Abstract
COVID-19 (SARS-CoV-2) has emerged as one of the worst pandemics that have tormented the globe due to its highly contagious nature. Even if the disease manifests fever-like symptoms mostly, the disease may progress to the pulmonary-hyper inflammatory phase, with severe pneumonia, hypoxia and subsequent multiple organ infection. This subsequently creates a huge burden to the health care systems across the globe for an immediate arrangement of ventilator facilities, oxygen supply and advanced health care. We evaluated the pathological similarity of COVID-19 with other airway obstructive disorders such as COPD and asthma and found typical mucus hypersecretion and mucus plugging in COVID-19 subjects. From several bronchoscopy and clinical autopsy carried out in COVID-19 patients, the overexpression of mucin gene was evident which play a significant role in mucus hypersecretion and accumulation, leading to airway obstruction and further to respiratory distress. In the present work, we highlight the need for intense research inputs to elucidate the exact role the mucus plays in worsening COVID-19 symptoms. This will further help to find a proper approach to quantify the airway mucus plugging in each patient and to develop an appropriate therapy either to inhibit mucus secretion or to improve mucus clearance through well-designed clinical trials.
Keywords: Airway mucus, Cytokine storm, Mucins, Mucus Hypersecretion, Respiratory distress, SARS CoV 2
Introduction
The novel coronavirus, SARS CoV2, came to light towards the end of 2019, had gripped the globe in a massive fever quarantine with a dashboard pointing towards the pandemic peak with 79,686,071 confirmed cases of COVID-19, including 3,899,172 deaths, reported to WHO as of June 2021[1]. The primary mode of transmission of the disease is thought to be through the droplets, where the viral S protein interacts with ACE receptor in the lungs of the host, with a basic reproduction number of 3, indicating the ability of the virus to infect another three from the index person [2]. The infected person remains more contagious or infectious during the initial days of illness due to heavy viral load in the airways and the viral load is linked with the severity of disease especially in the elderly and health workers [3]. This initial phase of infection manifests as fever, headache, myalgia, throat irritation and dry cough. An appropriate early immune response is sufficient to block the disease progression at the initial phase itself. But, a delayed or insufficient immune reaction leads to a pulmonary phase which manifests as viral pneumonia with hypoxia. In susceptible patients, abrupt or hyperinflammatory response worsens the disease to a complicated phase which is typically labelled as ‘cytokine storm’ characterized by the excess level of pro-inflammatory cytokines, ferritin and C-reactive protein leading to multiple organ failure, Acute respiratory distress syndrome (ARDS) or Severe hypoxemia, which are the serious conditions for intensive care such as invasive ventilation or other strategies to improve oxygenation [4].
The airway to the lungs is lined with epithelial tissue which is covered by mucus (5–50 μm thickness), which serves as the first barrier to the external environment. [5] This function is performed by trapping harmful pathogens and dust particles and then removing them via mucociliary clearance. The level of mucus in our body is regulated by two mechanisms: mucus secretory cells and mucus ciliary escalator [6]. The functional properties of mucus seems to depend upon mucin, one of the major macromolecular constituent of mucus. They are responsible for providing mucus with viscosity and elastic properties. They are classified into two groups: secretory-based and membrane-tethered. In the lung tissue and lung airway, about 3 different secretory mucins (MUC2, MUC5AC and MUC5B) and 3 different membrane-tethered mucins (MUC1, MUC4, MUC16) are found to play a major role [7]. Along with the defensive role, mucin also performs functions such as encapsulating, holding and liberating biologically active molecules in signal transduction; inflammation, proliferation, differentiation and immune response regulation[8]. Besides their protective role, mucus and mucin are seen to play a role in many pulmonary obstructive disorders.
Upon evaluating many respiratory conditions such as Asthma, Chronic obstructive pulmonary disease(COPD) etc, it was observed that the mucus plugging of airways has a significant correlation with increased morbidity and mortality. In asthmatic patients, even after the sputum is expectorated, airway obstruction persists due to the mucus hypersecretion occurring in the distal airway. As the distal airways are too narrow, a slight elevation of mucus level may result in airway limitation and rapid decline of lung function[9]. In comparison with other respiratory conditions such as COPD and Cystic fibrosis (CF), mucus in an asthmatic patient is more viscous and tends to form mucus plugs. Autopsy studies have shown that mucus plug formation is the main reason for mortality in asthma patients[10]. In a study with 146 asthma patients, 67% of subjects showed a high mucus score (plugs in greater than 4 segments) with marked eosinophilia [11]. The pathophysiological phenotype in asthma is exhibited as airway inflammation, luminal mucus hypersecretion with MUC5B (low charge glycoform) and MUC5AC as the major mucin constituents (MUC5AC < lcgf MUC5B≫>≫MUC2), goblet cell hyperplasia and plasma exudation. The secretory mucins (MUC5B& MUC5AC) lining the airways are suspected to contribute towards mucus hypersecretion in asthmatic patients [12]. Two significant signaling pathways, IL-13/ IL-4 receptor α complex and epidermal growth factor are the inducers of MUC5AC secretion [13]. Strong clinical evidence is available to support the improvement of lung function in subjects with asthma by giving drugs targeting mucus secretion or synthesis [14]
Another important pulmonary pathology characterized by mucus hypersecretion is Chronic obstructive pulmonary disorder (COPD), a progressive disorder that manifests as a myriad of diseases. This is characterized by persistent airway obstruction which is related to the chronic inflammatory response of the airway and lungs towards toxic gas and particles. Inflammatory response and toxic particles cause injury to the airway, which triggers to undergo structural changes (airway remodelling). Bronchiolar smooth muscle hypertrophy, mural oedema, peribronchiolar oedema, mucus metaplasia and mucus hypersecretion (in airways less than 400 μm in diameter) are the common structural changes in COPD patients. In a study, it was noted that MUC-1 expression was remarkably increased in COPD patients when compared with that of healthy individuals of having normal lung function.MUC-1 is suspected of triggering airway remodelling and thereby increasing the number of mucus-producing cells [15]. Along with this, elevated levels of MUC5AC and MUC5B are seen in the sputum collected from COPD patients. When the sputum of smokers was examined, MUC5AC was found to be the predominant mucin, whereas, in COPD patients, the lower charged glycosylated form of MUC5B was abundant. Together with this, mucus clogging in airways increases the risk of airway inflammation, bacterial load and hence, chances of disease exacerbation. [16]. Therefore, techniques that can improve the mucociliary clearance in COPD patients work better to improve the exacerbations and poor health-related quality of life (HRQL), even though major therapies still focus on bronchodilators and anti-inflammatory glucocorticoids [17].
Evidence supporting the mucus hypersecretion in COVID-19 patients
From all the aforementioned evidence, it is clear that besides the protective role, abnormal or hypersecretion of mucus is associated with sudden deterioration of the functioning of lungs, increased rate of hospitalization and mortality in affected people. Reviewing the background of COVID-19, we found evidence linking mucus hypersecretion and disease severity. Blood analysis of COVID-19 patients in an early stage showed a normal WBC count mostly. As the disease progress, they exhibited significant neutrophilia and lymphopenia with a drastic increase in the level of pro-inflammatory cytokines such as IL-6, IL-10, and TNF-α. [18]. Therefore, as mentioned earlier, a severe COVID-19 infection can be indicated as a cytokine release syndrome which is characterized by elevated plasma levels of pro-inflammatory cytokines and markers. Several studies support that the pro-inflammatory cascades trigger overproduction of mucus with altered composition and impaired mucociliary clearance in infected respiratory epithelia, which ends up in further obstruction of airways and respiratory distress [19], [20]. A clinical autopsy conducted in two severe COVID-19 patients found that prominent mucus plugging with serous and fibrinoid exudation in all respiratory tracts, which was not a ventilator-induced lung injury. Bronchial mucus plugging and peribronchiolar metaplasia were evident in these COVID patients, which resembles the morphology of mucoid adenocarcinoma. The corona virus-infected alveolar macrophages acquire an aggregated phenotype with the massive release of cytokines which contributes to hyperinflammatory responses, hypoxemia, mucus plugging, and fibrosis in the respiratory tract [21]
Some studies suggested that neutrophils can form Neutrophil extracellular traps (NET) which can either detain the viral particles or exacerbate lung hyper inflammation in COVID-19 subjects [22]. Veras et al. reported elevated levels of NET in both the tracheal aspirate and plasma of 32 hospitalized severe COVID-19 patients and also found it in the lung tissue specimen during post-mortem analysis. They also observed prominent mucus plugging in all the studied subjects which revealed the link between neutrophilia and mucus hypersecretion. The hyperinflammatory and hyperimmune responses lead to NET overproduction and a higher level of neutrophil elastase which trigger modifications in mucus to become too thick and viscous with no antimicrobial properties. This promotes bacterial colonization, facilitates secondary infections, and eventually declining the respiratory functions of COVID-19 patients [23], [24].
A recent study thoroughly investigated the extent of mucus production in COVID-19 infected lung epithelial cells of both humans and macaques. H &E and periodic acid Schiff staining of bronchoalveolar lavage showed the presence of abundant mucus and linked carbohydrates. It was further confirmed by immunohistochemical staining which identified remarkably upregulated mucins mainly MUC 2, MUC5A and MUC5B. Later, they demonstrated the role of interferons in mucin overexpression. The study indicates that SARS CoV2 infection triggers the release of IFN-γ and IFN-β which activate aryl hydrocarbon receptor (AhR) signaling and stimulate mucin genes. In the initial stage, the synthesized mucus stick to the blood-gas barrier, increase its thickness and reduce the gas exchange, specifically oxygen exchange in the alveolar epithelial cells which manifest as silent hypoxia. But, as the disease worsens, the inflammatory exudates combine with the thick, viscous mucus and increases the barrier thickness to a greater extent which impairs the gas exchange of both O2 and CO2 which ultimately leads to critical illness [25].
Luigi Vetrugno and group, in 2020 described the imaging features of COVID-19 patients mainly by lung ultrasound and high-resolution computerized tomography (HRCT). This work reported the presence of pathological fluid in alveolar space, increased sputum volume and mucus hypersecretion in 40% of patients [26]. The formation of colloidal mucus plug was also evident in another imaging study published in the initial phases of the pandemic. This study evaluated the autopsy of 12 deceased patients and reported unique pulmonary manifestation characterized by fibromyxoid exudates and thick mucus plug formation, which was not observed in SARS-CoV-1[27]. From a series of autopsies conducted, it was noted that the lungs of the COVID-19 victims appeared heavy, firm, and edematous, with some haemorrhage areas and thick mucus into the airways, and the mediastinal lymph nodes were dimensionally augmented. [28].COVID-19 autopsies performed in Oklahoma, USA was also reported diffuse alveolar damage, chronic inflammation, mucus plugging and superimposed bacterial pneumonia [29]. Among the 38 autopsy samples of COVID-19 from northern Italy, 24 cases showed inflammatory infiltration with abundant neutrophils and lymphocytes in the alveolar lumina and importantly, dense mucoid material in the lumina of bronchi and bronchiolar branches [30]. Approximately, 40% of COVID-19 patients exhibited an increase in sputum volume [31] and mucus hypersecretion associated symptoms and 33% of COVID-19 autopsies detected severe mucoid tracheitis in the lower respiratory tract [32].
Another study showed that our immune system recognizes SARS CoV2 as an allergen and establishes an allergic immunity against the virus. This stimulates respiratory mucosal sensitivity and activation of SOX2 gene which leads to goblet cell hyperplasia and mucus hypersecretion as same as other allergic and inflammatory diseases [33].Apart from the reports depicting mucus plugging as an acute manifestation of COVID-19, a case report of painless-noninflammatory tracheal mucus hypersecretion in COVID-19 has been published in 2020, which was the first to suggest mucus plugging as one of the long term symptoms of COVID-19. They reported that the hyperstimulation of tracheal goblet cells by the coronavirus triggered mucus production and accumulation of mucus leading to hypoxemia and suffocation in patients though the patient did not have a severe COVID-19 infection [34].
The above-noted analysis gives a clear picture of the similarity in disease progression of COVID-19 with typical mucus hypersecretory diseases such as asthma and COPD. This is clear from several studies that factors like MUC5AC, MUC5B, MUC1 etc are overexpressed and the changes in them might be the reason behind the mucus adhesion, airway obstruction later leading to respiratory distress. Hence, the excess mucus load in the airways of COVID-19 patients is a prerequisite issue that is to be addressed, if not, it will make all the therapies ineffective like passing oxygen through a blocked path or even worsen hypoxia in patients.
Hypothesis
Based on the background information, we hypothesize that the drugs targeting elevated mucins or else implementing better ways to remove excess mucus clogs in airways can improve pulmonary complications in COVID-19 patients. This will improve the treatment outcome or drop off the need for ventilation and oxygen therapy and thereby, gearing faster recovery and get a better survival rate. Therefore, a proper consideration of the mucus plug in COVID-19 patients and an adequate therapy based on the pathological state can contribute greatly to reduce the hospital and socio-economic burden during this pandemic time.
Justification of the hypothesis
Evidence of ACE receptor-mediated entry of SARS CoV2 is numerous, while the virus can also utilize mucus, which is present in the outer and inner layer of lung epithelial cells for entry into host cells. Depending on the pattern and extent of glycosylation, mucin can either prevent or facilitate virus entry into epithelial cells [35], [36]. Sungnaket al in 2020 surveyed single-cell RNA sequencing data sets from valid resources and reported significant overexpression of SARS CoV2 entry factors such as ACE, TMPRSSS2 with detectable co-expression of mucin synthesizing genes in a nasal goblet and ciliated cells. Since it does not require lysis for release, SARS CoV2 is more likely to utilize inherent secretory pathways in goblet cells and they signify the role of nasal carriage, thereby mucin secretion in disease transmission [37]. Cantuti-castlevetri et al in 2020 evaluated the role of Neuropilin-1 as the host factor to facilitate SARS CoV2 entry after comparing published scRNA-seq data sets of human lung tissue and human olfactory epithelium. They reported abundant expression of NRP1 and NRP2 in almost all pulmonary and olfactory cells and later confirmed the results after detecting positive immunoreactivity for NRP1 in human autopsic tissues, specifically in the surface layer of human respiratory and olfactory epithelium [38]. Zhou R et al reported a positive correlation between NRP1and MUC 1 and found that MUC 1 can induce the expression of NRP1 [39]. All these studies justify the significance of evaluating mucin expression and its glycosylation to predict the extent of viral load in a host cell and susceptibility to COVID-19 infection, because of the involvement of mucus and its associated genes in facilitating viral entry into epithelial cells.
A team of more than 60 pathologists and technicians assembled to form a COVID-19 pathology team in Wuhan and Chongquing, China. The team performed intensive pathological diagnosis and research at the organ, tissue and molecular level on about 91 COVID-19 patients. The study led to the conclusion that COVID-19 infection resulted in multiple organ and tissue injury, especially in the lungs, which was characterized by a prominent and extensive pulmonary lesion. There were also significant pathological changes in the respiratory system as well; certain areas of the bronchial mucosa displayed epithelial exfoliation, mucin accumulation and mucin-plug formation. The team also found that most of the victims of COVID-19 infections had mucus plugs in the alveoli and the deep small airways as well, impairing ventilation. In such events of lower airway obstruction, providing ventilatory support would be like pumping water through a clogged pipe and will not resolve the issue and may even lead to further damage[40]. A single-cell RNA sequence analysis (scRNA-seq) was conducted by Jiangping He et al in 2020, on the transcriptome profile of 8356 cells by subjecting them through downstream analysis. For the study, the public pulmonary scRNA–seq data set of 8 healthy donors were used as a control against COVID-19 samples. When they compared the differential expressing genes of a healthy donor and COVID-19 patient, they noted that there is an increase in the expression of gel-forming mucin (GFM), MUC5AC and MUC2B as well as membrane-tethered protein MUC4, MUC16 and MUC20[41].
A study was conducted by WenjuLu et al, in 2020 at the Guangzhou Institute of Respiratory Health (GIRH) on sixteen COVID-19 patients to investigate the changes occurring in the lungs. The study identified that the overexpression of mucin genes was evident that play a key role in COVID-19 symptoms. [42]. For this study, an aspiration of respiratory tract mucus from critically ill COVID-19 patients was performed by bronchoscopy. Along with this, optical coherence tomography (OCT) was carried out to obtain the cross-sectional images of bronchioles. Then the sputum was subjected to component analysis and compared against the healthy sample. MUC5AC and MUC1 were analyzed using ELISA and MUC1 cytoplasmic tail was measured using western blotting. In comparison, it was noted that the MUC5AC and MUC1 levels were elevated in the COVID 19 patient and the OCT(Optical coherence tomography) results showed an obstruction in the small airways of the bronchioles. The mucus collected from the patient was highly viscous and concentrated and caused airway dehydration leading to mucus adhesion and blockage of the airway. The increased mucus level and viscosity may be due to elevated MUC5AClevel. The MUC1 and MUC1- cytoplasmic tail were found on the cytoplasmic side of the cell, indicating disruption of the alveolar epithelium of the airway. This disruption of the alveolar epithelium was confirmed by the evidence obtained from the pathological findings from the lung tissues of COVID-19 patients[42]
Importantly, Yixuan J Hou and the team explored SARS CoV2 pathogenesis and viral tropism along the human respiratory tract using a reverse genetics system. The study generated valuable reference data for future research, in which they identified the accumulation of aberrantly secreted MUC5BA in the alveolar region and they presented it as a remarkable feature of COVID-19. They performed Alcian Blue Periodic Acid Schiff staining (AB-PAS) in SARS CoV2 infected autopsy lungs for detecting mucin or mucin-like carbohydrates. They found considerable staining in the alveolar region of lung samples. Further, immunohistochemical analysis was performed to characterize the components of the stained area and found it as a mixture of secreted mucins, MUC5B and MUC5AC [43]. The aberrant accumulation of MUC5B in the alveolar parenchyma manifests the typical feature of idiopathic pulmonary fibrosis [44]. A detailed investigation of respiratory pathology was conducted by Yin W and team and reported remarkable mucus plugging in COVID-19 infected lungs. Histological staining revealed mucus accumulation in many distal bronchioles with goblet cell hyperplasia and hypoplasia of club cells. Importantly, the corona-infected respiratory epithelium showed MUC5AC gene overexpression and poor expression of MUC5B and CC16, which control mucus hypersecretion mucociliary clearance respectively, as compared with healthy controls. All these contribute to uncontrolled mucus production and accumulation in airways which may cause dyspnoea or even death, if not considered with caution. They also put forward a possibility of pushing mucus plugs deep into the alveoli during mechanical ventilation, which cover up most of the air exchange surface in the lungs and lead to severe hypoxia. These evidence indicates the involvement of mucin in exacerbating COVID-19 symptoms [45].
In addition to the aforementioned evidence depicting the role of mucus in COVID pathology, some studies suggest a positive treatment response and faster recovery with drug targeting mucus in COVID-19 patients. A single centred, retrospective observational study analyzed the sputum characteristics of severely ill COVID-19 patients with an average age of 66.7 years. Among the 41 patients included in the study, who are progressed to critical illness had increased neutrophil/lymphocyte ratio and importantly, had a higher percentage of grade 3 sticky sputum within 10 days of the treatment. But, the group which has received an early IV expectorant (greater than 270 g Ambroxol/day) and drainage in the prone position avoided progression to the critical stage and improved prognosis[46]. Furthermore, another single-centre, observational study retrospectively analyzed critical ill COVID-19 patients who were received mechanical ventilation. The computational and experimental analysis predicted that, in a healthy lung with surface tension, γ = 25dyn/cm and viscosity of mucus, µ=0.01 P, the airway opening pressure would be between 2.1 and 3.8 cmH2O, whereas, in a COVID-19 infected lung, the accumulated lower airway mucus with the same viscosity of medicinal glycerine or even peanut butter causes a sharp increase in airway opening pressure to 35–42 or 30–49 cmH2O. In a normal adult, the maximal transpulmonary pressure during spontaneous respiration is approximately 25–30 cmH2O, which is less than the predicted airway opening pressure in covid-19 infected, mucus plugged lungs. Thus, ventilation strategy might not be effective to open the airways, which worsens the hypoxia and suffocation, eventually lead to death [47].
Farooqui et al.evaluated 3 critical cases of COVID-19, who developed ARDS and received supportive mechanical ventilation. By considering the possibility of mucus overproduction and plugging due to either underlying COVID infection or supportive ventilation, they implemented proper airway hygiene management using mucolytics, bronchodilators, and tracheal suctioning. This strategy prevented acute complications in the ICU and also long-term sequelae of infection [48]. Importantly, the trace element, zinc is being mostly evaluated as a prophylactic or supportive treatment for COVID-19 because of its direct anti-viral and immune-boosting properties [49]. Together with this, the potential of zinc to activate P2X receptor and stimulate ciliary beat frequency help to establish proper mucociliary clearance in COVID-19 patient and therefore, may prevent the disease occurrence or avoid respiratory complications in COVID-19 patients [50], [51].
With more than 12 active COVID-19 clinical trials and the proven involvement of zinc deficiency in the pathogenesis of about 16% of deep respiratory infections confer remarkable value to zinc as a promising supplement against COVID-19 [52]. Likewise, a single centred case series evaluated the combined administration of dornase alpha and albuterol to five mechanically ventilated COVID-19 patients through an inline nebulizer system. Dornase alpha as a recombinant human DNase 1, digested the DNA from neutrophil extracellular traps in the mucoid sputum and cleared the mucus plugs interfering with the ventilation. All the five patients under study were successfully extubated and recovered with no drug-associated toxicity[53].
The above-discussed observations suggest that the cytokine storm associated mucus plugging in airways makes COVID-19, a mucus hypersecretory disease to some extent, which makes them unique from the previous coronavirus family. Therefore, significant research input is needed to clarify the exact role of mucus in COVID-19 pathogenesis to understand whether it is protective or harmful. In the present work, we hypothesize that the therapies which inhibit mucus hypersecretion or improve mucociliary clearance are found to be relieving asthma, COPD and cystic fibrosis and thereby, reduce mortality. The same techniques can be extrapolated to COVID-19 patients as they exhibit twinning pathophysiological phenotype such as mucus plugging, airway inflammation etc. (Table 1, Table 2 , Fig. 1, Fig. 2 ).
Table 1.
Therapies targeting mucus synthesis, secretion and clearence against COVID-19.
| Sl.no | Therapy/technique | Outcome |
|---|---|---|
| Existing Therapies | ||
| a) | Mucolytics | |
| Carbo-cysteine |
|
|
| N-acetyl cysteine |
|
|
| b) | Expectorants | |
| Guaifenesin |
|
|
| Iodide containing expectorant (Potassium iodide) |
|
|
| c) | Mucoactive Drugs | |
| Bronchodilators |
|
|
| d) | Osmolar agents | |
| Mannitol |
|
|
| Hypertonic saline |
|
|
| Novel Therapies[55] | ||
| a) | CXCR2 antagonist(AZD5069) | CXCR2 antagonist inhibits neutrophil movement and thereby inhibit mucin secretion. |
| b) | EGFR Tyrosine kinase inhibitor (Gefitinib) | Reduces mucin synthesis and goblet cell hyperplasia. |
| c) | P38inhibitor (Acumapimod) | Inhibit mucin secretion by airway epithelium through inhibiting MARCKs protein. |
| d) | Anti-IDO antibody, clone 10.1 | IDO is an enzyme that is responsible for converting tryptophan to kynurenines. It is upregulated by interferon γ. By inhibiting this mechanism, MUC gene expression via KYN AhRsignalling pathway is down-regulated. |
| e) | Statins (simvastatin) | Inhibits airway mucus production and goblet cell hyperplasia. |
| f) | Bio-11006 | It is an analogue of Myristoylated –N terminal sequence(MANS) and Inhibits MARCKS. |
| Techniques to removes mucus from the airway[56] | ||
| a) | Chest physiotherapy (CPT) |
|
| b) | Flutter valve |
|
| c) | High-frequency chest wall oscillator (HFCWO) |
|
| d) | Positive Expiratory Pressure (PEP) device |
|
Table 2.
Ongoing clinical trials targeting mucus secretory pathways against COVID-19.
| Drug | Mechanism of Action | clinical trial | Expected Outcome | Title of the study & NCT No |
|---|---|---|---|---|
| N-Acetyl Cysteine | Mucolytic | Phase 2 | Loose thickened mucus | A Study of N-acetylcysteine in Patients With COVID-19 Infection: NCT04374461 |
| Aerosolized Dornase Alpha | Mucolytic | Phase 3 | Facilitate mucus clearance | Nasal Irrigation to Reduce COVID-19 Morbidity: NCT04355364 |
| Infliximab | TNF-α inhibitor | Phase 2 | Improved oxygenation in hypoxia patients | A Phase 2 Trial of Infliximab in Coronavirus Disease 2019 (COVID-19): NCT04443881 |
| Anakinra | IL-1 Receptor antagonist | Completed | Reduce hyperinflamation& respiratory distress | Clinical Trial of the Use of Anakinra in Cytokine Storm Syndrome Secondary to Covid-19 (ANA-COVID-GEAS): NCT04443881 |
| Anti-interleukin Drug and their combination | Siltuximab –IL-6 inhibitor Toclizumab –IL-6 inhibitor Anakinra –IL-1 inhbitor |
Phase 3 | Improvement in oxygenation | Treatment of COVID-19 Patients With Anti-interleukin Drugs (COV-AID): NCT04330638 |
| Anti-interleukin Drugs | Toclizumab – IL-6 inhibitor Infliximab – TNF-α inhibitor |
Recruiting | Patient clinical status improvement and improvement in oxygenation | Comparison of Tocilizumab Versus Tocilizumab/Infliximab in Patients With COVID-19-associated Cytokine Storm Syndrome |
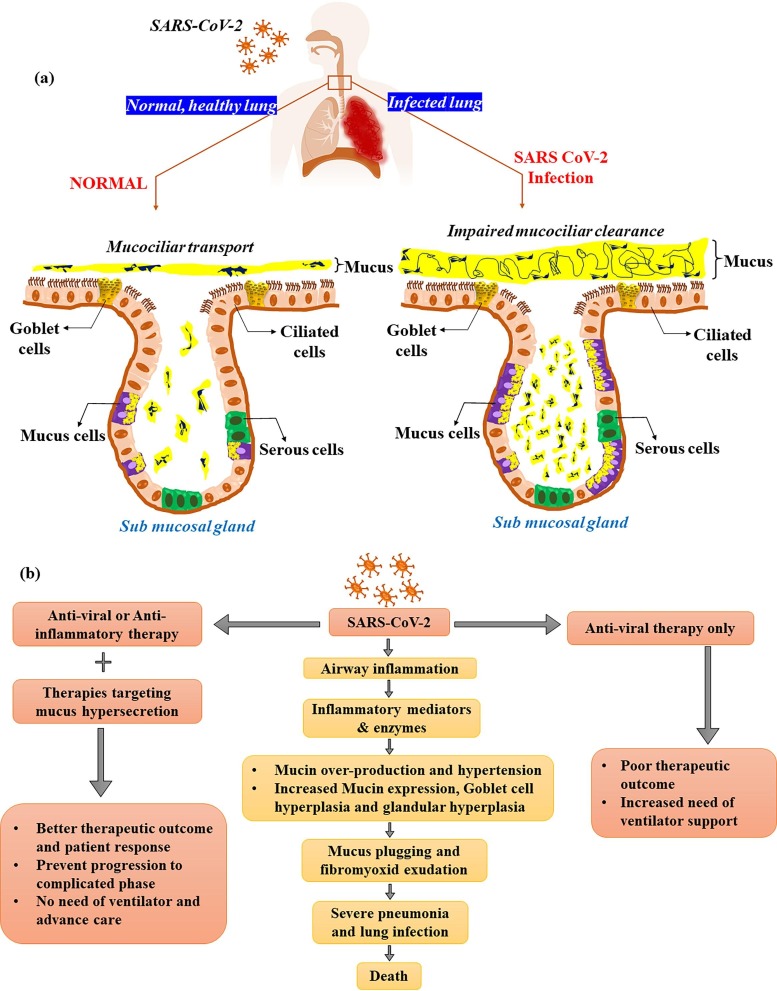
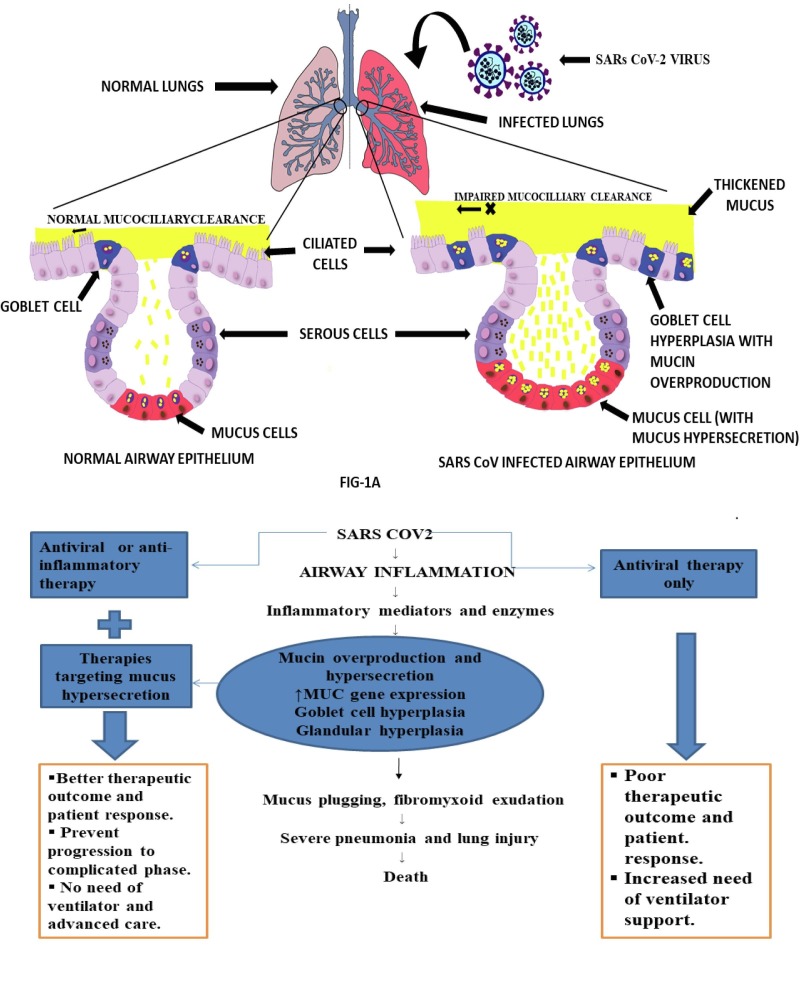
Fig. 1.
a) RESPONSE OF AIRWAY SECRETORY CELLS: NORMAL Vs SARS CoV2. The lung airway is lined with epithelial tissue, that is coated by a mucus layer. The mucus layer protects the lungs from various external agents such as pathogens and dust particle by trapping and expel through mucociliary action. The elasticity and viscous property of the mucus is governed by mucin, glycosylated macromolecular constituents of the mucin. In a normal airway epithelium, goblet cells and mucus cells are responsible for mucin synthesis and secretion and hence control the thickness and nature of the mucus layer. The airway epithelium of lungs performs a variety of critical function including, maintenance of lung fluid balance, metabolism and/or clearance of inhaled agents, attraction and activation of inflammatory cells in response to injury and the regulation of airway smooth muscle function via secretion of numerous mediators. The airway epithelium is also the primary site of contact for various external agents that may trigger mucus hypersecretion and cause airway obstruction. In a SARS CoV-2 infected airway epithelium, the mucin secretion is upregulated as a result of a cascade of events originating from cytokine storm. Additionally with goblet cell hyperplasia and mucous cell hypertrophy results in viscous mucus secretion. The virus also causes ciliary dysfunction, leading to impaired mucociliary clearance. This together with excess mucus productionlead to mucus plug formation and airway clogging. The clogging increases the risk of airway inflammation and bacterial load thereby, causing disease exacerbation leading to severeacute respiratory distress syndrome (ARDS). b) POTENTIAL ROLE OF MUCUS TARGETING IN IMPROVING LUNG FUNCTION OF COVID-19 PATIENTS. We postulate that the drugs targeting elevated mucins to remove excess mucus clogging in airways can improve pulmonary complications in COVID patient. This will subsequently improve efficiency or decrease the need for ventilation and oxygen therapy and also hastening recovery rate and improve the survival rate.
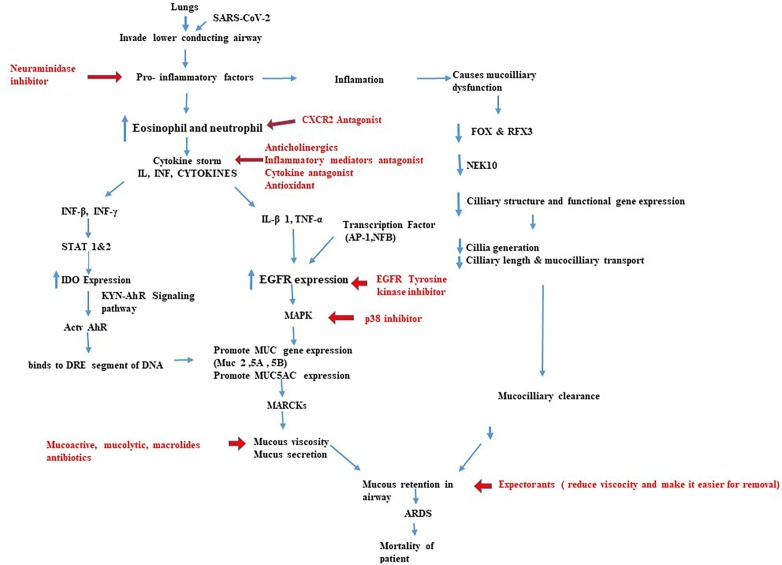
Fig. 2.
DRUGS TARGETING POTENTIAL SIGNALLING PATHWAYS REGULATING MUCIN SYNTHESIS, MUCUS SECRETION AND MUCOCILIARY CLEARANCE. The flowchart represents the various pathways that can be targeted to decrease mucus accumulation in the airway and reduce mortality in COVID-19 patients. Mucus hypersecretion may occur due to increased mucin synthesis. Aberrant expression of mucin synthesizing genes and mucus hypersecretionoccur as a result of cytokine storm in the hyperinflammatory phase of COVID-19 infection. This will further lead to the activation of various signallings such as IDO dependent KYN AhRsignalling pathway and EGFRmediated MAPK pathway. This flowchart represents important signalling pathways involved in mucus production and suggests the drugs targeting various signalling molecule, which helps to modulate mucus hypersecretion in COVID-19 patients.
Conclusion
As the medical community across the globe is trying to find out a suitable therapy for COVID-19, a multitude of drug development paradigms are being evaluated day by day. Mucus plugging, airway inflammation and subsequent disease exacerbation are found in most of the complicated cases of COVID-19. This further point out the necessity of targeting mucus plugs along with the antiviral therapies, which may help to prevent the disease progression, limit the need for ventilator support and its associated advanced hospital care. This may gradually help to reduce the socio-economic burden of this pandemic. We highlight with this hypothesis that the need for intense research inputs to elucidate the exact role of mucus plugs in worsening COVID-19 symptoms. Additionally, it is suggested to find out a proper approach to quantify the mucin expression and its pattern of glycosylation in patients. This will further facilitate the prediction of disease susceptibility and exacerbation and subsequently validate the efficacy of existing as well as novel therapies targeting mucin against COVID-19 through well-designed clinical trials.
CRediT authorship contribution statement
Sarath S. Kumar: Data curation, Formal analysis, Methodology, Resources, Writing – original draft, Software. Aiswarya Binu: Data curation, Formal analysis, Methodology, Resources, Writing – original draft, Software. Aswathy.R. Devan: Data curation, Formal analysis, Methodology, Resources, Writing – original draft. Lekshmi.R. Nath: Conceptualization, Funding acquisition, Investigation, Project administration, Validation, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
We express our sincere gratitude to Dr Shanthikumar. V. Nair, Dean of Research, Amrita Vishwa Vidyapeetham and Dr Sabitha M, Principal, Amrita School of Pharmacy for the facilities provided to carry out the work.
Funding: We acknowledge the support of the Amrita Rapid-Response-Research COVID 19 (ARCH) initiative by Amrita Vishawa Vidyapeetham to LRN and DST INSPIRE PhD fellowship (IF 190226) to ARD.
Data availability statement: The authors confirm that the data supporting the findings of this study are available within the article.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. (2021). Retrieved 25 June 2021, from https://covid19.who.int/.
- 2.Joseph S., Nair B., Nath L.R. The Ineluctable Role of ACE-2 Receptors in SARS COV-2 Infection and Drug Repurposing as a Plausible SARS COV-2 Therapy: A Concise Treatise. Curr Mol Med. 2021;21 doi: 10.2174/1573405617666210204212024. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y., Li L. SARS-CoV-2: virus dynamics and host response. Lancet Infect Dis. 2020;20(5):515–516. doi: 10.1016/S1473-3099(20)30235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baby B., Devan A.R., Nair B., Lekshmi R., Nath “The Impetus of COVID -19 in Multiple Organ Affliction Apart from Respiratory Infection: Pathogenesis, Diagnostic Measures and Current Treatment Strategy. Infect Disord - Drug Target. 2020;20:1. doi: 10.2174/1871526520999200905115050. [DOI] [PubMed] [Google Scholar]
- 5.Fahy J.V., Dickey B.F. Airway mucus function and dysfunction. N Engl J Med. 2010;363(23):2233–2247. doi: 10.1056/NEJMra0910061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu W., Zheng J. The function of mucins in the COPD airway. Current Respirat Care Rep. 2013;2(3):155–166. doi: 10.1007/s13665-013-0051-3. [DOI] [Google Scholar]
- 7.Roy M.G., Livraghi-Butrico A., Fletcher A.A., McElwee M.M., Evans S.E., Boerner R.M., et al. Muc5b is required for airway defence. Nature. 2014;505:412–416. doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratan C., Cicily K.D.D., Nair B., Nath L.R. MUC glycoproteins: Potential biomarkers and molecular targets for cancer therapy. Curr Cancer Drug Targets. 2021;21(2):132–152. doi: 10.2174/1568009620666201116113334. [DOI] [PubMed] [Google Scholar]
- 9.Evans C.M., Kim K., Tuvim M.J., Dickey B.F. Mucus hypersecretion in asthma: causes and effects. CurrOpinPulm Med. 2009 Jan;15(1):4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunican E.M., Watchorn D.C., Fahy J.V. Autopsy and Imaging Studies of Mucus in Asthma. Lessons Learned about Disease Mechanisms and the Role of Mucus in Airflow Obstruction Journal Article. Ann Am Thora Soc. 2018;15(Supplement_3):S184–S191. doi: 10.1513/AnnalsATS.201807-485AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunican EM, Elicker BM, Gierada DS, et al. Mucus plugs in patients with asthma linked to eosinophilia and airflow obstruction. J Clin Investigat 2018; 128(3):997-1009. DOI: 10.1172/jci95693. [DOI] [PMC free article] [PubMed]
- 12.Young H.W.J., Williams O.W., Chandra D., Bellinghausen L.K., Pérez G., Suárez A., et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37(3):273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishikawa N., Mazur W., Toljamo T., Vuopala K., Rönty M., Horimasu Y., et al. Ageing and long-term smoking affects KL-6 levels in the lung, induced sputum and plasma. BMC Pulm Med. 2011;11(1) doi: 10.1186/1471-2466-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laforest L., Van Ganse E., Devouassoux G., El Hasnaoui A., Osman L.M., Bauguil G., et al. Dispensing of antibiotics, antitussives and mucolytics to asthma patients: a pharmacy-based observational survey. Respir Med. 2008;102(1):57–63. doi: 10.1016/j.rmed.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Kirkham S., Kolsum U., Rousseau K., Singh D., Vestbo J., Thornton D.J. MUC5B is the major mucin in the gel phase of sputum in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178(10):1033–1039. doi: 10.1164/rccm.200803-391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DI Stefano A., Lusuardi A.M., Balbo P., Vecchio C., Maestrelli P., et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med. 1998;158(4):1277–1285. doi: 10.1164/ajrccm.158.4.9802078. [DOI] [PubMed] [Google Scholar]
- 17.Bhowmik A., Chahal K., Austin G., Chakravorty I. Improving mucociliary clearance in chronic obstructive pulmonary disease. Respir Med. 2009;103(4):496–502. doi: 10.1016/j.rmed.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 18.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen Y., Huang S., Kang J., Lin J., Lai K., Sun Y., et al. Management of airway mucus hypersecretion in chronic airway inflammatory disease: Chinese expert consensus (English edition) Int J Chron Obstruct Pulmon Dis. 2018;13:399–407. doi: 10.2147/COPD.S144312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X., Ma X. Acute respiratory failure in COVID-19: is it “typical” ARDS? Crit Care. 2020;24(1):198. doi: 10.1186/s13054-020-02911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C., Xie J., Zhao L., Fei X., Zhang H., Tan Y., et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBioMedicine. 2020;57:102833. doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borges L., Pithon-Curi T.C., Curi R., Hatanaka E., Vago J. COVID-19 and Neutrophils: The Relationship between Hyperinflammation and Neutrophil Extracellular Traps. Mediators Inflamm. 2020;2020:1–7. doi: 10.1155/2020/8829674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6):e20200652. doi:10.1084/jem.20200652. [DOI] [PMC free article] [PubMed]
- 24.Flavio ProtasioVeras, Marjorie Cornejo Pontelli, Camila Meirelles Silva, et al. SARS-CoV-2–triggered neutrophil extracellular traps mediate COVID-19 pathology. J Exp Med 7. 2020. 217 (12): e20201129. doi: https://doi.org/10.1084/jem.20201129. [DOI] [PMC free article] [PubMed]
- 25.Liu Y., Lv J., Liu J., Li M., Xie J., Lv Q., et al. Mucus production stimulated by IFN-AhR signaling triggers hypoxia of COVID-19. Cell Res. 2020;30(12):1078–1087. doi: 10.1038/s41422-020-00435-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vetrugno L., Baciarello M., Bignami E., Bonetti A., Saturno F., Orso D., et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1) doi: 10.1186/s13089-020-00185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oli Smith, https://www.express.co.uk/news/world/1255250/Coronavirus-vaccine breakthrough-COVID-19-autopsies-doctors 14 march 2020, accessed on 12 October 202065.
- 28.Maiese A, Manetti AC, La Russa R, et al. Autopsy findings in COVID-19-related deaths: a literature review [published online ahead of print, 2020 Oct 7]. Forensic Sci Med Pathol. 2020;1-18. doi:10.1007/s12024-020-00310-8. [DOI] [PMC free article] [PubMed]
- 29.Barton L.M., Duval E.J., Stroberg E., Ghosh S., Mukhopadhyay S. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol. 2020;153(6):725–733. doi: 10.1093/ajcp/aqaa062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carsana L., Sonzogni A., Nasr A., Rossi R.S., Pellegrinelli A., Zerbi P., et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20(10):1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vetrugno L., Baciarello M., Bignami E., Bonetti A., Saturno F., Orso D., et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020;12(1) doi: 10.1186/s13089-020-00185-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77(2):198–209. doi: 10.1111/his.v77.210.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khashkhosha H.K., Elhadi M. A hypothesis on the role of the human immune system in covid-19. Med Hypotheses. 2020;143:110066. doi: 10.1016/j.mehy.2020.110066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manckoundia, P., &Franon, E. Is Persistent Thick Copious Mucus a Long-Term Symptom of COVID-19?. Eur J Case Rep Int Med; 2020: 7(12). 002145. https://doi.org/10.12890/2020_002145. [DOI] [PMC free article] [PubMed]
- 35.Bose M., Mitra B., Mukherjee P. Mucin signature as a potential tool to predict susceptibility to COVID‐19. Physiol Rep. 2021;9 doi: 10.14814/phy2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bose M., Mukherjee P. Microbe-MUC1 Crosstalk in Cancer-Associated Infections. Trends Mol Med. 2020;26(3):324–336. doi: 10.1016/j.molmed.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Lung Biological Network H.C.A., Sungnak W., Huang N., Bécavin C., Berg M., Queen R., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantuti-Castelvetri L., Ojha R., Pedro L.D., Djannatian M., Franz J., Kuivanen S., et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020;370(6518):856–860. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou R., Curry J.M., Roy L.D., Grover P., Haider J., Moore L.J., et al. A novel association of neuropilin-1 and MUC1 in pancreatic ductal adenocarcinoma: role in induction of VEGF signaling and angiogenesis. Oncogene. 2016;35(43):5608–5618. doi: 10.1038/onc.2015.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bian X.-W., The COVID-19 Pathology Team, Yao X.-H., Ping Y.-F., Yu S., Shi Y., et al. Autopsy of COVID-19 patients in China. Natl Sci Rev. 2020;7:1414–1418. doi: 10.1093/nsr/nwaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He J., Cai S., Feng H., Cai B., Lin L., Mai Y., et al. Single-cell analysis reveals bronchoalveolar epithelial dysfunction in COVID-19 patients. Protein Cell. 2020;11(9):680–687. doi: 10.1007/s13238-020-00752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu W., Liu X., Wang T., Liu F., Zhu A., Lin Y., et al. Elevated MUC1 and MUC5AC mucin protein levels in airway mucus of critical ill COVID-19 patients. J Med Virol. 2021;93(2):582–584. doi: 10.1002/jmv.v93.210.1002/jmv.26406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hou Y.J., Okuda K., Edwards C.E., Martinez D.R., Asakura T., Dinnon K.H., et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell. 2020;182(2):429–446.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans C.M., Fingerlin T.E., Schwarz M.I., Lynch D., Kurche J., Warg L., et al. Idiopathic Pulmonary Fibrosis: A Genetic Disease That Involves Mucociliary Dysfunction of the Peripheral Airways. Physiol Rev. 2016;96(4):1567–1591. doi: 10.1152/physrev.00004.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin W., Cao W., Zhou G., Wang L., Sun J., Zhu A., et al. Analysis of pathological changes in the epithelium in COVID-19 patient airways. ERJ Open Res. 2021;7(2):00690-2020. doi: 10.1183/23120541.00690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang, Yu et al. Sputum characteristics and airway clearance methods in patients with severe COVID-19. Medicine. 2020; 99.46 :e23257.doi:10.1097/MD.0000000000023257. [DOI] [PMC free article] [PubMed]
- 47.Chen Z., Zhong M., Jiang L., et al. Effects of the Lower Airway Secretions on Airway Opening Pressures and Suction Pressures in Critically Ill COVID-19 Patients: A Computational Simulation. Ann Biomed Eng. 2020;48(12):3003–3013. doi: 10.1007/s10439-020-02648-0(1,2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farooqi F.I., Morgan R.C., Dhawan N., Dinh J., Yatzkan G., Michel G. Airway Hygiene in COVID-19 Pneumonia: Treatment Responses of 3 Critically Ill Cruise Ship Employees. Am J Case Rep. 2020:21. doi: 10.12659/AJCR.926596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joachimiak M.P. Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Negl Trop Dis. 2021;15 doi: 10.1371/journal.pntd.0008895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skalny A., Rink L., Ajsuvakova O., Aschner M., Gritsenko V., Alekseenko S., et al. Zinc and respiratory tract infections: Perspectives for COVID-19 (Review) Int J Mol Med [Internet]. 2020 doi: 10.3892/ijmm10.3892/ijmm.2020.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodworth B.A., Zhang S., Tamashiro E., Bhargave G., Palmer J.N., Cohen N.A. Zinc Increases Ciliary Beat Frequency in a Calcium-Dependent Manner. Am J Rhinol Allergy. 2010;24(1):6–10. doi: 10.2500/ajra.2010.24.3379. [DOI] [PubMed] [Google Scholar]
- 52.Wessels I., Rolles B., Rink L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber A.G., Chau A.S., Egeblad M., Barnes B.J., Janowitz T. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: a case series. Mol Med. 2020;26(1):91. doi: 10.1186/s10020-020-00215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncan F. Rogers & Peter J. Barnes Treatment of airway mucus hypersecretion, Ann Med; 2006: 38:2, 116-125, DOI: 10.1080/07853890600585795. [DOI] [PubMed]
- 55.Ha E.V.S., Rogers D.F. Novel Therapies to Inhibit Mucus Synthesis and Secretion in Airway Hypersecretory Diseases. Pharmacology. 2016;97(1–2):84–100. doi: 10.1159/000442794. [DOI] [PubMed] [Google Scholar]
- 56.Osadnik C.R., McDonald C.F., Jones A.P., Holland A.E. Airway clearance techniques for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD008328.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]