Abstract
Microorganisms cause variety of diseases that constitutes a severe threat to mankind. Due to the upsurge of many infectious diseases, there is a high requirement and demand for the development of safety products finished with antimicrobial properties. The study involves the antimicrobial activity of natural cotton coated with copper iodide capped with Hibiscus rosa-sinensis L. flower extract (CuI-FE) which is rich in anthocyanin, cyanidin-3-sophoroside by ultrasonication method. The coated and uncoated cotton fabric was characterised through XRD, SEM, AFM, tensile strength and UV–Visible spectroscopic techniques. XRD confirmed the formation of CuI particles, SEM showed that CuI-FE was prismatic in shape. The average size of CuI-FE particles was found to be 552.45 nm. Anti-bacterial studies showed copper iodide particles to be a potent antimicrobial agent. AFM images confirmed the rupture of bacterial cell walls in the presence of prismatic CuI-FE. In-vitro cytotoxicity investigation of CuI-FE was performed against cancer and spleen cell lines to evaluate the cell viability. Cytotoxicity analysis revealed the IC50 value of 233.93 μg/mL in the presence of CuI-FE. Molecular docking study was also carried out to understand the interaction of CuI-FE with COVID-19 main protease. This paper has given an insight on the usage of CuI-FE coated on the cotton fabric that has proved to have strong inhibition against the nano ranged bacterial, cancerous cell line and a strong interaction with the COVID-19 protease. Such eco-friendly material will provide a safe environment even after the disposable of medical waste from the infectious diseases like influenza and current pandemic like COVID-19.
Keywords: Green synthesis, Cyanidin-3-sophoroside, CuI, Cotton fabric, Covid-19 main protease
1. Introduction
Cotton fabric plays an important role in our everyday life. It has been extensively used for clothing applications since many years. The flexibility and comfort of cotton fabric make it an outstanding and far & widely used textile in the world owing to its exclusive qualities such as restoration, eco-friendly, gentleness, hypoallergenic and hygroscopic nature [1,2]. Cotton is the most significant textile crop in numerous countries around the globe. Wool, silk, nylon may have been around longer in some countries, but cotton has excelled them all in adaptability and versatility [3].
In the course of Corona Virus Disease-2019 (COVID-19), there has been rapid rise in the use of Personal Protective Equipment (PPE) kits by the frontline workers and sanitation bodies to decrease the probability of infections [4]. The efficient management of corona virus infectious waste including PPEs has been recognized as a major area of concern worldwide. PPE kit, include face masks, face shields, goggles, hand gloves, gowns, head and shoe cover which are all made of plastic and they decrease the danger of an individual using them from contracting the infection. However, the extreme usage and consumption of such single-use plastics have become a severe threat to human health and natural ecosystem. In view of immediate and urgent preventive measures taken, it is apparent that the used PPEs waste is expected to multiply in many folds and will create hassle in the present waste management systems posing a serious threat to the environment if not handled properly on time [5,6]. Therefore, there is a need of the hour to find an eco-friendly alternative which can effectively replace the single use plastics and control the pollution in the environment.
The infection caused by various pathogens should be controlled as the proliferation of microorganisms on fabric could lead to dramatic effects such as deterioration of textile strength, mutilation and odors which can contaminate the fabric as well as endanger the wearer with diseases such as serious skin infections [7]. Generally, the influence of the microorganism on textile material could be understood by two major approaches, that is assimilation and degradation. In fact, their metabolites disclosed the spots on the surface of fabric, the generation of bubbles on colored surface of the fabric, stimulation of breaking of bonds in fibrous materials and adverse effects on mass loss, mechanical strength, variation of chemical properties, etc [8].
Health care concern related with the upsurge of infectious diseases by various microorganisms has augmented the need for the fabrication of an effective multifunctional textile. In this context, antimicrobial textile has been established to forbid the growth of bacteria on the textile to avoid adverse hygiene effect on wearer. Despite the excellent properties of cotton materials, they also provide a good atmosphere for microbial proliferation, due to their wide surface area and capability to maintain dampness. In order to control such issues, a broad range of chemicals have been used to produce antimicrobial property to cotton materials [[9], [10], [11]]. Metal-based nanostructures provided novel functionality and capability to develop pristine textile in terms of physical, chemical, and biological properties have been studied as one of the most hopeful routes to make antimicrobial textile. Currently, metallic nanoparticles such as Ag [12,13], ZnO [14], Cu based compounds [15,16] and TiO2 [17] utilized in the development of antimicrobial textile owing to its potential antimicrobial activity. The coating and deposition of these nanoparticles onto the substrate involved various procedures such as dip coating, ultrasonication, pad-dry cure method, in-situ chemical reduction, covalent bonding methods etc [[12], [13], [14], [15], [16], [17], [18]] It has been reported the ultrasonication coating is of high practical advantage of being rapid, simple and economically viable single-step reaction. It is also eco-friendly and involves ‘green’ chemistry principles as the method is free from toxic materials [13,17]. Now, American Environmental Protection Agency (EPA) has registered copper as the first and only metal with antimicrobial properties, which kills 99.9% of most pathogens within 2 h contact [19]. The features of copper metal are comparable to those of other expensive metals with antibacterial activity, such as silver and gold [20].
Copper (I) compounds have been reported to show better anti-bacterial and anti-viral activity as compared to metallic copper and copper (II) compounds [[21], [22], [23], [24], [25]]. For ages, copper and its compounds have been known to be effective against a variety of microbial pathogens. Copper compounds have been used for the inactivation of avian influenza virus (copper metal &Cu (II) compounds) [[26], [27], [28], [29], [30]].
Copper iodide (CuI) has acquired the interest of many researchers with its applications in diverse fields as it shows exceptional properties [[31], [32], [33], [34], [35], [36]]. Various physical, chemical and electrochemical methodologies have been reported regarding the synthesis of CuI [[37], [38], [39], [40], [41]] which can effectually generate CuI of even size and good crystalline nature, but the process require toxic raw materials, chemical reducing agents, complex synthetic steps or high temperature which leads to ecological and biological menace. Therefore, it is essential to investigate a neat, facile, and non-polluting route to produce CuI at viable conditions. Green synthesis is economical, expeditious, and efficient which characteristically produces nanostructures with a wide range of shapes. The usage of extracts from plants or fruits as reducing, stabilizing and capping agents have become the majorly concentrated area as they effectively produce copper iodide in a much greener and ecologically friendly ways [[42], [43], [44], [45], [46]].
The present study involves the coating of Hibiscus rosa-sinensis L. flower extract reduced CuI (CuI-FE) as an antimicrobial finish onto natural cotton by ultrasonication method. CuI-FE coated cotton has been characterized by various physical methodologies and the antibacterial activity of it was tested against major skin infection causing bacteria such as E. coli and S. faecalis by Agar Disc-diffusion method. The effect of CuI-FE on bacterial morphology was analysed by Atomic Force Microscopy. Invitro cytotoxicity was performed to study the impact CuI-FE on cell viability of EAC, DLA and rat spleen cells. Furthermore, studies on molecular docking were performed to understand the binding interaction between CuI-FE and COVID-19 main protease.
2. Material and methods
2.1. Materials used
Hibiscus flowers were collected from Stella Maris College campus. AnalR-Grade CuSO4.5H2O and KI were procured from Spectrum and Fischer Scientific India Pvt. Ltd respectively and were employed as obtained. Muller-Hinton agar and Nutrient broth were secured from Hi-Media, India. The bacterial cultures were purchased from Hubert enviro care systems Pvt. Ltd. Natural cotton fiber was purchased from a local store.
2.2. Preparation of FE
The extract of Hibiscus flower was prepared in water by the same procedure previously reported by us [[47], [48], [49]].
2.3. Preparation of CuI-FE
CuI was synthesized using Hibiscus flower extract by reacting 1:2 M solutions of CuSO4. 5H2O and KI respectively, followed by addition of FE extract by an approach reported by us earlier [[47], [48], [49]].
2.4. Qualitative phytochemical screening of Hibiscus rosa-sinensis L. Flower extract
The presence of some phytoconstituents in the extract was studied by standard phytochemical methods. Phytochemical analysis of alkaloids, anthocyanins, flavonoids, polyphenols, saponins and tannins were performed according to the tests described in the literature [50,51]. Table 1 represents the tests conducted for confirming the presence of phytochemicals.
Table 1.
Standard qualitative tests for screening the presence of Phytochemicals.
| Phytochemicals | Tests | Reagents | Results | Reference |
|---|---|---|---|---|
| Alkaloids | Hager's test | Hager's reagent | Prominent yellow ppt | [51] |
| Polyphenols | Ferric chloride test | 2% FeCl3 | Blackish green coloration | [50] |
| Flavonoids | Ammonia test | 1% NH3 | Yellow color | [51] |
| Anthocyanin | Ammonia test | 2 N HCl, NH3 | Purplish blue | [50] |
| Terpenoids | Salkowski test | 0.5 mL CHCl3, 1 mL Conc. H2SO4 | Reddish brown coloration at the interface | [51] |
| Tannins | Lead acetate test | 10% Lead acetate | Bulky white ppt | [51] |
| Saponins | Foam test | Distilled water | Persistent foam | [51] |
2.5. GC-MS analysis
The separation of chemicals was done using Helium as the carrier gas at a constant flow rate of 1 mL/min on a fused silica column packed with Elite-5MS (5 percent biphenyl 95 percent dimethylpolysiloxane, 30 m × 0.25 mm IDx 250 m df). The injector temperature was kept at 260 °C throughout the chromatographic run. 1 L of floral extract was put into the instrument, with the following oven temperature: 60 °C for 2 min, followed by 300 °C at a rate of 10 °C min-1 and maintained at 300 °C for 6 min. The mass detector was set to 230 °C for the transfer line& the ion source and 70 eV for the ionisation mode electron impact, with a scan length of 0.2 s and a scan interval of 0.1s.The fragment from 40 to 600 Da were collected. Using the GC-MS NIST (2008) library, the spectra of the separated compounds was compared to the spectrum of standard compounds in a database.
2.6. In vitro cytotoxicity analysis
CuI-FE was analysed for short term in vitro cytotoxicity using Ehrlich Ascites Carcinoma (EAC) cells, Dalton's Lymphoma Ascites cells (DLA) and rat spleen cells. This study was done at Amala Cancer Research Centre Society (A Society registered T.C.Act, XII of 1955sl.No. 56 of 1984), it is a registered society which follows all rules of its licensing committee for performing of we confirm that all methods were carried out in accordance with regulations. We also confirm that all experimental protocols were approved by a licensing committee. The tumour cells were washed three times with PBS or normal saline after being removed from the peritoneal cavity of tumor-bearing mice. The cell viability was determined using the trypan blue exclusion method. Phosphate buffered saline was used to make up to 1 mL of viable cell suspension (1 × 106 cells in 0.1 mL) in the tubes holding varied amounts of the substances. As a control, a tube holding simply the cell suspension was employed. At 37 °C, the test tubes were incubated for 3 h. The cell solution was then mixed with 0.1 mL of 1% trypan blue for 2–3 min before being put into a hemocytometer. The dye colour of trypan blue is taken up by the dead cells, while the dye colour is not taken up by the living cells. Separate counts of labeled and unstained cells were made.
For rat spleen cells, the rat was sacrificed using carbon dioxide anesthesia and the spleen tissue was taken out. In RPMI complete medium, it was shattered into single cell suspension and filtered with a mesh fabric. The cells thus collected were washed thrice and suspended in known volume of RPMI complete medium containing antibiotics and counted. Viable cell suspension (1 × 106 cells in 0.1 mL) was added to tubes containing various sample concentrations, and the volume was increased to 1 mL using RPMI medium. The tube containing only the cell suspension was used as the control. The test tubes were incubated for 3 h at 37 °C. The cell solution was then mixed with 0.1 mL of 1% trypan blue for 2–3 min before being put into a hemocytometer. The number of stained and unstained cells were individually counted [52].
2.7. Coating of CuI-FE onto natural cotton fabric
The cotton fabric was made into 5 × 5 cm2 pieces and dipped in the solution of CuI-FE dissolved in acetonitrile (1 mg/mL) and ultrasonicated for 30 min. The CuI-FE treated cotton was washed with ethanol and subsequently with deionized water quite a few times to remove any unbound particles. The washed cotton was then dried at 60 °C and characterized [13,17].
2.8. Antibacterial activity of CuI-FE incorporated cotton
Antibacterial susceptibility of natural cotton coated with CuI-FE was scrutinized by Agar disc diffusion method [53,54]. A Gram -ve E. coli and Gram +ve S. faecalis were employed as the test microbes. Mother cultures were sub-cultured in Nutrient broth for 24 h at 37 °C. The sub-cultured organism was swabbed uniformly on agar plate using sterile cotton swabs. The CuI-FE coated and uncoated cotton (1 × 1 cm2) were gently kept on inoculated solidified agar gel plates. The plates were left for 24 h at 37 °C for incubation. The zone of inhibition produced by the indicator bacterial strains was measured.
2.9. Cell morphology study
The effect of CuI-FE on the cell membrane was investigated by AFM. 40 μL of bacterial suspension treated with CuI-FE (sample) and the untreated one (control) was applied onto a sterilized glass surface and air dried. The sample spots were then rinsed gently with de-ionized water to get rid of any impurities and air dried again [55].
2.10. Molecular docking of cyanidin-3-sophoroside capped CuI and prediction of binding sites of CuI to the COVID-19 main protease
Cyanidin-3-sophoroside is an anthocyanin of Hibiscus-rosa-sinensis L. The activity of Cyanidin-3-sophoroside bound CuI against the COVID-19 main protease protein was predicted through the molecular docking approach. The 2-dimensional structure of Cyanidin-3-sophoroside was downloaded from PubChem and optimized with OPLS force field 2005 using ligprep module of Schrodinger. The target protein Mpro with an ID-6LU7 was retrieved from PDB, energy minimized and processed with OPLS2005 force field using the protein synthesis wizard of Schrodinger. The receptor grid was produced for the active site of the target and molecular docking of the protein and compound were performed with the glide module of Schrodinger [56].
The metal binding sites of COVID-19 protease was identified from MIB [57] database that works by comparing the metal ion binding sites based on a template alignment to predict the metal binding sites on query protein. A threshold score is set above the alignment scores for both sequence-based structure-based metal binding sites of proteins [58].
2.11. Characterization techniques
The prepared Hibiscus flower extract was analysed using GC-MS (PerkinElmer, Clarus 680, Clarus 600 (EI)). The fabricated CuI-FE coated and uncoated cotton was delineated using Bruker D8 advance P-XRD, SEM (FEI-Quanta FEG 200 F & Oxford INCA Energy 250 Microanalysis system), Alpha-T and Jasco UV–Vis Spectrophotometer, V-50 and Hounsfield Universal testing Machine, 50 KN-S. Bacterial topography was studied using AFM (NTEGRA, NT-MDT RUSSIA).
3. Results and discussion
3.1. Qualitative phytochemical analysis of Hibiscus rosa-sinensis L
The results from the qualitative tests performed for Hibiscus rosa-sinensis L. flower extract represented in Table 2 , indicated that it contained alkaloids, anthocyanins, flavonoids, polyphenols and terpenoids which are the main phytochemical groups [50].
Table 2.
Qualitative analysis of Phytochemicals present in Hibiscus rosa-sinensis L. flower extract.
| S.No. | Chemical Test | Extract |
|---|---|---|
| 1. | Alkaloids | + |
| 2. | Polyphenols | + |
| 3. | Flavonoids | + |
| 4. | Anthocyanin | + |
| 5. | Terpenoids | + |
| 6. | Tannins | - |
| 7. | Saponins | – |
(+) Present; (−) Absent.
3.2. GC-MS analysis of FE
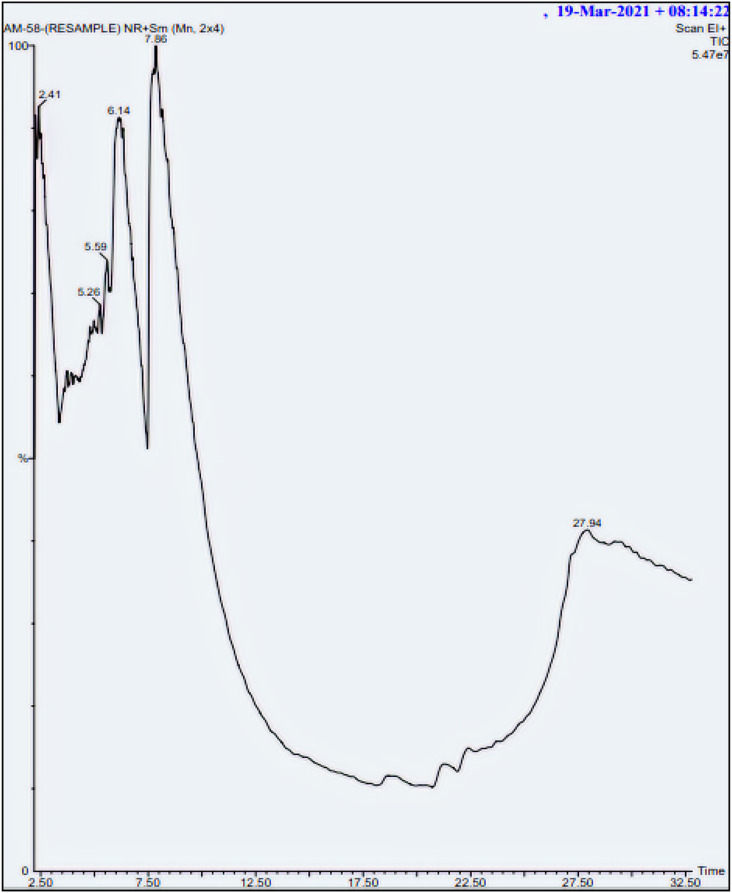
GC-MS analysis performs a crucial role in recognizing the nature of various active compounds present in medicinal flowers. The active components from FE in ethyl acetate were examined by GC-MS analysis (Fig. 1 ). Table 3 lists the principal components in the extract, along with their chemical formula, retention time (RT), molecular weight (MW), and peak area (percentage). Four active principles were recognized in FE through GC-MS. Pentanoic acid, 2- (Aminooxy)- (78.47%), Tetradecane, 1-chloro- (7.21%), Methane carbothiolic acid (7.16%) and Methoxyacetic acid, Pentyl Ester (7.14%). These are the major phytochemicals which bring about various pharmacological activities such as anti-fertility, anti-oxidant, antimicrobial, antimutagenic, anti-inflammation, anticancer, hepato-protective, Diuretic, Antiasthma activities, etc. Hibiscus rosa-sinensis L. flower petals have medicinal value due to the presence of these major constituents [59,60].
Fig. 1.
GC-MS Chromatogram of Extract of Hibiscus rosa-sinensis L. flower (FE).
Table 3.
Phytocomponents identification in FE by GC-MS.
| S.No. | RT Retention Time | Compound name | Molecular formula | Molecular weight | Peak area in percentage |
|---|---|---|---|---|---|
| 1. | 7.61 | Methanecarbothiolic acid | C2H4OS | 76 | 7.168 |
| 2. | 8.23 | Methoxyacetic acid, Pentyl Ester | C8H16O3 | 160 | 7.144 |
| 3. | 27.82 | Pentanoic acid, 2- (Aminooxy)- | C5H11O3N | 133 | 78.474 |
| 4. | 29.49 | Tetradecane, 1-chloro- | C14H29Cl | 232 | 7.214 |
Among the identified 4 compounds, pentanoic acid, 2- (Aminooxy)- was found to be the major bioactive compound of FE which show 78.47% peak area with retention time of 27.82. Pentanoic acid, 2- (Aminooxy)- is an aromatic phenolic group of salicylic acid. It is the principal compound present in the extract which contribute to the reduction of Cu2+ to Cu+, with the acid performing as a good reducing agent as it is rich in hydroxyl groups and good dispersant owing to their resulting C O groups that enhances the stability and capping abilities [61,62]. Thus, the extract of Hibiscus rosa-sinensis L. flower act as effective reducing, stabilizing and capping agent in the synthesis of CuI-FE.
3.3. Characterization of CuI-FE
3.3.1. Structural analysis
Fig. 2 shows the XRD patterns of CuI-FE. It shows the X-ray diffraction peaks at 2θ values of 25.64°, 29.66°, 42.34°, 50.07°, 52.46°, 61.33°, 67.51°, 69.51° and 77.49° corresponding to (111), (200), (220), (311), (400), (331), (420) and (422) planes of CuI nanoparticles [47]. The XRD data was found to match well with literature (JCPDS card no., 82-2111) [49]. It was clear that the peaks were well-defined and no other impurities were present signifying the high crystallinity and purity of the compound. The XRD powder data of CuI-FE was identified to fit in the fcc lattice system. The ‘a’ value and the average crystallite size was calculated to be 6.0357 Å and 89.01 nm respectively [47].
Fig. 2.
XRD of CuI-FE.
3.3.2. Morphological studies
The surface morphology and elemental analysis was done by SEM and EDAX. CuI-FE appeared to be triangular prism-like in shape with sharp edges Fig. 3 . The mean size of the prismatic particles was found to be 552.45 nm. The EDAX analysis ( Fig. 3) of CuI-FE revealed the occurrence of Cu and I elements, signifying that the synthesized product is of high purity [47].
Fig. 3.
a). SEM images of CuI-FE. b). EDAX of CuI-FE.
3.4. In-vitro cytotoxicity analysis of CuI-FE
Cytotoxicity is an in-vitro test used to study the effect of the substance of interest on cell viability. Cytotoxicity tests are widely performed for nanoparticles as they pave the way for its use in the biomedical field. Ehrlich carcinoma and Dalton's Lymphoma Ascites cells are a type of undifferentiated carcinoma cells which are hyperdiploid at first, has an excellent transplantable capacity, no debilitation, rapid growth, a short life time, is 100 percent malignant, and lacks tumor-specific transplantation antigen. It looks like human tumours, which are the most chemotherapy-resistant. Hence, Ehrlich carcinoma and Dalton's Lymphoma Ascites cells was employed for the study.
The short-term in vitro cytotoxicity analysis results of CuI-FE at five different concentrations in ethanol are given in Table 4 . Linear trendline was drawn for each graph which was used to calculate the IC50 value against each cell line by regression analysis (Fig. 4 ). CuI-FE showed more cytotoxicity against the two cancer cell lines than the normal spleen cell culture. The percentage cell death observed for CuI-FE against rat spleen cell line, EAC and DLA at 200 μg/mL are 38.1%, 84%, and 83.9% respectively and the corresponding IC50 value as predicted by the linear regression analysis are 11.27 μg/mL, 36.53 μg/mL and 233.93 μg/mL respectively.
Table 4.
Cytotoxicity analysis of CuI-FE.
| Cell line | Percentage Cell death μg/mL |
||||||
|---|---|---|---|---|---|---|---|
| 2.5 | 5 | 10 | 20 | 50 | 100 | 200 | |
| Normal | – | – | 9.95 ± 1.34 | 13.6 ± 1.41 | 23.7 ± 1.52 | 32.5 ± 0.66 | 38.1 ± 1.89 |
| EAC | 26 ± 1.5 | 42 ± 0.3 | 52 ± 1.4 | 67 ± 0.9 | 71 ± 1.1 | 76 ± 2.7 | 84 ± 1.2 |
| DLA | 19.6 ± 1.7 | 34.7 ± 1.6 | 50.8 ± 1 | 54.1 ± 4 | 64.3 ± 0.6 | 77.2 ± 1 | 83.9 ± 1.9 |
Fig. 4.
Linear regression analysis for the cytotoxicity analysis of CuI-FE against a) normal cell b) EAC c) DLA.
For CuI-FE the highest toxicity was found against Ehrlich Ascites Carcinoma (EAC) and Dalton's Lymphoma Ascites cells (DLA) which caused a cell death of 84% and 83.9% at 200 μg/mL (Fig. 5 ). They were also observed to have the least IC50 values of 11.27 μg/mL and 36.53 μg/mL respectively. Both cancer lines (EAC and DLA) were more sensitive to CuI-FE than the rat spleen cell line. The IC50 value of 233.93 μg/mL for CuI-FE indicated its minimal effect on normal spleen cells and can be well used in therapeutic applications.
Fig. 5.
Percentage cell death of EAC, DLA and rat spleen cells at various concentrations of CuI-FE.
The nanoparticles were found to interact with microbial components in a variety of ways, such as contact by ROS, coordinate bond, electrostatic and hydrophobic reactions. Metal and metal compound nanoparticles are thought to operate on the virus's surface, physically inhibiting the virus's contact with host cells. Silver and copper compounds nanoparticle are found to be safe, efficient and eco-friendly which can be synthesized through green routes [[63], [64], [65]]. The high antiviral activity of copper iodide nanoparticles against feline calicivirus was due to Cu+ ions, by consequent ROS generation and capsid protein oxidation. CuFeO2 with good antiviral activity and good chemical stability in a weak acid condition has exhibited excellent inactivation of viral phage at the end of 4 h in the dark condition [23,29].
3.5. CuI-FE as an antibacterial finish on textiles
The cytotoxicity of CuI-FE against the normal cell line was less, that its propensity in biomedical applications can be promising. As a proof of the concept, it was thought worthwhile to coat it onto natural cotton as an antibacterial finish and study its antibacterial property.
3.5.1. XRD analysis of CuI-FE coated natural cotton
Fig. 6 exhibits the XRD patterns of cotton and CuI-FE coated natural cotton. Fig. 6 a) shows the X-ray diffractions at 2θ values of 15.1°, 16.3°, 23.2° and 34.6oattributing to the (110), (110), (200) and (004) planes of pure cotton respectively [13,66]. In addition to pure cotton peaks, the XRD of CuI-FE (Fig. 6 b)) coated cotton shows peaks at 25.19°, 30.08°, 43.04°, 50.89°, 62.07°, 68.16° and 77.97° which corresponds to (111), (200), (220), (311), (400), (331) and (422) planes of CuI nanoparticles. This confirms that CuI-FE was deposited on the cotton.
Fig. 6.
XRD of a) natural cotton and b) after coating of CuI-FE by Ultrasonication.
3.5.2. Surface morphology studies
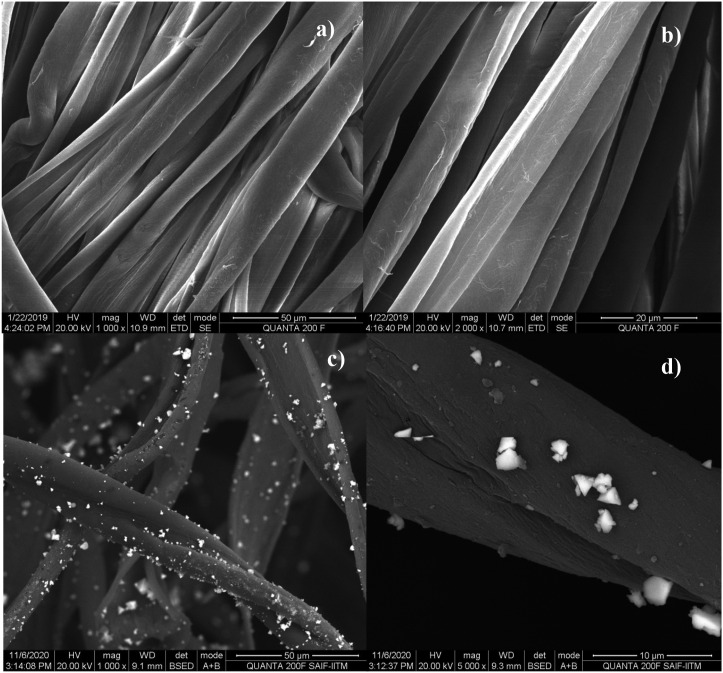
The surface morphology of CuI-FE coated cotton was probed with scanning electron microscope (SEM). The SEM images of CuI-FE treated cotton and untreated cotton are shown in Fig. 7 . The untreated cotton (Fig. 7 a) and b)) appear as smooth fibers whereas (Fig. 7 c) and d)) clearly shows the deposition of triangular CuI-FE on the surface of the cotton. The ultrasonication treatment helps in the dispersion of particles while coating. The coating attributed to the physical adsorption of CuI-FE on the cotton surface due to ultrasonication process.
Fig. 7.
SEM images of a), b) bare cotton and c), d) CuI-FE coated cotton.
3.5.3. UV–Vis analysis
The UV–Vis spectral analysis in the wavelength range of 400–800 nm was used to evaluate the coating of CuI-FE on cotton surface. Fig. 8 shows the UV–Vis spectra of CuI-FE treated and untreated cotton. The Surface Plasmon resonance peak corresponding to CuI has been noticed in the UV–Vis spectra which revealed the incorporation of CuI-FE in the cotton. Fig 8 a) shows the reflectance of CuI-FE treated and untreated cotton. It is inferred from the spectra that the uncoated sample exhibited greater reflectance values covering from 40 to 80%. For CuI-FE treated specimen, the value of reflectance was reduced to 20–50%, owing to the decrease in refractive index and roughness of the cotton surface. From Fig. 8 b) it was observed that the intensity of absorbance was lesser for uncoated cotton than that of the coated one. A wide absorption peak appeared around 405 nm for CuI-FE coated cotton, confirming the good adhesion of CuI-FE [67].
Fig. 8.
UV-DRS a) reflectance and b) absorbance spectra of the Uncoated and CuI-FE coated cotton.
3.5.4. Tensile testing
A force–extension plot is used to depict the tensile properties of a material. The tensile strength studies of the natural cotton before and after coating of CuI-FE are shown in Fig. 9 a) and b). The extension is directly proportional to the force applied. For both bare cotton and CuI-FE coated cotton, the fabric deforms initially with extension leading to increase in force. Thereafter, at the breakpoint, the force decreases as the fabric can no longer be elongated [68]. The tensile strength and elongation of uncoated cotton was 28.70 MPa and 20.01% respectively, whereas for CuI-FE coated cotton the tensile strength and elongation was measured to be 31.58 MPa and 21.00% respectively. It is observed that, with the incorporation of CuI-FE, the tensile strength and elongation properties of the cotton material increased. The deposition of CuI-FE on cotton was ascribed to the electrostatic interaction between the cotton substrate and CuI-FE [69]. It has been described that the hydrogen bond formation between substrate and substance being coated resulted in the enhancement of tensile strength [13,70]. In the present study the organic molecules surrounding CuI-FE may induce a hydrogen bond with the functional group of cotton which might be the cause for the increase in tensile strength. It was observed from the results that the coating CuI-FE on cotton fabric did not cause any considerable damage.
Fig. 9.
Tensile testing of a) bare cotton b) CuI-FE coated cotton.
3.6. Antibacterial activity of natural cotton coated with CuI-FE
The investigation of antibacterial properties of CuI-FE coated cotton was performed against Gram negative E. coli and Gram-positive S. faecalis using the disc diffusion technique. The untreated cotton was employed as a reference. Fig. 10 shows the antibacterial activity of CuI-FE coated and uncoated cotton against E. coli and S. faecalis. The CuI-FE incorporated cotton showed a significant inhibition zone around the cotton coated with CuI-FE. The zone of inhibition represents the antibacterial activity of CuI-FE treated cotton against both E. coli and S. faecalis. The inhibition zone formation evidently reveals that the CuI-FE coated cotton shows antibacterial activity owing to its effect on the bacterial cell membrane. The uncoated cotton used as reference did not show any antibacterial activity. The zone of inhibition diameter was determined to be 36 mm and 30 mm for E. coli and S. faecalis respectively which shows the higher sensitivity of E. coli towards CuI-FE coated cotton.
Fig. 10.
Antibacterial activity of CuI-FE coated cotton against E. coli and S. faecalis.
3.7. Atomic Force Microscopy
The changes observed in bacterial cell after treatment with CuI-FE were analyzed using AFM technique. Fig. 11 b) shows, E. coli on treatment with CuI-FE (200 μg/mL) has underwent reduction in thickness of the cell membrane and disruption of the same as compared to the untreated one Fig. 11 a). For S. faecalis also (compare Fig. 11 c) & 11 d)) the membrane was damaged with 200μg/mLof CuI-FE. Therefore, the AFM study confirms the bacterial cell damage by CuI-FE. The reduction in the thickness of the cell membrane was visualized to be more for E. coli treated with CuI-FE which can be attributed to the fact that the thin cell of gram negative E. coli makes the penetration of CuI-FE into it more easier whereas the presence of thick cell wall and an additional poly-glutamate capsule in gram positive S. faecalis makes it comparatively resistant towards CuI-FE [22]. It has been reported that the metal and the metal oxide nanoparticles change the structure of the cell membrane of the bacteria [[71], [72], [73]]. On the entry of CuI-FE into bacterial cells, the anthocyanin (capping agent from FE) deactivates the vital enzymes present in the cell membrane causing the damage of the cell membrane. CuI-FE being prismatic in shape with sharp edges is highly anisotropic in nature which elevates the cellular ROS level by the release of large number of Cu+ ions which on interaction with lipids, proteins and DNA causes inhibition of the overall bacterial viability [74].
Fig. 11.
AFM images of a) E. coli b) E. coli treated with CuI-FE c) S. faecalis and d) S. faecalis treated with CuI-FE.
4. Molecular docking studies of cyanidin-3-sophoroside capped CuI against COVID-19 main protease protein
Copper iodide particles have been proved to be potent microbicidal agents due to its high surface to volume ratio. Hence, they can find effective applications in the fabrication of face masks and PPE [75]. In the present work, the CuI particles have been found to show good anti-bacterial activity and anti-cancerous activity, hence it was thought worthwhile to do molecular docking study with the CuI-FE synthesized to act against COVID-19 virus.
The first stage of treatment is to eliminate the binding S protein to ACE2 receptor [76] which is E. coli expedited by transmembrane serine protease 2 (TMPRSS2) via protease activity.
In the COVID-19 main protease, 15 Cu binding sites were identified. The residues and the score of the same is shown in Table 5 . The score of the normal residues were identified as 0.22, whereas the Cu bound residues scored from 3.02 to 5.64. The highest score of 5.64 was obtained with 264 Met, upon Cu binding. The least score 3.02 was seen in 208 Leu, 264 His and 250 Leu. The lollipop plot shown in Fig. 12 a) shows the binding affinity of Cu with COVID-19 main protease and Fig. 12 b) shows the bound CuI with COVID-19 main protease.
Table 5.
Residues and scores upon Cu binding.
| Residues | Score |
|---|---|
| 41 His | 5.57 |
| 44 Cys | 5.57 |
| 49 Met | 5.574 |
| 102 Lys | 3.02 |
| 140 Phe | 4.485 |
| 145 Cys | 4.485 |
| 156 Cys | 3.14 |
| 163 His | 4.485 |
| 165 Met | 4.485 |
| 208 Leu | 3.02 |
| 246 His | 3.023 |
| 250 Leu | 3.023 |
| 264 Met | 5.64 |
| 268 Leu | 5.08 |
| 276 Met | 3.76 |
Fig. 12.
a) Binding affinity of Cu with the residues of COVID-19 main protease; b) Bound CuI with COVID-19 main protease.
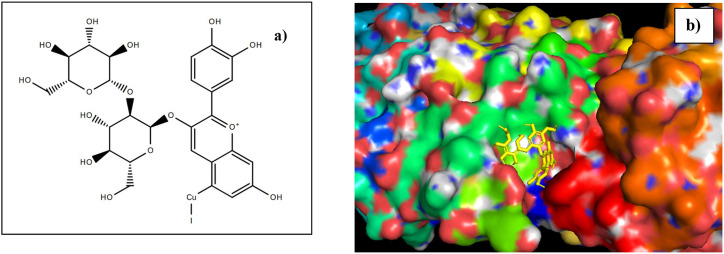
The docking of cyanidin-3-sophoroside capped CuI with COVID-19 main protease when compared with the original co-crystallized ligand, resulted in better binding energy. The complex disclosed a minimum binding energy of −80.34 kcal/mol and a binding score of −9.01. The interacting residues were Lys 102, His 164 and Gln 110 with a hydrogen bond distance of 3.12 Å, 2.67 Å and 2.45 Å respectively. The interactions of ligand and the anthocyanin capped CuI are represented in Table 6 . The structure of cyanidine-3-sophoroside capped CuI and the docked complex are represented in Fig. 13 a) and b) respectively. Therefore, from the better binding energy and interaction of cyanidine-3-sophoroside bound CuI with the COVID-19 main protease protein. Thus, CuI-FE can take up a crucial function in the treatment and management of the viral infections.
Table 6.
Interactions of Cyanidin-3-sophoroside capped CuI with COVID-19 main protease.
| Ligand | Binding energy Kcal/mol | Binding score | Interactions | H Bond Å |
|---|---|---|---|---|
| Co crystallized ligand N3 | −78.34 | −8.98 | (HO⋯O)GLY143z | 3.14 |
| (HO⋯O)HIS164 | 3.23 | |||
| Cyanidin-3-sophoroside capped CuI | −80.34 | −9.01 | LYS102(NH ….O) | 3.12 |
| (HO⋯O)HIS164 | 2.67 | |||
| (NH⋯O) GLN110 | 2.45 |
Fig. 13.
a) structure of Cyanidin-3-sophoroside bound CuI; b) Cyanidin-3-sophoroside docked within the binding site of COVID-19 main protease.
5. Conclusion
The synthesized CuI-FE was deposited onto the natural cotton to improve its multifunctional properties. The coating process of CuI-FE on cotton involved a simple ultrasonic treatment that assisted in the homogeneous dispersion of particles. CuI-FE was well adsorbed on the cotton surface due to the physical and chemical interactions. The cotton coated with CuI-FE exhibited good antibacterial activity against infections caused by broad spectrum bacteria. The cytotoxicity analysis of CuI-FE revealed that both the cancer cell lines (EAC and DLC) were more sensitive than spleen cells. The reduction of cell membrane thickness on treatment with CuI-FE indicated its effect on bacterial morphology.
The pandemic observed in 2019 has been very vulnerable and there is a need for face mask and PPE permanently in future. The current results are in line with the need for an efficient material (cotton coated with CuI-FE) to handle the current pandemic. Hence, highly efficient CuI-FE has been experimented using in-silico analysis with the main enzyme protease. Molecular docking studies confirmed the better interaction of CuI-FE with COVID-19 main protease protein. A preliminary lab scale analysis of the anti-bacterial activity of CuI-FE coated cotton has been performed successfully. By employing furthermore research involving techniques such as electrospinning might help to expand the practical use of CuI-FE coated cotton as an effective and environmentally friendly alternative in the manufacture of PPEs and other safety products in the future.
CRediT author statement
Archana.K.M: Investigation; Methodology; Formal analysis; Writing - Original Draft; Data curation; Resources.
Revathy Rajagopal: Conceptualization; Methodology; Validation; Supervision; Writing - Review & Editing.
K. Veena Gayathri: Conceptualization; Methodology; Validation; Supervision; Writing - Review & Editing.
Aishwarya S: Investigation, Data curation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to sincerely acknowledge DST-FIST, CRIST lab, Stella Maris College, Chennai; P-XRD lab, Department of Chemistry, IIT-Madras; SAIF, IIT-Madras, Chennai; PSG Institute of Advanced Sciences, Department of Chemistry Anna University, Amala Cancer Research Centre, Thrissur, VIT-SIF Lab, SAS, Chemistry Division for NMR and GC-MS Analysis, for their help with the sample characterizations and analyses. We would like to thank the management of Stella Maris College (Autonomous) and SEED for their support.
References
- 1.Taylor P.D.M. Commodity of the quarter: cotton. J Agric Food Inf. 2009;10:1–7. doi: 10.1080/10496500902802742. [DOI] [Google Scholar]
- 2.Tan L.Y., Sin L.T., Bee S.T., Ratnam C.T., Woo K.K., Tee T.T., et al. A review of antimicrobial fabric containing nanostructures metal-based compound. J Vinyl Addit Technol. 2019;25:E3–E27. doi: 10.1002/vnl.21606. [DOI] [Google Scholar]
- 3.Bedi J., Cororaton C. Cotton-textile-apparel sectors of India: situations and challenges faced. Food Policy. 2008;5:104. [Google Scholar]
- 4.Herron J.B.T., Hay-David A.G.C., Gilliam A.D., Brennan P.A. Personal protective equipment and Covid 19- a risk to healthcare staff? Br J Oral Maxillofac Surg. 2020;58:500–502. doi: 10.1016/j.bjoms.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patrício Silva A.L., Prata J.C., Walker T.R., Duarte A.C., Ouyang W., Barcelò D., et al. Increased plastic pollution due to COVID-19 pandemic: challenges and recommendations. Chem Eng J. 2021;405:126683. doi: 10.1016/j.cej.2020.126683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar H., Azad A., Gupta A., Sharma J., Bherwani H., Labhsetwar N.K., et al. COVID-19 Creating another problem? Sustainable solution for PPE disposal through LCA approach. Environ Dev Sustain. 2021;23:9418–9432. doi: 10.1007/s10668-020-01033-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandrasekar S., Vijayakumar S., Rajendran R. Application of chitosan and herbal nanocomposites to develop antibacterial medical textile. Biomed Aging Pathol. 2014;4:59–64. doi: 10.1016/j.biomag.2013.10.007. [DOI] [Google Scholar]
- 8.Calomiris J.J., Armstrong J.L., Seidler R.J. Association of metal tolerance with multiple antibiotic resistance of bacteria isolated from drinking water. Appl Environ Microbiol. 1984;47:1238–1242. doi: 10.1128/aem.47.6.1238-1242.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang F., Wu X., Chen Y., Lin H. Application of silver nanoparticles to cotton fabric as an antibacterial textile finish. Fibers Polym. 2009;10:496–501. doi: 10.1007/s12221-009-0496-8. [DOI] [Google Scholar]
- 10.Kan C.W., Lam Y.L. Low stress mechanical properties of plasma-treated cotton fabric subjected to zinc oxide-anti-microbial treatment. Materials (Basel) 2013;6:314–333. doi: 10.3390/ma6010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam Y.L., Kan C.W., Yuen C.W.M. Effect of metal oxide on anti-microbial finishing of cotton fabric. Bio Resources. 2012;7:3960–3983. doi: 10.15376/biores.7.3.3960-3983. [DOI] [Google Scholar]
- 12.Balashanmugam P., Kalaichelvan P.T. Biosynthesis characterization of silver nanoparticles using Cassia roxburghii DC. aqueous extract, and coated on cotton cloth for effective antibacterial activity. Int J Nanomed. 2015;10:87–97. doi: 10.2147/IJN.S79984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balamurugan M., Saravanan S., Soga T. Coating of green-synthesized silver nanoparticles on cotton fabric. J Coating Technol Res. 2017;14:735–745. doi: 10.1007/s11998-016-9894-1. [DOI] [Google Scholar]
- 14.Buşilə M., Muşat V., Textor T., Mahltig B. Synthesis and characterization of antimicrobial textile finishing based on Ag:ZnO nanoparticles/chitosan biocomposites. RSC Adv. 2015;5:21562–21571. doi: 10.1039/c4ra13918f. [DOI] [Google Scholar]
- 15.Karthick Raja Namasivayam S., Rabel A.M., Abhraham T. Antibacterial activity of chemogenic copper nanoparticles coated cotton fabrics against pyogenic bacteria isolated from post operative patients. Adv Mater Res. 2013;622:842–846. doi: 10.4028/www.scientific.net/AMR.622-623.842. [DOI] [Google Scholar]
- 16.Hasan R. Production of antimicrobial textiles by using copper oxide nanoparticles. Int J Contemp Res Rev. 2018;9:20195–20202. doi: 10.15520/ijcrr/2018/9/08/564. [DOI] [Google Scholar]
- 17.Perelshtein I., Applerot G., Perkas N., Grinblat J., Gedanken A. A one-step process for the antimicrobial finishing of textiles with crystalline TiO2 nanoparticles. Chem A Eur J. 2012;18:4575–4582. doi: 10.1002/chem.201101683. [DOI] [PubMed] [Google Scholar]
- 18.Xu Y., Li S., Yue X., Lu W. Nanosilver-cellulose antibacterials. Bio Resources. 2018;13:2150–2170. [Google Scholar]
- 19.Hans M., Erbe A., Mathews S., Chen Y., Solioz M., Mücklich F. Role of copper oxides in contact killing of bacteria. Langmuir. 2013;29:16160–16166. doi: 10.1021/la404091z. [DOI] [PubMed] [Google Scholar]
- 20.Usman M.S., El Zowalaty M.E., Shameli K., Zainuddin N., Salama M., Ibrahim N.A. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomed. 2013;8:4467–4479. doi: 10.2147/IJN.S50837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez A.C., Archana K.M., Rajagopal R. Green synthesis, characterization, catalytic and antibacterial studies of copper iodide nanoparticles synthesized using Brassica oleracea var. capitata f. rubra extract. Chem Data Collect. 2020;29:100538. doi: 10.1016/j.cdc.2020.100538. [DOI] [Google Scholar]
- 22.Pramanik A., Laha D., Bhattacharya D., Pramanik P., Karmakar P. A novel study of antibacterial activity of copper iodide nanoparticle mediated by DNA and membrane damage. Colloid Surf B Biointerfaces. 2012;96:50–55. doi: 10.1016/j.colsurfb.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 23.Qiu X., Liu M., Sunada K., Miyauchi M., Hashimoto K. A facile one- step hydrothermal synthesis of rhombohedral CuFeO2 crystals with antivirus property. Chem Commun. 2012;48:7365–7367. doi: 10.1039/C2CC33475E. [DOI] [PubMed] [Google Scholar]
- 24.Qiu X., Miyauchi M., Sunada K., Minoshima M., Liu M., Lu Y., et al. Hybrid Cu xO/TiO 2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano. 2012;6:1609–1618. doi: 10.1021/nn2045888. [DOI] [PubMed] [Google Scholar]
- 25.Fujimori Y., Sato T., Hayata T., Nagao T., Nakayam M., Nakayam T., et al. Novel antiviral characteristics of nanosized copper(i) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ Microbiol. 2012;78:951–955. doi: 10.1128/AEM.06284-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noyce J.O., Michels H., Keevil C.W. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl Environ Microbiol. 2007;73:2748–2750. doi: 10.1128/AEM.01139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Horie M., Ogawa H., Yoshida Y., Yamada K., Hara A., Ozawa K., et al. Inactivation and morphological changes of avian influenza virus by copper ions. Arch Virol. 2008;153:1467–1472. doi: 10.1007/s00705-008-0154-2. [DOI] [PubMed] [Google Scholar]
- 28.Borkow G., Lara H.H., Covington C.Y., Nyamathi A., Gabbay J. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob Agents Chemother. 2008;52:518–525. doi: 10.1128/AAC.00899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shionoiri N., Sato T., Fujimori Y., Nakayama T., Nemoto M., Matsunaga T., et al. Investigation of the antiviral properties of copper iodide nanoparticles against feline calicivirus. J Biosci Bioeng. 2012;113:580–586. doi: 10.1016/j.jbiosc.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Sucipto T.H., Churrotin S., Setyawati H., Kortaki T., Martal F., Soegijnato S. Antiviral activity of copper(ii)chloride dihydrate against dengue virus type-2 in Vitro cell. IJTID. 2017;6:84–87. doi: 10.20473/IJTID.V6I4.3806. [DOI] [Google Scholar]
- 31.Jiang Y., Gao S., Li Z., Jia X., Chen Y. Cauliflower-like CuI nanostructures: green synthesis and applications as catalyst and adsorbent. Mater Sci Eng B Solid-State Mater Adv Technol. 2011;176:1021–1027. doi: 10.1016/j.mseb.2011.05.023. [DOI] [Google Scholar]
- 32.Ghanbari M., Bazarganipour M., Salavati-Niasari M. Photodegradation and removal of organic dyes using cui nanostructures, green synthesis and characterization. Sep Purif Technol. 2017;173:27–36. doi: 10.1016/j.seppur.2016.09.003. [DOI] [Google Scholar]
- 33.Xu H.J., Liang Y.F., Cai Z.Y., Qi H.X., Yang C.Y., Feng Y.S. CuI-nanoparticles-catalyzed selective synthesis of phenols, anilines, and thiophenols from aryl halides in aqueous solution. J Org Chem. 2011;76:2296–2300. doi: 10.1021/jo102506x. [DOI] [PubMed] [Google Scholar]
- 34.Sepalage G.A., Meyer S., Pascoe A., Scully A.D., Huang F., Bach U., et al. Copper(I) iodide as hole-conductor in planar perovskite solar cells: probing the origin of J-V hysteresis. Adv Funct Mater. 2015;25:5650–5661. doi: 10.1002/adfm.201502541. [DOI] [Google Scholar]
- 35.Tavakoli F., Salavati-Niasari M., Mohandes F. Green synthesis of flower-like CuI microstructures composed of trigonal nanostructures using pomegranate juice. Mater Lett. 2013;100:133–136. doi: 10.1016/j.matlet.2013.02.114. [DOI] [Google Scholar]
- 36.Sabatini C., Mennito A.S., Wolf B.J., Pashley D.H., Renné W.G. Incorporation of bactericidal poly-acrylic acid modified copper iodide particles into adhesive resins. J Dent. 2015;43:546–555. doi: 10.1016/j.jdent.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sekkal W., Zaoui A. Monte Carlo study of transport properties in copper halides. Phys B Condens Matter. 2002;315:201–209. doi: 10.1016/S0921-4526(01)01043-2. [DOI] [Google Scholar]
- 38.Feraoun H., Aourag H., Certier M. Theoretical studies of substoichiometric CuI. Mater Chem Phys. 2003;82:597–601. doi: 10.1016/S0254-0584(03)00318-3. [DOI] [Google Scholar]
- 39.Ferhat M., Zaoui A., Certier M., Dufour J.P., Khelifa B. Electronic structure of the copper halides CuCl, CuBr and CuI. Mater Sci Eng B. 1996;39:95–100. doi: 10.1016/0921-5107(95)01518-3. [DOI] [Google Scholar]
- 40.Chen D., Wang Y., Lin Z., Huang J., Chen X., Pan D., et al. Growth strategy and physical properties of the high mobility p-type cui crystal. Cryst Growth Des. 2010;10:2057–2060. doi: 10.1021/cg100270d. [DOI] [Google Scholar]
- 41.Dhere S.L., Latthe S.S., Kappenstein C., Mukherjee S.K., Rao A.V. Comparative studies on p-type CuI grown on glass and copper substrate by SILAR method. Appl Surf Sci. 2010;256:3967–3971. doi: 10.1016/j.apsusc.2010.01.058. [DOI] [Google Scholar]
- 42.Meng L.R., Mo R., Zhou H., Wang G., Chen W., Wang D., et al. Synthesis of luminescent cubic phase one-dimensional CuI nanostructures in solution. Cryst Growth Des. 2010;10:3387–3390. doi: 10.1021/cg9015417. [DOI] [Google Scholar]
- 43.Tanji A., Akai I., Kojima K., Karasawa T., Komatsu T. Exciton transitions in the hexagonal CuI microcrystallites grown on polymers. J Lumin. 2000;87:516–518. doi: 10.1016/S0022-2313(99)00274-4. [DOI] [Google Scholar]
- 44.Tennakone K., Kumara G.R.R.A., Kottegoda I.R.M., Perera V.P.S., Aponsu G.M.L.P., Wijayantha K.G.U. Deposition of thin conducting films of CuI on glass. Sol Energy Mater Sol Cells. 1998;55:283–289. doi: 10.1016/S0927-0248(98)00117-2. [DOI] [Google Scholar]
- 45.Liu Y., Zhan J., Zeng J., Qian Y., Tang K., Yu W. Ethanolthermal synthesis to γ-Cul nanocrystals at low temperature. J Mater Sci Lett. 2001;20:1865–1867. doi: 10.1023/A:1012849522970. [DOI] [Google Scholar]
- 46.Kariper I.A. CuI film produced by chemical extraction method in different media. Mater Res. 2016;19:991–998. doi: 10.1590/1980-5373-MR-2016-0067. [DOI] [Google Scholar]
- 47.Archana K.M., Yogalakshmi D., Rajagopal R. Application of green synthesized nanocrystalline CuI in the removal of aqueous Mn(VII) and Cr(VI) ions. SN Appl Sci. 2019;1 doi: 10.1007/s42452-019-0544-y. [DOI] [Google Scholar]
- 48.Akhtar F.Z., Archana K.M., Krishnaswamy V.G., Rajagopal R. Remediation of heavy metals (Cr, Zn) using physical, chemical and biological methods: a novel approach. SN Appl Sci. 2020;2 doi: 10.1007/s42452-019-1918-x. [DOI] [Google Scholar]
- 49.Indubala E., Dhanasekar M., Sudha V., Malar E.J.P., Divya P., Sherine J., et al. L-Alanine capping of ZnO nanorods: increased carrier concentration in ZnO/CuI heterojunction diode. RSC Adv. 2018;8:5350–5361. doi: 10.1039/c7ra12385j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Obouayeba A.P., Diarrassouba M., Soumahin E.F., Kouakou H. Phytochemical analysis , purification and identification of Hibiscus anthocyanins. J Pharm Chem Biol Sci. 2015;3:156–168. [Google Scholar]
- 51.Vastrad J V., Byadgi S A. Phytochemical screening and antibacterial activity of Hibiscus rosa - sinensis leaf extracts. Int J Curr Microbiol Appl Sci. 2018;7:3329–3337. doi: 10.20546/ijcmas.2018.703.384. [DOI] [Google Scholar]
- 52.Hussein R.A., Mohsin A.J. Trypan blue exclusion assay verifies in vitro cytotoxicity of new cis-platinum (II) complex in human cells. Baghdad Sci J. 2019;16:555–559. doi: 10.21123/bsj.2019.16.3.0555. [DOI] [Google Scholar]
- 53.Shateri-Khalilabad M., Yazdanshenas M.E., Etemadifar A. Fabricating multifunctional silver nanoparticles-coated cotton fabric. Arab J Chem. 2017;10:S2355–S2362. doi: 10.1016/j.arabjc.2013.08.013. [DOI] [Google Scholar]
- 54.Balamurugan M., Saravanan S., Ohtani N. Synthesis of uniform and high density silver nanoparticles by using peltophorum pterocarpum flower extract. Palliat Support Care. 2014;1584:1–8. doi: 10.1557/opl.2014.279. [DOI] [Google Scholar]
- 55.Eaton P., Fernandes J.C., Pereira E., Pintado M.E., Xavier Malcata F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy. 2008;108:1128–1134. doi: 10.1016/j.ultramic.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 56.Aishwarya S., Gunasekaran K., Sagaya Jansi R., Sangeetha G. From genomes to molecular dynamics - a bottom up approach in extrication of SARS CoV-2 main protease inhibitors. Comput toxicol. 2021;18:100156. doi: 10.1016/j.comtox.2021.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu C.H., Lin Y.F., Lin J.J., Yu C.S. Prediction of metal ion-binding sites in proteins using the fragment transformation method. PLoS One. 2012;7:1–12. doi: 10.1371/journal.pone.0039252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Y.F., Cheng C.W., Shih C.S., Hwang J.K., Yu C.S., Lu C.H. MIB: metal ion-binding site prediction and docking server. J Chem Inf Model. 2016;56:2287–2291. doi: 10.1021/acs.jcim.6b00407. [DOI] [PubMed] [Google Scholar]
- 59.Ruth Christiya C., Thoufikana Evaluation of bioactive compounds in the edible Hibiscus rosa-sinensis L flower petals by gc-ms. World J Pharm Res. 2018;7:403–409. doi: 10.20959/wjpr201813-12028. [DOI] [Google Scholar]
- 60.Vijayakumar S., Morvin Yabesh J.E., Arulmozhi P., Praseetha P.K. Identification and isolation of antimicrobial compounds from the flower extract of Hibiscus rosa-sinensis L: in silico and in vitro approaches. Microb Pathog. 2018;123:527–535. doi: 10.1016/j.micpath.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 61.Kumar H., Bhardwaj K., Kuča K., Kalia A., Nepovimova E., Verma R., et al. Flower-based green synthesis of metallic nanoparticles: applications beyond fragrance. Nanomaterials. 2020;10 doi: 10.3390/nano10040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y.S., Chang Y.C., Chen H.H. Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. J Food Drug Anal. 2018;26:649–656. doi: 10.1016/j.jfda.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mokhtarzadeh A., Eivazzadeh-Keihan R., Pashazadeh P., Hejazi M., Gharaatifar N., Hasanzadeh M., et al. Nanomaterial-based biosensors for detection of pathogenic virus. TrAC - Trends Anal Chem. 2017;97:445–457. doi: 10.1016/j.trac.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vaze N., Pyrgiotakis G., McDevitt J., Mena L., Melo A., Bedugnis A., et al. Inactivation of common hospital acquired pathogens on surfaces and in air utilizing engineered water nanostructures (EWNS) based nano-sanitizers. Nanomed Nanotechnol Biol Med. 2019;18:234–242. doi: 10.1016/j.nano.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deshmukh S.P., Patil S.M., Mullani S.B., Delekar S.D. Silver nanoparticles as an effective disinfectant: a review. Mater Sci Eng C. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kwak W.G., Oh M.H., Gong M.S. Preparation of silver-coated cotton fabrics using silver carbamate via thermal reduction and their properties. Carbohydr Polym. 2015;115:317–324. doi: 10.1016/j.carbpol.2014.08.070. [DOI] [PubMed] [Google Scholar]
- 67.Sun M., Hu J., Zhai C., Zhu M., Pan J. A p-n heterojunction of CuI/TiO2 with enhanced photoelectrocatalytic activity for methanol electro-oxidation. Electrochim Acta. 2017;245:863–871. doi: 10.1016/j.electacta.2017.06.035. [DOI] [Google Scholar]
- 68.Asayesh A., Jeddi A.A.A. Modeling the creep behavior of plain woven fabrics constructed from textured polyester yarn. Text Res J. 2010;80:642–650. doi: 10.1177/0040517509343816. [DOI] [Google Scholar]
- 69.Tang B., Li J., Hou X., Afrin T., Sun L., Wang X. Colorful and antibacterial silk fiber from anisotropic silver nanoparticles. Ind Eng Chem Res. 2013;52:4556–4563. doi: 10.1021/ie3033872. [DOI] [Google Scholar]
- 70.Sherazy E.H., Saad M.M., Kobesy O.M., Alsaid A.A., Nermin M., Aly Characterization of the tensile strength properties of hybrid sandwich composites. Int Des J. 2015;5:607–614. [Google Scholar]
- 71.hong Sun X., tong Zhou T., hong Wei C., qing Lan W., Zhao Y., Pan Y jie, et al. Antibacterial effect and mechanism of anthocyanin rich Chinese wild blueberry extract on various foodborne pathogens. Food Control. 2018;94:155–161. doi: 10.1016/j.foodcont.2018.07.012. [DOI] [Google Scholar]
- 72.You J., Zhang Y., Hu Z. Bacteria and bacteriophage inactivation by silver and zinc oxide nanoparticles. Colloids Surfaces B Biointerfaces. 2011;85:161–167. doi: 10.1016/j.colsurfb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 73.Zhang L., Jiang Y., Ding Y., Povey M., York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids) J Nanoparticle Res. 2007;9:479–489. doi: 10.1007/s11051-006-9150-1. [DOI] [Google Scholar]
- 74.Archana K.M., Rajalakshmi S., Kumar P.S., Krishnaswamy V.G., Rajagopal R., Kumar D.T., et al. Effect of shape and anthocyanin capping on antibacterial activity of CuI particles. Environ Res. 2021;200:111759. doi: 10.1016/j.envres.2021.111759. [DOI] [PubMed] [Google Scholar]
- 75.Konda A., Prakash A., Moss G.A., Schmoldt M., Grant G.D., Guha S. Aerosol filtration efficiency of common fabrics used in respiratory cloth masks. ACS Nano. 2020;14:6339–6347. doi: 10.1021/acsnano.0c03252. [DOI] [PubMed] [Google Scholar]
- 76.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]