Abstract
PURPOSE
We present a physician survey of the impact of 21-gene Breast Recurrence Score test results on treatment decisions in clinical practice in Latin America.
METHODS
This prospective survey enrolled consecutive patients at 14 sites in Argentina, Colombia, Mexico, and Peru who had routine 21-gene testing. Physician surveys captured patient and tumor characteristics and treatment decisions before and after 21-gene test results. The survey spanned the period before and after Trial Assigning Individualized Options for Treatment (TAILORx) results reported (June 2018). Overall net percent change in adjuvant chemotherapy recommendations was estimated, and asymptotic 95% CIs with continuity correction were calculated. The proportion with a change between pretest treatment recommendation and actual treatment received was calculated overall and by Recurrence Score groups per TAILORx.
RESULTS
Between March 2015 and December 2019, the survey was completed for 647 patients; 20% were node-positive. The mean patient age was 54 years (24-85 years); 55% were postmenopausal; 17%, 63%, and 20% had grade 1, 2, and 3 tumors, respectively; and 30% had tumors > 2 cm. Recurrence Score (RS) results were as follows: 20% RS 0-10, 56% RS 11-25, and 24% RS 26-100. Overall, chemotherapy recommendations fell by a relative proportion of 39% (95% CI, 33.4 to 44.3) after 21-gene testing (33% decrease in node-negative and 55% decrease in node-positive). Among node-negative patients, the relative decrease in chemotherapy recommendations was 28% (95% CI, 18.9 to 39.5) before TAILORx and 36% (95% CI, 28.4 to 43.7) after.
CONCLUSION
To our knowledge, this large survey of 21-gene test practice patterns was the first conducted in Latin America and showed the relevance of 21-gene testing in low- and medium-resource countries to minimize chemotherapy overuse and underuse in breast cancer. The results showed substantial reductions in chemotherapy use overall—especially after TAILORx reported—indicating the practice-changing potential of that study.
INTRODUCTION
Breast cancer is the most common cancer and a leading cause of cancer death among women worldwide, including Latin America.1-3 Although the incidence rate of breast cancer is lower in Latin America than in North America and Western Europe,1 the mortality rate for breast cancer in Latin America is relatively higher and has been rising since the 1990s even as the rates have remained stable in high-income regions.4 Approximately 41% of breast cancer diagnoses in Latin America are made at advanced stages of disease (stage III and/or IV),5 in contrast to the 8%-23% of advanced diagnoses made in European countries6 and the 6% of metastatic diagnoses made in the United States.7 The considerable clinical burden of breast cancer in Latin America underscores the urgent need for strategies to improve outcomes for these patients.
CONTEXT
Key Objective
The study examined the effect of 21-gene test results on treatment decisions for 647 women with breast cancer treated at 14 sites in Argentina, Colombia, Mexico, and Peru.
Knowledge Generated
The mean age was 54 years; 55% of women were postmenopausal. High-risk clinicopathologic features included 20% node-positive, 20% grade 3 tumors, and 30% > 2 cm in size. Before 21-gene testing, on the basis of clinicopathologic features, 325 of 647 women (50%) were recommended chemotherapy. Samples were sent for 21-gene testing; 20% had Recurrence Score (RS) 0-10, 56% had RS 11-25, and 24% had RS 26-100. With RS results available, 199 patients were recommended chemotherapy, representing a 39% relative reduction (19% absolute reduction) in chemotherapy recommendations.
Relevance
Our findings showed the relevance of 21-gene testing in low- and medium-resource countries to minimize chemotherapy overuse and underuse in breast cancer.
Efforts to improve breast cancer care across Latin America depend on an understanding of practice patterns in breast cancer management. The factors that affect how physicians manage their patients with breast cancer are myriad and include type of insurance (public or private), patient socioeconomic status, country-specific medical policy, and level of access to treatments.2,8,9 In one study in Argentina that explored factors affecting prescribing practices in breast cancer, clinicopathologic factors such as age, nodal status, estrogen receptor (ER) and progesterone receptor (PR) status, human epidermal growth factor receptor 2 (HER2) status, and expression of the Ki67 proliferation marker influenced physicians' decisions to prescribe hormonal therapy with or without chemotherapy. Use of multigene assays to assess tumor biology was not named as an influential factor, however, primarily because of limited availability related to cost.8
The 21-gene Oncotype DX Breast Recurrence Score test (Genomic Health Inc, a wholly owned subsidiary of Exact Sciences Corp, Redwood City, CA) is a multigene assay validated as a prognosticator of 10-year risk of recurrence and a predictor of adjuvant chemotherapy benefit in patients with ER+, HER2–, node-negative, or node-positive early breast cancer who receive 5 years of hormonal therapy.10-17 Importantly, the primary analyses of the landmark Trial Assigning Individualized Options for Treatment (TAILORx) trial in node-negative breast cancer, reported in June 2018, showed that the cohort with Recurrence Score (RS) 0-25 derived little to no benefit from adjuvant chemotherapy. An exploratory analysis found, however, that patients ≤ 50 years with RS 11-25 may derive some benefit.16,18 The clinical utility of the Breast Recurrence Score test, or the capacity of the test to change clinical practice and improve outcomes of patients with node-negative or node-positive breast cancer in a cost-effective manner, has been demonstrated in numerous studies conducted worldwide.19-38 One such study conducted in Mexico (N = 96; node-negative and node-positive breast cancer) showed that knowledge of RS results led to a 28% reduction in recommendations for chemotherapy at one center.24 Another study (N = 551; node-negative and node-positive breast cancer) showed that physicians in Peru made treatment recommendations in line with RS results such that the rate of chemotherapy recommendations increased with RS groups of higher risk (13% with RS 0-17, 77% with RS 18-30, and 98% with RS 31-100).39
The promising but limited data on the clinical utility of the Breast Recurrence Score test in Latin America highlight the need for further research on the potential health and economic impact of the test in this region. In this study, we presented the results of a physician survey to assess the clinical utility of the Breast Recurrence Score test in routine practice across 14 centers in five countries in Latin America.
METHODS
This was a multicenter, prospective, observational physician survey. The primary objective of this physician survey was to provide a descriptive, qualitative assessment of patients with breast cancer who receive Oncotype DX Breast Recurrence Score testing as part of their routine care. Specific descriptive analyses aimed to characterize (1) the patterns of use of the Oncotype DX Breast Recurrence Score test in routine care across demographic and clinicopathologic variables (age, tumor size, tumor grade, and nodal status), (2) the distribution of RS results across all patients and subgroups stratified by clinicopathologic variables, (3) the effect of RS results on treatment planning as measured by the change in treatment recommendations before and after RS results are available (all patients and by nodal status), (4) the effect of RS results on physicians' level of confidence in their treatment recommendations as measured by the change in physicians' stated level of confidence before and after RS results are available, and (5) the association between the RS result and treatment decision as a function of clinicopathologic variables.
Participating physicians had to be medical oncologists or breast surgeons practicing in Latin America who make adjuvant treatment recommendations for patients with breast cancer, provide consent to participate, and complete questionnaires both before and after the Breast Recurrence Score test for each patient. The physician survey was completed for consecutive patients who met the following eligibility criteria: (1) male or female at least 18 years of age diagnosed with ER+, HER2–, node-negative, or node-positive breast cancer; (2) data available on age, tumor size, tumor grade, tumor histology, nodal status, ER and PR status by immunohistochemistry (IHC), and HER2 status by IHC and/or fluorescence in situ hybridization; (3) Breast Recurrence Score test ordered as part of routine care (either self-funded by patients or covered as part of a state-funded research grant); and (4) written and signed informed consent provided. Patients who received hormonal therapy or chemotherapy before Breast Recurrence Score testing were ineligible. If available, other clinicopathologic characteristics collected included menopausal status, tumor histology, lymphovascular invasion, and Ki67 by IHC.
Clinical and treatment decision data (chemotherapy, hormonal therapy, or chemotherapy plus hormonal therapy) were collected before and after the Breast Recurrence Score test was ordered in the course of routine care. Treatment recommendations made before testing were based on physician-patient discussions that included available clinicopathologic information and patient preferences. Treatment recommendations made after testing also included RS results. Attending physician's confidence in treatment recommendation before Breast Recurrence Score testing and 2-3 months after test results received were recorded.
All analyses were descriptive in nature unless otherwise indicated. Descriptive statistics included, as appropriate, frequency counts and percentages in contingency tables; means, standard deviations (SDs), medians, quartiles, and ranges; Pearson and Spearman correlation coefficients; and concordance percentages and other measures of association. McNemar's test was conducted on paired preassay and postassay recommendation for chemotherapy. Trend in chemotherapy recommendation by increasing results in five-unit categories was assessed using the Cochran-Armitage trend test at both preassay and postassay recommendations. Statistics included point estimates and asymptotic 95% CIs with continuity correction when appropriate.40 CIs calculated below 0% or above 100% were set to the appropriate boundary. All hypothesis tests were conducted at a two-sided α level of .05.
RESULTS
Between March 2015 and December 2019, the survey was completed for 647 patients across 14 sites in Argentina, Colombia, Mexico, and Peru. Patient demographics and disease characteristics are given in Table 1. The mean age was 54 years (SD 12 years), and 55% were postmenopausal. Approximately 20% of patients had node-positive breast cancer (N1mi, N1, or N2). The mean tumor size was 1.9 cm (SD 1.0 cm), and approximately 30% had tumors larger than 2 cm. Almost 20% of patients had grade 3 tumors.
TABLE 1.
Patient Baseline Clinical and Pathologic Characteristics
The distribution of RS results by nodal status is shown in Figure 1. Using RS groups defined by RS 0-17, RS 18-30, and RS 31-100, 52% of all patients had RS 0-17, ranging from 51% to 64% depending on nodal status. Using TAILORx RS groups (RS 0-10, RS 11-25, and RS 26-100), 76% of patients had RS 0-25, ranging from 74% to 85% depending on nodal status.
FIG 1.

Distribution of RS results by (A) risk groups of RS 0-17, RS 18-30, and RS 31-100 and (B) Trial Assigning Individualized Options for Treatment risk groups of RS 0-10, RS 11-25, and RS 26-100. RS, Recurrence Score.
The distributions of RS results by nodal status and several clinicopathologic factors (tumor size, tumor grade, age, and KI67%) are shown in Figure 2. There was a wide range of RS results for every category of every clinicopathologic factor. For example, patients with high-risk clinicopathologic features (large tumor size, high grade, younger age, and higher Ki67%) or low-risk features (small tumor size, low grade, older age, and lower Ki67%) have RS results that span low to high genomic risk.
FIG 2.
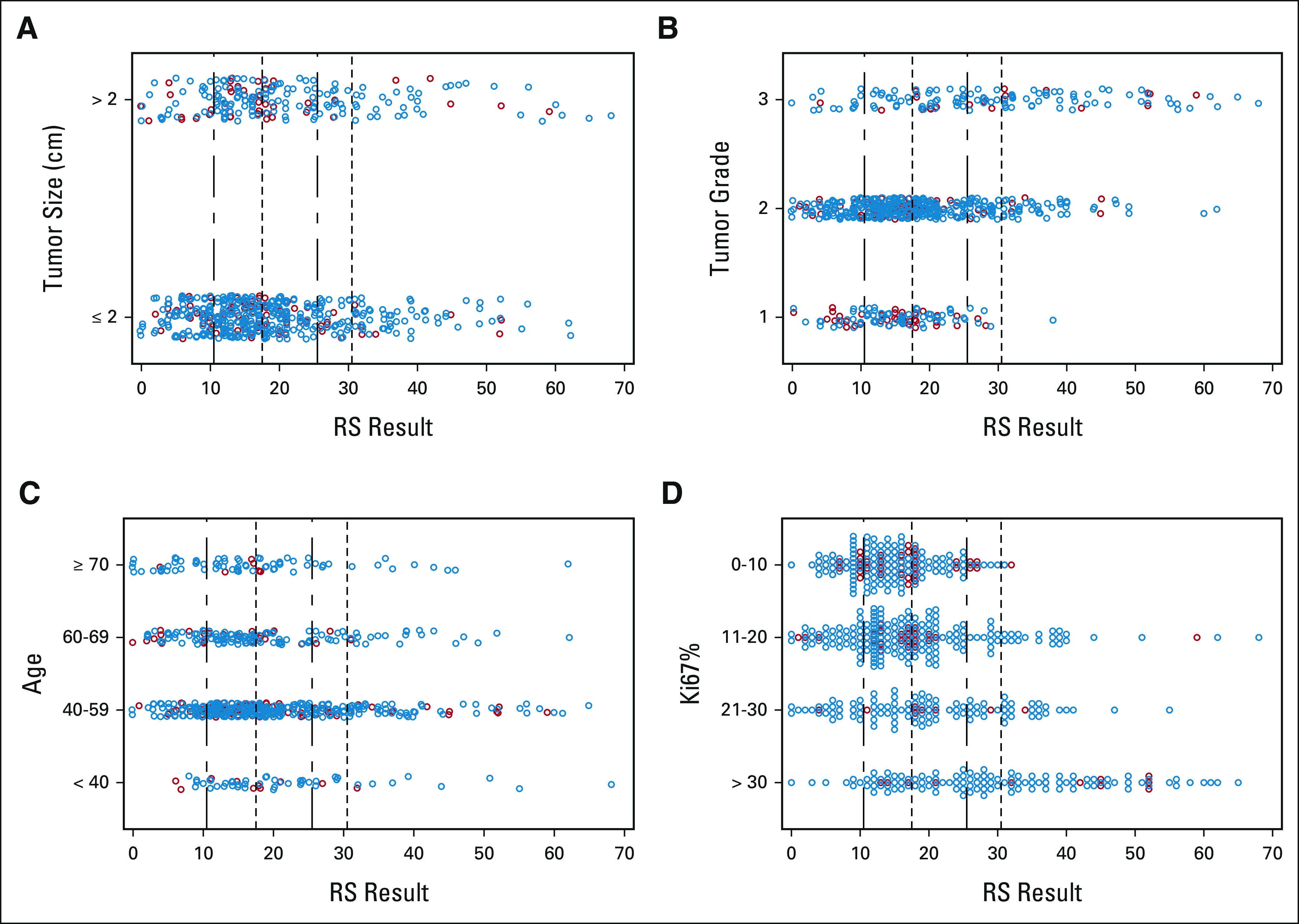
Distribution of RS results by (A) tumor size, (B) tumor grade, (C) age, and (D) Ki67%. Nodal status is marked by color: node-negative (blue circles), micrometastases and 0-3 positive nodes (blue circles), and 4-9 positive nodes (red circles). Vertical dotted-dashed lines mark the Trial Assigning Individualized Options for Treatment cutpoints of RS 11 and RS 26; vertical dotted lines mark the traditional cutpoints of RS 18 and RS 31. RS, Recurrence Score.
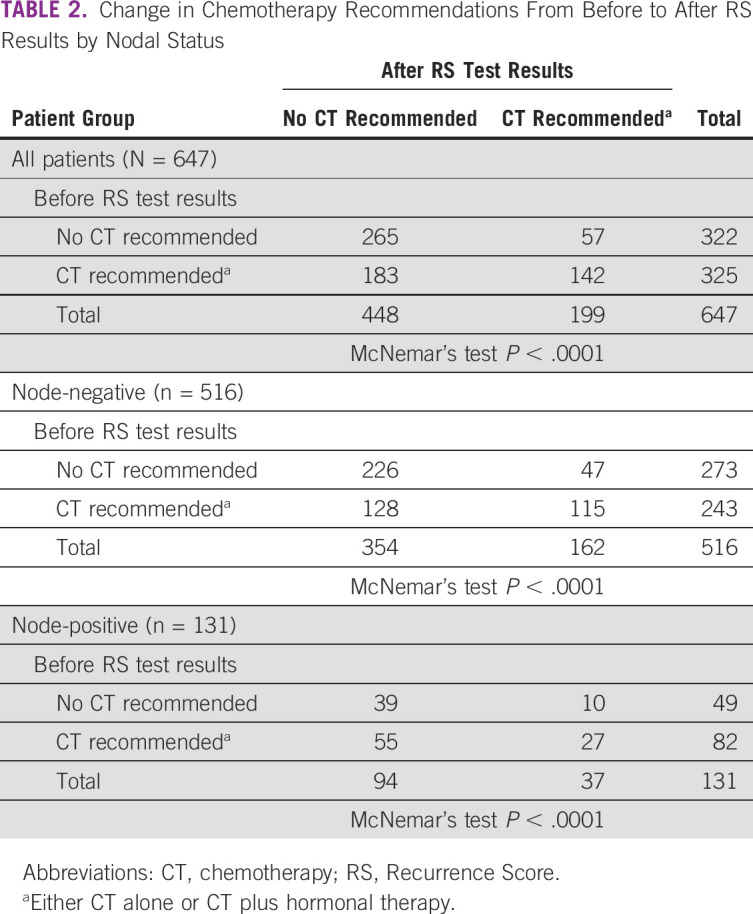
After Breast Recurrence Score testing, 37% (n = 240) of all patients, 34% (n = 175) of patients with node-negative breast cancer, and 50% (n = 65) of patients with node-positive breast cancer had a change in treatment recommendation. Most of the changes in treatment recommendations after Recurrence Score testing resulted in a reduction in chemotherapy recommendations (Table 2). Before Breast Recurrence Score testing, 50% (n = 325) of all patients had a chemotherapy recommendation; after testing, 31% (n = 199) did, which represents a 39% (95% CI, 33.4 to 44.3) relative reduction and a 19% absolute reduction in chemotherapy recommendations. Before testing, 47% (n = 243) of patients with node-negative breast cancer had a chemotherapy recommendation; after testing, 31% (n = 162) did, which represents a 33% (95% CI, 25.3 to 43.7) relative reduction and a 16% absolute reduction in chemotherapy recommendations.
TABLE 2.
Change in Chemotherapy Recommendations From Before to After RS Results by Nodal Status
The percent reduction in chemotherapy recommendations for patients with node-negative breast cancer varied by the time period before or after the primary analyses from the TAILORx trial reported: 28% (95% CI, 15.9 to 48.3) before TAILORx reported and 36% (95% CI, 25.8 to 49.1) after (Data Supplement). Before TAILORx, net reductions in chemotherapy recommendations were made in patients with RS 0-20; after TAILORx, net reductions were made in patients with RS 0-25 (Data Supplement). These results remained consistent when assessed by age in patients with node-negative breast cancer. After TAILORx, chemotherapy recommendations were reduced in patients with RS 0-25, regardless of age group (≤ 50 or > 50 years; Data Supplement).
Before testing, 63% (n = 82) of patients with node-positive breast cancer had a chemotherapy recommendation; after testing, 28% (n = 37) did, which represents a 55% (95% CI, 42.1 to 70.7) relative reduction and a 34% absolute reduction in chemotherapy recommendations. The percent reduction varied by extent of nodal involvement: 74% (95% CI, 50.1 to 100 [CI was calculated to exceed 100% and set to the appropriate boundary]) for N1mi, 49% (95% CI, 31.5 to 73.5) for N1, and 50% (95% CI, 20.5 to 97.7) for N2. The percent reduction in node-positive patients was similar before and after TAILORx reported (Data Supplement).
The proportion of patients with chemotherapy recommendations before and after Breast Recurrence Score testing is shown by RS result in Figure 3. Before testing, when only clinicopathologic factors were used to guide treatment recommendations, a considerable proportion of patients with lower RS results had recommendations for chemotherapy and a proportion with higher RS results had no recommendation for chemotherapy. After RS testing, chemotherapy recommendations were reduced for those with lower RS results and increased for those with higher results.
FIG 3.
Proportion of patients recommended CT before (pretest) and after receiving RS results (post-test), by RS result. CT, chemotherapy; RS, Recurrence Score.
Before RS testing, 322 (50%) of patients had recommendations excluding chemotherapy. After testing, 57 (18%) of these patients had their recommendations changed to include chemotherapy, including 47 patients (17%) with node-negative and 10 patients (20%) with node-positive breast cancer (Table 2).
Physicians rated their level of confidence in the treatment decisions made for each patient before and after RS testing (Fig 4). Before testing, physicians felt strongly confident (45%) or somewhat confident (37%) in 82% of their decisions. After RS testing, physicians felt strongly confident (81%) or somewhat confident (17%) in 98% of their treatment decisions.
FIG 4.

Physician self-reported confidence in treatment recommendations (A) before and (B) after 21-gene testing.
DISCUSSION
This survey was the largest prospective study conducted in Latin America to evaluate the clinical utility of the Oncotype DX Breast Recurrence Score test. After the clinical validation of RS results as a predictor of adjuvant chemotherapy benefit in node-negative and node-positive breast cancer,11,14 prospective clinical utility studies conducted worldwide showed how physicians applied the RS results to recommend and prescribe chemotherapy more judiciously to patients with node-negative and node-positive breast cancer.21,22,33,35,41 In this study, we observed a reduction in chemotherapy recommendations after Breast Recurrence Score testing across all groups, indicating that the clinical utility of the test across nodal status endures in Latin America. Furthermore, the post–TAILORx treatment recommendations observed in our study for node-negative breast cancer were generally reflected clinical practice guidelines that advise chemotherapy for patients with RS 26-100 of any age, consideration of chemotherapy for patients with RS 16-25 who are premenopausal, and no chemotherapy for patients with RS 0-25 who are postmenopausal or RS 0-15 who are premenopausal.42,43
To our knowledge, this was the first clinical utility study to assess physician practice patterns since the publication of the TAILORx results, which further refined the RS cutpoint for prediction of adjuvant chemotherapy benefit.16 The patient population of this study differed from that of TAILORx,15-17 with greater proportions of patients who were ≤ 50 years of age (43% v 31%) and who had tumors > 2 cm in size (∼30% v 25%), PR-negative status (21% v 10%), and node-positive disease (20% v 0%). Patient differences notwithstanding, physicians made fewer chemotherapy recommendations for patients with node-negative breast cancer after TAILORx reported, especially in patients with RS 0-25 and regardless of age group (≤ 50 or > 50 years), consistent with TAILORx results.16,18 This suggests how practice-changing the TAILORx results were to physicians in Latin America and how very comfortable these physicians were in applying the learnings from TAILORx to their clinical practices. Indeed, 98% of physicians reported being strongly or somewhat confident in the treatment decisions they made with RS results.
There were 131 patients with nodal involvement (N1mi, N1, or N2) in our study, including 20 (15%) with N2 breast cancer. This proportion was larger than the proportion with ≥ 4 positive nodes in the SEER registry (4%).44 Our study did not assess reasons for the higher rate of Breast Recurrence Score testing among patients with more nodal involvement, but we note that many of the patients in this study paid out of pocket for testing, which may account at least in part for this relatively large proportion of patients with N2 breast cancer. In this group with nodal involvement, we observed a 55% relative reduction in chemotherapy recommendations after Breast Recurrence Score testing, which is higher than the percent differences documented in other decision impact studies done with patients with node-positive breast cancer. For example, de Boer et al (n = 50) noted a 32% relative reduction after RS results, Eiermann et al (n = 122) a 38% relative reduction, and Torres et al (n = 67) a 34% relative reduction.21,22,37 The reasons for the larger reduction in chemotherapy recommendations observed in this study were not explored but may reflect differences in regional clinical practice, greater clinical utility of Breast Recurrence Score testing in higher risk patient cohorts in Latin America than in other countries, or an evolution of clinical practice over time during which physicians worldwide have sought evidence-based strategies to appropriately de-escalate treatment in selected patients.
A combination of prospective clinical trial results and real-world evidence supports appropriate de-escalation for patients with node-positive breast cancer but low genomic risk as determined by the Breast Recurrence Score test.44-46 In the PlanB clinical trial, patients with 1-3 positive nodes and RS 0-11 who received endocrine therapy alone (n = 110) had 94.4% disease-free survival at 5 years (compared with 94.2% for 238 patients with node-negative breast cancer).45 In the population-based Clalit Health Services registry (n = 709), the 5-year freedom from distant recurrence was 97.3% for 518 patients with node-positive breast cancer and RS 0-17 who were treated with endocrine therapy alone.46 In the SEER registry (N = 6,768), the 5-year breast cancer-specific survival ranged from 95.1% to 99.4% for those with RS 0-17, depending on the extent of nodal involvement (micrometastases and 1-3 positive nodes). Adjuvant chemotherapy use was reported in 18%-41% of patients in this SEER cohort, depending on the extent of nodal involvement.44 The Breast Recurrence Score test has been validated to predict chemotherapy benefit in the node-positive setting, and multiple clinical utility and registry studies have now demonstrated the opportunity to spare chemotherapy in those with low RS results. The prospective Treatment for Positive Node, Endocrine Responsive Breast Cancer (RxPONDER; SWOG S1007) clinical trial recently reported no evidence that the Breast RS result predicts relative chemotherapy benefit within the RS 0-25 range.47-49 This was not surprising, given that the SWOG S8814 study predicted significant chemotherapy benefit in the RS 31-100 group in an analysis that included the full range of RS results (RS 0-100). RxPONDER did find that relative chemotherapy benefit with RS 0-25 depends on menopausal status, with premenopausal patients deriving benefit from chemotherapy treatment.49 These findings could have affected the treatment decisions made by the physicians and patients in our study had they been reported earlier, and further investigations will be needed to understand how the RxPONDER results may affect clinical practice.
We found wide distributions of RS results across all categories of clinicopathologic factors, namely, age, tumor size, tumor grade, and Ki67%. These factors are often used alone without genomic information to estimate risk of recurrence and make chemotherapy decisions. A set of uniform criteria for low versus high clinicopathologic risk was not prespecified for the participating sites, so pretest treatment recommendations were based on physician-determined estimates of clinicopathologic risk. Our observations suggest that clinicopathologic features and RS results provide complementary information that can be considered together for risk estimation and treatment decision making. As observed in Figure 3, a considerable proportion of patients with low RS results were recommended chemotherapy before testing, on the basis of clinicopathologic factors and/or patient preference. In the National Surgical Adjuvant Breast and Bowel Project B-20 analysis, clinical variables including age, tumor size, and tumor grade did not independently predict chemotherapy benefit in patients with node-negative breast cancer who had a RS result.11 The RS results therefore provide information beyond what can be gleaned from clinicopathologic factors alone that can be used to guide treatment decisions. Importantly, the RS result is validated to predict chemotherapy benefit for patients with node-negative and node-positive breast cancer.11,12,14
In summary, our study documented a 39% overall reduction in chemotherapy recommendations after Breast Recurrence Score testing, regardless of nodal status. Physicians overall reported increased confidence in treatment recommendations with RS results. This study demonstrates the clinical utility of the Breast Recurrence Score test among patients with higher risk clinical characteristics, including node-positive patients, in Latin America.
ACKNOWLEDGMENT
The authors thank Anna Lau, PhD, for editorial support of manuscript development.
Henry L. Gomez
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Roche, Novartis, AstraZeneca, Bristol Myers Squibb, Lilly
Research Funding: MSD Oncology
Aníbal R. Núñez De Pierro
Honoraria: Roche, Genomic Health Inc
Consulting or Advisory Role: Laboratorio Roche Argentina, Genomic Health
Speakers' Bureau: Roche, Genomic Healh Inc, Omics SRL
Travel, Accommodations, Expenses: Labotorio Roche Argentina, Genomic Health, Omics SRL
Lisandro L. B. Gil
Stock and Other Ownership Interests: Centro de Mastologia (CEMA)
Mauricio Lema-Medina
Honoraria: Roche, Bristol Myers Squibb/Medarex, Boehringer Ingelheim, AstraZeneca, AbbVie, Lilly
Speakers' Bureau: Roche, Bristol Myers Squibb/Medarex, Boehringer Ingelheim, Lilly, AbbVie, AstraZeneca
Research Funding: Pfizer, Bristol Myers Squibb/Medarex
Travel, Accommodations, Expenses: Bristol Myers Squibb/Medarex, Roche
Raquel G. Cwilich
Employment: ABC Medical Center
Leadership: ABC Medical Center
Sergio C. Oliveira
Employment: Exact Sciences
Travel, Accommodations, Expenses: Exact Sciences
Debbie M. Jakubowski
Employment: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Calvin Chao
Employment: Exact Sciences, Tempus
Leadership: Exact Sciences, Tempus
Stock and Other Ownership Interests: Exact Sciences, Tempus
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at 2020 ASCO Annual Meeting, virtual, May 29-31, Abstract No. e12539.
SUPPORT
Supported by Genomic Health Inc (a wholly owned subsidiary of Exact Sciences Corp) for the establishment and maintenance of this registry in Latin America.
AUTHOR CONTRIBUTIONS
Conception and design: Juan E. Bargallo-Rocha, Roberto J. Billinghurst, Ignacio L. McLean, Mauricio Leon, Roberto E. Castaño, Sergio C. Oliveira, Debbie M. Jakubowski, Calvin Chao
Financial support: Roberto J. Billinghurst, Calvin Chao
Administrative support: Roberto J. Billinghurst
Provision of study materials or patients: Juan E. Bargallo-Rocha, Roberto J. Billinghurst, Federico A. Coló, Lisandro L. B. Gil, Carola Allemand, Ignacio L. McLean, Raquel G. Cwilich, Silvia G. Falcon, Roberto E. Castaño
Collection and assembly of data: Henry L. Gomez, Juan E. Bargallo-Rocha, Roberto J. Billinghurst, Federico A. Coló, Lisandro L. B. Gil, Carola Allemand, Ignacio L. McLean, Mauricio Lema-Medina, Fernando Herazo-Maya, Francisco J. Terrier, Mauricio Leon, Silvia G. Falcon, Roberto E. Castaño, Sergio C. Oliveira, Debbie M. Jakubowski, Calvin Chao
Data analysis and interpretation: Henry L. Gomez, Juan E. Bargallo-Rocha, Roberto J. Billinghurst, Aníbal R. Núñez De Pierro, Ignacio L. McLean, Raquel G. Cwilich, Mauricio Leon, Sergio C. Oliveira, Debbie M. Jakubowski, Calvin Chao
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Henry L. Gomez
Consulting or Advisory Role: AstraZeneca
Speakers' Bureau: Roche, Novartis, AstraZeneca, Bristol Myers Squibb, Lilly
Research Funding: MSD Oncology
Aníbal R. Núñez De Pierro
Honoraria: Roche, Genomic Health Inc
Consulting or Advisory Role: Laboratorio Roche Argentina, Genomic Health
Speakers' Bureau: Roche, Genomic Healh Inc, Omics SRL
Travel, Accommodations, Expenses: Labotorio Roche Argentina, Genomic Health, Omics SRL
Lisandro L. B. Gil
Stock and Other Ownership Interests: Centro de Mastologia (CEMA)
Mauricio Lema-Medina
Honoraria: Roche, Bristol Myers Squibb/Medarex, Boehringer Ingelheim, AstraZeneca, AbbVie, Lilly
Speakers' Bureau: Roche, Bristol Myers Squibb/Medarex, Boehringer Ingelheim, Lilly, AbbVie, AstraZeneca
Research Funding: Pfizer, Bristol Myers Squibb/Medarex
Travel, Accommodations, Expenses: Bristol Myers Squibb/Medarex, Roche
Raquel G. Cwilich
Employment: ABC Medical Center
Leadership: ABC Medical Center
Sergio C. Oliveira
Employment: Exact Sciences
Travel, Accommodations, Expenses: Exact Sciences
Debbie M. Jakubowski
Employment: Exact Sciences
Stock and Other Ownership Interests: Exact Sciences
Calvin Chao
Employment: Exact Sciences, Tempus
Leadership: Exact Sciences, Tempus
Stock and Other Ownership Interests: Exact Sciences, Tempus
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin 68394–4242018 [DOI] [PubMed] [Google Scholar]
- 2.Justo N, Wilking N, Jonsson B, et al. A review of breast cancer care and outcomes in Latin America Oncologist 18248–2562013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cazap E.Breast cancer in Latin America: A map of the disease in the region Am Soc Clin Oncol Ed Book 38451–4562018 [DOI] [PubMed] [Google Scholar]
- 4.Azamjah N, Soltan-Zadeh Y, Zayeri F.Global trend of breast cancer mortality rate: A 25-year study Asian Pac J Cancer Prev 202015–20202019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Lemos LLP, Carvalho de Souza M, Pena Moreira D, et al. Stage at diagnosis and stage-specific survival of breast cancer in Latin America and the Caribbean: A systematic review and meta-analysis. PLoS One. 2019;14:e0224012. doi: 10.1371/journal.pone.0224012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walters S, Maringe C, Butler J, et al. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000-2007: A population-based study Br J Cancer 1081195–12082013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Cancer Institute . SEER Cancer Stat Facts: Female Breast Cancer. 2020. https://seer.cancer.gov/statfacts/html/breast.html [Google Scholar]
- 8.Eraso Y. Factors influencing oncologists' prescribing hormonal therapy in women with breast cancer: A qualitative study in Cordoba, Argentina. Int J Equity Health. 2019;18:35. doi: 10.1186/s12939-019-0936-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costanzo MV, Nervo A, Lopez C, et al. Adjuvant breast cancer treatment in Argentina: Disparities between prescriptions and funding requirements—A survey. J Clin Oncol. 2008;26 suppl; abstr 17571. [Google Scholar]
- 10.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer N Engl J Med 3512817–28262004 [DOI] [PubMed] [Google Scholar]
- 11.Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer J Clin Oncol 243726–37342006 [DOI] [PubMed] [Google Scholar]
- 12.Geyer CE, Jr, Tang G, Mamounas EP, et al. 21-Gene assay as predictor of chemotherapy benefit in HER2-negative breast cancer. NPJ Breast Cancer. 2018;4:37. doi: 10.1038/s41523-018-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: A TransATAC study J Clin Oncol 281829–18342010 [DOI] [PubMed] [Google Scholar]
- 14.Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: A retrospective analysis of a randomised trial Lancet Oncol 1155–652010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer N Engl J Med 3732005–20142015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer N Engl J Med 379111–1212018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sparano JA, Gray RJ, Makower DF, et al. Clinical outcomes in early breast cancer with a high 21-gene Recurrence Score of 26 to 100 assigned to adjuvant chemotherapy plus endocrine therapy: A secondary analysis of the TAILORx randomized clinical trial JAMA Oncol 6367–3742019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer N Engl J Med 3802395–24052019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lo SS, Mumby PB, Norton J, et al. Prospective multicenter study of the impact of the 21-gene Recurrence Score assay on medical oncologist and patient adjuvant breast cancer treatment selection J Clin Oncol 281671–16762010 [DOI] [PubMed] [Google Scholar]
- 20.Ademuyiwa FO, Miller A, O'Connor T, et al. The effects of Oncotype DX recurrence scores on chemotherapy utilization in a multi-institutional breast cancer cohort Breast Cancer Res Treat 126797–8022011 [DOI] [PubMed] [Google Scholar]
- 21.Eiermann W, Rezai M, Kummel S, et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use Ann Oncol 24618–6242013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Boer RH, Baker C, Speakman D, et al. The impact of a genomic assay (Oncotype DX) on adjuvant treatment recommendations in early breast cancer Med J Aust 199205–2082013 [DOI] [PubMed] [Google Scholar]
- 23.Geffen DB, Abu-Ghanem S, Sion-Vardy N, et al. The impact of the 21-gene Recurrence Score assay on decision making about adjuvant chemotherapy in early-stage estrogen-receptor-positive breast cancer in an oncology practice with a unified treatment policy Ann Oncol 222381–23862011 [DOI] [PubMed] [Google Scholar]
- 24.Bargallo JE, Lara F, Shaw-Dulin R, et al. A study of the impact of the 21-gene breast cancer assay on the use of adjuvant chemotherapy in women with breast cancer in a Mexican public hospital J Surg Oncol 111203–2072015 [DOI] [PubMed] [Google Scholar]
- 25.Hochheiser L, Hornberger J, Turner M, et al. Multi-gene assays: Effect on chemotherapy use, toxicity and cost in estrogen receptor-positive early stage breast cancer J Comp Eff Res 8289–3042019 [DOI] [PubMed] [Google Scholar]
- 26.Pomponio M, Keele L, Hilt E, et al. Impact of 21-gene expression assay on clinical outcomes in node-negative </= T1b breast cancer Ann Surg Oncol 271671–16782020 [DOI] [PubMed] [Google Scholar]
- 27.Bacchi CE, Prisco F, Carvalho FM, et al. Potential economic impact of the 21-gene expression assay on the treatment of breast cancer in Brazil Rev Assoc Med Bras 56186–1912010 [DOI] [PubMed] [Google Scholar]
- 28.Vataire AL, Laas E, Aballea S, et al. Cost-effectiveness of a chemotherapy predictive test [in French] Bull Cancer 99907–9142012 [DOI] [PubMed] [Google Scholar]
- 29.Dreyfus C, Ballester M, Gligorov J, et al. Impact of the 21-gene assay in decision-making during multidisciplinary breast meeting: A French experience [in French] Gynecol Obstet Fertil 43780–7852015 [DOI] [PubMed] [Google Scholar]
- 30.Bargallo-Rocha JE, Lara-Medina F, Perez-Sanchez V, et al. Cost-effectiveness of the 21-gene breast cancer assay in Mexico Adv Ther 32239–2532015 [DOI] [PubMed] [Google Scholar]
- 31.Hannouf MB, Xie B, Brackstone M, et al. Cost-effectiveness of a 21-gene Recurrence Score assay versus Canadian clinical practice in women with early-stage estrogen- or progesterone-receptor-positive, axillary lymph-node negative breast cancer. BMC Cancer. 2012;12:447. doi: 10.1186/1471-2407-12-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masucci L, Torres S, Eisen A, et al. Cost-utility analysis of 21-gene assay for node-positive early breast cancer Curr Oncol 26307–3182019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davidson JA, Cromwell I, Ellard SL, et al. A prospective clinical utility and pharmacoeconomic study of the impact of the 21-gene Recurrence Score assay in oestrogen receptor positive node negative breast cancer Eur J Cancer 492469–24752013 [DOI] [PubMed] [Google Scholar]
- 34.Gligorov J, Pivot XB, Jacot W, et al. Prospective clinical utility study of the use of the 21-gene assay in adjuvant clinical decision making in women with estrogen receptor-positive early invasive breast cancer: Results from the SWITCH study Oncologist 20873–8792015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamauchi H, Nakagawa C, Yamashige S, et al. Societal cost-effectiveness analysis of the 21-gene assay in estrogen-receptor-positive, lymph-node-negative early-stage breast cancer in Japan. BMC Health Serv Res. 2014;14:372. doi: 10.1186/1472-6963-14-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albanell J, Svedman C, Gligorov J, et al. Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer Eur J Cancer 66104–1132016 [DOI] [PubMed] [Google Scholar]
- 37.Torres S, Trudeau M, Gandhi S, et al. Prospective evaluation of the impact of the 21-gene Recurrence Score assay on adjuvant treatment decisions for women with node-positive breast cancer in Ontario, Canada Oncologist 23768–7752018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry LR, Stojadinovic A, Swain SM, et al. The influence of a gene expression profile on breast cancer decisions J Surg Oncol 99319–3232009 [DOI] [PubMed] [Google Scholar]
- 39.Ruiz R, Morante Z, Namuche F, et al. Evaluation of Oncotype DX testing and subsequent treatment choices in the Latin American setting. Cancer Res. 2019;79 abstr P3-08-17. [Google Scholar]
- 40.Newcombe RG.Improved confidence intervals for the difference between binomial proportions based on paired data Stat Med 172635–26501998 [PubMed] [Google Scholar]
- 41.Gligorov J, Dohollou N, Mouysset JL, et al. The 21-gene assay in the decision impact assessment of ER+, HER2- breast cancer: A French real life prospective study. Presented at the San Antonio Breast Cancer Symposium, San Antonio, TX, 2016.
- 42.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: Breast Cancer (V2.2021) 2021. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [Google Scholar]
- 43.Andre F, Ismaila N, Henry NL, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: ASCO Clinical Practice Guideline update-integration of results from TAILORx J Clin Oncol 371956–19642019 [DOI] [PubMed] [Google Scholar]
- 44.Roberts MC, Miller DP, Shak S, et al. Breast cancer-specific survival in patients with lymph node-positive hormone receptor-positive invasive breast cancer and Oncotype DX Recurrence Score results in the SEER database Breast Cancer Res Treat 163303–3102017 [DOI] [PubMed] [Google Scholar]
- 45.Nitz U, Gluz O, Christgen M, et al. Reducing chemotherapy use in clinically high-risk, genomically low-risk pN0 and pN1 early breast cancer patients: Five-year data from the prospective, randomised phase 3 West German Study Group (WSG) PlanB trial Breast Cancer Res Treat 165573–5832017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stemmer SM, Steiner M, Rizel S, et al. Clinical outcomes in ER+ HER2-, node-positive breast cancer patients who were treated according to the Recurrence Score results: Evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3:32. doi: 10.1038/s41523-017-0033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Southwest Oncology Group A phase III, randomized clinical trial of standard adjuvant endocrine therapy +/- chemotherapy in patients with 1-3 positive nodes, hormone-responsive and HER2-negative breast cancer according to Recurrence Score. 2011 https://www.swog.org/clinical-trials/s1007 [Google Scholar]
- 48.ClinicalTrials.Gov Tamoxifen citrate, letrozole, anastrozole, or exemestane with or without chemotherapy in treating patients with invasive RxPONDER breast cancer. 2011 https://clinicaltrials.gov/ct2/show/NCT01272037 [Google Scholar]
- 49.Kalinsky K, Barlow WE, Meric-Bernstam F, et al. First results from a phase III randomized clinical trial of standard adjuvant endocrine therapy ± chemotherapy in patients with 1-3 positive nodes, hormone receptor-positive and HER2-negative breast cancer with Recurrence Score ≤25: SWOG S1007 (RxPONDER) Presented at the San Antonio Breast Cancer Symposium, San Antonio, TX, December 8-December 12, 2020.




