Supplemental Digital Content is available in the text.
Abstract
Background.
While liver transplantation (LT) with neoadjuvant chemoradiation is increasingly utilized for the management of unresectable cholangiocarcinoma (CCA), data on post-LT survival are limited.
Methods.
We identified 844 patients who underwent LT (2002–2019) for nonincidental (CCA listing) or incidental (CCA on explant, not at listing) CCA in the Scientific Registry of Transplant Recipients. Kaplan–Meier and multivariable proportional hazards regression methods evaluated the effects of patient characteristics, donor type, transplant era (before/after 2010), and center volume (center-level CCALTs/active year) on the risk of graft failure and patient mortality.
Results.
One center performed >12 CCALTs/y, and the rest performed ≤4. Five-year graft survival was 50.6%. Multivariable models demonstrated laboratory model of end-stage liver disease ≥40 versus <15 and center volumes of 1, >1 to ≤2, and >2 to ≤4 CCALTs/y compared to >12 were associated with increased risk of graft failure and mortality (all P ≤ 0.002). Extra vessel use was associated with center volume. Among all recipients, extra vessel use occurred in 55.4% of CCALTs performed at the highest volume center and in 14.0% of cases at centers having ≤4 CCAs/y (P < 0.05).
Conclusions.
Center volume-related differences in outcomes and extra vessel use highlight the importance of establishing a unified, effective treatment protocol and the potential utility of regionalization of LT for CCA.
INTRODUCTION
Hilar cholangiocarcinoma (CCA) was considered a contraindication to liver transplantation (LT) until the introduction of neoadjuvant radiochemotherapy reported in the early 2000s by the University of Nebraska (protocol initiated in 1987)1 and the Mayo Clinic Rochester (protocol initiated in 1993).2 Subsequent implementation and optimization of this concept by the Mayo Clinic Rochester allowed for the broader utilization of LT for hilar CCA with improved survival that eventually became known as the “Mayo” protocol.3-8 In light of these results, effective January 2010, the United Network for Organ Sharing (UNOS) Board of Directors decided that Model of End-Stage Liver Disease (MELD) score exception points would be allocated to hilar CCA patients listed for LT only after completion of neoadjuvant treatment.9,10
The small number of candidates with potentially transplantable hilar CCA and the complexity of the neoadjuvant protocol limits the total number of CCA LTs performed in the United States. Although many centers offer LT for CCA, only a few have accumulated an extensive experience and the reproducibility of their outcomes by less-experienced centers remains questionable. A 2012 study of 12 centers in the United States reported an 5-y post-LT recurrence-free survival of 65% with no detectable difference in outcomes between the highest volume center versus the remaining 11 centers (68% versus 60%, respectively).11 However, using Scientific Registry of Transplant Recipients (SRTR) data (2002–2017), we recently reported estimated 5-y graft survival to be 50.1% after deceased donor LT (DDLT) among 212 patients with CCA.12 It is apparent that the outcomes of certain well-experienced centers may deviate significantly from the national average, yet no data are available to elucidate the factors contributing to this discrepancy.
The present study aimed to: (1) quantify post-LT graft and patient survival in CCA patients in the MELD era and (2) evaluate the effects of CCA LT center volume, transplant era, donor type, and patient characteristics on post-LT graft and patient survival.
MATERIALS AND METHODS
Data Source, Inclusion Criteria, and Data Encoding
This study used data from the SRTR. The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This study was approved by the Vanderbilt Institutional Review Board.
We identified the records, in the September 2019 release of the SRTR Standard Analysis Files, of all adults (age ≥ 18 y) undergoing a primary DDLT or living donor liver transplant (LDLT) between March 2002 and August 2019 with a primary or secondary diagnosis of CCA. Recipients were considered to have a nonincidental CCA, if CCA was a listing diagnosis and was also recorded posttransplant. Recipients were considered to have an incidental CCA if they were listed for another indication but CCA was identified only among transplant recipient diagnosis variables.
Center-level CCA volume was defined as the number of CCA LTs per unique center per active year (the number of calendar years during which a given center performed at least 1 CCA LT between March 2002 and August 2019). After inspecting the positively skewed frequency distribution (percentile ranks and extremes), the 94 unique centers performing CCA LTs at any time during the study period were classified based on their CCA LT volume per active year as: 1 (lower extreme, 43rd percentile rank), >1 to ≤2 (44th to 92nd percentile ranks), >2 to ≤4 (93rd to 99th percentile ranks), and >12 (upper extreme, 1.1% of centers; a single center), and as either ≤4 or >12. These center volume classification variables were linked with individual recipient records via SRTR center codes. Laboratory MELD at the time of LT was treated as a continuous variable and was also stratified as <15, 15–19, 20–24, 25–29, 30–34, 35–39, and ≥40. Waiting time (in months) was computed from transplant and listing dates and treated as a continuous variable. Transplant era was dichotomized as before or after January 18, 2010 based on the UNOS policy regarding MELD exception points for candidates with CCA.10 Use of extra vessels during the transplant procedure, which was recorded in the SRTR database as no, yes, unknown, not applicable, or missing (ie, nothing recorded), was encoded for a secondary analysis as no, yes, not applicable, and unknown/missing.
Statistical Analysis
Data were summarized and between-group comparisons of recipient, donor, and technical characteristics were performed using parametric and nonparametric methods, as appropriate. The Kaplan–Meier method with log-rank tests was used to evaluate graft survival after LT for CCA and the unadjusted effects of donor type, incidental or nonincidental diagnosis, and center-level CCA LT volume strata on graft survival. Multivariable Cox proportional hazards regression models evaluated the effects of center-level CCA volume strata, waiting time, age, gender, donor type (LDLT or DDLT), incidental or nonincidental diagnosis of CCA, laboratory MELD strata, whether MELD exception points were granted, transplant era, and whether there was an MELD exception by era interaction effect on graft and patient survival. Dataset development and statistical analyses were performed using SPSS Statistics software (version 26.0, IBM, Armonk, NY) and Stata (version 15.1, StataCorp, College Station, TX). All tests were 2-sided and P values <0.05 were considered statistically significant.
RESULTS
The study cohort consisted of 844 CCA LT recipients, of whom 432 (51.2%) received DDLT and 100 (11.8%) LDLT for nonincidental CCA, and 286 (33.9%) received DDLT and 26 (3.1%) LDLT for incidental CCA (Table 1). In general, incidental CCA LT recipients were older and had higher MELD scores and longer waiting times than nonincidental CCA LT recipients (all P < 0.001). The annual number of CCA LTs and the number of centers performing LT for CCA in a given year increased significantly over the period of this study (both overall temporal trend rho ≥ 0.85), with the greatest rate of increase for both measures occurring in the pre-2010 era (Figure 1). The majority of CCA LT recipients (71.6%) received MELD exception points and two-thirds of all CCA LTs were performed after implementation of the January 2010 exception pathway. Overall graft survival is depicted in Figure 2. Among all 844 recipients, overall 1-, 3-, and 5-y graft survival point estimates were 83.1%, 61.8%, 50.6%, respectively. In unadjusted analysis, graft survival after DDLT for incidental CCA was reduced compared to both DDLT and LDLT for nonincidental CCA (both P = 0.003) (Figure 3).
TABLE 1.
Cohort characteristics
| Variable | Incidental living donor (n = 26) | Incidental deceased donor (n = 286) | Nonincidental living donor (n = 100) | Nonincidental deceased donor (n = 432) | P | Total (n = 844) |
|---|---|---|---|---|---|---|
| Age (y) | 53.5 ± 12.3 | 57.1 ± 9.7a,b | 50.3 ± 11.5a | 53.4 ± 11.1b | <0.001 | 54.3 ± 10.9 |
| Gender | 0.24 | |||||
| Female | 11 (42.3) | 72 (25.2) | 31 (31.0) | 123 (28.5) | 237 (28.1) | |
| Male | 15 (57.7) | 214 (74.8) | 69 (69.0) | 309 (71.5) | 607 (71.9) | |
| MELD exception | 13 (50.0)a | 173 (60.5)b | 66 (66.0)c | 352 (81.5)a,b,c | <0.001 | 604 (71.6) |
| Transplant era | 0.23 | |||||
| Through January 17, 2010 | 8 (30.8) | 97 (33.9) | 42 (42.0) | 135 (31.3) | 282 (33.4) | |
| Beginning January 18, 2010 | 18 (69.2) | 189 (66.1) | 58 (58.0) | 297 (68.8) | 562 (66.6) | |
| Laboratory MELD score strata | ||||||
| <15 | 17 (65.4) | 126 (44.1) | 81 (81.0) | 273 (63.2) | 497 (58.9) | |
| 15–19 | 4 (15.4) | 52 (18.2) | 13 (13.0) | 63 (14.6) | 132 (15.6) | |
| 20–24 | 4 (15.4) | 41 (14.3) | 6 (6.0) | 57 (13.2) | 108 (12.8) | |
| 25–29 | 1 (3.8) | 25 (8.7) | 0 (0.0) | 15 (3.5) | 41 (4.9) | |
| 30–34 | 0 (0.0) | 14 (4.9) | 0 (0.0) | 9 (2.1) | 23 (2.7) | |
| 35–39 | 0 (0.0) | 14 (4.9) | 0 (0.0) | 9 (2.1) | 23 (2.7) | |
| ≥40 | 0 (0.0) | 14 (4.9) | 0 (0.0) | 6 (1.4) | 20 (2.4) | |
| Laboratory MELD score | 12.9 ± 6.0a | 18.0 ± 9.9a,b | 10.4 ± 4.8b | 13.7 ± 7.9b | <0.001 | 14.8 ± 8.7 |
| Center-level CCA LTs per active year | <0.001 | |||||
| 1 | 3 (11.5)a | 54 (18.9)b,c | 2 (2.0)a,b | 25 (5.8)c | 84 (10.0) | |
| >1 to ≤2 | 15 (57.7)a | 159 (55.6)b | 17 (17.0)a,b | 147 (34.0)a,b | 338 (40.0) | |
| >2 to ≤4 | 4 (15.4) | 63 (22.0)a | 5 (5.0)a | 126 (29.2)a | 198 (23.5) | |
| >12 | 4 (15.4)a | 10 (3.5)a,b | 76 (76.0)a,b | 134 (31.0)b | 224 (26.5) | |
| Waiting time (mo) | 12.4 ± 16.4a | 9.3 ± 18.6b | 4.4 ± 3.0a,b | 6.9 ± 6.2 | 0.001 | 7.6 ± 12.2 |
Tables entries are mean ± SD or frequency (%).
Column percentages or means differ (P < 0.05) a vs a, b vs b, and c vs c.
CCA, cholangiocarcinoma; LT, liver transplant; MELD, Model for End-Stage Liver Disease.
FIGURE 1.
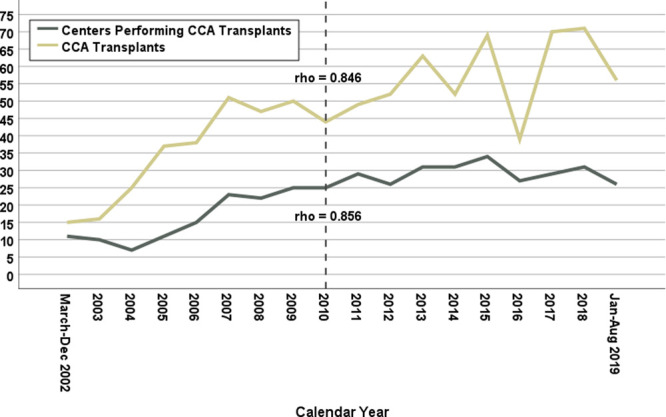
Temporal trends of numbers of liver transplants performed for cholangiocarcinoma (gold line) and of transplant centers performing liver transplant for cholangiocarcinoma (gray line) by calendar year. Both metrics showed a significant overall increase with the steepest increases occurring before 2010. CCA, cholangiocarcinoma.
FIGURE 2.
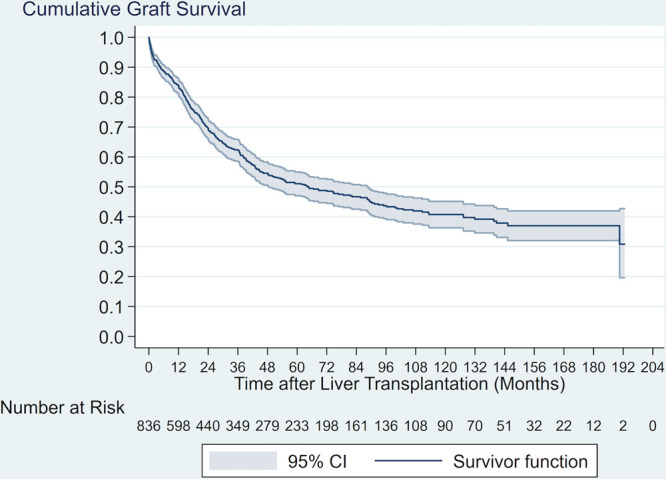
Kaplan–Meier graft survival curve in cholangiocarcinoma liver transplant recipients. CI, confidence interval.
FIGURE 3.
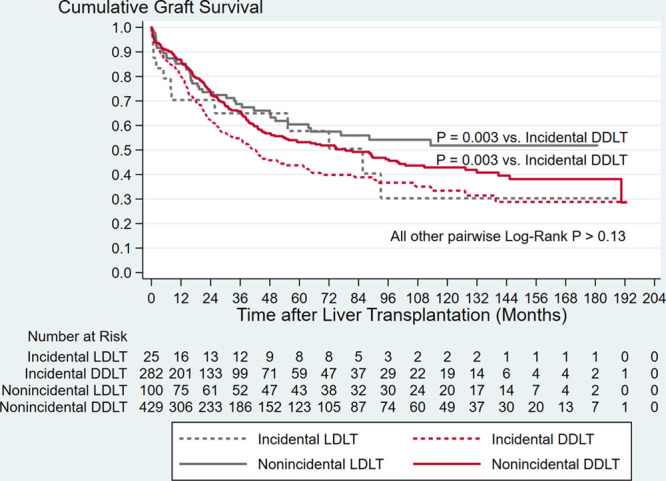
Kaplan–Meier graft survival curves demonstrating differences by donor type and by incidental vs nonincidental cholangiocarcinoma diagnosis. Graft survival after deceased donor liver transplant for incidental cholangiocarcinoma was inferior compared to both deceased donor and living donor liver transplant for nonincidental cholangiocarcinoma. DDLT, deceased donor LT; LDLT, living donor liver transplant; LT, liver transplantation.
Among the 94 unique centers performing CCA LT at any time over the course of this study, the median number of active CCA LT years was 3.0 (minimum, 0.7; maximum, 17.7). Classifying each LT record based on its associated center’s CCA experience stratum demonstrated that 26.5% of all CCA LTs were associated with a single center that performed >12 (specifically, 12.7) CCA LTs per active year, with 50% of all CCA LTs being performed at centers performing ≤ 2 per active year (Table 1). Multivariable models (Table 2 and Table S1, SDC, http://links.lww.com/TXD/A317) demonstrated that center experience and MELD were independently associated with graft and patient survival (main effects P ≤ 0.002). Laboratory MELD ≥40 was associated with increased risk of graft failure and patient mortality compared to MELD <15. In comparison to (the single center) performing >12 CCA LTs per active year, center experiences of 1, >1 to ≤2, and >2 to ≤4 CCA LTs per active year were associated with between 1.8 and 2.3 times the overall risk of graft failure (all P ≤ 0.002) and between 1.9 and 2.6 the overall risk of patient mortality (all P ≤ 0.002). The overlapping confidence intervals for the hazard ratios between the 3 lower center experience strata indicated an absence of an ordinal effect of increasing risk compared to >12 CCA LTs per active year. In unadjusted analysis, the highest center volume stratum (>12 CCA LTs per active year) was associated with superior graft survival compared to the 3 lower center volume strata combined (≤4 CCA LTs per active year) (P < 0.001). Based on the method of confidence intervals, a significant difference in survival was apparent at approximately 6 mo post-LT, which persisted over time (Figure 4). Among all 844 recipients, point estimates of 1-, 3-, and 5-y graft survival were 90.0%, 73.1%, and 66.2% for persons transplanted at the center performing >12 CCA LTs per active year. Corresponding point estimates were 80.7%, 57.5%, and 44.3% for persons transplanted at centers performing ≤4 CCA LTs per active year.
TABLE 2.
Multivariable cox proportional hazards regression model for the risk of graft failure
| Variable | Estimate | P | Hazard ratio | 95% CI | |
|---|---|---|---|---|---|
| Lower bound | Upper bound | ||||
| Age (y) | −0.01 | 0.30 | 1.00 | 0.99 | 1.01 |
| Male gender (ref: female) | −0.18 | 0.13 | 0.84 | 0.67 | 1.05 |
| Living donor (ref: deceased) | 0.06 | 0.72 | 1.07 | 0.76 | 1.50 |
| Incidental CCA (ref: no) | 0.09 | 0.43 | 1.10 | 0.87 | 1.39 |
| MELD exception (ref: no) | −0.13 | 0.48 | 0.88 | 0.62 | 1.25 |
| Recent era (ref: before January 18, 2010) | −0.20 | 0.30 | 0.82 | 0.57 | 1.19 |
| MELD exception by era interaction effect | 0.18 | 0.44 | 1.19 | 0.77 | 1.86 |
| Laboratory MELD score (ref: <15) | 0.001 | ||||
| 15–19 | 0.32 | 0.03 | 1.37 | 1.02 | 1.83 |
| 20–24 | 0.32 | 0.053 | 1.38 | 0.995 | 1.91 |
| 25–29 | 0.37 | 0.12 | 1.45 | 0.91 | 2.32 |
| 30–34 | 0.03 | 0.93 | 1.03 | 0.49 | 2.19 |
| 35–39 | −0.15 | 0.70 | 0.86 | 0.41 | 1.81 |
| ≥40 | 1.27 | <0.001 | 3.56 | 1.99 | 6.38 |
| Center-level CCA LTs per active year (ref: >12) | <0.001 | ||||
| 1 | 0.85 | <0.001 | 2.33 | 1.52 | 3.57 |
| >1 and ≤2 | 0.66 | <0.001 | 1.93 | 1.41 | 2.65 |
| >2 and ≤4 | 0.58 | 0.002 | 1.78 | 1.24 | 2.58 |
| Waiting time (mo) | 0.00 | 0.81 | 1.00 | 0.99 | 1.01 |
CCA, cholangiocarcinoma; CI, confidence interval; LT, liver transplantation; MELD, Model for End-Stage Liver Disease; ref, reference group.
FIGURE 4.
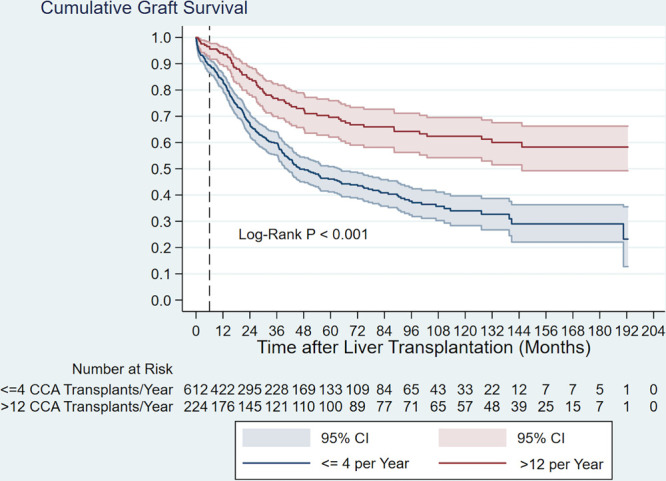
Kaplan–Meier graft survival curves demonstrating differences in outcomes by center volume strata. Graft survival was better overall among transplants that were performed at the center performing >12 cholangiocarcinoma transplants per activey, with the curves diverging at approximately month 6. CCA, cholangiocarcinoma; CI, confidence interval.
A secondary analysis demonstrated that center experience was associated with the use of extra vessels during the transplant procedure (Figure 5). Proportions of transplants across reported extra vessel use categories (yes, no, not applicable, and unknown/missing) differed by whether the transplant was performed at a center in the highest CCA volume stratum (>12 per active year) or in any of the lower strata (≤4 per active year) (overall chi-square test P < 0.001). Tests of column percentages for each extra vessel use category demonstrated that the use of extra vessels, which was reported among 55.4% of recipients transplanted at the highest volume center, was significantly higher (P < 0.05) compared to the 14.0% of recipients transplanted at centers performing ≤4 CCA LTs per active year. Not using extra vessels was specifically reported among 21.9% of recipients transplanted at the highest volume center and among 59.7% of recipients transplanted at centers performing ≤4 CCA LTs per active year (P < 0.05).
FIGURE 5.
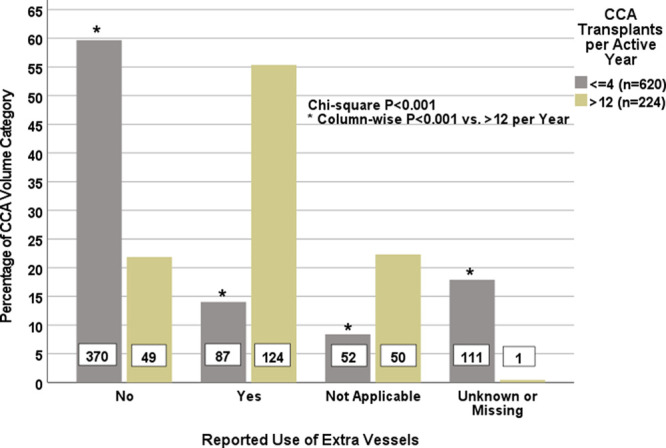
Extra vessel use (yes, no, not applicable, and unknown/missing) within cholangiocarcinoma transplant volume categories (>12 or ≤4 per active year). Percentages total 100% within each volume category. Asterisks indicate statistically significant (P < 0.05) comparisons between volume categories for each response category. CCA, cholangiocarcinoma.
DISCUSSION
In January of 2010, UNOS approved a MELD exception scheme for the treatment of hilar CCA with LT based on initial reports demonstrating a 5-y survival of 76%–82%.3-5,9,10 To qualify for an exception, a candidate has to meet the following criteria: unresectable malignant-appearing hilar stricture or tumor radial diameter <3 cm without regional or distant metastasis and either biopsy or cytology confirmed malignancy, a carbohydrate antigen 19-9 >100 U/mL without cholangitis or aneuploidy and receipt of neoadjuvant chemoradiation with imaging workup and staging laparotomy before LT.3-8,13,14 Since the first implemented exception, over 90 centers have performed an LT for hilar CCA. Although LT for hilar CCA represents <1% of all adult LTs performed in the United States (based on OPTN data: 111,506 adult LTs performed between 2002 and 2019), it is important to identify factors associated with favorable outcomes for this unique group of patients undergoing a complex pre-LT selection and treatment protocol.
In the present study, we found center experience to be a key covariable associated with graft survival. This is in accordance with a smaller study of 155 LTs for CCA in which experience was stratified based on the overall number of LT for CCA performed over a 7-y study period (2010–2017).15 The less-experienced group (n = 23 centers; <6 CCA LTs per center over the study period) demonstrated an increased risk of patient mortality compared to the well-experienced group (n = 7 centers; ≥6 CCA LTs per center over the study period).15 However, no difference in patient survival was seen between the highest volume center and the group of other well-experienced centers.15 In contrast, we defined experience as center-level CCA LTs performed per active year. This allowed adjustment for personnel and programmatic changes that could affect or transfer experience between centers. Only 1 center in the United States performed >12 CCA recipients per active year with all other centers transplanting 4 or fewer recipients per active year. We found the risk of graft failure in each of the lower-volume center strata to be nearly double that of the solitary high-volume center when adjusting for several clinically important covariates. We also found that a marginal increase in the number of CCA patients transplanted per active year did not lead to a significant reduction in the risk of graft failure.
The importance of center experience on post-LT outcomes is likely multifactorial. The successful transplantation of a candidate with hilar CCA requires careful pre-LT coordination among multiple teams outside of the immediate transplant sphere including medical oncology, radiation oncology, and advanced biliary endoscopy to comply with the UNOS requirement for neoadjuvant treatment before LT.10,13 The Mayo Clinic neoadjuvant treatment protocol includes: (1) high-dose external beam radiation (4500 cGy); (2) intravenous 5-fluorouracil; (3) either high-dose brachytherapy or stereotactic body radiation therapy (3000 cGy) or proton-beam therapy if brachytherapy cannot be delivered; and (4) oral capecitabine maintenance administered to eligible patients while on the LT waitlist.8,16 However, UNOS does not specify the exact neoadjuvant protocol resulting in considerable variability among transplant centers. In the 2012 multicenter study of LT for CCA, only 3 of 12 centers (25%) utilized the Mayo Clinic protocol with an unknown impact on long-term recurrence-free survival.11
Additionally, there are unique technical challenges with the transplant operation of CCA related to the irradiated field which damages the endothelial cells and the arterial wall.17,18 The initial experience utilizing the irradiated recipient hepatic artery during CCA LT was associated with an increased incidence of hepatic artery thrombosis. This experience resulted in the routine use of arterial conduits when available for DDLT.2 The short recipient portal vein resulting from the oncologically driven low hilar dissection in combination with radiation injury resulted in an increased incidence of portal vein thrombosis. The experience resulted in the routine use of deceased donor iliac vein extension conduits for LDLT recipients.19 Despite these technical adjustments, 21% of patients had hepatic artery complications, and 22% had portal vein thrombosis.20 In our study, we corroborated the frequent use of extra vessels by the highest volume center and infrequent use of extra vessels by all other centers. Additionally, the accumulated experience of LT for CCA at Mayo Clinic Rochester led them to the implementation of a close follow-up protocol including surveillance with duplex Doppler ultrasonography on post-LT days 0, 1, 4, 7, 21, at 4 mo and then annually to promptly diagnose and intervene on vascular complications and avoid graft failure.8,19,20 These nonstandard technical variations and the early post-LT vigilance for potential vascular complications are the result of an accumulated experience and may partly explain the immediate divergence of graft survival curves in our study, reflecting early perioperative morbidity and mortality.
Our study has several strengths including a robust methodology to dynamically address the impact of center volume changes over time. Additionally, our analysis included all recipients with a CCA diagnosis, but examined graft survival specific to incidental versus nonincidental diagnosis and by donor type, to capture all primary CCA LTs performed in the United States over a 17+ y study period. The incidental DDLT, in fact, had lower graft survival compared to the nonincidental diagnosis groups. Our analysis then adjusted for incidental and nonincidental CCA diagnosis, as well as for whether DDLT or LDLT. We also examined the differences in utilization of extra vessels during the transplant procedure based on center volume as a plausible explanation for differing outcomes. However, transplant registries lack robust detail for several variables of interest including whether there is underlying primary sclerosing cholangitis versus de novo CCA, hilar versus intrahepatic CCA, tumor size, administration of neoadjuvant treatment and the specifications of the regimen which may vary across centers, carbohydrate antigen 19-9 level, more detailed transplant procedure variations, and consistent reporting of tumor recurrence data. More detailed data are needed for patients listed and/or transplanted for CCA to better understand the risk factors associated with poor survival and factors that may ensure a successful treatment approach.
In conclusion, our findings demonstrate that center experience is significantly associated with survival after LT for CCA. With the exception of laboratory MELD score, recipient characteristics, donor type, transplant era, waiting time, and time of diagnosis (incidental or not incidental) were not identified as risk factors for graft failure or patient mortality. The limited number of suitable candidates with CCA that is treatable with LT has resulted in a concentrated experience at a single center with other centers in the United States performing substantially fewer CCA LTs per active year. The significant difference in the utilization of extra vessels between the highest volume center and all other centers suggests an experience-based change of practice by the highest volume center(s), which may explain the early divergence in post-LT graft survival. Improved communication and emulation of the practices associated with successful CCA LT at high-volume centers or regionalization of care may improve outcomes after LT for CCA.
Supplementary Material
Footnotes
Published online 22 March, 2021
I.A.Z. participated in analysis and interpretation of data, manuscript drafting and critical revision, and final approval. M.A.R., L.K.M., and M.I. participated in analysis and interpretation of data, critical revision, and final approval. S.A.R. participated in acquisition, analysis and interpretation of data, critical revision, and final approval. I.D.F. participated in project conception/design, acquisition, analysis and interpretation of data, critical revision, and final approval. S.P.A. participated in project conception/design, analysis and interpretation of data, manuscript drafting and critical revision, and final approval.
The authors declare no funding or conflicts of interest.
As a condition of the SRTR data use agreement for Standard Analysis Files, we note the following. The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002; 2:774–779 [DOI] [PubMed] [Google Scholar]
- 2.De Vreede I, Steers JL, Burch PA, et al. Prolonged disease-free survival after orthotopic liver transplantation plus adjuvant chemoirradiation for cholangiocarcinoma. Liver Transpl. 2000; 6:309–316 [DOI] [PubMed] [Google Scholar]
- 3.Heimbach JK, Gores GJ, Haddock MG, et al. Liver transplantation for unresectable perihilar cholangiocarcinoma. Semin Liver Dis. 2004; 24:201–207 [DOI] [PubMed] [Google Scholar]
- 4.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005; 242:451–458. Discussion 458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heimbach JK, Gores GJ, Haddock MG, et al. Predictors of disease recurrence following neoadjuvant chemoradiotherapy and liver transplantation for unresectable perihilar cholangiocarcinoma. Transplantation. 2006; 82:1703–1707 [DOI] [PubMed] [Google Scholar]
- 6.Rosen CB, Darwish Murad S, Heimbach JK, et al. Neoadjuvant therapy and liver transplantation for hilar cholangiocarcinoma: is pretreatment pathological confirmation of diagnosis necessary? J Am Coll Surg. 2012; 215:31–38. Discussion 38–40 [DOI] [PubMed] [Google Scholar]
- 7.Darwish Murad S, Kim WR, Therneau T, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology. 2012; 56:972–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan EK, Taner T, Heimbach JK, et al. Liver transplantation for peri-hilar cholangiocarcinoma. J Gastrointest Surg. 2020; 24:2679–2685 [DOI] [PubMed] [Google Scholar]
- 9.Gores GJ, Gish RG, Sudan D, et al. ; MELD Exception Study Group. Model for end-stage liver disease (MELD) exception for cholangiocarcinoma or biliary dysplasia. Liver Transpl. 2006; 1212 Suppl 3S95–S97 [DOI] [PubMed] [Google Scholar]
- 10.United Network for Organ Sharing Liver/Intestine, News.. Submitting standardized MELD/ PELD exception scores. 2010. Available at https://unos.org/news/submitting-standardized-meldpeld-exception-scores/. Accessed February 10, 2021
- 11.Darwish Murad S, Kim WR, Harnois DM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012; 143:88–98.e3. Quiz e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ziogas IA, Hickman LA, Matsuoka LK, et al. Comparison of wait-list mortality between cholangiocarcinoma and hepatocellular carcinoma liver transplant candidates. Liver Transpl. 2020; 26:1112–1120 [DOI] [PubMed] [Google Scholar]
- 13.Organ Procurement and Transplantation Network.. Policy 9: Allocation of livers. 9.5.A Requirements for Cholangiocarcinoma (CCA) MELD or PELD Score Exceptions. 2021. Available at https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Accessed February 10, 2021
- 14.Mantel HT, Westerkamp AC, Adam R, et al. ; European Liver and Intestine Transplant Association (ELITA). Strict selection alone of patients undergoing liver transplantation for hilar cholangiocarcinoma is associated with improved survival. PLoS One. 2016; 11:e0156127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitajima T, Hibi T, Moonka D, et al. Center experience affects liver transplant outcomes in patients with hilar cholangiocarcinoma. Ann Surg Oncol. 2020; 27:5209–5221 [DOI] [PubMed] [Google Scholar]
- 16.Lehrke HD, Heimbach JK, Wu TT, et al. Prognostic significance of the histologic response of perihilar cholangiocarcinoma to preoperative neoadjuvant chemoradiation in liver explants. Am J Surg Pathol. 2016; 40:510–518 [DOI] [PubMed] [Google Scholar]
- 17.Fajardo LF, Prionas SD, Kowalski J, et al. Hyperthermia inhibits angiogenesis. Radiat Res. 1988; 114:297–306 [PubMed] [Google Scholar]
- 18.Halle M, Christersdottir T, Bäck M. Chronic adventitial inflammation, vasa vasorum expansion, and 5-lipoxygenase up-regulation in irradiated arteries from cancer survivors. Faseb J. 2016; 30:3845–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan EK, Rosen CB, Heimbach JK, et al. Living donor liver transplantation for perihilar cholangiocarcinoma: outcomes and complications. J Am Coll Surg. 2020; 231:98–110 [DOI] [PubMed] [Google Scholar]
- 20.Mantel HT, Rosen CB, Heimbach JK, et al. Vascular complications after orthotopic liver transplantation after neoadjuvant therapy for hilar cholangiocarcinoma. Liver Transpl. 2007; 13:1372–1381 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


