0.86 |
| Tocilizumab | 1.32 | 0.52–3.33 | 0.56 |
| Vasopressors | 1.22 | 0.69–2.18 | 0.5 |
| TPN | 0.35 | 0.1–1.23 | 0.1 |
| Reduced model | |||
| Tracheostomy | 0.37 | 0.15–0.92 | 0.03 |
| Gender | 2.69 | 1.6–4.5 | <0.001 |
| Time until defined critically ill | 0.93 | 0.87–0.99 | 0.03 |
| CPAP/BiPAP | 0.27 | 0.14–0.54 | <0.001 |
| TPN | 0.36 | 0.11–1.16 | 0.086 |
Abbreviations: OR- odds ratio; HR- hazard ratio; CI- confidence interval; BP- blood pressure; IL6- interleukin 6; CPAP- continuous positive airway pressure; BiPAP- bilevel positive airway pressure; TPN- total parenteral nutrition.
In order to investigate whether there is an optimal cut-off value for the most efficient day to perform a tracheostomy, we used ROC analysis and Youden index. There tended to be a lower death rate among patients in which the procedure was performed after 8.5 days (4 death events out of 21 patients) compared with those who conducted tracheostomy earlier (5 death events out of 9 patients; p = 0.08).
4. Discussion
The decision if and when to perform tracheostomy among COVID-19 patients is uneasy for medical teams. The lack of sufficient data regarding the best timing of performance, the best method, short and long term influence on patients alongside the high risk of health-care workers infection, arises concerns regarding the necessity of the procedure. Current guidelines suggest individualized decision making, taking into account potential risks and benefits for the patient; risks posed to health-care workers during ICU hospitalization, and later on medical rehabilitation facilities, other patients, and families [15], [20].
This multicentral case-control study sheds light on the advantages of tracheostomy for COVID-19 patients. The two groups of patients we investigated had similar characteristics, with a predominance of males with multiple comorbidities. We found that patients who received tracheostomy were more likely to survive the hospitalization period, even though they were more prone to complications such as ARDS and secondary infections, probably associated with the more extended hospitalization period of this group of patients. Their longer ICU stay enabled the use of a variety of experimental treatment modalities, such as the drugs Remdesivir and Tocilizumab. When confronting the use of these drugs with tracheostomy on a multivariate analysis, tracheostomy had a statistically significant favorable influence on patients' outcomes, alongside female gender, more extended period until considered critically ill, and the use of noninvasive ventilation before intubation.
Volo et al. studied 23 COVID 19 patients who underwent tracheostomies. They found that the overall mortality of these patients was lower than the overall mortality of COVID 19 patients admitted to the ICU (18% vs 53%, respectively) [21]. Floyd et al. conducted 38 tracheostomies and concluded tracheostomy is of utility in these patients due to the high rate of weaning from the ventilator (55.2%), decannulation (13.2%), and low death rate (5.3%) [22]. Our findings are in accordance, demonstrating only 30% death events of patients undergoing tracheostomy compared with 61.6% of the control group.
When evaluating the long term impacts of tracheostomy of critically ill patients, it seems that it is not always in the patient's best interests. Vargas et al. followed non-COVID-19 patients who required tracheostomy after a long period of mechanical ventilation. They concluded that at least half do not survive longer than one year, and at one year less than 12% were functionally independent [12], [23]. The rapid outbreak and spreading of SARS-COVID 19 virus do not allow long term observation of any intervention used, tracheostomy included. However, we contend with high virus-related death events rates of patients worldwide, and favor interventions that could benefit our patients. Nonetheless, long term follow-up of patients undergoing tracheostomy, their long term survival, and their influence on their quality of life will need to be examined in future studies.
At the time of writing this article, we conducted a literature search and found 144 articles and guidelines regarding tracheostomy for COVID-19 patients. Most of which describe authors' experience with operation techniques under biosafety isolation, considerations for patient selection focusing on the proper timing. Only a few studies discussed short term results of patients undergoing tracheostomy. To the best of our knowledge this is the first published case-control study of COVID-19 patients who underwent tracheostomy, allowing us to present the benefits of tracheostomy in patients' management.
As discussed above, patients who underwent tracheostomy in our study had similar characteristics with patients who did not go through tracheostomy, and were ventilated after a comparable period of time. However, a more significant portion of this group survived. Even after confronting tracheostomy with other treatments this group received, in a multivariant analysis, tracheostomy had statistically significant advantages. We do take into account that patients in the control group died after a mean duration of five days from ventilation and that the mean period for tracheostomy was 13 days. Thus, this group of patients did not have a real opportunity to enjoy the benefits of tracheostomy. On the other hand, these patients who deteriorated and died so rapidly were probably very sick and could not go through operation or resuscitate afterward.
This study is limited due to its multicentral nature. Different medical teams treated patients, got treatment decisions, and conducted operations. Thus, the cohort is not homogenous. It is essential to conduct further studies and corroborate the value of tracheostomy in the treatment or critically ill COVID-19 patients.
5. Conclusion
Tracheostomy allows for more prolonged survival for gradually deteriorating critically ill COVID-19 patients. This should be integrated into the medical teams' considerations when debating whether or not to conduct tracheostomy.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The completion of data collection of this study could not have been possible without the participation and assistance of “The Israeli COVID-19 team”, physicians from all participating hospitals. Their contribution is sincerely appreciated and highly acknowledged.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA - J Am Med Assoc. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet (London, England) 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO; 2020. Novel Coronavirus – China. [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the Coronavirus Disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020:323. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.AAO position statement: tracheotomy recommendations during the COVID-19 pandemic n.d. https://www.entnet.org/content/aao-position-statement-tracheotomy-recommendations-during-covid-19-pandemic. (accessed July 14, 2020).
- 7.Griffiths J., Barber V.S., Morgan L., Young J.D. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330:1243. doi: 10.1136/bmj.38467.485671.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adly A., Youssef T.A., El-Begermy M.M., Younis H.M. Timing of tracheostomy in patients with prolonged endotracheal intubation: a systematic review. Eur Arch Otorhinolaryngol. 2018;275:679–690. doi: 10.1007/s00405-017-4838-7. [DOI] [PubMed] [Google Scholar]
- 9.Takhar A., Walker A., Tricklebank S., Wyncoll D., Hart N., Jacob T., et al. Recommendation of a practical guideline for safe tracheostomy during the COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2020;277:2173–2184. doi: 10.1007/s00405-020-05993-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skoog H., Withrow K., Jeyarajan H., Greene B., Batra H., Cox D., et al. Tracheotomy in the SARS-CoV-2 pandemic. Head Neck. 2020;42:1392–1396. doi: 10.1002/hed.26214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mecham J.C., Thomas O.J., Pirgousis P., Janus J.R. Utility of tracheostomy in patients with COVID-19 and other special considerations. Laryngoscope. 2020 doi: 10.1002/lary.28734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGrath B.A., Brenner M.J., Warrillow S.J., Pandian V., Arora A., Cameron T.S., et al. Tracheostomy in the COVID-19 era: global and multidisciplinary guidance. Lancet Respir Med. 2020;8:717–725. doi: 10.1016/S2213-2600(20)30230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CR L., NR D., L A., U C., A S., S S. Use of tracheostomy during the COVID-19 pandemic: American College of Chest Physicians/American Association for Bronchology and Interventional Pulmonology/Association of Interventional Pulmonology Program Directors Expert Panel Report. Chest. 2020 doi: 10.1016/J.CHEST.2020.05.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferri E., Boscolo Nata F., Pedruzzi B., Campolieti G., Scotto di Clemente F., Baratto F., et al. Indications and timing for tracheostomy in patients with SARS CoV2-related. Eur Arch Otorhinolaryngol. 2020;277:2403–2404. doi: 10.1007/s00405-020-06068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stubington T.J., Mallick A.S., Garas G., Stubington E., Reddy C., Mansuri M.S. Tracheotomy in COVID-19 patients: optimizing patient selection and identifying prognostic indicators. Head Neck. 2020;42:1386–1391. doi: 10.1002/hed.26280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angel L., Kon Z.N., Chang S.H., Rafeq S., Palasamudram Shekar S., Mitzman B., et al. Novel percutaneous tracheostomy for critically ill patients with COVID-19. Ann Thorac Surg. 2020 doi: 10.1016/j.athoracsur.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mattioli F., Fermi M., Ghirelli M., Molteni G., Sgarbi N., Bertellini E., et al. Tracheostomy in the COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2020;277:2133–2135. doi: 10.1007/s00405-020-05982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fluss R., Faraggi D., Reiser B. Estimation of the youden index and its associated cutoff point. Biom J. 2005;47:458–472. doi: 10.1002/bimj.200410135. [DOI] [PubMed] [Google Scholar]
- 19.Israel ministry of health n.d. https://t.me/s/MOHreport (accessed July 14, 2020).
- 20.Schultz M.J., Pattnaik R., Dondorp A.M. Walking the line between benefit and harm from tracheostomy in COVID-19. Lancet Respir Med. 2020;8:656–657. doi: 10.1016/S2213-2600(20)30231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volo T., Stritoni P., Battel I., Zennaro B., Lazzari F., Bellin M., et al. Elective tracheostomy during COVID-19 outbreak: to whom, when, how? Early experience from Venice, Italy. Eur Arch Otorhinolaryngol. 2020 doi: 10.1007/s00405-020-06190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floyd E., Harris S.S., Lim J.W., Edelstein D.R., Filangeri B., Bruni M. Early data from case series of tracheostomy in patients with SARS-CoV-2. Otolaryngol Neck Surg. 2020 doi: 10.1177/0194599820940655. [DOI] [PubMed] [Google Scholar]
- 23.Vargas M., Sutherasan Y., Brunetti I., Micalizzi C., Insorsi A., Ball L., et al. Mortality and long-term quality of life after percutaneous tracheotomy in intensive care unit: a prospective observational study. Minerva Anestesiol. 2018;84:1024–1031. doi: 10.23736/S0375-9393.18.12133-X. [DOI] [PubMed] [Google Scholar]