Dear editor
Neurological complications of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection are now well-recognized. Commonly reported syndromes include cerebrovascular disease, encephalopathy, acute disseminated encephalomyelitis, and para/post-infectious encephalitis [1]. Herein, we report a case of post-COVID-19 opsoclonus-myoclonus syndrome and encephalopathy associated with leucine-rich glioma-inactivated 1 (LGI-1) antibodies.
1. Case report
A 50-year-old left-handed male with no significant past medical history presented in November 2020 following a large cryptogenic left middle cerebral artery (MCA) territory infarction, later complicated by malignant MCA syndrome requiring hemicraniectomy. He underwent thrombectomy for a distal left M1 occlusion, and thereafter remained on clopidogrel 75 mg daily and atorvastatin 40 mg daily. Following the infarct he had residual right hemiparesis and right homonymous hemianopia, and required rehabilitation. He had been making good progress with therapy, was communicating normally and could tolerate a normal diet.
In late January 2021 he was exposed to SARS-CoV-2. Four days later he became febrile (temperature 38.5 °C), and SARS-CoV-2 was identified on RT-PCR of a nasopharyngeal swab specimen. On day 5 of his illness he became tachypnoeic (respiratory rate 24/min) and hypoxaemic (oxygen saturations 89%); C-reactive protein was raised at 230 mg/L and lymphocyte count was 0.7 × 109/L. A CT pulmonary angiogram showed bilateral consolidation and no pulmonary embolism. He was treated with low-flow oxygen, dexamethasone 6 mg daily and piperacillin/tazobactam. On day 7, he developed abnormal eye movements and jerks of the non-paretic left upper and lower limbs. On examination he was confused, distressed and unable to speak or follow commands. Opsoclonus and limb myoclonus was evident (Video 1) and a provisional diagnosis of COVID-19 related encephalitis with opsoclonus-myoclonus syndrome (OMS) was made. He was treated with levetiracetam 750 mg twice daily, clonazepam 1 mg daily and 3 days of intravenous methylprednisolone 1 g daily.
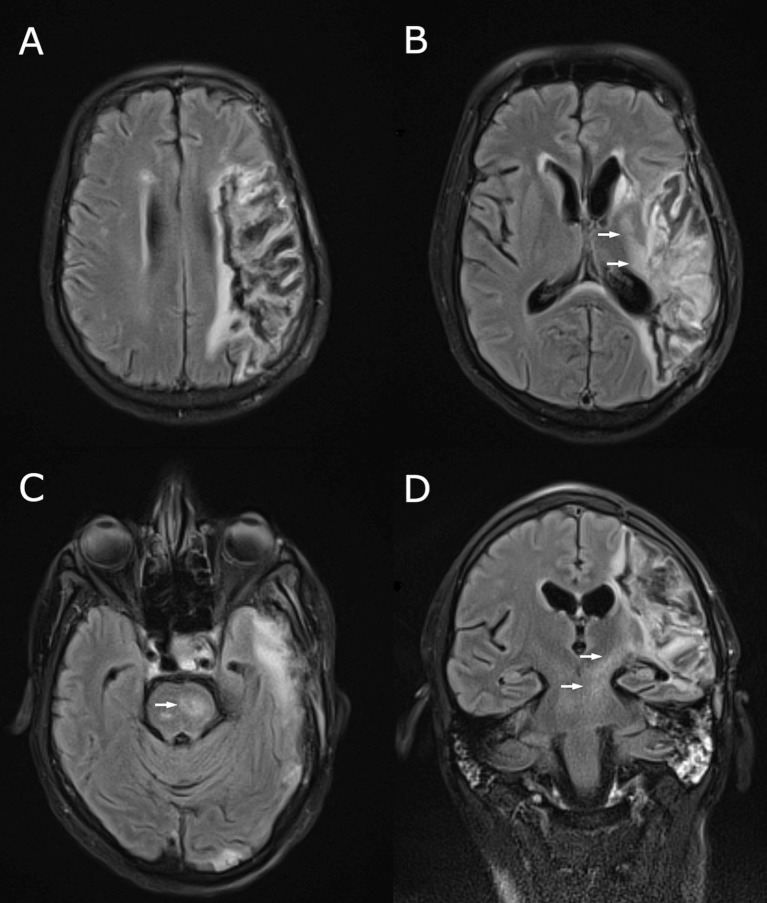
Brain MRI showed changes in keeping with prior MCA infarction, with Wallerian degeneration extending into the left internal capsule and brainstem (Fig. 1 ). Cerebrospinal fluid (CSF) was acellular, with protein = 0.566 g/L (0.15–0.45) and glucose = 4.3 mmol/L (serum 7.3 mmol/L). CSF PCR was negative for herpes simplex virus, varicella zoster virus, enterovirus and SARS-CoV-2. Antibodies to N-methyl-d-aspartate receptor (NMDA-R), LGI-1 and contactin-associated specific-protein 2 (CASPR-2) were requested on both serum and CSF. Antibodies to gamma-aminobutyric acid-B (GABA-B), alpha-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor 1 and 2, glycine receptor, glutamic acid decarboxylase (GAD), Hu, Yo, Ri and gangliosides were requested on serum. An electroencephalogram showed left frontotemporal slow waves and no epileptiform discharges. CT of the chest, abdomen and pelvis showed no evidence of malignancy.
Fig. 1.
MRI brain performed following onset of opsoclonus-myoclonus. Fluid attenuated inversion recovery (FLAIR) sequences in the axial (A-C) and coronal (D) planes show the chronic left MCA territory infarct, with Wallerian degeneration involving the corticospinal tract within the internal capsule and brainstem (arrows).
Over the following 11 days, the opsoclonus and myoclonus resolved, and he became able to intermittently follow simple commands. On day 27 however, the opsoclonus and myoclonus recurred, and he became more confused. On the same day, serum LGI-1 antibody returned as positive. CSF LGI-1 and other autoantibodies were negative. He was given a further 3 days of intravenous methylprednisolone 1 g daily followed by a tapering course of oral prednisolone. Within 10 days of restarting steroids, the opsoclonus resolved and myoclonus improved, and he recovered the ability to converse and follow commands. Serum LGI-1 antibodies were repeated and remained positive. Over several weeks, his cognition improved to the point where he could follow complex commands, read a newspaper and discuss current events (Video 2).
2. Discussion
As the worldwide pandemic continues, reports of ‘traditional’ antibody-mediated neurological disorders complicating COVID-19 have begun to surface. These include anti-NMDA-R encephalitis [2], anti-CASPR-2 encephalitis [3], anti-myelin associated glycoprotein (MOG)-associated encephalitis [4] and Bickerstaff encephalitis [5]. Antibody-negative limbic encephalitis has also been described, as has Guillain-Barre syndrome and its variants [1,6]. The patient described in this case developed opsoclonus-myoclonus and encephalopathy associated with LGI-1 antibodies, with onset 6 days after the start of COVID-19 symptoms. To our knowledge, this is the first reported case of possible post-infectious anti-LGI-1 encephalitis complicating COVID-19.
The significance of LGI-1 antibody positivity with respect particularly to the movement disorder aspect of this case remains unclear. Anti-LGI-1 encephalitis typically causes limbic encephalitis with cognitive impairment, behavioural disturbance and seizures (classically facio-brachial dystonic seizures) [7,8]. OMS is a rare, usually immune-mediated (commonly paraneoplastic/para-infectious) disorder characterised by multidirectional, chaotic, conjugate ocular saccades, multifocal myoclonus and commonly ataxia. OMS is not considered part of the phenotypic spectrum of anti-LGI-1 encephalitis, and while it may have represented an atypical manifestation in this instance, we feel on balance that OMS was more likely a manifestation of COVID-19 associated brainstem encephalitis [9,10].
In contrast, steroid-responsive cognitive decline is typical of anti-LGI-1 encephalitis [7,8]. Indeed, while all patients with anti-LGI-1 related disease experience cognitive impairment, the presence of other features such as seizures, MRI abnormalities and CSF pleocytosis is more variable [11]. Further, and in contrast to some other autoimmune encephalitides (such as anti-NMDA receptor encephalitis), sensitivity of anti-LGI1 antibody detection is generally higher in serum than CSF, as in this case [8]. In our patient, the steroid-responsive ‘double-dip’ in cognitive function suggested a sustained immune process, and was the clinical clue to search for another aetiology.
The pathophysiologic processes underlying anti-LGI-1 antibody production in this context may include epitope exposure as a result of the initial COVID-19 encephalitis or indeed COVID-19-induced molecular mimicry. Such antibody-driven neurological syndromes following infectious encephalitis are well described [12]. Alternative explanations would include the presence of a bystander antibody (unrelated to the clinical presentation) or antibody production in response to antigen liberation from the previous stroke. However, we believe that the latter scenarios are less likely, given the steroid-responsive nature of the encephalopathy (in keeping with anti-LGI-1 encephalitis), the rarity of ‘false positive’ LGI-1 antibodies in healthy controls and other disease cohorts [13], and the time course of symptom development relative to the ischaemic insult.
Though some uncertainty remains about the pathogenic relevance of anti-LGI-1 antibodies in this case, this report potentially expands the spectrum of para/post-infectious autoimmune neurological syndromes associated with SARS-CoV-2 infection. Clinicians should consider autoimmune encephalitides in the differential diagnosis of COVID-19 associated encephalopathy, especially if patients do not follow the typical clinical trajectory or experience secondary decline after a period of apparent improvement.
Day 7 of illness. There is marked opsoclonus and myoclonus, more prominent on the non-paretic left side. The patient is encephalopathic and unable to speak.
Following a prolonged course of steroids, the patient is able to speak appropriately and easily follows commands. There is now no opsoclonus, and myoclonus is minimal.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethical compliance statement
This case report did not require institutional review board approval. The patient provided written consent for publication. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. We have reviewed the Elsevier Policy on the Use of Images or Personal Information of Patients or other Individuals, and confirm that our report is compliant with this policy.
Acknowledgements
None.
References
- 1.Ellul M., Benjamin L., Singh B., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burr T., Barton C., Doll E., Lakhotia A., Sweeney M. N-methyl-D-aspartate receptor encephalitis associated with COVID-19 in a toddler. Pediatr. Neurol. 2021;114:75–76. doi: 10.1016/j.pediatrneurol.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guilmot A., Maldonado Slootjes S., Sellimi A., et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2021;268:751–757. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peters J., Alhasan S., Vogels C.B.F., Grubaugh N.D., Farhadian S., Longbrake E.E. MOG-associated encephalitis following SARS-COV-2 infection. Mult. Scler. Relat. Disord. 2021;50:102857. doi: 10.1016/j.msard.2021.102857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llorente Ayuso L., Torres Rubio P., Beijinho do Rosário R.F., Giganto Arroyo M.L., Sierra-Hidalgo F. Bickerstaff encephalitis after COVID-19. J. Neurol. 2021;268:2035–2037. doi: 10.1007/s00415-020-10201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pizzanelli C., Milano C., Canovetti S., et al. Autoimmune limbic encephalitis related to SARS-CoV-2 infection: case-report and review of the literature. Brain Behav. Immun. Health. 2021;12:100210. doi: 10.1016/j.bbih.2021.100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irani S.R., Alexander S., Waters P., et al. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma-inactivated-1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan’s syndrome and acquired neuromyotonia. Brain. 2010;133:2734–2748. doi: 10.1093/brain/awq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Sonderen A., Thijs R.D., Coenders E.C., et al. Anti-LGI1 encephalitis: clinical syndrome and long-term follow-up. Neurology. 2016;87:1449–1456. doi: 10.1212/WNL.0000000000003173. [DOI] [PubMed] [Google Scholar]
- 9.Emamikhah M., Babadi M., Mehrabani M., et al. Opsoclonus-myoclonus syndrome, a post-infectious neurologic complication of COVID-19: case series and review of the literature. J. Neuro-Oncol. 2021;27:26–34. doi: 10.1007/s13365-020-00941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah P.B., Desai S.D. Opsoclonus myoclonus ataxia syndrome in the setting of COVID-19 infection. Neurology. 2021;96:33. doi: 10.1212/WNL.0000000000010978. [DOI] [PubMed] [Google Scholar]
- 11.Ariño H., Armangué T., Petit-Pedrol M., et al. Anti-LGI1-associated cognitive impairment. Neurology. 2016;87(8):759–765. doi: 10.1212/WNL.0000000000003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armangue T., Moris G., Cantarín-Extremera V., et al. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology. 2015;85:1736–1743. doi: 10.1212/WNL.0000000000002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani S.R., Gelfand J.M., Al-Diwani A., Vincent A. Cell-surface central nervous system autoantibodies: clinical relevance and emerging paradigms. Ann. Neurol. 2014;76:168–184. doi: 10.1002/ana.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Day 7 of illness. There is marked opsoclonus and myoclonus, more prominent on the non-paretic left side. The patient is encephalopathic and unable to speak.
Following a prolonged course of steroids, the patient is able to speak appropriately and easily follows commands. There is now no opsoclonus, and myoclonus is minimal.