Abstract
Background.
Despite the revolutionary role of direct-acting antivirals for hepatitis C virus (HCV), the treatment timing for liver transplant candidates remains controversial. We hypothesize that deferring treatment until after liver transplantation improves access to a larger and higher-quality donor pool without a detrimental impact on post-liver transplantation outcomes.
Methods.
This single-center study includes recipients that underwent deceased-donor liver transplant with HCV as the primary indication January 1, 2014, to December 31, 2018. For recipients that were untreated (n = 87) versus treated (n = 42) pre-LT, we compared post-LT mortality using Cox regression with inverse probability of treatment-weighted data.
Results.
Among pre-LT untreated recipients, 95% were willing to accept an HCV+ donor, and 44.8% received a positive HCV antibody and nucleic acid amplification test (NAT) liver. Among pre-LT treated recipients, 5% were willing to accept an HCV+ donor, and 100% received a negative HCV antibody and NAT liver. The median calculated model for end-stage liver disease at transplant was similar between pre-LT untreated (13, IQR = 9–22) and treated recipients (11, IQR = 8–14) (P = 0.1). Pre-LT treated recipients received livers from older (47 y old versus 37, P < 0.01) and higher body mass index donors (30.2 versus 26.6; P = 0.04) and spent longer on the waiting list (319 d 180, P < 0.001). Unadjusted post-LT mortality at 1 year was higher in the pre-LT treated recipients (14.6% versus 3.5%, P = 0.02). After adjusting for recipient factors, pre-LT treated recipients trended toward a 3.9 times higher risk of mortality compared with the pre-LT untreated recipients (adjusted hazard ratio = 0.973.8615.4) (P = 0.06).
Conclusions.
Deferring HCV treatment improves access to higher-quality donors and may improve post-LT survival.
INTRODUCTION
The approval of direct-acting antivirals (DAAs) in 2013 revolutionized the care of hepatitis C virus (HCV) positive patients due to the higher rates of sustained virologic response.1 Before DAAs, the proportion of people with chronic HCV with cirrhosis was projected to increase from 25% in 2010 to 45% in 2030,2 so the increased effectiveness of DAAs has changed the course of the disease. Currently, HCV is the third leading indication for liver transplantation (LT).3 Based on organ transplantation procurement network (OPTN) data, 21.4% of the people on the liver waiting list in the United States had HCV.4 Although immediate treatment of de novo infection with DAAs is standard of care, treatment timing pretransplant versus deferring until after transplant remains controversial.5
The major advantage to pre-LT treatment is potential clinical improvement, decreased morbidity, and potential elimination of the need for transplant.6,7 Despite the potential benefits, many clinicians defer treatment until post-LT, in hopes that delaying LT may expand access to HCV-positive donor organs and thus reduce waitlist time.6 Additionally, pre-LT treatment may not result in clinically insignificant improvement or preclude the need for transplant due to morbidity from end-stage liver disease sequelae. Because Scientific Registry of Transplant Recipient (SRTR) data do not include treatment status before LT, literature about the comparative outcomes of patients who have been treated pre-LT versus post-LT is lacking, which limits evidence-based clinical decision making about appropriate treatment timing.
We sought to investigate the relationship between pre-LT HCV treatment and donor quality as well as posttransplant survival. We hypothesized that delaying treatment to post-LT would result in LT with higher donor quality and improved post-LT survival.
MATERIALS AND METHODS
Study Population
We studied 129 HCV-positive recipients who underwent deceased-donor liver transplant at our institution between January 1, 2014, and December 31, 2018. Patients were excluded if they were successfully treated and then transplanted with an HCV-positive donor under experimental HCV donor-positive/recipient-negative trials. We excluded transplant recipients who had simultaneous liver kidney transplant or were HIV coinfected. We augmented the cohort with registry data to obtain the additional variables. With regards to center-specific characteristics in 2019, the median MELD at transplant was 29,8 and there were 103 deceased donors,9 12 HCV-positive recipients,9 and 18 HCV-positive donor acceptances.10
Data Source
This study used data from the SRTR. We linked SRTR data using donor ID with our center’s electronic medical record data. The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the OPTN. The HRSA, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This dataset has previously been described elsewhere.11 This study was approved by our Institutional Review Board (IRB00215148).
Recipient demographic data included age, sex, race, and insurance type. Recipient clinical data included body mass index (BMI), diabetes, hepatocellular carcinoma (HCC), calculated and allocation model for end-stage liver disease (MELD) at time of transplant, willingness to accept an HCV-positive donor, and time on waitlist. Donor demographic data included age, sex, and race. Donor clinical data included BMI, donor risk index, diabetes, liver fibrosis stage, HCV-antibody (HCV-ab) status, HCV nucleic acid amplification test (HCV NAT), public health service infectious risk donors, and cold ischemia time.
HCV Treatment
Our cohort of LT candidates with a history of HCV was divided into those that were successfully treated pre-LT versus untreated pre-LT. To be classified as pre-LT treated, an individual had documentation of having received HCV treatment and undetectable levels of HCV RNA levels immediately before transplant. Because the decision on whether or not to treat is typically made by the referring providers before evaluation at our transplant center, we did not have detailed information regarding decision-making factors, medications, or treatment dates.
Post-LT Mortality
We estimated post-LT mortality at 1 y for both pre-LT untreated and pre-LT treated recipients using the Kaplan-Meier method. We applied inverse probability of treatment weighting using a propensity score to make pre-LT untreated versus pre-LT treated recipients comparable with regards to age, sex, and race.12 Using logistic regression, we calculated the probability of receiving treatment to compute the propensity score. The variables for the preliminary model were selected by drawing a conceptual framework.13 Using an a priori conceptual framework, likelihood ratio tests, and Akaike Information Criterion, the final model comprises recipient age, recipient sex, and recipient race. To check whether the 2 populations were comparable, we calculated standardized differences between pre-LT untreated versus pre-LT treated recipients, both before and after weighting. Standardized differences of <0.1 after weighting represented a balance between groups. We also used Cox proportional hazards regression models on weighted data to quantify the adjusted hazard ratio (aHR) of pre-LT untreated compared with pre-LT treated recipients. In addition, we presented standardized cumulative incidence curves adjusted for recipient age, sex, and race, by using the weighted population.
Statistical Analysis
We compared categorical variables between pre-LT untreated versus pre-LT treated using Fisher’s exact test and continuous variables using Wilcoxon rank-sum test. Confidence intervals are reported as per the method of Louis and Zeger.14 All analyses were performed using Stata 14.2/MP for Linux (College Station, TX).
RESULTS
Study Population
Among 129 LT recipients, 87 recipients (67.4%) were pre-LT untreated in comparison to 42 recipients (32.6%) who were pre-LT treated (Table 1). Among pre-LT untreated recipients, 83 (95%) were willing to accept an HCV-positive donor. Of the 87 pre-LT untreated recipients, 39 (44.8%) received an HCV-Ab+/NAT+ liver, 27 (31.0%) received an HCV-Ab-/NAT− liver, 19 (21.8%) received an HCV-Ab+/NAT− liver, and 2 (2.3%) received an HCV-Ab-/NAT+ liver. Among pre-LT treated, 5% were willing to receive an HCV-positive donor. Of the 42 pre-LT treated recipients, 100% received an HCV-Ab-/NAT− liver. Pre-LT untreated and treated recipients were similar in age, sex, race, insurance type, BMI, and diabetes. Although the calculated MELD was similar between the pre-LT untreated and treated recipients (13 versus 11, P = 0.1), the allocation MELD was higher in the pre-LT treated recipients (28 versus 29, P < 0.001). The pre-LT treated recipients had a significantly higher proportion of HCC patients (54% versus 86%, P < 0.001) and spent longer on the waiting list before transplant (180 versus 319 d, P < 0.001). Donor sex, race, donor risk index, diabetes, fibrosis, and cold ischemia time were similar between the 2 groups; however, pre-LT treated recipients received livers from older (47 y old versus 37, P < 0.01) and higher BMI donors (BMI 30.2 versus 26.6, P = 0.04) (Table 1).
TABLE 1.
Recipient and donor characteristics stratified by timing of HCV treatment
| Untreated(n = 87) | Treated(n = 42) | P | |
|---|---|---|---|
| Recipient characteristics | |||
| Age at transplant, median (IQR) | 59 (54, 64) | 62 (57, 65) | 0.2 |
| Male, N (%) | 67 (77) | 34 (81) | 0.7 |
| Race, N (%) | 0.7 | ||
| White or Asian | 64 (74) | 29 (69) | |
| Black | 23 (26) | 13 (31) | |
| Private insurance, N (%) | 37 (43) | 23 (55) | 0.3 |
| BMI, median (IQR) | 27.4 (24.4, 31.5) | 27.2 (24.7, 30.0) | 0.7 |
| Diabetes, N (%) | 17 (20) | 7 (17) | 0.8 |
| HCC, N (%) | 47 (54) | 36 (86) | <0.001 |
| Calculated MELD at transplant, median (IQR) | 13 (9,22) | 11 (8, 14) | 0.1 |
| Allocation MELD at transplant, median (IQR) | 28 (22, 29) | 29 (28, 30) | <0.001 |
| Willing to Accept HCV+ donor, N (%) | 83 (95%) | 2 (5%) | <0.001 |
| Time on waitlist, d, median (IQR) | 180 (30, 277) | 319 (245, 545) | <0.001 |
| Donor characteristics | |||
| Age at procurement, median (IQR) | 37 (31, 49) | 47 (41, 58) | <0.01 |
| Male, N (%) | 50 (57) | 30 (71) | 0.2 |
| Race, N (%) | |||
| White or other | 64 (74) | 27 (64) | 0.3 |
| Black | 23 (26) | 15 (36) | |
| BMI, median (IQR) | 26.6 (23.1, 31.6) | 30.2 (24.8, 33.9) | 0.04 |
| DRI, median (IQR) | 1.32 (1.14, 1.58) | 1.43 (1.14, 1.73) | 0.2 |
| Diabetes, N (%) | 13 (15) | 8 (19) | 0.6 |
| Fibrosis, N (%) | 0.2 | ||
| None | 31 (67) | 14 (82) | |
| Mild | 14 (30) | 2 (12) | |
| Moderate | 1 (2) | 0 | |
| Bridging | 0 | 1 (6) | |
| High macrovesicular steatosisa, N (%) | 4 (8.3)n = 48 | 4 (20.0)n = 20 | 0.2 |
| HCV-antibody status, positive, N (%) | 58 (67) | 0 | <0.001 |
| HCV NAT status, positive, N (%) | 41 | 0 | <0.001 |
| PHS infectious risk donor, N (%) | 58 (67) | 8 (19) | <0.001 |
| Cold ischemia time, median (IQR) | 4.5 (3.5, 6.0) | 4.0 (3.0, 5.3) | 0.2 |
aMacrovesicular steatosis ≥30%.
Bolded text presents P < 0.05.
BMI, body mass index; DAA, direct-acting antiviral; DRI, donor risk index; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; IQR, interquartile range; MELD, model for end-stage liver disease; NAT, nucleic acid amplification test; PHS, public health service.
Post-LT Mortality
Mortality was higher in the pre-LT treated recipients compared with pre-LT untreated recipients (14.6% versus 3.5%, P = 0.02) (Figure 1). Overall, pre-LT treated recipients had a 4.5 times higher risk of post-LT mortality at 12 months compared with the pre-LT untreated recipients (HR = 1.124.4817.9) (P = 0.03). After adjusting for recipient age, sex, and race, pre-LT treated recipients trended toward a 3.9 times higher risk of mortality compared with the pre-LT untreated recipients (aHR = 0.973.8615.4) (P = 0.06).
FIGURE 1.
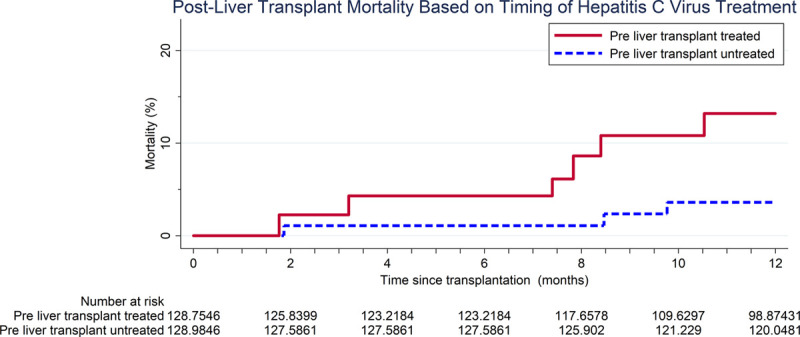
Postliver transplant mortality based on timing of hepatitis C virus treatment.
DISCUSSION
In this single-center retrospective cohort study of 129 LT recipients with a history of HCV, we found pre-LT treated recipients received livers from lower quality donors compared with pre-LT untreated recipients. Further, we found a trend toward a 3.9 times higher risk of 12-month mortality for pre-LT treated patients compared with pre-LT untreated patients, suggesting that deferring treatment not only increases the likelihood of receiving a higher quality liver graft but may also provide survival benefit.
These results are consistent with prior literature demonstrating the advantages of not treating HCV before LT. Coilly et al describe pre-LT treated patients being in “MELD purgatory” because their MELD improves with treatment, but they still require transplant.5,15,16 This “MELD purgatory” of pre-LT treated patients may explain the increased wait time seen in our study. Additionally, Daniel et al highlight the negative consequences of pre-LT HCV treatment, including potentially limiting the donor pool to HCV-negative recipients.7 This remains a concern at many centers in the United States, as HCV-positive donor to HCV-negative recipients remains experimental and not routine at many institutions across the country. Despite the fact that we do perform HCV-positive to HCV-negative transplants, we still saw shorter waitlist times for untreated recipients at our center, suggesting that there are potentially additional advantages to deferring treatment. Shorter waitlist time may be one of the major factors contributing to the trend in improved post-LT survival for pre-LT untreated recipients.
Our hypotheses and findings are important to consider in the context of the United States opioid epidemic. Donor deaths related to overdose have increased from 1.1% in 2000 to 13.4% in 2017, and the population with HCV is increasingly younger.17-19 These changes are leading to a larger donor pool of young,20 HCV-positive donors potentially with less fibrosis.19 This increased access is 1 possible explanation for the younger, healthier organs received by the pre-LT untreated recipients seen in our results. Importantly, the proportion of patients willing to accept HCV-positive organs was only 5% in the treated group, but prior studies have shown patient reluctance to accept high-risk organs even when better outcomes with high-risk organs have been demonstrated.21 We anticipate that patients’ willingness to accept HCV-positive organs in the future will continue to increase.
The effect of donor BMI on survival is unclear in the literature, so the interpretation of the lower donor BMI in the pre-LT untreated recipients is complicated. Bloom et al demonstrated that a lower donor BMI predicted early graft survival.22 Although other studies have found that high BMI does not significantly impact graft primary nonfunction23 nor predict transplant outcomes.24 Notably, Steggerda et al evaluated 60 200 transplants and demonstrated biopsies are being utilized at higher rates in donors with high BMI and improve donor selection.25 Therefore, higher BMI in the pre-LT untreated recipients does not necessarily explain worse post-LT outcomes as biopsies are commonly leveraged in this patient population.
A limitation of this single-center study includes constraints to generalizability due to the retrospective nature and small sample size. A multicenter consortium would provide a larger sample size, thus increasing the power and allowing for more granular subgroup analyses, for example, with patients with HCC. Second, the study was susceptible to selection bias since HCV treatment typically happens before referral to our transplant center. Therefore, we did not have data related to pre-LT decision making (eg, pretreatment MELD), treatment type, or treatment dates. This information would have helped to be more specific about the reasoning and appropriateness in the decision to treat before LT, such as treatment at earlier point in disease when MELD was lower. Given this limitation, we determined the least biased approach was to only evaluate patients that underwent transplant. Therefore, we, unfortunately, did not have waitlist mortality data.
In conclusion, we present the findings of a retrospective single-center studying examining the effect of HCV treatment before LT on post-LT outcomes. We found that deferring treatment to after LT does not negatively affect post-LT survival and is associated with access to higher quality donor livers and shorter waitlist time. Our findings support deferring HCV treatment until after LT.
ACKNOWLEDGMENTS
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government. The data reported here have been supplied by the Hennepin Healthcare Research Institute as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Footnotes
Published online 22 March, 2021
The authors declare no conflicts of interest.
This work was supported by grant number K01KD101677 (Massie) and K23DK115908 (Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). This work was supported by grant number K24AI144954 (Segev) from the National Institute of Allergy and Infectious Diseases (NIAID).
A.T.S. participated in research design, writing of the article, performance of the research, and data analysis. T.I. participated in writing of the article and data analysis. S.W. participated in research design and writing of the article. J.P.H., C.M.D., A.B.M., and D.L.S. participated in research design. C.S. participated in performance of the research. A.G. participated in research design and performance of the research. J.M.GW. participated in research design, writing of the article, performance of the research.
REFERENCES
- 1.Chung RT, Ghany MG, Kim AY, et al. Hepatitis C guidance 2018 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Clin Infect Dis. 2018; 67:1477–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis GL, Alter MJ, El-Serag H, et al. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010; 138:513–521, 521.e1 [DOI] [PubMed] [Google Scholar]
- 3.Cholankeril G, Ahmed A. Alcoholic liver disease replaces hepatitis C virus infection as the leading indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2018; 16:1356–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organ Procurement and Transplantation Network. National data. US Department of Health and Human Services website. 2020. Available at https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/. Accessed September 17, 2020
- 5.Bunchorntavakul C, Reddy KR. Treat chronic hepatitis C virus infection in decompensated cirrhosis—pre- or post-liver transplantation? The ironic conundrum in the era of effective and well-tolerated therapy. J Viral Hepat. 2016; 23:408–418 [DOI] [PubMed] [Google Scholar]
- 6.Chhatwal J, Samur S, Kues B, et al. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology. 2017; 65:777–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel KE, Said A. Considerations when treating hepatitis C in a cirrhotic transplant candidate. Curr Gastroenterol Rep. 2018; 20:20. [DOI] [PubMed] [Google Scholar]
- 8.Organ Procurement and Transplantation Network. Median MELD at transplant by 250 nautical mile circles around liver transplant. 2020. Available at https://optn.transplant.hrsa.gov/media/3667/mts_distribution_document_2020mar5.pdf. Accessed October 12, 2020
- 9.Organ Procurement and Transplantation Network. Center data—OPTN. Published October 27, 2020. Available at https://optn.transplant.hrsa.gov/data/view-data-reports/center-data/. Accessed October 28, 2020
- 10.Scientific Registry of Transplant Recipients (SRTR). Find and compare transplant programs. 2020. Available at https://www.srtr.org/. Accessed October 28, 2020
- 11.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant. 2014; 14:1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin PC, Stryhn H, Leckie G, et al. Measures of clustering and heterogeneity in multilevel Poisson regression analyses of rates/count data. Stat Med. 2018; 37:572–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrier I, Platt RW. Reducing bias through directed acyclic graphs. BMC Med Res Methodol. 2008; 8:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Louis TA, Zeger SL. Effective communication of standard errors and confidence intervals. Biostatistics. 2009; 10:1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coilly A, Roche B, Duclos-Vallée JC, et al. Optimum timing of treatment for hepatitis C infection relative to liver transplantation. Lancet Gastroenterol Hepatol. 2016; 1:165–172 [DOI] [PubMed] [Google Scholar]
- 16.Carrion AF, Khaderi SA, Sussman NL. Model for end-stage liver disease limbo, model for end-stage liver disease purgatory, and the dilemma of treating hepatitis C in patients awaiting liver transplantation. Liver Transpl. 2016; 22:279–280 [DOI] [PubMed] [Google Scholar]
- 17.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006-2012. Clin Infect Dis. 2014; 59:1411–1419 [DOI] [PubMed] [Google Scholar]
- 18.Jalal H, Buchanich JM, Roberts MS, et al. Changing dynamics of the drug overdose epidemic in the United States from 1979 through 2016. Science. 2018; 361:eaau1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand CM, Bowring MG, Thomas AG, et al. The drug overdose epidemic and deceased-donor transplantation in the United States: a National Registry Study. Ann Intern Med. 2018; 168:702–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg DS, Blumberg E, McCauley M, et al. Improving organ utilization to help overcome the tragedies of the opioid epidemic. Am J Transplant. 2016; 16:2836–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowring MG, Holscher CM, Zhou S, et al. Turn down for what? Patient outcomes associated with declining increased infectious risk kidneys. Am J Transplant. 2018; 18:617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bloom MB, Raza S, Bhakta A, et al. Impact of deceased organ donor demographics and critical care end points on liver transplantation and graft survival rates. J Am Coll Surg. 2015; 220:38–47 [DOI] [PubMed] [Google Scholar]
- 23.Yoo HY, Molmenti E, Thuluvath PJ. The effect of donor body mass index on primary graft nonfunction, retransplantation rate, and early graft and patient survival after liver transplantation. Liver Transpl. 2003; 9:72–78 [DOI] [PubMed] [Google Scholar]
- 24.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006; 6:783–790 [DOI] [PubMed] [Google Scholar]
- 25.Steggerda JA, Kim IK, Malinoski D, et al. Regional variation in utilization and outcomes of liver allografts from donors with high body mass index and graft macrosteatosis: a role for liver biopsy. Transplantation. 2019; 103:122–130 [DOI] [PubMed] [Google Scholar]


