Abstract
BACKGROUND
Expandable cages are often used to reconstruct cervical corpectomies but there are few long-term follow-up studies with large numbers.
OBJECTIVE
To analyze the clinical and radiographic results of cervical corpectomy reconstructed with expandable cages for degenerative stenosis.
METHODS
We performed a retrospective analysis of 78 patients with degenerative cervical stenosis treated with a corpectomy reconstructed with an expandable cage. We evaluated the clinical and radiographic outcomes, as well as complications of the procedure at a minimum 2-yr follow-up.
RESULTS
There was a decrease in the visual analog scale pain average from 75 mm to 8.5 mm (P = .02); a decrease in the Neck Disability Index average from 55% to 12% (P = .009); and improvement in the Japanese Orthopaedic Association average from 12 to 14 points (P = .01). There was a change in cervical lordosis (Cobb method) average from −9.3° to −15.1° (P = .002), without significant loss of lordosis (P = .63). The fusion rate, by criteria of the Cervical Spine Research Society (CSRS), was low: using dynamic X-rays – 50% (n = 39/78) and using computed tomography (CT) – 47.4% (n = 37/78). A total of 11 patients (14.1%) suffered complications.
CONCLUSION
To our knowledge, this is the largest series (78) with a minimum 2-yr follow-up in the literature and the first using the dynamic radiographic and CT criteria endorsed by the CSRS. Using these criteria, our fusion rates were much lower than all previous reports in the literature. Despite this, patient-reported outcomes were reasonable. There was a relatively low incidence of perioperative complications, most of which were likely not implant-specific and there was only 1 case of implant failure.
Keywords: Cervical spine, Spinal canal stenosis, Ventral decompression, Corpectomy, Transbody fusion, Telescopic prostheses
ABBREVIATIONS
- ACDF
anterior cervical discectomy and fusion
- CSRS
Cervical Spine Research Society
- JOA
japanese orthopaedic association
- NDI
Neck Disability Index
- PEEK
polyether ether ketone
Anterior cervical corpectomy and fusion is a well-accepted surgical technique to decompress the spinal cord in cases of cord compression due to various pathologies, including those with kyphotic malalignment.1-3 Various corpectomy reconstructive techniques have been described utilizing autograft, allograft, mesh, and expandable cages.4-9 Expandable cages can be lengthened postimplantation to fill the defect and improve alignment.10,11 Despite the increasing popularity of expandable cages, there are relatively few reports in the literature describing long-term outcomes. Importantly, to our knowledge, no study has critically examined the fusion rates using the proven accurate and stringent criteria endorsed by the Cervical Spine Research Society (CSRS).12 This is important because expandable cages are relatively bulky mechanical devices that leave little room for bone graft. The purpose of this study was to assess the outcomes and fusion rates following cervical corpectomies reconstructed with titanium expandable cages.
METHODS
Study Design
This was a retrospective single-center study.
Inclusion Criteria
We included all patients treated with an expandable cage with their last follow-up between 2009 and 2019, with a minimum 2-yr follow-up. The ethics committee at Irkutsk State Medical University approved the study. Voluntary consent was obtained. We included patients with stenosis (canal < 12 mm) at 2 contiguous levels with radiculopathy and/or myelopathy requiring a corpectomy. Most also had foraminal stenosis (vertical size < 4 mm.). The cervical alignment was either hypolordotic, kyphotic, or neutral at the operative level.
Exclusion Criteria
We excluded tandem stenosis, hyperlordosis, asymptomatic degeneration, single-level disease, osteoporosis, previous cervical operations, traumatic, oncologic or inflammatory cervical disease, or other concomitant disease at the index levels.
Study Conditions
Surgical interventions were carried out by 1 surgical team at Department of Neurosurgery of the Irkutsk Railway Clinical Hospital; we used a Caspar distractor (Germany), an operating microscope, and intraoperative X-ray navigation (Siemens, Germany). A left-sided approach was used. Following corpectomy, the posterior longitudinal ligament was resected (Figure 1A). An ADD-plus expandable cage (Urlich, Germany, no conflicts by authors) was placed with screw fixation (Figure 1B). The expandable cage does not allow for the use of bone grafts, as there is nothing to prevent graft from falling posteriorly into the cord. All screws were fixed-angle ones. Postoperatively, all patients wore compression stockings and ambulated within 1 to 2 d. Patients were followed for a minimum of 2 yr.
FIGURE 1.
Intraoperative photographs. A, prepared site for the implant; B, appearance of the ADD-plus expandable prosthesis.
Study Data
General Information
Demographics (gender, age, body mass index [BMI], and American Society of Anesthesiology [ASA] score), duration of surgery, Estimated blood loss, and postoperative course were used.
Clinical Outcomes
Visual analog scale (VAS) pain score, Neck Disability Index (NDI), modified Japanese Orthopaedic Association (JOA) scale, and complications were used.
Radiographic Outcomes
Sagittal Cobb angles, disc degeneration using Pfirrmann grades,13 magnetic resonance imaging (MRI) facet degeneration using Fujiwara's
classification,14 and fusion assessment using the criteria adopted by CSRS12: (1) interspinous processes motion <1 mm on 150% magnified flection-extension X-rays with >4 mm of motion at an adjacent nonoperated level or (2) the presence of bridging bone across the graft into adjacent endplates and bridging bone outside of the graft or cage and no lucent lines (defined as radiolucent line extending >50% of the cortical-host bone interface) according to computed tomography (CT) scans. The radiographs and CT were evaluated by 2 independent experts (neurosurgeon and radiologist), blinded to patient information and uninvolved in the care of the patient. The expert agreement was statistically assessed using Kappa statistics (Graph Pad Software, Inc., USA).
Statistical Analysis
Statistical data were obtained using the Statistica-8 database processing program. The distribution pattern was based on the Shapiro-Wilk, Kolmogorov-Smirnov, and Liljefors tests. Taking into account the presence of significant differences according to these tests (P < .05), the distribution was considered to be different from the normal, in connection with which the assessment of the significance of the differences in the sample sets was made according to the criteria of nonparametric statistics. Differences were considered significant at P < .05. The data were presented as the median, the values of the 1st and 3rd quartiles – Me (Q25, Q75). The following nonparametric statistics criteria were used: the Mann-Whitney test for intersubgroup comparison.
RESULTS
Data are presented in Table 1. A total of 78 patients (48 men, 61.5% and 30 women, 38.5%) aged 58 (47; 72) yr were included in the study. The follow-up period was 60 mo (32; 78). The majority of patients (62.8%) were categorized as ASA II anesthetic risk. The most common level involved a C6 corpectomy (52.6%).
TABLE 1.
General Information and Perioperative Parameters in the Study Group of Patients
| Characteristic | Study group (n = 78) | |
|---|---|---|
| Age (years) | 58 (47;72) | |
| Gender | Male | 48 (61.5%) |
| Female | 30 (38.5%) | |
| BMI (kg/m2) | 23.4 (21.6; 26.8) | |
| Smoking status | 4 (5.1%) | |
| Physical status by ASA | II | 49 (62.8%) |
| III | 26 (33.4%) | |
| IV | 3 (3.8%) | |
| Corpectomy level | C4 | 2 (2.5%) |
| C5 | 22 (28.2%) | |
| C6 | 41 (52.6%) | |
| C7 | 13 (16.7%) | |
| Compression symptoms | Radiculopathy | 78 (100%) |
| Myelopathy | 23 (29.5%) | |
| Duration of surgery (min) | 155 (120; 210) | |
| Total blood loss (ml) | 170 (140; 225) | |
| Mobilization (days) | 1 (1; 2) | |
| Duration of inpatient treatment after surgery (days) | 10 (9; 11) | |
There was a significant decrease in the severity of pain as measured by VAS pain scores from 75 mm (65; 89) to 20 mm (8; 30) at discharge (P = .003) and to 8.5 mm (4; 17) at final follow-up (P = .02) (Figure 2).
FIGURE 2.
Change in the VAS pain score postoperatively.
Postoperatively, there was a significant decrease in the NDI score from 55% (48; 70) to 24% (14; 26) at discharge (P = .01) and to 12% (10; 18) at the final long-term follow-up (P = .009) at 60 mo (32; 78) (Figure 3).
FIGURE 3.
Change in the NDI score in the study group of patients.
Modified JOA scores also improved from 12 (10; 13) points to 14 (13; 15) points at final follow-up (P = .01).
Complications are presented in Table 2. Three patients had a retropharyngeal hematoma that required surgical drainage without further complications. One patient had a superficial infection that resolved with local antiseptics and antibiotics. Two patients with symptomatic adjacent segment pathology underwent anterior cervical discectomy and fusion (ACDF). None of above was felt to be directly related to the cage. The following, however, were thought to be possibly related to the implant: 4 patients with neck pain without neurological deficits were treated with laser facet denervation. The one complication thought to be directly related to the implant occurred in 1 patient whose upper pair of screws lost fixation with associated partial destruction of the body of the overlying vertebra – in this case, a pseudarthrosis was noted on CT. This required revision with an additional level corpectomy and utilization of a longer expandable cage followed by posterior stabilization.
TABLE 2.
Reported Complications in the Study Group
| Complication type | Study group (n = 78) |
|---|---|
| Postoperative hematoma formation | 3 (3.8%) |
| Surgical site infection | 1 (1.3%) |
| Symptomatic degeneration of the adjacent level | 2 (2.5%) |
| Clinically significant facet syndrome in the operated segment | 4 (5.1%) |
| Instability of the cage | 1 (1.3%) |
Radiological parameters are presented in Table 3. The interobserver agreement, evaluated using kappa statistics, was good to excellent.
TABLE 3.
Interobserver Agreement for the Study Group
| Study group (n = 78) | ||
|---|---|---|
| Criteria | Kappa ± SE | 95% CI |
| Cobb angle at the C2-C7 level before operation | 0.968 ± 0.022 | 0.925-1.000 |
| Cobb angle at the C2-C7 level last follow-up | 0.873 ± 0.043 | 0.788-0.958 |
| Changes in the adjacent segment IVD according to Pfirrmann C. before operation | 0.921 ± 0.035 | 0.852-0.989 |
| Changes in the adjacent segment IVD according to Pfirrmann C. at last follow-up | 0.857 ± 0.046 | 0.767-0.947 |
| Changes in the adjacent segment FJ according to Fujiwara A. before operation | 0.952 ± 0.027 | 0.899-1.000 |
| Changes in the adjacent segment FJ according to Fujiwara A. at last follow-up | 0.984 ± 0.016 | 0.953-1.000 |
| Assessment of interspinous process motion on dynamic radiographs at last follow-up | 0.825 ± 0.050 | 0.727-0.924 |
| Assessment of bridging bone according to CT scans | 0.794 ± 0.054 | 0.687-0.900 |
FJ, facet joint; IVD, intervertebral disk.
The C2-C7 lordotic Cobb angles significantly increased postoperatively from −9.3° (−6.1; −12.5) to −15.1° (−13.2; −16.4) at long-term follow-up (P = .002) without significant loss of alignment throughout the follow-up period (P = .63).
There was no significant degeneration of the adjacent levels using the Pfirrmann disc and Fujiwara facet joint classifications (P = .25 and P = .81, respectively).
The fusion rate was low at a minimum 24-mo follow-up. Using the X-ray criteria, it was 50% (n = 39), and using the CT criteria, it was 47.4% (n = 37). Of note, when we had initially assessed the fusion rate, we used the Bridwell criteria and found that 92.3% were fused. In the clinical series, there was only 1 pseudoarthrosis case that was symptomatic: a patient with instability of the instrumentation that required revision surgery.
We performed a subgroup analysis of long-term clinical outcomes and radiological data between patients with CT-verified fusion (n = 37) and nonunion (n = 41). Long-term NDI score results were 10% (8; 16) and 12% (10; 18), respectively (P = .18), modified JOA score 14 (14; 15) points and 13 (13; 14) points, respectively (P = .32), and the C2-C7 lordotic Cobb angle was −15.4° (−13.9; −16.6) and −14.9° (−13.0; −15.9) respectively (P = .47). The clinical and radiographic outcomes were not statistically significantly different at 60 mo follow-up (32; 78).
Figures 4 to 8 show a C6 corpectomy and reconstruction.
FIGURE 4.
Cervical X-rays of patient B before surgery. A, A-P; B, lateral.
FIGURE 8.
CT of the cervical spine of patient B, 3 yr postoperatively. A, sagittal image; B, axial image at the level of the C6 corpectomy. This case has no extra-cage bridging bone and therefore was assessed as a nonunion case.
FIGURE 5.
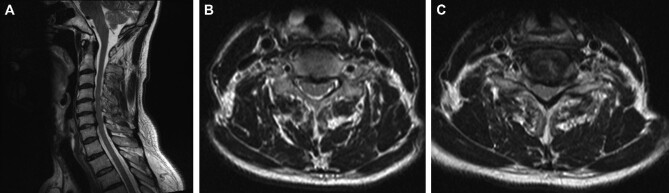
MRI of the cervical spine of patient B before surgery. A, sagittal projection; B, axial image at C5-C6; C, axial image at C6-C7.
FIGURE 6.
Cervical X-rays of patient B, postoperatively. A, A-P; B, lateral.
FIGURE 7.
MRI of the cervical spine of patient B, 3 yr postoperatively. A, sagittal image; B, axial image at C5-C6; C, axial image at C6-C7.
DISCUSSION
Cervical corpectomy is a well-established technique to treat cervical stenosis.15,16 Surgeons use a wide range of implants to reconstruct the spine including bone, fixed and expandable titanium, and polyether ether ketone (PEEK) cages.17 Autografts have a high fusion rate, but there are risks of graft migration and donor site complications.18 PEEK or titanium cages are popular but once the cage is placed, no further alignment changes can be made.19 Expandable prostheses have also become popular because they allow for further lordotic correction of deformities.20 The main negative aspect of these devices is that there is little room for bone grafting, which can impact the fusion rate.7 There are relatively few reports in the literature regarding the use of expandable cages to reconstruct cervical corpectomies.6-9,11,21-23 Most have short-term follow-up, and the results are often contradictory. We undertook this study to critically examine our results using an expandable cage. We assessed patient-reported outcomes, alignment, adjacent level degeneration, and fusion status. For fusion status, we utilized the most rigorous criteria in the literature, endorsed by the CSRS. The plain radiographic criteria are: <1 mm of interspinous process motion on a >150% magnified image with >4 mm of motion at an adjacent, unoperated level. The CT criteria are: extra-cage bridging bone on sagittal and coronal reconstructed views. Below, we summarize the literature and compare them with our findings.
Fusion Rate
Depending on the type of expandable implant used, fusion is reported to vary from 79% to 100% at 9 to 41 mo follow-up.7 The lowest previously-reported expandable cage fusion rate was 79% of 48 patients at 2 yr.9 This is significantly higher than ours (50% using X-rays and 47.4% using CT). It should be noted, however, that none of the previous papers on this topic used the stringent CSRS-endorsed criteria that we used.12 In fact, when we initially assessed the fusion rate, we used the Bridwell criteria and found a fusion rate of 92.3%. This would have made our fusion rates comparable to what is reported in the literature. However, we believe that our low rate is likely to be more of an accurate representation of what one might expect from expandable cages than previous reports in the literature, which used methods that have since been proven to be inaccurate. Fusion rates are obviously dependent upon the methodology used to assess fusion. The CSRS critically examined all of the fusion criteria reported in the literature and concluded that the one that they endorsed was the most accurate one.
Radiographic Alignment
Previous reports have noted that expandable cages allow for lordotic correction, achieving an average lordosis of 4° to 22° and an average kyphosis correction from 3° to 11.6°. Expandable cages have a lower risk of damage to the endplates than fixed ones.18,24 Subsidence occurred in 0% to 43% of cases, none of which required surgical treatment.7,25
We also found that C2-C7 lordosis significantly increased postoperatively from −9.3° (−6.1; −12.5) to −15.1° (−13.2; −16.4) at long-term follow-up without significant loss of alignment throughout the follow-up period.
Complications
The most common complications with expandable prostheses include dysphagia, dysphonia, surgical site infection, pseudarthrosis, subsidence, fracture of the proximal or distal vertebra, neck pain, and deformity at the operated segment.6,9,20,23 In some cases, due to hyperextension or distraction, facet pain or transient C5 palsy can occur.21
We found a relatively low complication rate. There was 1 implant-specific complication that resulted in instability requiring revision decompression and stabilization with corpectomy, use of a larger expandable cage, and posterior stabilization. Other complications included 3 cases of retropharyngeal hematoma requiring surgical evacuation, 1 case of a superficial infection treated with local antiseptics and antibiotics, 2 patients with symptomatic adjacent segment pathology requiring ACDF, and 4 patients with neck pain from the operated segments that were treated with laser denervation of the facet joints.
Outcomes
The literature documents good outcomes with expandable cages. König reported on 6 corpectomies reconstructed with the ADD-plus implant, the same one we used. Their JOA scores improved from an average of 12.0 to 14.5, with no subsidence.22
A retrospective analysis of 50 corpectomy cases treated with titanium expandable cages found significant improvement in preoperative pain at 3 mo, although there was no significant improvement in motor and sensory exam. In the study group, 2 complications were noted in the form of postoperative hematoma and C5 radiculopathy.4
In a multicenter study, 114 patients with multilevel stenosis were treated with corpectomy, reconstructed with autologous bone and plate, and a titanium expandable prosthesis with a plate or a titanium mesh cage filled with autologous bone and a plate. A total of 12 mo postoperatively, according to the Nurick scale, 73 patients achieved partial improvement and 41 showed complete improvement. As far as pain, medication usage, and return to activities, 62 reported excellent results, 48 reported good, and 4 reported satisfactory results.23
Waschke reported on 48 corpectomy patients reconstructed with expandable cages at an average 2-yr follow-up. Average VAS pain scores decreased 2.9 cm. Radiculopathy completely or partially resolved in 85% and Nurick scale improved in 60%.9
We also found similarly improved outcomes. VAS neck pain scores decreased from 75 mm (65-89) to 20 mm (8-30) at discharge and to 8.5 mm (4-17) at final follow-up. NDI scores decreased from 55% (48-70) to 24% (14-26) at discharge and to 12% (10-18) at the final follow-up at an average of 60 mo (range: 30-81). Modified JOA scores also improved from 12 (10-13) points to 14 (13-15) points at final follow-up.
Study Limitations
First, this is a retrospective study, with all the inherent limitations. Second, we utilized only 1 implant. Therefore, the results may not be generalizable to other implants. But we believe that the majority of these titanium implants are similar in design and none allow for much bone grafting. Therefore, we believe that our low fusion rates are likely to be representative of most such implants. Further, it is well-known that titanium bonds to bone better than PEEK. Therefore, one might expect that, if anything, PEEK expandable cages might be associated with an even lower rate of fusion than what we found. A third limitation is that all the operations were performed by 1 surgical team. Therefore, our results may not be generalizable. Fourth, although we only included cases with a minimum 2-yr follow-up, it is possible that with even longer follow-up, we may find more implant-related complications. Nevertheless, our series has one of the longest follow-up periods on this topic. Finally, we do not have a control group. Therefore, we do not know if other implants might result in better or worse outcomes.
Despite all of the above, we believe that our study has considerable merit. First, this is one of the largest series in the literature. Second, it has one of the longest follow-up periods. Third, unlike many previous studies, we used patient-reported outcomes (VAS, NDI, and modified JOA). Most importantly, unlike all previous studies on this topic, we used the most modern and rigorous plain radiographic and CT fusion-assessment criteria, both of which are endorsed by the CSRS. As such, we believe that our finding that the fusion rate is low, even with a minimum 2-yr follow-up, is an important and novel finding that surgeons need to be made aware of.
CONCLUSION
We performed a retrospective analysis of 1-level corpectomies reconstructed with an expandable titanium cage. To our knowledge, this is the largest series (78) with a minimum 2-yr follow-up in the literature. We found that the fusion rate was poor and far below the rates reported in the literature: 50% (n = 39/78) using dynamic plain radiographs and 47.4% (n = 37/78) using CT criteria. However, we used rigorous criteria that had never been used previously for expandable cages. In fact, the criteria that all the other studies on this topic have utilized have been shown to be inaccurate in a publication endorsed by the CSRS. This calls into question the validity of the fusion rates that are in the current literature. We believe that this is one of the most important findings of this study.
Despite the poor fusion rates, we found that the vast majority of our patients did well with significant improvement in their patient-reported outcome measures and relatively low complication rates.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Contributor Information
Vadim A Byvaltsev, Department of Neurosurgery, Irkutsk State Medical University, Irkutsk, Russia; Department of Neurosurgery, Railway Clinical Hospital, Irkutsk, Russia; Department of Traumatology, Orthopedics and Neurosurgery, Irkutsk State Medical Academy of Postgraduate Education - A Branch of the Federal State Budgetary Educational Institution of Continuing Professional Education “Russian Medical Academy of Continuing Professional Education” of the Ministry of Health of the Russian Federation, Irkutsk, Russia.
Andrei A Kalinin, Department of Neurosurgery, Irkutsk State Medical University, Irkutsk, Russia; Department of Neurosurgery, Railway Clinical Hospital, Irkutsk, Russia.
Marat A Aliyev, Asfendiyarov Kazakh National Medical University, Almaty, Kazakhstan.
Nurzhan O Azhibekov, Asfendiyarov Kazakh National Medical University, Almaty, Kazakhstan.
Valerii V Shepelev, Department of Neurosurgery, Irkutsk State Medical University, Irkutsk, Russia.
K Daniel Riew, Department of Orthopedic Surgery, Columbia University, New York, New York, USA; Department of Neurological Surgery, Weill Cornell Medical School, New York, New York, USA.
COMMENTS
Fusion rates were examined for the use of expandable cages in reconstruction after cervical corpectomy in this single-institution, retrospective study of 78 patients with degenerative cervical spine disease. Using CSRS criteria to define fusion, the authors found a fusion rate of 50% via dynamic X-rays and 47.4% via CT. These fusion rates are significantly lower based upon previous literature reports. Using Bridwell criteria, the fusion rate was 92%. A lower fusion rate may be expected for metallic, expandable cages with limited space for bone graft and with the potential for loosening/subsidence. This study emphasizes several key points. There is no concensus in the literature and there are heterogeneous methods to assess spinal fusion. Some methods are much less rigorous. Additionally, despite poor fusion rates based upon strict criteria, there was only one patient that the authors felt had a symptomatic pseudoarthrosis requiring revision surgery. Thus, patients demonstrating radiographic pseudoarthrosis still demonstrated acceptable outcomes. This point highlights the necessity to follow patients perhaps more closely and perhaps over a longer period of time if a radiographic pseudoarthrosis is suspected. Finally, this study emphasizes that clinical and radiographic outcomes following spinal fusion surgery need to be considered both independently, but also in conjunction to implement the appropriate treatment strategy for each individual patient. I applaud the authors in their use of strict criteria to assess spinal fusion in this study. It is my biased opinion that there is an overestimation of spinal fusion rates in the literature based upon less than rigorous criteria.
Timothy F. Witham
Baltimore, Maryland, USA
This is an excellent manuscript retrospectively examining fusion rates in anterior cervical corpectomy with reconstruction using a titanium expandable cage. The fusion rates were found to be (expectedly) low. It would be very interesting to know if there was any difference in outcome (especially functional outcome) between patients with fusion and patients with non-union. In the US, almost all the patients with poor outcome and a non-union will end up having some sort of revision, making this an extremely relevant piece of information.
Christopher Wolfla
Milwaukee, Wisconsin, USA
REFERENCES
- 1.Bakhsheshian J, Mehta VA, Liu JC. Current diagnosis and management of cervical spondylotic myelopathy. Global Spine J. 2017;7(6):572-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asher AL, Devin CJ, Kerezoudis Pet al. Comparison of outcomes following anterior vs posterior fusion surgery for patients with degenerative cervical myelopathy: an analysis from quality outcomes database. Neurosurgery. 2019;84(4):919-926. [DOI] [PubMed] [Google Scholar]
- 3.Wang T, Wang H, Liu S, An HD, Liu H, Ding WY. Anterior cervical discectomy and fusion versus anterior cervical corpectomy and fusion in multilevel cervical spondylotic myelopathy: a meta-analysis. Medicine (Baltimore). 2016;95(49):e5437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatter C, Persson O, Burström G, Edström E, Elmi-Terander A. Anterior cervical corpectomy and fusion for degenerative and traumatic spine disorders, single-center experience of a case series of 119 patients. Oper Neurosurg. 2020;20(1):8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen Z, Lu T, Wang Y, Liang H, Gao Z, He X. Anterior cervical corpectomy and fusion and anterior cervical discectomy and fusion using titanium mesh cages for treatment of degenerative cervical pathologies: a literature review. Med Sci Monit. 2018;24(Sep):6398-6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaïri F, Aboukais R, Thines L, Allaoui M, Assaker R. Relevance of expandable titanium cage for the treatment of cervical spondylotic myelopathy. Eur Spine J. 2012;21(8):1545-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elder BD, Lo SF, Kosztowski TAet al. A systematic review of the use of expandable cages in the cervical spine. Neurosurg Rev. 2016;39(1):1-11. [DOI] [PubMed] [Google Scholar]
- 8.Auguste KI, Chin C, Acosta FL, Ames CP. Expandable cylindrical cages in the cervical spine: a review of 22 cases. J Neurosurg Spine. 2006;4(4):285-291. [DOI] [PubMed] [Google Scholar]
- 9.Waschke A, Kaczor S, Walter J, Duenisch P, Kalff R, Ewald C. Expandable titanium cages for anterior column cervical reconstruction and their effect on sagittal profile: a review of 48 cases. Acta Neurochir. 2013;155(5):801-807. [DOI] [PubMed] [Google Scholar]
- 10.Nigro L, Tarantino R, Donnarumma P, Santoro A, Delfini R. A case of cervical tuberculosis with severe kyphosis treated with a winged expandable cage after double corpectomy. J Spine Surg. 2017;3(2):304-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarantino R, Nigro L, Donnarumma P, Rullo M, Santoro A, Delfini R. Cervical reconstruction techniques. After adequate selection of the patient report of a series of 34 patients treated with winged expandable cages. Neurosurg Rev. 2017;40(2):281-286. [DOI] [PubMed] [Google Scholar]
- 12.Rhee JM, Chapman JR, Norvell DC, Smith J, Sherry NA, Riew KD. Radiological determination of postoperative cervical fusion: a systematic review. Spine (Phila Pa 1976). 2015;40(13):974-991. [DOI] [PubMed] [Google Scholar]
- 13.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001;26(17):1873-1878. [DOI] [PubMed] [Google Scholar]
- 14.Fujiwara A, Lim TH, An HSet al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976). 2000;25(23):3036-3044. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Chen J, Cao Cet al. Anterior versus posterior approach for the therapy of multilevel cervical spondylotic myelopathy: a meta-analysis and systematic review. Arch Orthop Trauma Surg. 2019;139(6):735-742. [DOI] [PubMed] [Google Scholar]
- 16.Byvaltsev VA, Stepanov IA, Riew DK. Mid-term to long-term outcomes after total cervical disk arthroplasty compared with anterior diskectomy and fusion: a systematic review and meta-analysis of randomized controlled trials. Clin Spine Surg. 2020;33(5):192-200. [DOI] [PubMed] [Google Scholar]
- 17.Jiang SD, Jiang LS, Dai LY. Anterior cervical discectomy and fusion versus anterior cervical corpectomy and fusion for multilevel cervical spondylosis: a systematic review. Arch Orthop Trauma Surg. 2012;132(2):155-161. [DOI] [PubMed] [Google Scholar]
- 18.Weber MH, Fortin M, Shen Jet al. Graft subsidence and revision rates following anterior cervical corpectomy: a clinical study comparing different interbody cages. Clin Spine Surg. 2017;30(9):E1239-E1245. [DOI] [PubMed] [Google Scholar]
- 19.Tundo F, Avila MJ, Willard Let al. Spinal alignment, surgery, and outcomes in cervical deformity: a practical guide to aid the spine surgeon. Clin Neurol Neurosurg. 2019;185(Oct):105496. [DOI] [PubMed] [Google Scholar]
- 20.Rajshekhar V, Arunkumar MJ, Kumar SS. Changes in cervical spine curvature after uninstrumented one- and two-level corpectomy in patients with spondylotic myelopathy. Neurosurgery. 2003;52(4):799-805. [DOI] [PubMed] [Google Scholar]
- 21.Arts MP, Peul WC. Vertebral body replacement systems with expandable cages in the treatment of various spinal pathologies: a prospectively followed case series of 60 patients. Neurosurgery. 2008;63(3):537-545. [DOI] [PubMed] [Google Scholar]
- 22.König SA, Spetzger U. Distractable titanium cages versus PEEK cages versus iliac crest bone grafts for the replacement of cervical vertebrae. Minim Invasive Ther Allied Technol. 2014;23(2):102-105. [DOI] [PubMed] [Google Scholar]
- 23.Doria C, Mosele GR, Balsano M, Maestretti G, Caggiari G. Anterior decompression and plate fixation in treatment of cervical myelopathy: a multicentric retrospective review. Acta Orthop Traumatol Turc. 2018;52(3):185-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shriver MF, Lewis DJ, Kshettry VR, Rosenbaum BP, Benzel EC, Mroz TE. Pseudoarthrosis rates in anterior cervical discectomy and fusion: a meta-analysis. Spine J. 2015;15(9):2016-2027. [DOI] [PubMed] [Google Scholar]
- 25.Buser Z, Brodke DS, Youssef JAet al. Synthetic bone graft versus autograft or allograft for spinal fusion: a systematic review. J Neurosurg Spine. 2016;25(4):509-516. [DOI] [PubMed] [Google Scholar]