Abstract
Yellow warblers (Setophaga petechia) use referential ‘seet’ calls to warn mates of brood parasitic brown-headed cowbirds (Molothrus ater). In response to seet calls during the day, female warblers swiftly move to sit tightly on their nests, which may prevent parasitism by physically blocking female cowbirds from inspecting and laying in the nest. However, cowbirds lay their eggs just prior to sunrise, not during daytime. We experimentally tested whether female warblers, warned by seet calls on one day, extend their anti-parasitic responses into the future by engaging in vigilance at sunrise on the next day, when parasitism may occur. As predicted, daytime seet call playbacks caused female warblers to leave their nests less often on the following morning, relative to playbacks of both their generic anti-predator calls and silent controls. Thus, referential calls do not only convey the identity or the type of threat at present but also elicit vigilance in the future to provide protection from threats during periods of heightened vulnerability.
Keywords: alarm calling, episodic-like memory, host–parasite interactions, referential alarm calling
1. Introduction
Diverse lineages of mammals and birds exhibit referential communication: signals refer to specific objects in the environment, and others receiving such signals respond accordingly, revealing that these listeners understand what is being signalled [1,2]. For example, referential alarm calls in most species studied alert conspecifics of specific predatory threats, such as aerial-hunting versus ground-approaching predators, and the signal alone is sufficient to elicit escape responses by conspecifics appropriate for the type of predator [3]. More rarely, referential alarm calls specifically denote the presence of a very different threat: obligate brood parasitic birds that lay their eggs in the nests of other species [1]. When hosts detect anti-parasitic referential calls, they immediately respond with mobbing [4] or by physically protecting the nest from the parasite [5]. Whereas direct interactions with brood parasites have been shown to increase host nest vigilance on the following day [6], it remains unknown whether referential alarm calls also convey the ongoing risk of parasitism, thereby modifying the future behaviours of hosts until or at the time of day when parasitism is most likely to occur.
Yellow warblers (Setophaga petechia, hereafter ‘warblers’) express a suite of anti-parasitic behaviours, including referential signals [5,7]. These ‘seet’ calls refer specifically to brood parasitic brown-headed cowbirds (Molothrus ater, hereafter ‘cowbirds’), whereas ‘chip’ calls generically alert mates to diverse predators and intruding conspecifics [5,8]. In response to experimental exposure to either parasitic stimuli (e.g. live or model female cowbirds, playbacks of cowbird calls) or referential seet calls, female warblers return to sit tightly on the nest (figure 1; [5]). That is, conspecific seet calls alone elicit the same immediate defensive response that live cowbirds do. To date, published experiments have been performed during daylight hours, when the actions of warblers may prevent cowbirds from examining nest contents and removing host eggs (reviewed in [9]). Yet, such a response does not prevent parasitism per se because cowbirds typically lay their eggs at dawn, just before sunrise [10]. Could the detection of seet calls during the day also signal future parasitism risk, leading female warblers to engage in vigilance at dawn on the following day?
Figure 1.
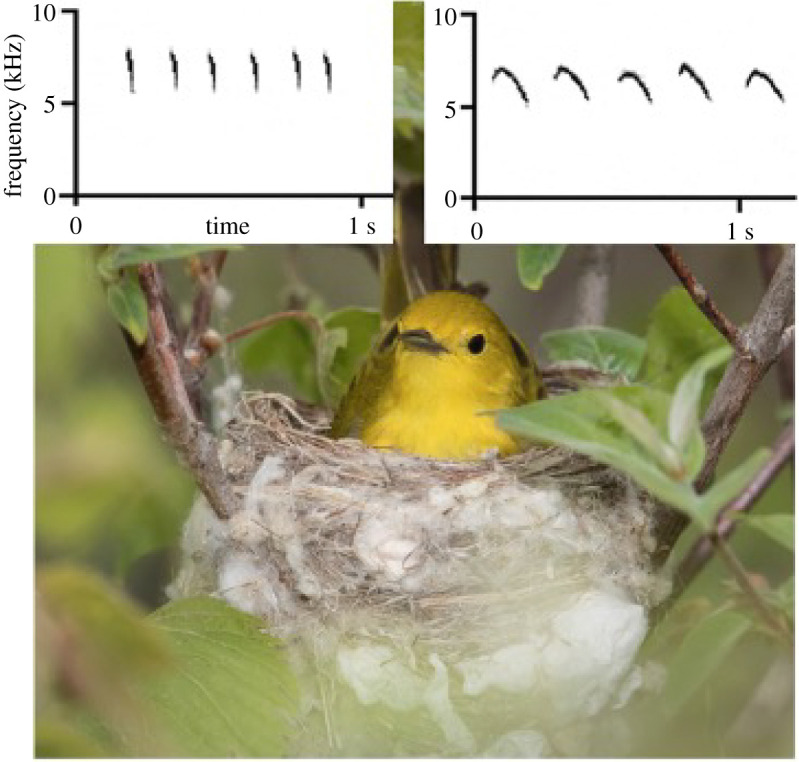
Female yellow warbler on the nest (photo credit with permission: M. Dunn of RoadsEndNaturalist); insets: spectrograms of chip (left) versus seet (right) calls with frequency (y-axis) and time (x-axis) indicated.
The ability to use personal experiences from the past to alter current or immediate future behaviour occurs across diverse animal lineages (e.g. [11]). In yellow warblers, differences in the meaning of referential seet calls versus generic anti-predator chip calls provide us with the opportunity to test longer term impacts of current communication signals in two divergent reproductive contexts: brood parasitism risk (seets) versus generic territorial and nest-predation threats (chips). We hypothesize that the perception of referential seet calls involves the mental representation of the parasitism-specific threat [1] and here we test whether the detection of seet calls alters future vigilance of females specifically at the time when increased nest attendance would be most adaptive. By contrast, no specific pattern of altered future behaviour is expected in response to the generic chip alarm calls; as a general threat signal, chip calls should not predictably alter vigilance at a specific (future) time of day.
2. Material and methods
During May and June in 2020 and 2021, we conducted sequential daily playback experiments and early morning observations at nests (N = 27) of unmarked yellow warbler pairs, located at three study sites (greater than or equal to 5 km apart) in east-central Illinois, USA. Here, cowbird parasitism rates on warbler nests vary between 30 and 40% [8]. For three consecutive days during the first half of the incubation period, we exposed nesting pairs between 8.00 and 10.00 h to a single 10 min daily treatment. We collected partial data from three nests owing to depredation between visits. All but two nests received a silent control (no playback) on Day 1 (the other two received them on Day 3) and we then randomly assigned seet or chip call playbacks on Days 2 and 3. For call treatments, we broadcast a randomly selected exemplar at a sound level of approximately 70 dB (from 1 m) from a FOXPRO NX4 game caller (Lewistown, PA, USA), placed approximately 10 m from the nest on the ground. We used five unique, 10 min exemplar files of each call type, which were each composed of calls from 2 or 3 different individuals interspersed with 2–6 s of silence; all were sourced from our previous study [7] and filtered out below 0.5 kHz, which is much lower in range than either of our stimulus types (figure 1).
The following morning (n = 74 nest-mornings total), we recorded female nest attendance data for 80 min (20 min before to 60 min after local sunrise). We note that we did not collect behavioural data for this study immediately following or during the course of the day of each playback. We quantified pauses in the female's incubation behaviour by tallying the number of times she left and by the total duration that she was off the nest per observation period. Observations were conducted directly by personnel situated at a distance of greater than or equal to 5 m (n = 51 nest-mornings) and/or by a set of Thermochron iButton (Whitewater, WI, USA) temperature loggers placed inside the nest cup (n = 46 nest-mornings) (following [12]). Females were scored as off-nest when observed flying away or when iButton temperatures decreased by more than 1°C relative to the temperature fluctuations recorded by a second iButton positioned approximately 1 m below the nest. Females were scored as back on the nest when they were seen returning or iButton temperatures increased by more than 1°C. We based these iButton criteria on the positive relationship between our two metrics of nest attendance (times off the nest: Spearman's ρ = 0.71, p = 0.0002; duration off the nest: ρ = 0.56, p = 0.007) from mornings when both data collection methods were employed simultaneously (n = 23 nest-mornings at N = by more than 10 nests). When both collection methods were used at nests, we analysed data collected by the method employed during each of three mornings and the observation data when both methods were available for the full experimental extent. During visual observations, we also recorded whether males attended to females by visiting and, typically, feeding them on the nest.
We combined our two direct response metrics (i.e. the number of departures from and cumulative time off the nest) through a principal component analysis, extracting the first PC score (PC1: both eigenvectors = 0.71, eigenvalue = 1.2, variance explained = 61%) to represent the females' morning nest departure behavioural responses to playbacks. To assess the effect of treatment (silence, chip or seet) on nest attendance during the following morning, we used a general linear mixed model (GLMM) with playback type and experimental order as predictors, PC1 as the response variable and nest ID as a random effect. We assessed post hoc differences between playbacks using corrected Student's t-test p-values. Finally, we examined whether male nest visitation itself was related to female nest departure behaviours (PC1), playback treatment and the order of experimentation, with nest ID as a random effect. Parametric statistical tests were conducted in JMP 12.0 (SAS Inc., Cary, USA) while non-parametric tests were calculated using an online calculator (https://www.socscistatistics.com/tests/spearman/). We set α = 0.05 for all analyses.
3. Results
Female warblers’ nest departure behaviours (PC1) on the mornings following playbacks on the previous day varied by playback treatments (F2,46 = 7.8, p = 0.0012) but not with experimental order (F1,46 = 1.2, p = 0.28). Post hoc comparisons revealed fewer nest departures following seet calls (mean ± s.d.: −0.57 ± 0.75) versus both chip calls (0.27 ± 1.26; t46 = −3.9, p = 0.0003) and silence (0.24 ± 1.06; t46 = −2.1, p = 0.043), and no difference between chip calls and silence (t46 = 0.87, p = 0.39) (figure 2).
Figure 2.

Female yellow warblers showed reduced nest departure behaviours (PC1) on the morning following playbacks of referential seet calls than following either chip calls or silent controls; lower values of PC1 reflect more time spent on and fewer departures from the nest. Each grey line connects data points sourced from the same nest; the playback treatment's order (order) of presentation is indicated by the different colours of the jittered data points. The empty black triangles indicate the mean for each treatment. Full and partial datasets per nest are all included in this figure.
For the subset of nest-mornings (n = 50) for which visual observations were made, male nest visitation was not statistically related to playback treatment (F2,37 = 1.2, p = 0.32) or experimental order (F1,40 = 2.4, p = 0.13), but was negatively related to female nest departures (with the regression equation as: PC1 = 0.54−0.16x male nest visits; F1,38 = 10.7, p = 0.0023).
4. Discussion
Yellow warblers are one of a handful of host species known to have referential calls denoting the presence of brood parasites around the nest [5,6]. When female warblers detect seet calls, they respond immediately with cowbird-specific nest-protection behaviours [5] (figure 1). Here, we discovered that females also perceived these calls to provide information about future risk at dawn when female cowbirds lay their eggs. We detected more intensive nest attendance (fewer and shorter nest departures; PC1) on the morning after playbacks of referential seet calls relative to generic chip calls or silent controls (figure 2). Specifically, female warblers left less often and spent less total time off their nest following the previous day's experimental exposure to seet calls. Thus, these anti-parasitic calls conveyed additional meaning about parasitism risk, causing warblers to be more vigilant at the nest during the following morning at the time when parasitism may occur. In turn, higher female nest attendance was statistically linked to more male at-nest (feeding) visits, but the males' behaviour was not caused by the experimental treatments. Finally, we did not assess in this study whether fewer nest departures would in fact effectively reduce parasitism by blocking female cowbirds’ assessments of nest content [13] or their access to lay into the nest [14] in the early mornings.
We propose that female yellow warblers possess the capacity to respond to referential alarm calls adaptively by altering their behaviours to face future threats of brood parasitism. The time elapsed between our alarm call playbacks on one day and the response patterns detected on the next morning at the nest suggests that female warblers have either continued to engage in or specifically recalled events that occurred in the past (the day before) when making decisions to stay in or leave their nests the following dawn. These patterns of behaviour could result from lower level cognitive mechanisms (e.g. semantic memory, instinct) and ongoing (afternoon and overnight) heightened motivation to sit longer on the nest following seet call playbacks. Alternatively, yellow warblers may specifically engage in mental time travel, recalling the past event of detecting seet calls to influence nest vigilance on the next day [10,15]. Mental time travel requires complex cognitive capabilities, including episodic-like memory [15] as well as the ability to act outside of current motivational state [15]. Some argue [16] against the latter ability in animals, suggesting that animals cannot anticipate or plan for future events. Although the present study does not address the specific cognitive mechanisms involved, our experimental design minimized possible differences in reproductive stage-relevant breeding motivation by testing warbler nests solely during early incubation, a time when female hosts are mostly on their nests to incubate their eggs. Additionally, we played the two types of alarm calls in random order to the same hosts on successive days to avoid treatment bias in daily motivational differences as incubation progressed. Nevertheless, further experimentation should specifically aim at understanding whether the decision to protect the nest at dawn in response to seet calls the day before involves the recollection of distant past events and the capacity to flexibly plan for the future. Specifically, do cues from parasitic cowbird calls on one day alter ongoing nest vigilance until the next morning or do they cause a specifically timed future vigilance by female warblers at the next dawn? Could responses to seet call or cowbird stimuli persist even further into the future? Is heightened nest attendance on the morning after a cowbird or seet call encounter adaptive to effectively preventing parasitism? Answers to these questions would help to establish the cognitive mechanisms and selective pressures underlying the decisions of female yellow warblers to engage in nest vigilance.
Studies of episodic-like memory of mental time travel have primarily focused on foraging contexts (e.g. [15]), yet reproductive contexts also provide rich opportunities that explore these concepts. For example, captive brood parasitic brown-headed cowbirds engage in complex cognition consistent with mental time travel within the context of their own egg-laying decisions [17]. We, therefore, propose both that our data [18] on female yellow warbler responses to anti-parasitic seet calls be replicated and that the birds' responses be studied across multiple time points following playbacks to explore more fully the nature and time-course of their referential alarm calls and anti-parasitic responses.
Acknowledgements
The Champaign County Forest Preserve District, the Vermilion County Conservation District, and the Illinois Department of Natural Resources allowed us to use their parks. We also thank Alex Di Giovanni for fieldwork, Alec Luro, Nico Adreani, and Matt Louder for R help, and Alison Bell, Jeff Hoover, Wendy Schelsky, and the editors and referees for constructive comments.
Ethics
We thank the University of Illinois Committee on Natural Areas, the Champaign County Forest Preserve District, the Vermilion County Conservation District, and the Illinois Department of Natural Resources for permitting us to use their parks for our research. We received institutional (no. 19032), state (no. NH20.6220) and federal (no. 23681) permits to conduct this work with vertebrate animals.
Data accessibility
The data forming the basis of these analyses are made available for open access through the Figshare.com dataset: https://doi.org/10.6084/m9.figshare.14879580.v1 [18].
Authors' contributions
S.A.G. conceived the study; S.L.L., J.K.E., M.E.H., C.S.W., T.J.B. and S.A.G. participated in its design; S.L.L. directed and S.L.L., J.K.E., K.S., S.K.W. and M.E.H. carried out fieldwork; S.L.L., M.E.H. and T.J.B. conducted data analysis; M.E.H., S.A.G. and C.S.W. drafted the manuscript, and all authors critically edited and revised the text. All authors approved the final version of the manuscript, and agree to be held accountable for the content therein.
Competing interests
We declare we have no competing interests.
Funding
The project was funded by the American Ornithological Society (to S.L.L.), the Illinois Ornithological Society (to S.L.L.), the National Geographic Society (NGS-60453R-19 to M.E.H.), the School of Integrative Biology (Clark Research Support Grant, Lebus Fund Award, Dissertation Travel Grant to S.L.L.) at the University of Illinois/Urbana-Champaign, and the USA National Science Foundation (IOS no. 1952726 to S.A.G. and IOS no. 1953226 to M.E.H.). Additional support was provided by the Center for Advanced Study at the University of Illinois and the Wissenschaftskolleg zu Berlin, Germany (to M.E.H.).
References
- 1.Gill SA, Bierema AMK. 2013On the meaning of alarm calls: a review of functional reference in avian alarm calling. Ethology 119, 449-461. ( 10.1111/eth.12097) [DOI] [Google Scholar]
- 2.Suzuki TN. 2016Semantic communication in birds: evidence from field research over the past two decades. Ecol. Res. 31, 307-319. ( 10.1007/s11284-016-1339-x) [DOI] [Google Scholar]
- 3.Seyfarth R, Cheney D, Marler P. 1980Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim. Behav. 28, 1070-1094. ( 10.1016/S0003-3472(80)80097-2) [DOI] [Google Scholar]
- 4.Feeney WE, Medina I, Somveille M, Heinsohn R, Hall ML, Mulder RA, Stein JA, Kilner RM, Langmore NE. 2013Brood parasitism and the evolution of cooperative breeding in birds. Science 341, 1506-1508. ( 10.1126/science.1240039) [DOI] [PubMed] [Google Scholar]
- 5.Gill SA, Sealy SG. 2004Functional reference in an alarm signal given during nest defence: seet calls of yellow warblers denote brood-parasitic brown-headed cowbirds. Behav. Ecol. Sociobiol. 56, 71-80. ( 10.1007/s00265-003-0736-7) [DOI] [Google Scholar]
- 6.Feeney WE, Langmore NE. 2015Superb fairy-wrens (Malurus cyaneus) increase vigilance near their nest with the perceived risk of brood parasitism. Auk 132, 359-364. ( 10.1642/AUK-14-218.1) [DOI] [Google Scholar]
- 7.Lawson SL, Enos JK, Mendes NC, Gill SA, Hauber ME. 2020Heterospecific eavesdropping on an anti-parasitic referential alarm call. Commun. Biol. 3, 143. ( 10.1038/s42003-020-0875-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawson SL, Enos JK, Mendes NC, Gill SA, Hauber ME. 2021Pairing status moderates both the production of and responses to anti-parasitic referential alarm calls in male yellow warblers. Ethology 127, 385-394. ( 10.1111/eth.13139) [DOI] [Google Scholar]
- 9.Lawson SL, Enos JK, Antonson ND, Gill SA, Hauber ME. 2021Do hosts of avian brood parasites discriminate parasitic vs. predatory threats? A meta-analysis. Adv. Study Behav. 53, 63-95. ( 10.1016/bs.asb.2021.03.002) [DOI] [Google Scholar]
- 10.Neudorf DLH, Sealy SG. 1994Sunrise nest attentiveness in hosts of the brown-headed cowbird. Condor 96, 162-169. ( 10.2307/1369073) [DOI] [Google Scholar]
- 11.Dere E, Dere D, de Souza Silva MA, Huston JP, Zlomuzica A.. 2018Fellow travellers: working memory and mental time travel in rodents. Behav. Brain Res. 352, 2-7. ( 10.1016/j.bbr.2017.03.026) [DOI] [PubMed] [Google Scholar]
- 12.Ospina EA, Merrill L, Benson TJ. 2018Incubation temperature impacts nestling growth and survival in an open-cup nesting passerine. Ecol. Evol. 8, 3270-3279. ( 10.1002/ece3.3911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strausberger BN. 1998Temporal patterns of host availability, brown-headed cowbird brood parasitism, and parasite egg mass. Oecologia 116, 267-274. ( 10.1007/s004420050588) [DOI] [PubMed] [Google Scholar]
- 14.Ellison K, Sealy SG. 2007Small hosts infrequently disrupt laying by brown-headed cowbirds and bronzed cowbirds. J. Field Ornithol. 78, 379-389. ( 10.1111/j.1557-9263.2007.00126.x) [DOI] [Google Scholar]
- 15.Clayton NS, Dickinson A. 1998Episodic-like memory during cache recovery by scrub jays. Nature 395, 272-274. ( 10.1038/26216) [DOI] [PubMed] [Google Scholar]
- 16.Baciadonna L, Cornero FM, Emery NJ, Clayton NS. 2020Convergent evolution of complex cognition: insights from the field of avian cognition into the study of self-awareness. Learn. Behav. 49, 9-22. ( 10.3758/s13420-020-00434-5) [DOI] [PubMed] [Google Scholar]
- 17.White DJ. 2020Avian egg timers: female cowbirds judge past, present, and future when making nest parasitism decisions. Front. Ecol. Evol. 8, 203. ( 10.3389/fevo.2020.00203) [DOI] [Google Scholar]
- 18.Lawson SL, Enos JK, Wolf CS, Stenstrom K, Winnicki SK, Benson TJ, Hauber ME. 2021Data from Lawson et al. Biology Letters 2021. Figshare ( 10.6084/m9.figshare.14879580.v1) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Lawson SL, Enos JK, Wolf CS, Stenstrom K, Winnicki SK, Benson TJ, Hauber ME. 2021Data from Lawson et al. Biology Letters 2021. Figshare ( 10.6084/m9.figshare.14879580.v1) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data forming the basis of these analyses are made available for open access through the Figshare.com dataset: https://doi.org/10.6084/m9.figshare.14879580.v1 [18].


