Abstract
Background and aims
The coronavirus disease 2019 (COVID-19) pandemic is severely threatening and challenging public health worldwide. Epidemiological studies focused on the influence of outdoor air pollution (AP) on COVID-19 risk have produced inconsistent conclusions. We aimed to quantitatively explore this association using a meta-analysis.
Methods
We searched for studies related to outdoor AP and COVID-19 risk in the Embase, PubMed, and Web of Science databases. No language restriction was utilized. The search date entries were up to August 13, 2021. Pooled estimates and 95% confidence intervals (CIs) were obtained with random-/fixed-effects models. PROSPERO registration number: CRD42021244656.
Results
A total of 35 articles were eligible for the meta-analysis. For long-term exposure to AP, COVID-19 incidence was positively associated with 1 μg/m3 increase in nitrogen dioxide (NO2; effect size = 1.042, 95% CI 1.017–1.068), particulate matter with diameter <2.5 μm (PM2.5; effect size = 1.056, 95% CI 1.039–1.072), and sulfur dioxide (SO2; effect size = 1.071, 95% CI 1.002–1.145). The COVID-19 mortality was positively associated with 1 μg/m3 increase in nitrogen dioxide (NO2; effect size = 1.034, 95% CI 1.006–1.063), PM2.5 (effect size = 1.047, 95% CI 1.025–1.1071). For short-term exposure to air pollutants, COVID-19 incidence was positively associated with 1 unit increase in air quality index (effect size = 1.001, 95% CI 1.001–1.002), 1 μg/m3 increase NO2 (effect size = 1.014, 95% CI 1.011–1.016), particulate matter with diameter <10 μm (PM10; effect size = 1.005, 95% CI 1.003–1.008), PM2.5 (effect size = 1.003, 95% CI 1.002–1.004), and SO2 (effect size = 1.015, 95% CI 1.007–1.023).
Conclusions
Outdoor air pollutants are detrimental factors to COVID-19 outcomes. Measurements beneficial to reducing pollutant levels might also reduce the burden of the pandemic.
Keywords: Air pollution, COVID-19 incidence, COVID-19 mortality, Meta-analysis, Observational study
1. Introduction
Coronavirus disease 2019 (COVID-19) is induced by a previously undiscovered coronavirus, designated as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Lu et al., 2020). Since SARS-CoV-2 was first discovered in December 2019, as of April 25, 2021, more than four million fatalities have been attributed to COVID-19, accounting for approximately 2% of the infected cases (>214 million) (Guan et al., 2020; World Health Organization, 2021). Although the pandemic has now lasted for more than 1.5 years, there are thousands of new cases and deaths daily worldwide with few regions spared (World Health Organization, 2021). The pathogenicity, lethality, rapid propagation, unexpected sequelae (such as decreased olfactory function and neuronal damage) of COVID-19, as well as the continuous emergence of more lethal and highly infectious variant clusters, are seriously threatening and challenging public health (Barrantes, 2020; Brann et al., 2020; China Daily, 2021). Notably, vaccines cannot 100% protect people from SARS-CoV-2 infection (Lopez Bernal et al., 2020). Therefore, exploring the factors influencing COVID-19 transmission and mortality have significant implications for the prevention and intervention strategies concerning this pandemic.
Generally, both individual susceptibility and ambient conditions contribute to COVID-19 transmission and mortality (De Angelis et al., 2021; Pranata et al., 2021). The ambient air quality index (AQI) is determined by airborne carbon monoxide (CO), nitrogen dioxide (NO2), ozone (O3), particulate matter with diameter <10 μm (PM10) and <2.5 μm (PM2.5), and sulfur dioxide (SO2) (Ministry of Ecology and Environment of the People's Republic of China, 2021). Globally, air pollution (AP) is the fourth greatest source of lethality (Health Effects Institute, 2020). Several epidemiological studies have linked AP to the COVID-19 pandemic (De Angelis et al., 2021; Stieb et al., 2020; Travaglio et al., 2021). However, the conclusions of individual studies are inconsistent. For example, positive associations between long-term exposure to PM2.5 and COVID-19 incidence were identified (De Angelis et al., 2021; Travaglio et al., 2021) but another study found no such association (Stieb et al., 2020).
No meta-analysis has yet quantitatively focused on the influence of AP on COVID-19 risk and there are inconsistent conclusions of individual studies, while the pandemic is spreading vigorously. To explore the quantitative effect of outdoor AP on COVID-19 incidence and mortality, we conducted the first meta-analysis on the association of AP-COVID-19, to inspire relevant decision-makers to formulate more effective strategies for prevention and control of this unprecedented pandemic.
2. Methods
We conducted the meta-analysis according to the PRISMA and MOOSE guidelines (Supplementary Tables 1–2) (Moher et al., 2009; Stroup et al., 2000). Registered PROSPERO number: CRD42021244656.
2.1. Study search
We searched for studies related to AP and COVID-19 incidence or mortality in three databases: Embase, PubMed, and Web of Science. The search term was constructed on the basis of (‘air quality’ OR ‘CO’ OR ‘NO2’ OR ‘nitrogen oxides’ OR ‘O3’ OR ‘PM10’ OR ‘PM2.5’ OR ‘SO2’) AND (‘COVID-19’), detailed in Supplementary Table 3. The search date entries were up to August 13, 2021, with no language restriction on the search. Additionally, we manually searched related references for enlargement of selection scope. The study search was done independently by two authors (S-TZ and JL), and any disagreement was resolved by a third author (Y-HZ).
2.2. Study selection
There was no language restriction on study selection. The research inclusion criteria follow: peer-reviewed; the study subjects were human; original research; observational study (cohort, ecological, time-series, cross-sectional, or case-control); the exposure was AQI, CO, NO2, nitrogen oxides (NOx), O3, PM10, PM2.5, or SO2; the outcome was COVID-19 incidence and/or mortality; COVID-19 with medical diagnostic criteria; with effect size and 95% confidence intervals (CIs) of the relationship between AP and COVID-19 risk; and effect size of the relationship between AP and COVID-19 incidence and/or mortality was expressed as dose-response.
The research exclusion criteria follow: not peer-reviewed; the study subjects were non-human; non-original research (review or meta-analysis); no effect size or 95% CI; no dose-response relationship; and data with obvious errors. After duplications were excluded, the title, abstract, and full text were evaluated separately, and finally included in the study if they met the criteria. The study selection was done independently by two authors (S-TZ and HX-Y), and any disagreement was resolved by a third author (Y-HZ).
2.3. Data extraction
The extracted terms including the first author, country, type of exposure window (long- or short-term), the number of lag days when the type of exposure window was short-term, exposure (AQI, CO, NO2, NOx, O3, PM10, PM2.5, or SO2), outcome (COVID-19 incidence and/or mortality), pollutant dose, effect size, and 95% CI. In addition, publication year, exposure window duration, research type, number of cases and participants, adjusted covariates, pollutant concentration, and pollutant measurement method were extracted. The data were extracted independently by two authors (S-TZ and LL), and any disagreement was resolved by a third author (Y-HZ).
2.4. Risk of bias (quality) assessment
The quality of the included studies was evaluated based on nine parameters suggested by the Agency for Healthcare Research and Quality (Lam et al., 2017; Zeng et al., 2015). These included sample acquisition, inclusion criteria, research period, uninformed evaluators, outcome evaluation, exclusion criteria, covariate control, handling of missing information, and information integrity. Answer each question according to the characteristics of each article. Each parameter was addressed based on the responses from the articles. The “yes” response was equivalent to one point, while the “no” response corresponded to zero points. Scores of 1–3, 4–6, and 7–9 indicated high, moderate, and low risk of bias, respectively.
2.5. Statistical analysis
The following formula was used to standardize the effect sizes (Shah et al., 2013):
where effect size(standardized) and effect size(original) represent the effect size of the association between air pollutants and COVID-19 risk with the Increment(standardized) and Increment(original) of pollutant concentration, respectively. The unit of concentration for air pollutants NO2, O3, PM10, PM2.5, or SO2 used by the Ministry of Ecology and Environment of the People's Republic of China was μg/m3 (Ministry of Ecology and Environment of the People's Republic of China, 2021). Thus, 1 μg/m3 served as the Increment(standardized) for NO2, O3, PM10, PM2.5, and SO2, as well as for CO and NOx. The random- and fixed-effects models were used to determine the effect size when heterogeneity was >50% and ≤50%, respectively (Ades et al., 2005; Fellmeth et al., 2018). An I-squared (I2) test was used to detect heterogeneity between studies, categorized into low-, medium-, and high-heterogeneity groups based on 50% and 75% boundaries (Higgins et al., 2003). The subgroup analysis was based on the following three aspects: whether the pollutant concentration prediction model was used, whether incidence and mortality have been normalized with respect to the population, and the national income level (high- or low-/middle level). Meta-regression was used to determine whether the three aforementioned factors were the source of heterogeneity. Publication bias of included articles was detected by Egger's test (Egger et al., 1997). Sensitivity analysis was performed for each meta-analysis when the included effect size was greater than that for the two items (Trikalinos et al., 2006). The number of studies was reduced by one at a time to see if the pooled results after the exclusion were significantly different from the overall pooled results, that is, whether the excluded studies made an impact on the overall results. If the effect size obtained after the exclusion was not statistically different from the overall pooled effect size, the effect of a given study on the overall effect size was not statistically significant and the overall result was robust. If the value obtained after a study was excluded was very different from the total pooled result, the study had an impact on the overall result, which was not robust. In addition, the evidence confidence was evaluated by GRADE guidelines (Murad et al., 2017). We used Stata (version 15.0, StataCorp LLC, USA) for statistical analysis, with significance determined as P < 0.05 (two-sided).
3. Results
3.1. Study search and selection
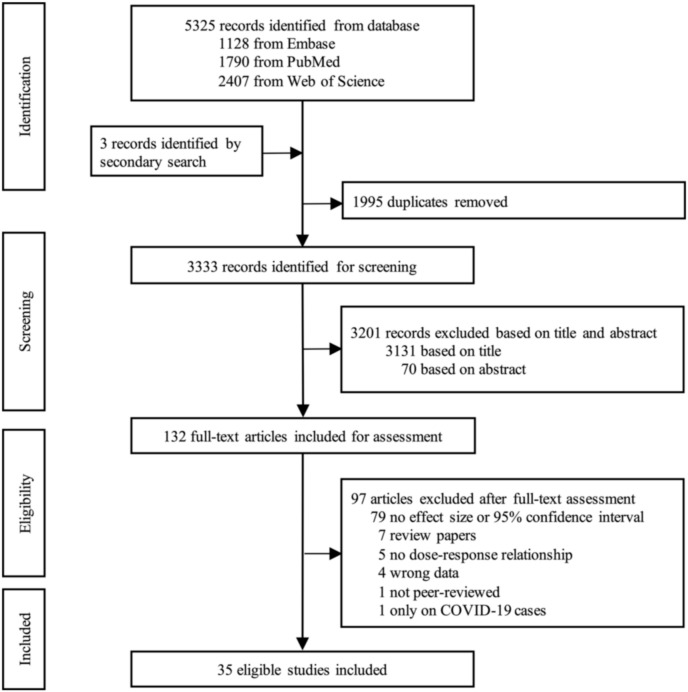
There were 1128, 1790, and 2407 items retrieved from the Embase, PubMed, and Web of Science databases, respectively. Three items were also manually identified for the selection scope enlargement. First, 1995 duplicates were excluded and then 3131 and 70 records were excluded based on the title and abstract, respectively. Full texts of 132 records were evaluated and in the end 35 articles that met the criteria were included in the study (Fig. 1 ) (Adhikari and Yin, 2020; Azuma et al., 2020; Berg et al., 2021; Cabrera-Cano et al., 2021; Coker et al., 2020; Dales et al., 2021; De Angelis et al., 2021; Elliott et al., 2021; Fang et al., 2021; Hoang and Tran, 2020; Hoang et al., 2020; Huang and Brown, 2021; Jiang et al., 2020; Konstantinoudis et al., 2021; Linares et al., 2021; Lorenzo et al., 2021; Lu et al., 2021; Ma et al., 2021; Qeadan et al., 2021; Petroni et al., 2020; Rodriguez-Villamizar et al., 2020; Sahoo, 2021; Sanchez-Piedra et al., 2021; Setti et al., 2020; Stieb et al., 2020; Travaglio et al., 2021; Valdés Salgado et al., 2021; Wang et al., 2020; Wu et al., 2020a, 2020b; Xu et al., 2020; Zhang et al., 2021; Zheng et al., 2021; Zhou et al., 2021; Zhu et al., 2020).
Fig. 1.
Literature selection.
3.2. Study characteristics
Characteristics of the included studies are detailed in Table 1 and Supplementary Tables 4–6. The 35 included articles were published in 2020–2021. Of these, 15 studies came from low-/middle-income countries (Chile [n = 2], China [n = 10], Colombia [n = 1], India [n = 1], and Mexico [n = 1]) and 20 from high-income countries (Canada [n = 1], Germany [n = 1], Italy [n = 3], Japan [n = 1], Korea [n = 2], Singapore [n = 1], Spain [n = 2], UK [n = 3], and USA [n = 6]). There were three (8.6%, 3/35) cohort studies, 17 (48.6%) ecological studies, and 15 (42.9%) time-series studies. All COVID-19 case information came from official reports from governments of countries or localities. All studies were adjusted for covariates such as humidity and temperature. Eight studies (22.9%) used evaluation models to assess the concentration of air pollutants on the basis of data from monitoring stations. A total of 29 (82.9%) studies had a low risk of bias and six (17.1%) had a moderate risk of bias. Ten studies had a normalized incidence and mortality with respect to the population. The latest collection of COVID-19 case information occurred on December 31, 2020. A total of 10 and 15 studies reported the association between long- and short-term exposure to air pollutants and COVID-19 incidence, respectively. In addition, 12 and two studies reported the association between long- and short-term exposure to air pollutants and COVID-19 mortality, respectively.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Study | Country | Design | Period | Outcome | Outcome normalized respect to the population | Pollutant | Risk of bias |
|---|---|---|---|---|---|---|---|
| Long-term exposure | |||||||
| Berg et al. (2021) | USA | Ecological | March 1 to August 31, 2020 | COVID-19 incidence, COVID-19 mortality | NA | PM2.5 | Low |
| Cabrera-Cano et al. (2021) | Mexico | Ecological | February to June 2020 | COVID-19 mortality | 1.09/100,000–76.97/100,000 | NO2, PM2.5 | Low |
| Coker et al. (2020) | Italy | Ecological | January 1 to April 30, 2020 | COVID-19 mortality | NR | PM2.5, O3, PM10 | Low |
| De Angelis et al. (2021) | Italy | Ecological | February 20 to April 16, 2020 | COVID-19 incidence | NR | NO2, PM10, PM2.5 | Low |
| Elliott et al. (2021) | UK | Cohort | January 31 to September 21, 2020 | COVID-19 mortality | NR | NOx, PM10, PM2.5 | Low |
| Fang et al. (2021) | USA | Ecological | Up to September 12, 2020 | COVID-19 incidence | NR | PM2.5 | Low |
| Huang and Brown (2021) | Germany | Ecological | Up to September 13, 2020 | COVID-19 incidence | NA | NO2, PM10, PM2.5, SO2 | Low |
| Jiang et al. (2020) | China | Cohort | January 25 to February 29, 2020 | COVID-19 incidence | NR | PM2.5 | Low |
| Konstantinoudis et al. (2021) | UK | Ecological | March 2 to June 30, 2020 | COVID-19 mortality | NR | NO2, PM2.5 | Low |
| Qeadan et al. (2021) | USA | Ecological | Up to June 2, 2020 | COVID-19 mortality | NR | PM2.5 | Moderate |
| Petroni et al. (2020) | USA | Ecological | Up to July 11, 2020 | COVID-19 mortality | NR | O3, PM2.5 | Low |
| Rodriguez-Villamizar et al. (2020) | Colombia | Ecological | Up to July 17, 2020 | COVID-19 mortality | Mean 0.75/1,000,000 | PM2.5 | Low |
| Sanchez-Piedra et al. (2021) | Spain | Ecological | February 3 to July 14, 2020 | COVID-19 mortality | NR | NO2, PM2.5 | Moderate |
| Stieb et al. (2020) | Canada | Ecological | Up to May 13, 2020 | COVID-19 incidence | Mean 114.0/100,000 | PM2.5 | Low |
| Travaglio et al. (2021) | UK | Ecological | Cases: up to April 26, 2020; deaths: up to April 31, 2020 | COVID-19 incidence, COVID-19 mortality | NR | NO2, NOx, O3, PM2.5, PM10 | Low |
| Valdés Salgado et al. (2021) | Chile | Ecological | 2020 | COVID-19 incidence, COVID-19 mortality | Mean: incidence 4501/100,000, mortality 71/100,000. | PM10, PM2.5 | Low |
| Wu et al. (2020a) | USA | Ecological | Up to June 18, 2020 | COVID-19 mortality | Mean 15.5/100,000 | PM2.5 | Low |
| Wu et al. (2020b) | China | Ecological | Up to April 21, 2020 | COVID-19 incidence | NR | NO2, O3, PM10, PM2.5 | Low |
| Zheng et al. (2021) | China | Ecological | December 31, 2019 to March 6, 2020 | COVID-19 incidence | NR | CO, NO2, O3, PM10, PM2.5, SO2 | Low |
| Short-term exposure | |||||||
| Adhikari and Yin (2020) | USA | Time-series | March 1 to April 20, 2020 | COVID-19 incidence, COVID-19 mortality | NR | PM2.5, O3 | Low |
| Azuma et al. (2020) | Japan | Cohort | March 13 to April 6, 2020 | COVID-19 incidence | Median: 132.5/1,000,000 | NO2, PM2.5 | Low |
| Dales et al. (2021) | Chile | Time-series | March 16 to August 31, 2020 | COVID-19 mortality | NR | CO, NO2, O3, PM2.5 | Low |
| Hoang and Tran (2020) | Korea | Time-series | February 24 to September 12, 2020 | COVID-19 incidence | NR | CO, NO2, O3, PM2.5, PM10, SO2 | Low |
| Hoang et al. (2020) | Korea | Time-series | February 24 to May 5, 2020 | COVID-19 incidence | NR | CO, NO2, SO2 | Low |
| Linares et al. (2021) | Spain | Time-series | February 1 to December 31, 2020 | COVID-19 incidence | NA | NO2, O3, PM10 | Low |
| Lorenzo et al. (2021) | Singapore | Time-series | January 23 to April 6, 2020 | COVID-19 incidence | NR | CO, NO2, O3, PM2.5, PM10, SO2 | Low |
| Lu et al. (2021) | China | Time-series | January 20 to February 29, 2020 | COVID-19 incidence | NR | NO2, O3, PM2.5, SO2 | Moderate |
| Ma et al. (2021) | China | Time-series | January 21 to February 29, 2020 | COVID-19 incidence | NR | NO2, PM2.5, PM10, SO2 | Moderate |
| Sahoo (2021) | India | Time-series | January 30 to April 23, 2020 | COVID-19 incidence | NR | NO2, PM2.5, PM10, SO2 | Moderate |
| Setti et al. (2020) | Italy | Time-series | February 24th to March 13th, 2020 | COVID-19 incidence | Less and most polluted provinces: median 3 and 26 cases over 100,000 inhabitants, respectively. | PM10 | Moderate |
| Wang et al. (2020) | China | Time-series | January 1 to March 2, 2020 | COVID-19 incidence | NR | PM10, PM2.5 | Low |
| Xu et al. (2020) | China | Time-series | January 29 to February 15, 2020 | COVID-19 incidence | NR | AQI | Low |
| Zhang et al. (2021) | China | Time-series | January 1 to April 6, 2020 | COVID-19 incidence | NR | AQI, CO, NO2, O3, PM2.5, PM10, SO2 | Low |
| Zhou et al. (2021) | China | Time-series | January 15 to March 18, 2020 | COVID-19 incidence | NR | CO, NO2, O3, PM2.5, SO2 | Low |
| Zhu et al. (2020) | China | Time-series | January 23 to February 29, 2020 | COVID-19 incidence | NR | CO, NO2, O3, PM10, PM2.5, SO2 | Low |
Abbreviations: AQI, ambient air quality index; CO, carbon monoxide; NA, not available; NO2, nitrogen dioxide; NOx, nitrogen oxides; NR, not reported. O3, ozone; PM10, particulate matter with diameter <10 μm; PM2.5, particulate matter with diameter <2.5 μm; SO2, sulfur dioxide.
3.3. Long-term exposure to air pollutants and COVID-19
3.3.1. Long-term exposure to air pollutants and COVID-19 incidence
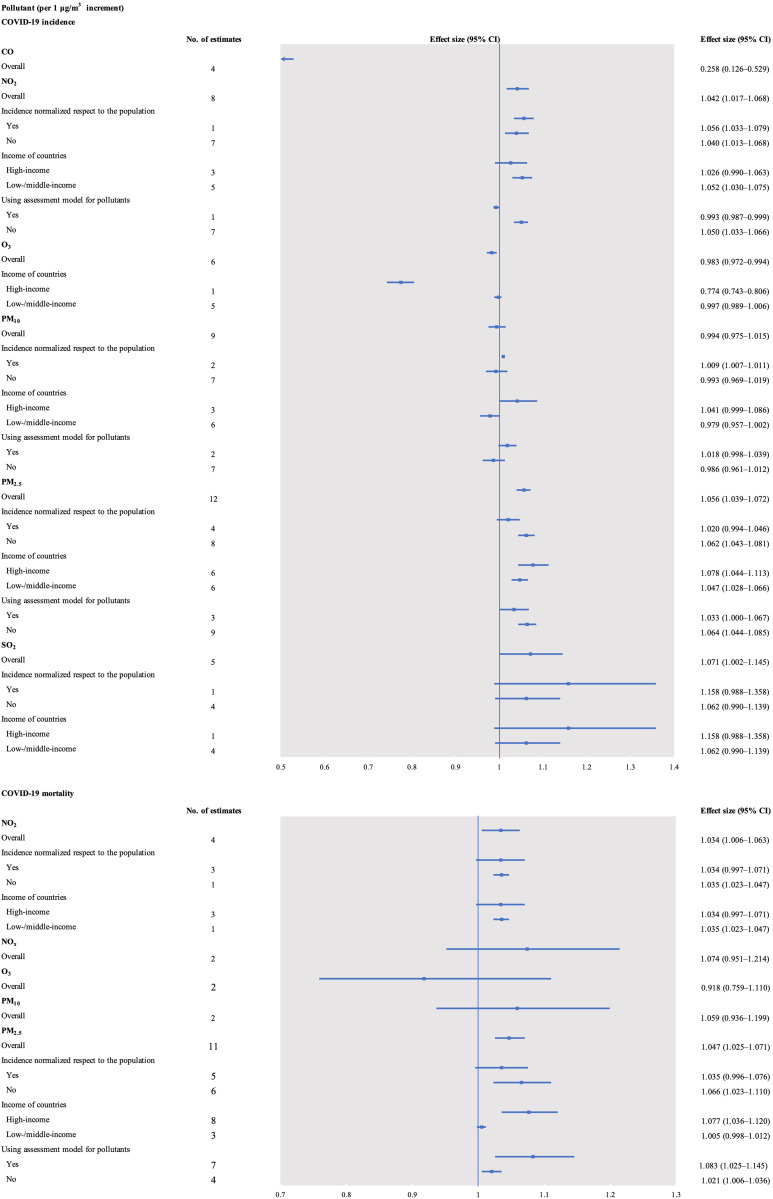
The COVID-19 incidence was positively associated with a 1 μg/m3 increase in NO2 (effect size = 1.042, 95% CI 1.017–1.068, I2 = 98.3%), PM2.5 (effect size = 1.056, 95% CI 1.039–1.072, I2 = 94.9%), and SO2 (effect size = 1.071, 95% CI 1.002–1.145, I2 = 97.2%). No association was found between long-term exposure to PM10 and COVID-19 incidence (effect size = 0.994, 95% CI 0.975–1.015, I2 = 98.9%). However, COVID-19 incidence was negatively associated with a 1 μg/m3 increase in CO (effect size = 0.258, 95% CI 0.126–0.529, I2 = 99.4%) and O3 (effect size = 0.983, 95% CI 0.972–0.994, I2 = 98.4%; Fig. 2 , Supplementary Tables 7–11, and Supplementary Figs. 1–6).
Fig. 2.
Forest plots of the association between long-term exposure to air pollutants and COVID-19 risk. CO, carbon monoxide; NO2, nitrogen dioxide; NOx, nitrogen oxides; O3, ozone; PM10, particulate matter with diameter <10 μm; PM2.5, particulate matter with diameter <2.5 μm; SO2, sulfur dioxide.
Subgroup analysis showed that the association between PM2.5 and COVID-19 incidence was still positive when subgroup analysis was performed by country income (high- or low-/middle-income). The association between NO2 and COVID-19 incidence was positive in studies from low-/middle-income countries (effect size = 1.052, 95% CI 1.030–1.075), but there was no association for high-income countries (effect size = 1.026, 95% CI 0.990–1.063). The association between NO2 and COVID-19 incidence was positive when subgroup analysis used normalized incidence with respect to the populations (“yes” or “no”). Subgroup analysis showed that the association between PM10 and COVID-19 incidence was significant in studies with normalized incidence with respect to the populations (effect size = 1.009, 95% CI 1.007–1.011).
3.3.2. Long-term exposure to air pollutants and COVID-19 mortality
The COVID-19 mortality was positively associated with a 1 μg/m3 increase in NO2 (effect size = 1.034, 95% CI 1.006–1.063, I2 = 97.5%) and PM2.5 (effect size = 1.047, 95% CI 1.025–1.071, I2 = 86.3%). No association was found between long-term exposure to NOx (effect size = 1.074, 95% CI 0.951–1.214, I2 = 90.9%), O3 (effect size = 0.918, 95% CI 0.759–1.110, I2 = 99.0%), or PM10 (effect size = 1.059, 95% CI 0.936–1.199, I2 = 85.3%; Fig. 2, Supplementary Tables 12–13, and Supplementary Figs. 7–11).
Subgroup analysis showed that the association between NO2 and COVID-19 mortality was positive in studies from low-/middle-income countries (effect size = 1.035, 95% CI 1.023–1.047), but there was no association for high-income countries (effect size = 1.034, 95% CI 0.997–1.071). The association between PM2.5 and COVID-19 mortality was still positive when subgroup analysis was performed using the assessment model for pollutants (“yes” or “no”).
3.4. Short-term exposure to air pollutants and COVID-19
3.4.1. Short-term exposure to air pollutants and COVID-19 incidence
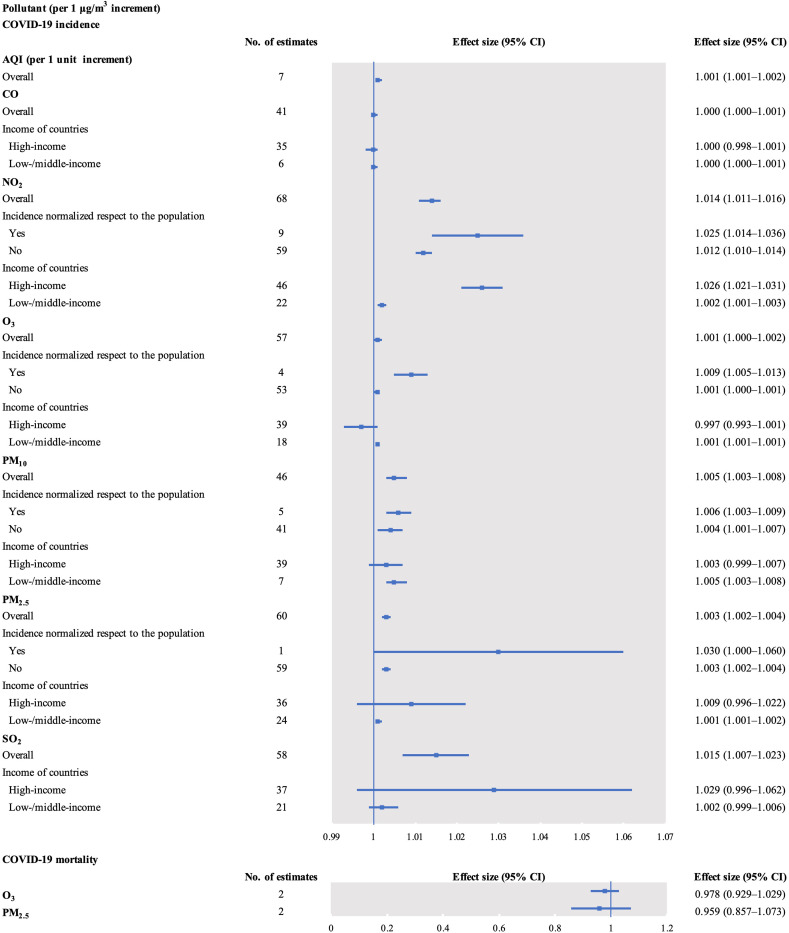
The COVID-19 incidence was positively associated with a 1 unit increase in AQI (effect size = 1.001, 95% CI 1.001–1.002) and a 1 μg/m3 increment in NO2 (effect size = 1.014, 95% CI 1.011–1.016), O3 (effect size = 1.001, 95% CI 1.000–1.002), PM10 (effect size = 1.005, 95% CI 1.003–1.008), PM2.5 (effect size = 1.003, 95% CI 1.002–1.004), and SO2 (effect size = 1.015, 95% CI 1.007–1.023). However, there was no association between COVID-19 incidence and short-term exposure to CO (effect size = 1.000, 95% CI 1.000–1.001; Fig. 3 , Supplementary Tables 14–19, and Supplementary Figs. 12–18).
Fig. 3.
Forest plots of the association between short-term exposure to air pollutants and COVID-19 risk. AQI, air quality index; CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; PM10, particulate matter with diameter <10 μm; PM2.5, particulate matter with diameter <2.5 μm; SO2, sulfur dioxide.
The association between NO2 and COVID-19 incidence was positive when subgroup analysis was performed considering country incomes (high-income: effect size = 1.026, 95% CI 1.021–1.031 and low-/middle-income: effect size = 1.002, 95% CI 1.001–1.003). The positive association persisted when subgroup analysis used normalized incidence with respect to the populations (“yes” or “no”). Subgroup analysis also showed a positive association between CO and COVID-19 incidence in studies from low-/middle-income countries (effect size = 1.000, 95% CI 1.000–1.001, P < 0.05). The association was also present with exposure to O3 (effect size = 1.001, 95% CI 1.001–1.001), PM10 (effect size = 1.005, 95% CI 1.003–1.008), and PM2.5 (effect size = 1.001, 95% CI 1.001–1.002). Subgroup analysis revealed a positive association between O3 and COVID-19 incidence in studies utilizing normalized incidence with respect to the populations (effect size = 1.009, 95% CI 1.005–1.013). The association between PM10 and COVID-19 incidence remained positive with subgroup analysis employing normalized incidence with respect to the populations (“yes” or “no”). This association persisted with exposure to PM2.5.
3.4.2. Short-term exposure to air pollutants and COVID-19 mortality
The COVID-19 mortality was not associated with 1 μg/m3 increase in O3 (effect size = 0.978, 95% CI 0.929–1.029) and PM2.5 (effect size = 0.959, 95% CI 0.857–1.073; Fig. 3, Supplementary Tables 20; Supplementary Figs. 19–20).
3.5. Publication bias, and robustness
Publication bias detection was performed for 15 associations identified in the present study. A total of 12 (80%) associations were without publication bias (Supplementary Table 21). Studies involving the association between COVID-19 incidence and long-term exposure to the air pollutants including CO, NO2, O3, PM10, PM2.5, and SO2, did not have a publication bias. There was also no publication bias in studies involving the association between COVID-19 incidence and short-term exposure to the following air pollutants: CO, O3, PM10, and SO2. Publication bias was found in studies involving the association between COVID-19 incidence and short-term exposure to PM2.5 or NO2, as well as between COVID-19 mortality and long-term exposure to PM2.5. It is likely that the bias was present because the meta-analysis did not include an exhaustive list of research studies on the topic. Researchers tend to report positive instead of negative results, while small-sample and low-quality studies are not easy to publish.
Studies involving the association between COVID-19 incidence and long-term exposure to NO2, PM10, PM2.5, and SO2 were robust. This means that a single study did not have a significant impact on the overall pooled results. The same was true for studies on association between COVID-19 mortality and long-term exposure to NO2 and PM2.5 and between COVID-19 incidence and short-term exposure to NO2, O3, PM10, PM2.5, and SO2 (Supplementary Figs. 21–35).
3.6. Evidence confidence
The evidence confidence for the association between COVID-19 incidence and long-term exposure to CO, NO2, O3, PM10, PM2.5, and SO2 was low. The results were similar for the association between COVID-19 mortality and long-term exposure to NO2 and PM2.5, between COVID-19 incidence and short-term exposure to CO, O3, PM10, and SO2, as well as between COVID-19 mortality and short-term exposure to O3 (Supplementary Table 22).
4. Discussion
The present study included 35 studies on the impact of air pollutants on COVID-19 incidence or mortality. It was determined that both long- and short-term exposure to NO2, PM2.5, and SO2 was associated with higher COVID-19 incidence. Long-term exposure to PM2.5 increased COVID-19 mortality. The COVID-19 incidence was positively associated with an increase in AQI. Short-term exposure to O3 and PM10 was associated with higher COVID-19 incidence. The burden of the pandemic in most regions worldwide is increasing, underscoring the importance and urgency of attenuating AP in order to address the situation.
A general warning concerning the Need for a Medical Reading of Environmental Data should be taken seriously (Iriti et al., 2020). Approximately 80% of European urban populations are exposed to air pollutant levels that are higher than those set by the World Health Organization (European Environment Agency, 2019). Significantly, air pollution increases all-cause mortality (Chen and Hoek, 2020). Epidemiology studies have confirmed that air pollution does not only increase the risk of respiratory diseases, such as lung cancer and influenza, but also increases the risk of breast cancer, cognitive diseases such as Alzheimer's disease, cerebrovascular diseases like stroke, and metabolic diseases like diabetes (Chen and Hoek, 2020; Chen et al., 2017; Guo et al., 2021; Liu et al., 2019; Tsai et al., 2019; Yang et al., 2019; Yu et al., 2020). Therefore, Iriti et al. (2020) have pointed out that the hygiene departments should study the environmental data and strengthen the cooperation with environmental agencies to jointly contribute to reducing the adverse impact of air pollution on human health.
Other systemic effects of COVID-19 such as neurological symptoms should not be underestimated (Bellocchio et al., 2020). The damage to the central nervous system is manifested as cephalodynia, cerebrovascular disorders like brain hemorrhage, encephalon diseases, lightheadedness, Rasmussen's syndrome with convulsions, and spinal cord inflammation. The symptoms of damage to the peripheral nervous system are manifested as acute inflammatory polyradiculoneuropathy, ataxy, hyposmia, hypogeusia, decreased chemosensory function, and skeletal muscle injury. In addition, Patel et al. (2020) have demonstrated that COVID-19 causes abnormality in gastrointestinal function, such as vomiting and diarrhea. COVID-19 complications also include cardiovascular injury, hepatosis, and myocardial damage (Zhang et al., 2020; Zheng et al., 2020).
In fact, the different restriction policies to contain the outbreak of COVID 19 for each country (for example lockdown period and entity), the population for each country, the healthcare capacity and access for each country, etc, could be confounder factors for the study. Moreover, non-COVID-related factors (e.g., meteorology and emissions from regional events) may play an important role in confounding short-term trends as well as the number of selected lag days, which were different among the considered studies.
Of the 35 included studies, ten had normalized incidence and mortality rates with respect to the population (Azuma et al., 2020; Berg et al., 2021; Cabrera-Cano et al., 2021; Huang and Brown, 2021; Linares et al., 2021; Rodriguez-Villamizar et al., 2020; Setti et al., 2020; Stieb et al., 2020; Valdés Salgado et al., 2021; Wu et al., 2020a). The heterogeneity among the studies when considering the association between long-term exposure to PM2.5 was lower (8.8%) in the subgroup with incidence normalized with respect to the population than in the subgroup without normalization (94.3%), indicating that normalized incidence with respect to the population decreases the heterogeneity among the studies. According to the subgroup analysis, long-/short-term exposure to NO2 had a promotive effect on COVID-19 incidence regardless of incidence normalization, which was similar to the overall pooled effect. The association between short-term exposure to PM10 or PM2.5 and COVID-19 incidence remained positive when subgroup analysis utilized normalized incidence with respect to the populations (“yes” or “no”), which was similar to the overall pooled effect. The association between long-term exposure to PM10 and COVID-19 incidence was significant in studies normalizing incidence with respect to the populations, which was different to the overall pooled effect. The association persisted with short-term exposure to O3. Normalizing incidence and mortality with respect to the population for pollutants that have not been demonstrated to promote the occurrence of COVID-19 can better reveal their adverse effects.
Healthcare capacity and access for each country, including number of doctors and medical equipment, such as hospital beds, respirators, and personal protective equipment, per capita, can serve as confounding factors. Six of the 35 included articles have taken into account healthcare capacity of the different countries (Berg et al., 2021; Coker et al., 2020; Konstantinoudis et al., 2021; Petroni et al., 2020; Rodriguez-Villamizar et al., 2020; Wu et al., 2020a). They considered per-capita hospital beds as proxy for healthcare capacity. Specifically, Rodriguez-Villamizar et al. (2020) have shown that decreasing COVID-19 mortality is associated with increasing hospital bed capacity. Coker et al. (2020), Petroni et al. (2020), and Wu et al. (2020a) have identified a null association between hospital bed capacity and COVID-19 mortality. In addition, intensive care unit bed capacity not associated with COVID-19 mortality was demonstrated by Konstantinoudis et al. (2021). However, the above six studies were from high-income countries, and the included literature from low-/middle-income countries has not taken into account their healthcare capacity. In addition, disproportionate national income tends to lead to inconsistencies in healthcare capacity and national ability to handle and control public health incidents. Subgroup analysis indicated that long-term exposure to NO2 was significantly associated with increased COVID-19 incidence or mortality in low-/middle-income countries. There was no such association in high-income countries. Similarly, a positive association between short-term exposure to CO and COVID-19 incidence has been demonstrated in studies from low-/middle-income countries, which persisted with exposure to O3, PM10, and PM2.5. Null association was shown in high-income countries. This means that low-/middle-income countries may face more severe air pollution challenges when responding to this public health emergency. Therefore, low-/middle-income countries should take their healthcare capacity into account.
In addition, due to high levels of pollution and inadequate disease surveillance, low-/middle-income countries are experiencing a more severe COVID-19 burden than high-income countries. However, apart from those from China, there were few included studies from other low-/middle-income countries (28.6% from China, 5.7% from Chile, 2.6% from Colombia, 2.6% from India, and 2.6% from Mexico). Hence, more epidemiological research linking AP and COVID-19 risk from low-/middle-income countries is needed, such as from Africa and India, to adequately examine the detrimental effect of AP on COVID-19 risk.
Subgroup analysis showed that the association between long-term exposure to PM2.5 and COVID-19 incidence remained positive when subgroup analysis evaluated countries’ income (high- or low-/middle-income). The association between short-term exposure to NO2 and COVID-19 incidence remained positive when subgroup analysis took country income into account. Although the air quality in high-income countries is relatively good, the impact of air pollution on COVID-19 risk cannot be ignored.
The association between long-term exposure to PM2.5 and COVID-19 mortality was positive when subgroup analysis used the assessment model for pollutants (“yes” or “no”). Adjusting for the confounding parameters that affect COVID-19 risk, such as population density and temperature, increased the accuracy of the relationship between AP and COVID-19 risk. In addition, the AP concentration assessed using a model that incorporated multiple parameters was closer to the true exposure level than the AP concentration released by the air monitoring station alone. The evidence presented here suggests that a less reliable exposure measurement method contributed to underestimation of the association between pollutants and COVID-19 risk.
In addition to the aforementioned factors, the heterogeneity among studies originated from the following aspects. First, with the development of the pandemic and the introduction of policies to lockdown and reduce social interactions in various countries, air quality has risen, resulting in large fluctuations in the concentration of air pollutants in distinct periods (Venter et al., 2020). Secondly, the scope of research objects covered by each study is diverse, including sample size and regions. Third, the incidence rates of the same ethnic group in different regions of the same country also differ as identified by Rodriguez-Diaz et al. (2020). Fourth, some studies originating in China excluded Wuhan (the epicenter) (Zheng et al., 2021; Zhou et al., 2021), while others did not. Fifth, when studying the impact of short-term exposure to air pollutants on the COVID-19 risk, the difference in the number of selected lag days is also a source of heterogeneity. Jiang et al. (2020) linked a 4-day lag of air pollutants with COVID-19 incidence, since the reference incubation period was 4 days. In contrast, Lorenzo et al. (2020) linked 8- and 15-day lags of air pollutants with COVID-19 incidence.
Zheng et al. (2021) confirmed that long-term exposure to NO2, PM10, and PM2.5 also facilitated severe COVID-19 incidence. Long-term NOx exposure increased both COVID-19 incidence and mortality according to Travaglio et al. (2021). Petroni et al. (2020) demonstrated that diesel PM was a malignant factor related to COVID-19 mortality (effect size = 2.82). Jiang and Xu (2020) identified AP as a detrimental factor for COVID-19 mortality. Smoking was significantly correlated with COVID-19 risk (Reddy et al., 2021). Long-/short-term PM10 and PM2.5 were positively correlated with case fatality rate (Yao et al., 2020a, 2020b, 2020b). Dating back to 2003, during the SARS epidemic period, Cui et al. (2003) that high AP was significantly correlated with case fatality rate risk of SARS (effect size = 2.18, 95% CI 1.31–3.65).
Setti et al. (2020) have proposed that PM is a potential SARS-CoV-2 vector and a promoting factor in SARS-CoV-2 transmission. Frontera et al. (2020) suggested that long-term exposure to air pollutants will cause overexpression of angiotensin-converting enzyme 2 (SARS-CoV-2's target), thereby leading to SARS-CoV-2 overloading. The other potential biological mechanism of the impact of AP on COVID-19 is inflammatory response and oxidative stress (Fiorito et al., 2017; Ogen, 2020; Olvera Alvarez et al., 2018; Wolf et al., 2016). On the one hand, air pollutants directly impair lung function through inflammation and oxidative stress, including decreased pulmonary defense function and phagocytic function of macrophages (Becker et al., 2003; Guarnieri and Sinha, 2014; Selley et al., 2020). On the other hand, air pollutants indirectly affect the occurrence and development of COVID-19 through a series of predisposing factors for COVID-19, such as diabetes and high blood pressure (Mantovani et al., 2020; Taylor et al., 2020). Air pollutants directly damage the vascular system, or indirectly damage it by stimulating inflammatory factors produced by the body, and affect the autonomic nervous system that regulates vascular functions, causing vascular endothelial dysfunction (Fiorito et al., 2018; Miller, 2014; Shanley et al., 2016). Air pollutants affect the insulin signaling pathway related to the onset of diabetes (Alderete et al., 2018; Lucht et al., 2019). Air pollutants also affect the methylation of genes related to the above mechanisms (Li et al., 2018).
Several advantages of these research results cannot be ignored. First, we conducted the first meta-analysis on the association between AP and COVID-19 incidence and mortality. Second, the effects of two types of AP exposure (long- and short-term) on the two outcomes of COVID-19 (incidence and mortality) were included, to comprehensively explore the relationship between AP and COVID-19 risk. Third, no language restriction was applied in the search and selection of items. Fourth, the included effects of AP on COVID-19 were all dose-response, which provides more accuracy than higher vs. lower comparisons. Fifth, all included studies had a low or moderate risk of bias. Finally, 89.5% of the studies of association between long-term AP exposure and COVID-19 risk were ecological, providing less variability in exposure of a population.
There were several disadvantages of this research. First, underestimation of COVID-19 cases might exist in some regions from where the included studies originated, due to inadequate disease surveillance. Second, although some studies controlled for confounders that might have affected outcomes, residual confounding caused by unknown, overlooked, and unmeasured confounders could not be eliminated. Third, diverse healthcare resources accessible to individuals, and authoritative intervention policies in various regions, biased the result validity to different degrees. Fourth, underestimation of the association between pollutants and COVID-19 risk was partly attributed to less-controlled confounders, less-reliable exposure measurement method, and underestimation of COVID-19 cases. Fifth, few studies were eligible for the pooled estimates for the association between pollutants and COVID-19 mortality. Finally, ecological fallacies are often unavoidable and individual-level associations cannot be estimated using ecological research.
5. Conclusions
Due to lethality and high infectiousness, COVID-19 remains a significant threat to public health worldwide. With the continuous spread of COVID-19, AP has been linked to the pandemic in several epidemiological studies, with inconsistent conclusions. Our results demonstrated that air pollutants are detrimental factors to COVID-19 outcomes. Highly polluted and severely COVID-19 burdened, low-/middle-income regions should perform epidemiological research to accurately estimate the detrimental effect of AP on the pandemic risk. The evidence presented here suggests that measurements beneficial to reducing pollutant levels might also reduce the burden of the pandemic.
Author contributions
Zhao Yu-Hong: Conceptualization, Methodology, Resources, Writing - Review & Editing, Supervision, Project administration, Funding acquisition. Chang Qing: Software, Formal analysis, Validation, Writing - Review & Editing, Supervision. Wu Qi-Jun: Conceptualization, Methodology, Writing - Review & Editing, Supervision. Zang Si-Tian: Software, Validation, Formal analysis, Investigation, Data curation, Writing - Original draft preparation, Writing - Review & Editing, Visualization. Luan Jie: Software, Validation, Formal analysis, Investigation, Data curation, Writing - Original draft preparation, Writing - Review & Editing, Visualization. Li Ling: Software, Validation, Formal analysis, Investigation, Data curation, Writing - Original draft preparation, Writing - Review & Editing. Yu Hui-Xin: Software, Validation, Formal analysis, Investigation, Data curation, Writing - Original draft preparation, Writing - Review & Editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank BioMed Proofreading LLC (http://www.biomedproofreading.com) and International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2021.112065.
Funding
This work was supported by the National Key R&D Program of China [grant number 2017YFC0907405]; the LiaoNing Revitalization Talents Program [grant number XLYC1802095]; the Key R&D Program of Liaoning Province [grant number 2019JH8/10300005]; and the Science and Technology Project of Liaoning Province [grant number 2019JH6/10400002].
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Ades A.E., Lu G., Higgins J.P. The interpretation of random-effects meta-analysis in decision models. Med. Decis. Making. 2005;25(6):646–654. doi: 10.1177/0272989X05282643. [DOI] [PubMed] [Google Scholar]
- Adhikari A., Yin J. Short-term effects of ambient ozone, PM and meteorological factors on COVID-19 confirmed cases and deaths in Queens, New York. Int. J. Environ. Res. Publ. Health. 2020;17(11):4047. doi: 10.3390/ijerph17114047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete T.L., Chen Z., Toledo-Corral C.M., Contreras Z.A., Kim J.S., Habre R., Chatzi L., Bastain T., Breton C.V., Gilliland F.D. Ambient and traffic-related air pollution exposures as novel risk factors for metabolic dysfunction and type 2 diabetes. Curr. Epidemiol. Rep. 2018;5(2):79–91. doi: 10.1007/s40471-018-0140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma K., Kagi N., Kim H., Hayashi M. Impact of climate and ambient air pollution on the epidemic growth during COVID-19 outbreak in Japan. Environ. Res. 2020;190:110042. doi: 10.1016/j.envres.2020.110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrantes F.J. Central nervous system targets and routes for SARS-CoV-2: current views and new hypotheses. ACS Chem. Neurosci. 2020;11(18):2793–2803. doi: 10.1021/acschemneuro.0c00434. [DOI] [PubMed] [Google Scholar]
- Becker S., Soukup J.M., Sioutas C., Cassee F.R. Response of human alveolar macrophages to ultrafine, fine, and coarse urban air pollution particles. Exp. Lung Res. 2003;29(1):29–44. doi: 10.1080/01902140303762. [DOI] [PubMed] [Google Scholar]
- Bellocchio L., Bordea I.R., Ballini A., Lorusso F., Hazballa D., Isacco C.G., Malcangi G., Inchingolo A.D., Dipalma G., Inchingolo F., Piscitelli P., Logroscino G., Miani A. Environmental issues and neurological manifestations associated with COVID-19 pandemic: new aspects of the disease? Int. J. Environ. Res. Publ. Health. 2020;17(21):8049. doi: 10.3390/ijerph17218049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K., Romer Present P., Richardson K. Long-term air pollution and other risk factors associated with COVID-19 at the census tract level in Colorado. Environ. Pollut. 2021;287:117584. doi: 10.1016/j.envpol.2021.117584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann D.H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I.C., Chou H.J., Fletcher R.B., Das D., Street K., de Bezieux H.R., Choi Y.G., Risso D., Dudoit S., Purdom E., Mill J., Hachem R.A., Matsunami H., Logan D.W., Goldstein B.J., Grubb M.S., Ngai J., Datta S.R. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020;6(31) doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Cano A.A., Cruz-de la Cruz J.C., Gloria-Alvarado A.B., Alamo-Hernandez U., Riojas-Rodriguez H. Association between Covid-19 mortality and atmospheric pollution in Mexican cities. Salud Publica Mex. 2021;63(4):470–477. doi: 10.21149/12355. [DOI] [PubMed] [Google Scholar]
- Chen J., Hoek G. Long-term exposure to PM and all-cause and cause-specific mortality: a systematic review and meta-analysis. Environ. Int. 2020;143:105974. doi: 10.1016/j.envint.2020.105974. [DOI] [PubMed] [Google Scholar]
- Chen G., Zhang W., Li S., Zhang Y., Williams G., Huxley R., Ren H., Cao W., Guo Y. The impact of ambient fine particles on influenza transmission and the modification effects of temperature in China: a multi-city study. Environ. Int. 2017;98:82–88. doi: 10.1016/j.envint.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- China Daily Virus variant on the move beyond India. 2021. http://www.chinadaily.com.cn/a/202105/12/WS609b2ec5a31024ad0babd557.html/ Available at: Accessed.
- Coker E.S., Cavalli L., Fabrizi E., Guastella G., Lippo E., Parisi M.L., Pontarollo N., Rizzati M., Varacca A., Vergalli S. The effects of air pollution on COVID-19 related mortality in Northern Italy. Environ. Resour. Econ. 2020:1–24. doi: 10.1007/s10640-020-00486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Zhang Z.F., Froines J., Zhao J., Wang H., Yu S.Z., Detels R. Air pollution and case fatality of SARS in the People's Republic of China: an ecologic study. Environ. Health. 2003;2(1):15. doi: 10.1186/1476-069X-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales R., Blanco-Vidal C., Romero-Meza R., Schoen S., Lukina A., Cakmak S. The association between air pollution and COVID-19 related mortality in Santiago, Chile: a daily time series analysis. Environ. Res. 2021;198:111284. doi: 10.1016/j.envres.2021.111284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis E., Renzetti S., Volta M., Donato F., Calza S., Placidi D., Lucchini R.G., Rota M. COVID-19 incidence and mortality in Lombardy, Italy: an ecological study on the role of air pollution, meteorological factors, demographic and socioeconomic variables. Environ. Res. 2021;195:110777. doi: 10.1016/j.envres.2021.110777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M., Davey S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Bodinier B., Whitaker M., Delpierre C., Vermeulen R., Tzoulaki I., Elliott P., Chadeau-Hyam M. COVID-19 mortality in the UK Biobank cohort: revisiting and evaluating risk factors. Eur. J. Epidemiol. 2021;36(3):299–309. doi: 10.1007/s10654-021-00722-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Environment Agency Air quality in Europe—2019 report. 2019. https://www.eea.europa.eu/publications/air-quality-in-europe-2019 Available at: Accessed.
- Fang F., Mu L., Zhu Y., Rao J., Heymann J., Zhang Z.F. Long-term exposure to PM2.5, facemask mandates, stay home orders and COVID-19 incidence in the United States. Int. J. Environ. Res. Publ. Health. 2021;18(12):6274. doi: 10.3390/ijerph18126274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellmeth G., Rose-Clarke K., Zhao C., Busert L.K., Zheng Y., Massazza A., Sonmez H., Eder B., Blewitt A., Lertgrai W., Orcutt M., Ricci K., Mohamed-Ahmed O., Burns R., Knipe D., Hargreaves S., Hesketh T., Opondo C., Devakumar D. Health impacts of parental migration on left-behind children and adolescents: a systematic review and meta-analysis. Lancet. 2018;392(10164):2567–2582. doi: 10.1016/S0140-6736(18)32558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito G., Vlaanderen J., Polidoro S., Gulliver J., Galassi C., Ranzi A., Krogh V., Grioni S., Agnoli C., Sacerdote C., Panico S., Tsai M.Y., Probst-Hensch N., Hoek G., Herceg Z., Vermeulen R., Ghantous A., Vineis P., Naccarati A., EXPOsOMICS consortium Oxidative stress and inflammation mediate the effect of air pollution on cardio- and cerebrovascular disease: a prospective study in nonsmokers. Environ. Mol. Mutagen. 2018;59(3):234–246. doi: 10.1002/em.22153. [DOI] [PubMed] [Google Scholar]
- Frontera A., Cianfanelli L., Vlachos K., Landoni G., Cremona G. Severe air pollution links to higher mortality in COVID-19 patients: the "double-hit" hypothesis. J. Infect. 2020;81(2):255–259. doi: 10.1016/j.jinf.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S., China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnieri M., Balmes J.R. Outdoor air pollution and asthma. Lancet. 2014;383(9928):1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q., Wang X., Gao Y., Zhou J., Huang C., Zhang Z., Chu H. Relationship between particulate matter exposure and female breast cancer incidence and mortality: a systematic review and meta-analysis. Int. Arch. Occup. Environ. Health. 2021;94:191–201. doi: 10.1007/s00420-020-01573-y. [DOI] [PubMed] [Google Scholar]
- Health Effects Institute . 2020. State of Global Air 2020. Special Report.https://www.stateofglobalair.org/ Available at: Accessed. [Google Scholar]
- Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T., Tran T.T.A. Ambient air pollution, meteorology, and COVID-19 infection in Korea. J. Med. Virol. 2021;93:878–885. doi: 10.1002/jmv.26325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T., Nguyen T.Q., Tran T.T.A. Short-term exposure to ambient air pollution in association with COVID-19 of two clusters in South Korea. Trop. Med. Int. Health. 2021;26(4):478–491. doi: 10.1111/tmi.13538. [DOI] [PubMed] [Google Scholar]
- Huang G., Brown P.E. Population-weighted exposure to air pollution and COVID-19 incidence in Germany. Spat. Stat. 2021;41:100480. doi: 10.1016/j.spasta.2020.100480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriti M., Piscitelli P., Missoni E., Miani A. Air pollution and health: the need for a medical reading of environmental monitoring data. Int. J. Environ. Res. Publ. Health. 2020;17(7):2174. doi: 10.3390/ijerph17072174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Xu J. The association between COVID-19 deaths and short-term ambient air pollution/meteorological condition exposure: a retrospective study from Wuhan, China. Air. Qual. Atmos. Health. 2020:1–5. doi: 10.1007/s11869-020-00906-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Wu X.J., Guan Y.J. Effect of ambient air pollutants and meteorological variables on COVID-19 incidence. Infect. Control Hosp. Epidemiol. 2020;41(9):1011–1015. doi: 10.1017/ice.2020.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinoudis G., Padellini T., Bennett J., Davies B., Ezzati M., Blangiardo M. Long-term exposure to air-pollution and COVID-19 mortality in England: a hierarchical spatial analysis. Environ. Int. 2021;146:106316. doi: 10.1016/j.envint.2020.106316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J., Lanphear B.P., Bellinger D., Axelrad D.A., McPartland J., Sutton P., Davidson L., Daniels N., Sen S., Woodruff T.J. Developmental PBDE exposure and IQ/ADHD in childhood: a systematic review and meta-analysis. Environ. Health Perspect. 2017;125(8) doi: 10.1289/EHP1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chen R., Cai J., Cui X., Huang N., Kan H. Short-term exposure to fine particulate air pollution and genome-wide DNA methylation: a randomized, double-blind, crossover trial. Environ. Int. 2018;120:130–136. doi: 10.1016/j.envint.2018.07.041. [DOI] [PubMed] [Google Scholar]
- Linares C., Culqui D., Belda F., López-Bueno J.A., Luna Y., Sánchez-Martínez G., Hervella B., Díaz J. Impact of environmental factors and Sahara dust intrusions on incidence and severity of COVID-19 disease in Spain. Effect in the first and second pandemic waves. Environ. Sci. Pollut. Res. Int. 2021:1–13. doi: 10.1007/s11356-021-14228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Chen G., Huo W., Wang C., Liu S., Li N., Mao S., Hou Y., Lu Y., Xiang H. Associations between long-term exposure to ambient air pollution and risk of type 2 diabetes mellitus: a systematic review and meta-analysis. Environ. Pollut. 2019;252(Pt B):1235–1245. doi: 10.1016/j.envpol.2019.06.033. [DOI] [PubMed] [Google Scholar]
- Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., Simmons R., Cottrell S., Roberts R., O'Doherty M., Brown K., Cameron C., Stockton D., McMenamin J., Ramsay M. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo J.S.L., Tam W.W.S., Seow W.J. Association between air quality, meteorological factors and COVID-19 infection case numbers. Environ. Res. 2021;197:111024. doi: 10.1016/j.envres.2021.111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Wu N., Jiang J., Li X. Associations of acute exposure to airborne pollutants with COVID-19 infection: evidence from China. Environ. Sci. Pollut. Res. Int. 2021:1–11. doi: 10.1007/s11356-021-14159-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht S., Hennig F., Moebus S., Führer-Sakel D., Herder C., Jöckel K.H., Hoffmann B., Heinz Nixdorf Recall Study Investigative Group Air pollution and diabetes-related biomarkers in non-diabetic adults: a pathway to impaired glucose metabolism? Environ. Int. 2019;124:370–392. doi: 10.1016/j.envint.2019.01.005. [DOI] [PubMed] [Google Scholar]
- Ma Y., Cheng B., Shen J., Wang H., Feng F., Zhang Y., Jiao H. Association between environmental factors and COVID-19 in Shanghai, China. Environ. Sci. Pollut. Res. Int. 2021;28(33):45087–45095. doi: 10.1007/s11356-021-13834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A., Byrne C.D., Zheng M.H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr. Metabol. Cardiovasc. Dis. 2020;30(8):1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.R. The role of oxidative stress in the cardiovascular actions of particulate air pollution. Biochem. Soc. Trans. 2014;42:1006–1011. doi: 10.1042/BST20140090. [DOI] [PubMed] [Google Scholar]
- Ministry of Ecology and Environment of the People's Republic of China Bulletin of China's ecological environment. 2021. http://english.mee.gov.cn Available at: Accessed.
- Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad M.H., Mustafa R.A., Schünemann H.J., Sultan S., Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid. Base Med. 2017;22(3):85–87. doi: 10.1136/ebmed-2017-110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to coronavirus (COVID-19) fatality. Sci. Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvera Alvarez H.A., Kubzansky L.D., Campen M.J., Slavich G.M. Early life stress, air pollution, inflammation, and disease: an integrative review and immunologic model of social-environmental adversity and lifespan health. Neurosci. Biobehav. Rev. 2018;92:226–242. doi: 10.1016/j.neubiorev.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K.P., Patel P.A., Vunnam R.R., Hewlett A.T., Jain R., Jing R., Vunnam S.R. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J. Clin. Virol. 2020;128:104386. doi: 10.1016/j.jcv.2020.104386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroni M., Hill D., Younes L., Barkman L., Howard S., Howell B.I., Mirowsky J., Collins M.B. Hazardous air pollutant exposure as a contributing factor to COVID-19 mortality in the United States. Environ. Res. Lett. 2020;15 doi: 10.1088/1748-9326/abaf86. [DOI] [Google Scholar]
- Pranata R., Lim M.A., Yonas E., Vania R., Lukito A.A., Siswanto B.B., Meyer M. Body mass index and outcome in patients with COVID-19: a dose-response meta-analysis. Diabetes Metab. 2021;47(2):101178. doi: 10.1016/j.diabet.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qeadan F., Mensah N.A., Tingey B., Bern R., Rees T., Madden E.F., Porucznik C.A., English K., Honda T. The association between opioids, environmental, demographic, and socioeconomic indicators and COVID-19 mortality rates in the United States: an ecological study at the county level. Arch. Publ. Health. 2021;79(1):101. doi: 10.1186/s13690-021-00626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy R.K., Charles W.N., Sklavounos A., Dutt A., Seed P.T., Khajuria A. The effect of smoking on COVID-19 severity: a systematic review and meta-analysis. J. Med. Virol. 2021;93(2):1045–1056. doi: 10.1002/jmv.26389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Diaz C.E., Guilamo-Ramos V., Mena L., Hall E., Honermann B., Crowley J.S., Baral S., Prado G.J., Marzan-Rodriguez M., Beyrer C., Sullivan P.S., Millett G.A. Risk for COVID-19 infection and death among Latinos in the United States: examining heterogeneity in transmission dynamics. Ann. Epidemiol. 2020;52:46–53. doi: 10.1016/j.annepidem.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Villamizar L.A., Belalcázar-Ceron L.C., Fernández-Niño J.A., Marín-Pineda D.M., Rojas-Sánchez O.A., Acuña-Merchán L.A., Ramírez-García N., Mangones-Matos S.C., Vargas-González J.M., Herrera-Torres J., Agudelo-Castañeda D.M., Piñeros Jiménez J.G., Rojas-Roa N.Y., Herrera-Galindo V.M. Air pollution, sociodemographic and health conditions effects on COVID-19 mortality in Colombia: an ecological study. Sci. Total Environ. 2021;756:144020. doi: 10.1016/j.scitotenv.2020.144020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo M.M. Significance between air pollutants, meteorological factors, and COVID-19 infections: probable evidences in India. Environ. Sci. Pollut. Res. Int. 2021;28(30):40474–40495. doi: 10.1007/s11356-021-12709-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Piedra C., Cruz-Cruz C., Gamiño-Arroyo A.E., Prado-Galbarro F.J. Effects of air pollution and climatology on COVID-19 mortality in Spain. Air Qual. Atmos. Health. 2021:1–7. doi: 10.1007/s11869-021-01062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley L., Schuster L., Marbach H., Forsthuber T., Forbes B., Gant T.W., Sandström T., Camiña N., Athersuch T.J., Mudway I., Kumar A. Brake dust exposure exacerbates inflammation and transiently compromises phagocytosis in macrophages. Metall. 2020;12(3):371–386. doi: 10.1039/c9mt00253g. [DOI] [PubMed] [Google Scholar]
- Setti L., Passarini F., De Gennaro G., Barbieri P., Licen S., Perrone M.G., Piazzalunga A., Borelli M., Palmisani J., Di Gilio A., Rizzo E., Colao A., Piscitelli P., Miani A. Potential role of particulate matter in the spreading of COVID-19 in Northern Italy: first observational study based on initial epidemic diffusion. BMJ Open. 2020;10(9) doi: 10.1136/bmjopen-2020-039338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A.S., Langrish J.P., Nair H., McAllister D.A., Hunter A.L., Donaldson K., Newby D.E., Mills N.L. Global association of air pollution and heart failure: a systematic review and meta-analysis. Lancet. 2013;382(9897):1039–1048. doi: 10.1016/S0140-6736(13)60898-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley R.P., Hayes R.B., Cromar K.R., Ito K., Gordon T., Ahn J. Particulate air pollution and clinical cardiovascular disease risk factors. Epidemiology. 2016;27(2):291–298. doi: 10.1097/EDE.0000000000000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb D.M., Evans G.J., To T.M., Brook J.R., Burnett R.T. An ecological analysis of long-term exposure to PM and incidence of COVID-19 in Canadian health regions. Environ. Res. 2020;191:110052. doi: 10.1016/j.envres.2020.110052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., Moher D., Becker B.J., Sipe T.A., Thacker S.B. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. J. Am. Med. Assoc. 2000;283 doi: 10.1001/jama.283.15.2008. 2008–2012. [DOI] [PubMed] [Google Scholar]
- Taylor E.H., Hofmeyr R., Torborg A., Tonder C.V., Biccard B. Risk factors and interventions associated with mortality or survival in adult COVID-19 patients admitted to critical care: a systematic review and meta-analysis. South. Afr. J. Anaesth. Analg. 2020;26(3):116–127. doi: 10.36303/SAJAA.2020.26.3.2428. [DOI] [Google Scholar]
- Travaglio M., Yu Y., Popovic R., Selley L., Leal N.S., Martins L.M. Links between air pollution and COVID-19 in England. Environ. Pollut. 2021;268:115859. doi: 10.1016/j.envpol.2020.115859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trikalinos T.A., Salanti G., Khoury M.J., Ioannidis J.P. Impact of violations and deviations in Hardy-Weinberg equilibrium on postulated gene-disease associations. Am. J. Epidemiol. 2006;163(4):300–309. doi: 10.1093/aje/kwj046. [DOI] [PubMed] [Google Scholar]
- Tsai T.L., Lin Y.T., Hwang B.F., Nakayama S.F., Tsai C.H., Sun X.L., Ma C., Jung C.R. Fine particulate matter is a potential determinant of Alzheimer's disease: a systemic review and meta-analysis. Environ. Res. 2019;177:108638. doi: 10.1016/j.envres.2019.108638. [DOI] [PubMed] [Google Scholar]
- Valdés Salgado M., Smith P., Opazo M.A., Huneeus N. Long-term exposure to fine and coarse particulate matter and COVID-19 incidence and mortality rate in Chile during 2020. Int. J. Environ. Res. Publ. Health. 2021;18(14):7409. doi: 10.3390/ijerph18147409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venter Z.S., Aunan K., Chowdhury S., Lelieveld J. COVID-19 lockdowns cause global air pollution declines. Proc. Natl. Acad. Sci. U. S. A. 2020;117(32):18984–18990. doi: 10.1073/pnas.2006853117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Liu J., Li Y., Fu S., Xu X., Li L., Zhou J., Liu X., He X., Yan J., Shi Y., Niu J., Yang Y., Li Y., Luo B., Zhang K. Airborne particulate matter, population mobility and COVID-19: a multi-city study in China. BMC Publ. Health. 2020;20(1):1585. doi: 10.1186/s12889-020-09669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Popp A., Schneider A., Breitner S., Hampel R., Rathmann W., Herder C., Roden M., Koenig W., Meisinger C., Peters A., KORA-Study Group Association between long-term exposure to air pollution and biomarkers related to insulin resistance, subclinical inflammation, and adipokines. Diabetes. 2016;65(11):3314–3326. doi: 10.2337/db15-1567. [DOI] [PubMed] [Google Scholar]
- World Health Organization Coronavirus disease (COVID-19) dashboard. 2021. https://covid19.who.int/ Available at: [PubMed]
- Wu X., Nethery R.C., Sabath M.B., Braun D., Dominici F. Air pollution and COVID-19 mortality in the United States: strengths and limitations of an ecological regression analysis. Sci. Adv. 2020;6(45) doi: 10.1126/sciadv.abd4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhan Q., Zhao Q. Long-term air pollution exposure impact on COVID-19 morbidity in China. Aerosol Air Qual. Res. 2020;20(1):200413. doi: 10.4209/aaqr.2020.07.0413. [DOI] [Google Scholar]
- Xu H., Yan C., Fu Q., Xiao K., Cheng J. Possible environmental effects on the spread of COVID-19 in China. Sci. Total Environ. 2020;731:139211. doi: 10.1016/j.scitotenv.2020.139211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Li S., Sun L., Zhang X., Cao Z., Xu C., Cao X., Cheng Y., Yan T., Liu T., Wang Y. Smog and risk of overall and type-specific cardiovascular diseases: a pooled analysis of 53 cohort studies with 21.09 million participants. Environ. Res. 2019;172:375–383. doi: 10.1016/j.envres.2019.01.040. [DOI] [PubMed] [Google Scholar]
- Yao Y., Pan J., Liu Z., Meng X., Wang W., Kan H., Wang W. Temporal association between particulate matter pollution and case fatality rate of COVID-19 in Wuhan. Environ. Res. 2020;189:109941. doi: 10.1016/j.envres.2020.109941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Pan J., Wang W., Liu Z., Kan H., Qiu Y., Meng X., Wang W. Association of particulate matter pollution and case fatality rate of COVID-19 in 49 Chinese cities. Sci. Total Environ. 2020;741:140396. doi: 10.1016/j.scitotenv.2020.140396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Zheng L., Jiang W., Zhang D. Exposure to air pollution and cognitive impairment risk: a meta-analysis of longitudinal cohort studies with dose-response analysis. J. Glob. Health. 2020;10 doi: 10.7189/jogh.10.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Zhang Y., Kwong J.S., Zhang C., Li S., Sun F., Niu Y., Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J. Evid. Base Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- Zhang C., Shi L., Wang F.S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Tang M., Guo F., Wei F., Yu Z., Gao K., Jin M., Wang J., Chen K. Associations between air pollution and COVID-19 epidemic during quarantine period in China. Environ. Pollut. 2021;268(Pt A):115897. doi: 10.1016/j.envpol.2020.115897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17(5):259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P., Chen Z., Liu Y., Song H., Wu C.H., Li B., Kraemer M.U.G., Tian H., Yan X., Zheng Y., Stenseth N.C., Jia G. Association between coronavirus disease 2019 (COVID-19) and long-term exposure to air pollution: evidence from the first epidemic wave in China. Environ. Pollut. 2021;276:116682. doi: 10.1016/j.envpol.2021.116682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J., Qin L., Meng X., Liu N. The interactive effects of ambient air pollutants-meteorological factors on confirmed cases of COVID-19 in 120 Chinese cities. Environ. Sci. Pollut. Res. Int. 2021;28(21):27056–27066. doi: 10.1007/s11356-021-12648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Xie J., Huang F., Cao L. Association between short-term exposure to air pollution and COVID-19 infection: evidence from China. Sci. Total Environ. 2020;727:138704. doi: 10.1016/j.scitotenv.2020.138704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.