Abstract
BACKGROUND
Spinal cord stimulation (SCS) is an effective treatment in chronic neuropathic pain, but its efficacy in complex regional pain syndrome (CRPS) needs to be proven.
OBJECTIVE
To study the outcome of SCS in CRPS as measured by trial success, explantation rate, complications, and changes in opioid and neuropathic pain medication use over a 4-yr follow-up.
METHODS
We retrospectively reviewed all medical records of 35 consecutive CRPS patients who underwent SCS trials at 2 hospitals during January 1998 to December 2016. The purchase data of opioids and neuropathic pain medication during January 1995 to March 2016 were retrieved from national registries.
RESULTS
Based on a 1-wk trial, permanent SCS was implanted in 27 (77%) patients. During the median follow-up of 8 yr, 8 (30%) SCS devices were explanted, of which 7 were because of inefficient pain relief. Complications leading to revision occurred in 17 (63%) patients: 8 electrode migrations or stimulation to the wrong area, 1 deep infection, 9 hardware malfunctions, 2 pulse generator discomforts, and 2 SCS replacements. None of the 6 patients using strong opioids discontinued their use during the 2-yr follow-up. The mean opioid dose increased nonsignificantly both in patients with SCS in permanent use (53 ± 150 morphine milligram equivalents morphine milligram equivalent (MME)/day to 120 ± 240 MME/day) and in patients who had SCS explanted (27 ± 72 MME/day to 57 ± 66 MME/day).
CONCLUSION
Despite the fact that CRPS patients were not able to discontinue or reduce their strong opioid or neuropathic pain medication use, 70% continued to use their SCS device during a median 8-yr follow-up.
Keywords: Complex regional pain syndrome, Spinal cord stimulation, Opioid, Neuropathic pain medication
ABBREVIATIONS
- CMM
conventional medical management
- CRPS
complex regional pain syndrome
- DDD
defined daily dose
- IPG
internal pulse generator
- KUH
Kuopio University Hospital
- MME
morphine milligram equivalent
- RSD
reflex sympathetic dystrophy
- SCH
Savonlinna Central Hospital
- SCS
spinal cord stimulation
- SII
Social Insurance Institution
- STROBE
STrengthening the Reporting of OBservational studies in Epidemiology
Complex regional pain syndrome (CRPS) is a chronic neuropathic disorder with a complex pathophysiology.1 The worldwide incidence of CRPS is unknown, but an estimated incidence rate of 26.6/100 000 life years has been reported in the population of the Netherlands. The risk of being affected is 3 times greater among females than in males.2 CRPS is often divided into 2 subcategories: CRPS I, which is formerly known as reflex sympathetic dystrophy (RSD), in which there is no evidence of nerve lesions, and CRPS II, which is formerly known as Causalgia, in which nerve lesions are present.3 Both types share similar symptoms, including constant pain, sensory, vasomotor, sudomotor, motor, and trophic changes, which form the diagnostic Budapest criteria for CRPS.4-6 The year that a CRPS diagnose is set, the median total costs per patient total $8508, from which $2077 are a result of pain prescriptions.7 Comorbidities are common in CRPS, and the disease substantially decreases the quality of life.8,9
Strong opioids are not recommended for pain management in CRPS because evidence of their efficiency in relieving neuropathic pain in CRPS is lacking; however, they are still widely used.10 Opioids have various adverse effects and can lead to opioid abuse, which has contributed to the ongoing opioid crisis. Therefore, we should favor alternative ways to manage pain and avoid indiscriminate pain prescriptions.11,12 Here, spinal cord stimulation (SCS) has been used for severe CRPS refractory to medical treatment. In prospective studies, there were statistically significant improvements in the visual analog scale (VAS) scores and reduced narcotic use after the beginning of SCS.13-15 Altogether, only a few long-term studies, including on medication use, have been made (Table 1).16-33 The initial costs may be high, but it is a cost-effective option among carefully selected patients.34 High-quality evidence of efficacy of SCS in CRPS is still lacking, and multicenter studies would be recommended because of the small number of patients in individual centers.10,35,36
TABLE 1.
Previous Studies on Spinal Cord Stimulation (SCS) in Complex Regional Pain Syndrome (CRPS)
| Reference year | Country | Type of study | Cases and groups | Mean age ± SD (yr) | Medication included | Outcome measures used | Relevant results |
|---|---|---|---|---|---|---|---|
| Mekhail (2020)26 | United States | Retrospective review | 420 cases Current smoker 177 Former smoker 51 Nonsmoker 192 |
43 ± 12 | - | Pain score | Tobacco cigarette smoking was associated with reduced SCS effectiveness for pain relief. |
| Levy (2020)31 | United States | Prospective randomized controlled trial | 145 cases DRG 73 SCS 72 |
DRG 53 ± 13 SCS 52 ± 12 |
- | PPR, POMS, VAS | Pain relief decreased significantly at 12 mo for SCS. Tonic SCS demonstrated therapy habituation at 9 and 12 mo. DRG stimulation produces more stable pain relief through 12 mo than SCS. |
| Risson (2018)25 | United States | Prospective cohort study | 33 cases | 48 range 23-68 |
- | PDI, VAS | Significant improvement in pain and disability. 65% improvement in PDI was observed subsequently. Preoperative VAS 9.4 ± 0.8 and postoperative VAS 2.86 ± 2.08 (P < .0001). An average reduction of 70% of painful symptoms after the surgical procedure. |
| Sanders (2016)28 | United States | Retrospective review | 46 CRPS of total 199 cases |
52 ± 14 | Opioids | Trial success, explantation rate, oral morphine equivalent (OME), NRS | Trial success 74.2%, explantation rate 8%. Statistically significant decrease in opioid use. OME baseline 43 ± 11; 12 mo 20 ± 7, P = .02. NRS baseline 8.3 ± 1.5; 6 mo 2.5 ± 1.7, P < .001; 12 mo 3.4 ± 1.8, P < .001. Overall satisfaction rate was 84%. |
| Hayek (2015)24 | United States | Retrospective review | 68 CRPS of total 342 cases | 54 ± 15 | - | Trial success, explantation rate, revision rate, complications | CRPS trial success 68%. Revision and explantation rate of 23.9%. Complications in 34.6% of total implants. |
| Chivukula (2014)23 | United States | Retrospective review | 36 CRPS of total 121 cases | 46 ± 12 | Pain medication | NRS, ADL, revision rate, complications | Mean pain reduction averaged 56.6%. |
| Geurts (2013)33 | The Netherlands | Prospective cohort study | 84 cases | 35 IQR 32-46 |
- | VAS, PGIC, explantation rate, revision rate, complications | At least 30% pain relief in 41% (95% CI: 27-55) of patients at end of follow-up. During 12 yr of follow-up 63% (95% CI: 41-85) of the implanted patients still use their SCS device at measured end point. Explantation rate 47%, revision rate 61%. |
| Kumar (2011)29 | Canada | Retrospective review | 25 cases | 51 range 32-82 |
Anticonvulsants, antidepressants, opioids, nonsteroidal anti-inflammatory drugs | VAS, ODI, BDI, EQ-5D, SF-36 | Medication usage decline > 25% in most of the patients. Baseline: VAS 8.4, ODI 70%, BDI 28, EQ-5D 0.30, and SF-36 24. 3 m: VAS 4.8, ODI 45%, BDI 15, EQ-5D 0.57, and SF-36 45. Last follow-up: VAS 5.6, ODI 50%, BDI 19, EQ-5D 0.57, and SF-36 40. In general, maximum improvement was recorded at follow-up at 3 mo. |
| Mekhail (2011)30 | United States | Retrospective review | 345 CRPS of total 707 cases | 46 ± 15 | - | Trial success, revision rate, complications | Trial success: CRPS 1 79%, CRPS 2 83%. 38% of patients had hardware related complications. 4.5% had documented infections. |
| Reig (2009)22 | Spain | Retrospective review | 40 CRPS of total 260 cases | 40 range 22-73 |
- | Four-point category verbal scale, VAS, complications | CRPS patients: 5% no pain relief, 40% poor pain relief, 47.5% good pain relief, and 7.5% excellent pain relief. 64% of all patients received a 50% or greater improvement in symptoms at the end of the last follow-up. Complication rate in CRPS group was 32.5%. |
| Kemler (2008)20, (2004)21, (2000)32 | The Netherlands | Prospective randomized controlled trial | 54 cases 36 SCS + PT 18 PT |
SCS + PT 40 ± 12 PT 35 ± 8 |
- | Trial success, GPE, NRS, health-related quality of life measures (%) Nottingham Health Profile, EQ-5D, Self-Rating Depression scale, VAS | Trial success 67%. GPE (P = .02) and pain relief (P = .06) in 20 patients with an implant exceeded those in 13 patients who received PT only, but effect of SCS diminishes over time and is no longer significant at 3 yr of follow-up. 95% of patients with an implant would repeat the treatment for the same result. During 5 yr 42% reoperation rate due to complications. |
| Kumar (2006)19 | Canada | Retrospective review | 32 CRPS of total 410 cases | 54 range 21-87 |
- | Trial success, long-term success, complications | CRPS trial success 87.5%, long-term success 72%. |
| Harke (2005)27 | Germany | Prospective cohort study | 29 cases | 50 ± 15 | - | VAS, PDI | With long‐term SCS combined with physiotherapy, the functional status and the quality of life could be significantly improved. |
| Forouzanfar (2004)18 | The Netherlands | Prospective cohort study | 36 cases 19 cervical 17 lumbar |
Cervical 38 range 26-55 lumbar 42 range 28-59 |
- | GPE, EQ-5D, HRQL | Pain intensity was reduced at 6 mo, 1 and 2 yr after implantation (P < .05). Complications and adverse effects occurred in 64%. All patients reported at least 50% pain reduction at 6 mo after implantation. After 1 and 2 yr of follow-up, there was a slight but significant increase in pain (VAS) indicating that the effect was declining. |
| Kemler (1999)17 | The Netherlands | Retrospective review | 23 cases | 39 range 24-54 |
- | VAS, GPE, trial success, explantation rate, complications | The mean pain score (VAS) had decreased from 7.9 to 5.4 (P < .001). Trial success 78%, explantation rate 17%. 50% suffered complications after implantation of the permanent SCS system. |
| Bennett (1999)16 | United States | Retrospective review | 101 cases Group I (30) single-lead quadripolar Group II (71) dual-lead octopolar |
Group I 44 ± 13 Group II 43 ± 12 |
– | VAS, overall satisfaction score | Significant reduction in VAS (P < .0001). Overall satisfaction scores were 70% in group I and 91% in group II (P < .05). |
The articles were selected from PubMed with the query ((spinal cord stimulation) and ((complex regional pain syndrome) or (reflex sympathetic dystrophy))), totaling 407 results, including 58 clinical studies of the long-term outcomes of SCS in CRPS. Studies with less than 20 CRPS patients, less than a 1-yr follow-up during SCS, conducted before year 1999, or without subgroup analyses of CRPS were discarded. The resulting 18 studies and outcomes relevant to the current study are presented.
EQ-5D = Questionnaire of health-related quality of life developed by the EuroQol Group. SF-36 = 36-Item Short Form Health Survey. SCS = spinal cord stimulation. CRPS = complex regional pain syndrome. DRG = dorsal root ganglion stimulation. PPR = percentage pain relief. POMS = profile of mood states. VAS = visual analog scale. PDI = pain disability index. NRS = numeric rating scale. ADL = activity of daily living. OME = oral morphine equivalent. PGIC = patients’ global impression of change. ODI = Oswestry Disability Index. BDI = Beck's Depression Inventory. PT = physiotherapy. GPE = global perceived effect. HRQL = health-related quality of life.
We present a retrospective collaborative analysis of CRPS patients treated with SCS during an 18-yr period with a median follow-up time of 8 yr. Our objective was to estimate the long-term outcome of SCS in CRPS by measuring the (1) effect on opioid and neuropathic pain medication use before and during SCS, (2) explantation rate, and (3) complications.
METHODS
Patients
The medical records of all 35 consecutive CRPS patients with SCS implantation were retrospectively reviewed. A total of 27 patients were treated at the Kuopio University Hospital (KUH) Neurosurgery and 8 patients at the Savonlinna Central Hospital (SCH) between January 1, 1998, and December 31, 2014. During the period, KUH neurosurgery provided acute and elective neurosurgical services for the 850 000 residents in Eastern and Central Finland, whereas SCH offered elective spine and pain surgery services for 43 000 people. Before SCS implantation, patients underwent conventional treatment with oral analgesics, physical therapy, sympathetic blockades, and other options for pain management. Other treatable pathologies were ruled out by a neurosurgeon or a pain physician. A median duration from the first symptoms to SCS implantation was 3 yr (range 1-13). All patients who had previously been treated with SCS or where the SCS device had been implanted elsewhere were excluded in the current study.
All permanent residents of Finland are entitled to health care and are covered by the Social Insurance Institution (SII) of Finland. The patients’ expenses are minor, and no selection based on economic status can be expected.
Clinical Evaluation
All details concerning the SCS treatment, revisions, and complications were evaluated from the medical charts. Age, gender, place of residence, duration of symptoms, site of pain, use of sympathetic blockades, and suspected precipitating factor that led to CRPS were included in the baseline characteristics. Follow-up data were gathered along the way, and all patients were followed up from medical records until December 31, 2016.
SCS Implantation
The SCS electrode was implanted in the epidural space of the spinal canal either by percutaneous approach or by surgical laminotomy. Surgical paddle leads (Resume 3586, Symmix 3982, Specify 2×4 3998, Specify 5-6-5 39 565, Medtronic, Dublin, Ireland) were implanted under general anesthesia and percutaneous leads (Pisces-Quad 3487A, Vectris 3873, Medtronic, Dublin, Ireland; Lamitrode-S, Octrode, St. Jude Medical, Plano, Texas) under local anesthesia. For surgical leads, implantation level was determined neuroanatomically by pain localization. Electrophysiologic guidance was not used for lead placement. Percutaneous leads were inserted either through the Tuohy cannula or, in the case of the Lamitrode-S electrode, with an Epiducer delivery system. For percutaneous leads, intraoperative testing was performed to confirm that paresthesia covered most of the pain area. When the leads were adjusted to the optimal position, they were fixated in place with an anchor. All patients went through the trial period (median 7 d, range 2-63), and those who reported adequate pain relief with sufficient coverage of the pain area received an internal pulse generator (IPG). To avoid additional operations, permanent SCS treatment continued with the same lead that was implanted for the trial period.
Statistical Analysis
SPSS version 27 (IBM Corporation, Armonk, New York) was used for statistical analyses. The data were analyzed by calculating the means and standard deviations for the normally distributed variables or medians, and the ranges were calculated for the other variables. A logistic regression analysis was used to predict successful trial stimulation, and a Cox regression analysis was used to analyze the variables associated with SCS explantation.
Medication Data
For each patient, we reclaimed the data of the purchased opioids and neuropathic pain medications from the SII of Finland. Under the supervision of the Finnish Parliament, SII is an independent institution that maintains a registry database of all permanent residents of Finland; it consists of prescribed medication, prescription dates, medication purchase dates, amounts, and prices. The retrieved opioid analgesics included the following strong opioids: fentanyl, hydromorphone, methadone, morphine, and oxycodone. Neuropathic pain medications included amitriptyline, duloxetine, gabapentin, nortriptyline, and pregabalin. We obtained purchase data 24 mo before and after the implantation of SCS devices. For the purposes of the present study, the purchased amount of neuropathic pain medication is represented as the defined daily dose (DDD), which is defined by the World Health Organization (WHO) as the assumed average maintenance dose per day for a drug used for its main indication in adults. Likewise, opioids were converted and represented as morphine milligram equivalent (MME), which allows for a comparison between different drugs.
Ethical Issues
Data collection was approved by the Institutional Review Board of KUH. The national registry data were merged with the approval of the Ministry of Social Affairs and Health of Finland. Informed consent was not required by Finnish legislation because the study was based on registry data, and patients were not contacted. STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines were used to ensure the reporting of this observational study.
RESULTS
CRPS History
The median age of the 35 patients at the end of the trial period was 51 yr (range 16-83), and 24 (69%) were female (Table 2). The median duration of pain before SCS was 3 yr (range 1-13). Of the patients, 18 (51%) suffered from upper limb pain, 16 (48%) from pain in lower limbs, and 1 (3%) from pelvic pain. The estimated incident that led to CRPS was conservatively treated bone fracture or other trauma in 17 (49%) patients, orthopedic surgery, or other operative trauma in 17 patients (49%), and in one patient, it was unknown. Sympathetic blockades were tried at least once in 24 (69%) patients, and the response to the treatment was good or better than before in 17 (71%) of them and poor or worse than before in six (25%) of them. Data were missing for one patient.
TABLE 2.
Patient Demographics of 35 Complex Regional Pain Syndrome Patients Treated Between January 1998 and December 2016 and Multivariate Analysis of the Variables Associated With Successful Trial Stimulation and Spinal Cord Stimulation Explantation
| Permanent SCS implanted n = 27 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Trial only n = 8 | SCS explanted n = 8 | SCS in use at the end of follow-up n = 19 | ||||||||
| All | % | OR (95% CI)a | P | All | % | HR (95% CI)b | P | All | % | |
| Gender | ||||||||||
| Female | 5 | 63 | 1 | 5 | 63 | 1 | 14 | 74 | ||
| Male | 3 | 37 | 1.8 (0.16-20) | .63 | 3 | 37 | n.d. | .32 | 5 | 26 |
| Age (mean ± SD) | 54 ± 11 | 0.95 (0.87-1.0) | .29 | 41 ± 13 | 0.38 (0.04-3.5) | .39 | 51 ± 13 | |||
| Location of pain | ||||||||||
| Arm(s) | 4 | 50 | 1 | 4 | 50 | 1 | 10 | 53 | ||
| Leg(s) | 3 | 38 | n.d. | 1.0 | 4 | 50 | n.d. | .63 | 9 | 47 |
| Pelvis | 1 | 12 | 0 | 0 | 0 | 0 | ||||
| Duration of pain in years before SCS (mean ± SD) | 3 ± 3 | 1.2 (0.77-1.7) | .50 | 4 ± 3 | n.d. | .33 | 5 ± 3 | |||
| Estimated incident leading to CRPS | ||||||||||
| Conservatively treated bone fracture or other trauma | 4 | 50 | 1 | 3 | 43 | 1 | 10 | 53 | ||
| Orthopedic surgery or other operative trauma | 4 | 50 | 2.1 (0.26-17) | .49 | 4 | 57 | n.d. | .49 | 9 | 47 |
| Missing | 1 | |||||||||
| Sympathetic blockade | ||||||||||
| Yes | 7 | 88 | 1 | 3 | 60 | 1 | 14 | 74 | ||
| No | 1 | 12 | 3.5 (0.22-54) | .38 | 2 | 40 | n.d. | .64 | 5 | 26 |
| Missing | 3 | |||||||||
| Response to sympathetic blockade | ||||||||||
| Good or better than before | 5 | 83 | 2 | 67 | 10 | 71 | ||||
| Poor or worse than before | 1 | 17 | 1 | 33 | 4 | 29 | ||||
| Not used or missing | 2 | 5 | 5 | |||||||
| Spinal segment of electrode | ||||||||||
| Cervical | 4 | 50 | 1 | 5 | 63 | 1 | 10 | 53 | ||
| Thoracal | 4 | 50 | n.d. | 1.0 | 3 | 37 | n.d. | .61 | 9 | 47 |
| Type of electrode | ||||||||||
| Surgical | 7 | 87 | 1 | 7 | 87 | 1 | 10 | 53 | ||
| Percutaneous | 1 | 13 | 6.0 (0.46-77) | .17 | 1 | 13 | n.d. | .84 | 9 | 47 |
SCS = spinal cord stimulation. n.d. = not defined. CRPS = complex regional pain syndrome.
aOdds ratio is calculated using multivariate logistic regression analysis for a successful trial.
bHazard ratio is calculated by using Cox regression analysis for SCS explantation.
Trial Stimulation
All 35 patients went through a trial period of a median of 7 d (range 2-63), and 27 (77%) of them received an IPG. The remaining 8 (23%) patients did not experience adequate pain relief and had their electrodes removed (Figure 1). Electrodes were placed in the cervical 19 (54%) or thoracic 16 (46%) segment, and most were surgical 24 (69%) electrodes. During the trial, one patient suffered from postoperative urinary retention. Otherwise, no revisions or complications occurred during the trial. In the multivariate logistic regression analysis, none of the variables—age, gender, location of pain (arm or leg/pelvis), estimated incident leading to CRPS, sympathetic blockade, spinal segment of electrode, or type of electrode—were associated with the success of the trial.
FIGURE 1.
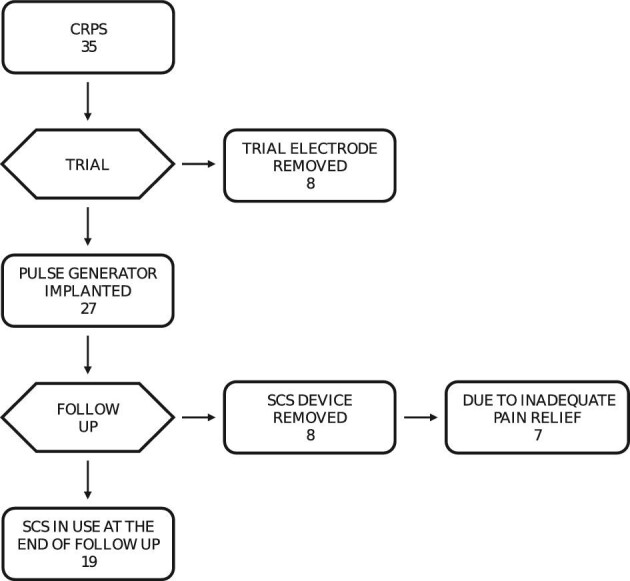
Flow chart of 35 consecutive CRPS patients who underwent spinal cord stimulation from 1998 to 2016.
Explantation Rate
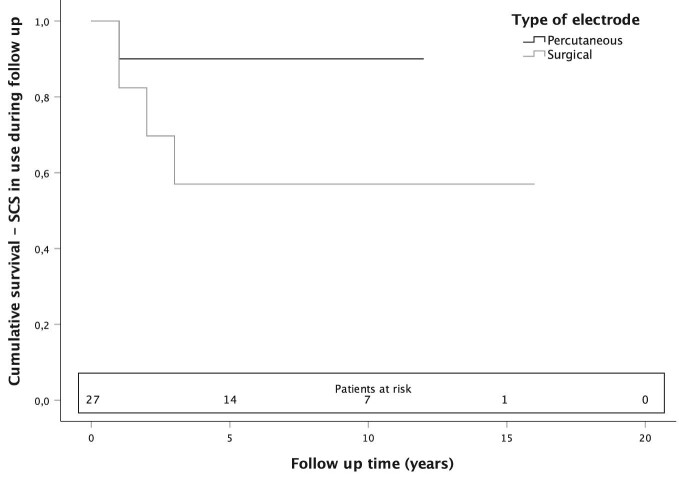
In 27 patients receiving a permanent SCS device after the trial, the mean follow-up was 6 yr (range 1-17, total 162 follow-up years). During follow-up, 8 (30%) SCS devices were explanted, of which 7 were because of inefficient pain relief and 1 because of pregnancy desire. Of the 10 percutaneously implanted electrodes, 1 (10%) was explanted, and of the 17 surgically implanted electrodes, 7 (41%) were explanted (Figure 2). The mean time for explantation was 2 yr (range 1-4). In the Cox regression analysis, none of the variables—age, gender, location of pain (arm or leg/pelvis), estimated incident leading to CRPS, sympathetic blockade, spinal segment of electrode, and type of electrode—was associated with the explantation of the SCS device (Table 2).
FIGURE 2.
Kaplan-Meier survival curve for all 27 CRPS patients with SCS device implanted after the trial period. The end point of the follow-up was SCS explantation because of inefficient pain relief in 7 patients and because of pregnancy desire in 1 patient.
Opioid Use
The obtained purchase data 24 mo before and after SCS show the mean daily MME of all 35 individual patients; these data were divided into 1-yr periods. During the 4-yr period, strong opioids were purchased at least once by 3 (38%) of the 8 patients in the trial-only group, 6 (75%) of the 8 patients in the explanted group, and 8 (42%) of the 19 patients in the permanent group (Table 3). There were no significant differences in opioid use between the groups before or after SCS (Table 4).
TABLE 3.
Patient Demographics of 35 Spinal Cord Stimulation (SCS) Patients and Purchased Opioids and Neuropathic Pain Medications Divided Into 1-Year Periods
| Mean daily opioid dose (MME) | Mean daily neuropathic medication dose (DDD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Age | Site of pain | Years from trauma to SCS | –2 yr | –1 yr | +1 yr | +2 yr | –2 yr | –1 yr | +1 yr | +2 yr | |
| Permanent 1 | Female | 43 | Left leg | 1 | 0 | 0 | 0 | 0 | 0.05 | 0 | 0 | 0 |
| Permanent 2 | Male | 40 | Right leg | 2 | 0 | 0 | 0 | 0 | 0 | 0.04 | 0 | 0 |
| Permanent 3 | Female | 52 | Right arm | 3 | 0 | 0 | 8 | 0 | 0.05 | 0 | 0.05 | 0.22 |
| Permanent 4 | Female | 50 | Both arms | 4 | 0 | 0 | 78 | 203 | 0.18 | 0.33 | 0.58 | 0.64 |
| Permanent 5 | Female | 46 | Left arm | 4 | 8 | 8 | 41 | 26 | 0.61 | 0 | 0 | 0 |
| Permanent 6 | Female | 58 | Right arm | 7 | 44 | 59 | 51 | 51 | 0 | 0.01 | 0 | 0 |
| Permanent 7 | Male | 83 | Right leg | 3 | 0 | 0 | 0 | 0 | 0.29 | 0.31 | 0.27 | 0.42 |
| Permanent 8 | Male | 56 | Left arm | 4 | 0 | 0 | 0 | 0 | 0.20 | 0 | 0 | 0 |
| Permanent 9 | Female | 47 | Right arm | 2 | 0 | 0 | 0 | 0 | 0.01 | 0.04 | 0 | 0 |
| Permanent 10 | Female | 26 | Right leg | 3 | 0 | 0 | 0 | 0 | 0.08 | 0 | 0 | 0 |
| Permanent 11 | Female | 42 | Both legs | 13 | 0 | 0 | 0 | 0 | 0.27 | 0.46 | 0.92 | 0.49 |
| Permanent 12 | Female | 58 | Left arm | 8 | 235 | 205 | 397 | 205 | 0.73 | 0.55 | 0.64 | 0.82 |
| Permanent 13 | Female | 68 | Left leg | 8 | 0 | 0 | 0 | 0 | 0.19 | 0.15 | 0.13 | 0.13 |
| Permanent 14 | Female | 60 | Right leg | 3 | 0 | 0 | 0 | 0 | 0.23 | 0.27 | 0.15 | 0 |
| Permanent 15 | Male | 37 | Right arm | 4 | 0 | 0 | 0 | 66 | 0.05 | 0.05 | 0.35 | 0.40 |
| Permanent 16 | Female | 47 | Right leg | 3 | 0 | 0 | 19 | 0 | 0.61 | 0.70 | 1.2 | 0.43 |
| Permanent 17 | Male | 56 | Left arm | 9 | 0 | 88 | 159 | 237 | 0.90 | 1.0 | 1.0 | 0.15 |
| Permanent 18 | Female | 61 | Right arm | 2 | 0 | 0 | 0 | 0 | 0.04 | 0 | 0 | 0 |
| Permanent 19 | Female | 42 | Left leg | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Explanted 1 | Male | 44 | Right arm | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Explanted 2 | Female | 41 | Right arm | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Explanted 3 | Female | 51 | Right arm | 9 | 0 | 0 | 0 | 47 | 0.35 | 1.3 | 1.6 | 1.4 |
| Explanted 4 | Male | 35 | Right leg | 2 | 10 | 6 | 4 | 0 | 0.72 | 0.91 | 0.92 | 0.26 |
| Explanted 5 | Female | 16 | Left leg | 6 | 0 | 0 | 1 | 0 | 0.21 | 0 | 0 | 0 |
| Explanted 6 | Male | 58 | Right arm | 6 | 0 | 0 | 60 | 0 | 0.17 | 0 | 0.12 | 0 |
| Explanted 7 | Female | 32 | Left leg | 2 | 0 | 0 | 0 | 86 | 0 | 1.7 | 0.37 | 0.33 |
| Explanted 8 | Female | 49 | Right leg | 4 | 88 | 66 | 29 | 26 | 1.2 | 1.0 | 0.79 | 0.61 |
| Trial 1 | Female | 54 | Right arm | 3 | 0 | 0 | 0 | 0 | 0.55 | 0.50 | 0.68 | 0.45 |
| Trial 2 | Female | 56 | Right leg | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Trial 3 | Male | 58 | Left leg | 2 | 0 | 0 | 0 | 0 | 0.06 | 0.04 | 0 | 0 |
| Trial 4 | Female | 59 | Left arm | 9 | 183 | 180 | 155 | 180 | 0.85 | 0.58 | 0.96 | 1.4 |
| Trial 5 | Female | 28 | Pelvis | 1 | 0 | 3 | 10 | 5 | 0.12 | 0 | 0.25 | 0.50 |
| Trial 6 | Male | 65 | Left arm | 2 | 0 | 0 | 0 | 0 | 0 | 0.04 | 0 | 0 |
| Trial 7 | Female | 52 | Right arm | 5 | 0 | 0 | 0 | 0 | 0.52 | 0.63 | 0.58 | 0.57 |
| Trial 8 | Male | 60 | Right leg | 1 | 0 | 2 | 2 | 0 | 0.04 | 0.88 | 0.87 | 0.69 |
| Summary (mean ± SD) | 49 ± 13 | 4.3 ± 2.9 | 16 ± 51 | 18 ± 48 | 29 ± 75 | 32 ± 67 | 0.13 ± 0.40 | 0.20 ± 0.53 | 0.16 ± 0.47 | 0.15 ± 0.44 | ||
Mean daily opioid dose (MME) = mean daily opioid dose purchased in morphine milligram equivalent (MME) during a 1-yr periods related to the SCS implantation date; mean daily neuropathic medication dose (DDD) = mean daily neuropathic medication purchased in defined daily dose (DDD) during a 1-yr periods related to the SCS implantation date; permanent = SCS implanted and in use throughout the follow-up period; explanted = SCS implanted but explanted before the end of the follow-up; trial = SCS trial only.
TABLE 4.
The Risk of Strong Opioid and Neuropathic Medication Use for the Explanted Group and the Trial Group Compared With the Permanent Group
| Permanent (n = 19) | Explanted (n = 8) | Trial only (n = 8) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | MME (mean ± SD) | n (%) | MME (mean ± SD) | OR (95% CI) | P c | n (%) | MME (mean ± SD) | OR (95% CI) | P c | |
| Strong opioid use before SCS (18-0 mo)a | 4 (21) | 53 ± 150 | 2 (25) | 27 ± 72 | 1.3 (0.18-8.7) | .82 | 3 (38) | 71 ± 200 | 2.3 (0.37-14) | .38 |
| Strong opioid use after SCS (6-24 mo)b | 8 (42) | 120 ± 240 | 5 (63) | 57 ± 66 | 2.3 (0.42-13) | .34 | 3 (38) | 66 ± 180 | 0.83 (0.15-4.5) | .82 |
| DDD (mean ± SD) | DDD (mean ± SD) | DDD (mean ± SD) | ||||||||
| Neuropathic medication use before SCS (18-0 mo)a | 16 (84) | 0.52 ± 0.57 | 6 (75) | 1.4 ± 0.90 | 0.6 (0.07-4.2) | .58 | 7 (88) | 0.73 ± 0.61 | 1.3 (0.12-15) | .83 |
| Neuropathic medication use after SCS (6-24 mo)b | 10 (53) | 0.82 ± 0.48 | 5 (63) | 1.2 ± 1.2 | 1.5 (0.28-8.1) | .64 | 5 (63) | 1.5 ± 0.68 | 1.5 (0.28-8.1) | .64 |
MME = mean daily opioid dose purchased in morphine milligram equivalent during an 18-mo period related to the SCS implantation date; DDD = mean daily neuropathic medication purchased in defined daily dose during an 18-mo period related to the SCS implantation date.
aAt least one purchase during the 18-mo period before SCS implantation.
bAt least one purchase during the 18-mo period starting at the 6-mo washout period after SCS implantation.
cP-values were calculated using Fischer's exact test.
Neuropathic Medication Use
Obtained data 24 mo before and after SCS shows purchased DDDs of all 35 patients and were divided into 1-yr periods. During the 4-yr period, neuropathic pain medication was purchased at least once by 7 (88%) of the 8 patients in the trial-only group, 6 (75%) of the 8 patients in the explanted group, and 18 (95%) of the 19 patients in the permanent group (Table 3). There were no significant differences in neuropathic medication use between the groups before or after SCS (Table 4).
Complications and Revisions
From trial period to the end of follow-up, 30 revisions were made in 17 (63%) out of the 27 patients with permanently implanted SCS device. Of the revisions, 3 (10%) were because of complications (2 IPG repositions because of local pain and 1 deep electrode infection), 9 (30%) were because of hardware malfunction (6 electrodes; 2 extension cables; 1 IPG), and 6 (20%) were because of electrode migration. Eight IPGs were replaced because of battery depletion. In 2 patients, the SCS devices were replaced with newer models, and electrodes were repositioned because of stimulation to the wrong area (Table 5).
TABLE 5.
All Complications and Revisions From Trial Period to the End of Follow-up of the 27 Patients With Permanently Implanted SCS Device
| Revisions and complications during SCS | |||
|---|---|---|---|
| SCS with | |||
| Type of revision/complication | Surgical paddle lead n = 17 | Percutaneous lead n = 10 | Total quantity |
| Deep infection (electrode) | 0 | 1 | 1 |
| Electrode repositioned because of | |||
| Migration | 1 | 2 | 3 |
| Stimulation to wrong area | 2 | 0 | 2 |
| Electrode replaced because of | |||
| Hardware malfunction | 5 | 1 | 6 |
| Migration | 1 | 2 | 3 |
| Extensions replaced because of hardware malfunction | 2 | 0 | 2 |
| IPG repositioned because of local pain | 1 | 1 | 2 |
| IPG replaced because of | |||
| End of the lifespan | 5 | 3 | 8 |
| Hardware malfunction | 1 | 0 | 1 |
| SCS device replaced | 0 | 2 | 2 |
| Total | 18 | 12 | 30 |
SCS = spinal cord stimulation. IPG = internal pulse generator.
DISCUSSION
This collaborative retrospective study of 35 CRPS patients shows the long-term outcome of SCS by measuring the success of the trial period, explantation rate, revision rate, use of opioids, and neuropathic pain medication. Despite the fact that patients were not able to discontinue or reduce their strong opioid or neuropathic pain medication use, 70% continued to use their SCS device during a mean 6-yr follow-up. The analysis consisted of an 18-yr period from 1998 to 2016, with a median follow-up time of 8 yr, and was based on medical records and national registry data.
Short-term success in SCS is often measured by the results of the trial period. Here, 77% of the patients experienced adequate pain relief during the trial and received IPG. This trial success rate is in line with previous studies.30,32,37,38 Still, no factors were found that could predict the success of the trial.
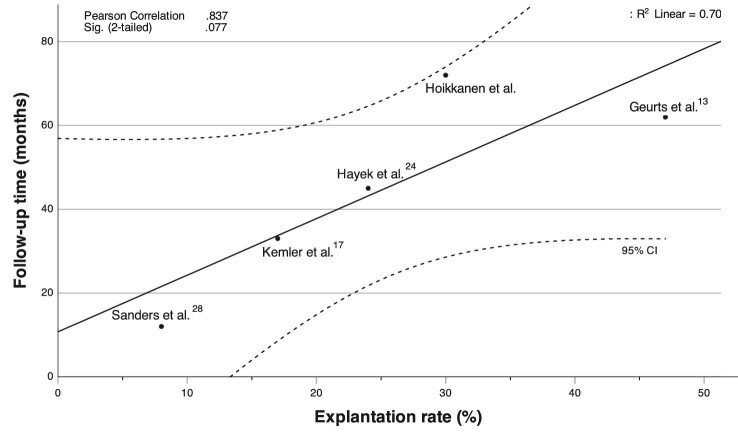
Long-term success in SCS is often evaluated by the explantation rate. Permanent SCS devices were removed from 30% of the patients who had had a successful trial period. The main reason for explantation was inefficient pain relief. This explantation rate was slightly higher compared with a recent study with failed back surgery syndrome patients. Among the studies containing CRPS patients, the result was relatively good with respect to the long follow-up time (Figure 3).17,24,28,33 As in previous reports, no factors predicting SCS device explantation were found.
FIGURE 3.
Comparison of explantation rates and follow-up times in studies of SCS in treatment of CRPS. Follow-up time was reported as median in Geurts33 and mean in other studies.17,24,28 Line of best fit was calculated with the least squares method and the 95% confidence intervals are presented as dotted lines.
In contrast to previous studies, opioid use remained the same or increased in different groups.28,39,40 No statistically significant differences occurred in the use of neuropathic medication, but generally, there was a downward trend. The corresponding results have been reported previously.39
We observed no differences between surgically or percutaneously implanted electrodes. In earlier studies, pain reduction in CRPS was not dependent on the type of waveform or frequency of stimulation.41 During the study period, in our hospitals, tonic stimulation was still in use for most patients and new modalities, including burst and high-frequency stimulation, were rarely used.
According to previous studies, SCS is a cost-effective treatment for chronic neuropathic pain patients despite high initial costs. Already after the first year of implantation, total costs were lower in group with SCS and conventional medical management (CMM) combined compared to patients refractory to CMM.42,43 However, longer observational periods than 12 to 24 mo used in most studies should be considered because loss of efficiency and explantation rates increase over time (Figure 3).
Complication and revision rates in earlier studies vary between 24% and 64%, and our study makes no exception with combined 63% revision and complication rate. Distinctly, higher revisions rates can be explained by longer follow-up time and consequent IPG depletion. Questionnaires show that culmination point in pain relief is achieved by 6 to 12 mo after SCS. Beyond that, effect of SCS diminishes over time. Together with increasing explantation rates by time, it is reasonable to say that 12- to 24-mo follow-up time is too short to evaluate long-term effect of SCS. In contrast to many previous reports, opioid consumption did not decrease in consequence of SCS in our patients. With our long follow-up time and accurate medication data, we provide additional information in the field (Table 1).
With short- and long-term success combined, 19 (54%) of the patients benefited from SCS. Generally, SCS is often the only choice left in CRPS patients who are still suffering from severe pain and for whom conventional treatments have failed.
Limitations of the Study
Because the retrospective nature of the current study, limitations are present. The neurosurgeons, medication, and criteria for permanent SCS have changed and vary between centers/care units. No information was available or used in the form of questionnaires about patient satisfaction, pain relief, or quality of life. In our study, we regard CRPS as a single disease entity instead of considering it as a pathophysiologically divergent syndrome that might respond in various ways to SCS treatment.
Strength of the Study
Only CRPS patients were included instead of mixing different pain etiologies. The follow-up time was long (up to 18 yr), and the size of the cohort was large (35 patients). The study was based on data from an extensive national prospectively collected registry database in Finland's public health care system. We had a complete day-to-day prescription drug purchasing history for every SCS patient. SCS is covered by the SII of Finland, and no selection based on the economic situation of each patient can be expected. There were no patients lost to follow-up because there were no deaths during the study period and in Finland, all contacts to health providers, including SCS revisions and explanations, are recorded in national registries and our database.
CONCLUSION
The current study involved an extremely long follow-up period with accurate follow-up data. Trial success and explantation rate in CRPS were comparable to other neuropathic pain indications. In contrast, CRPS patients were not able to discontinue or reduce their strong opioid use. Patient selection should be improved by developing novel predictive biomarkers.
Funding
This study is supported by State Research Funding of Kuopio University Hospital.
Disclosures
Dr Nissen has received travel funding from Medtronic and Abbott St Jude Medical. Ms Ikäheimo, Dr Huttunen, Dr Jyrkkänen, and Dr von und zu Fraunberg have received travel funding from Medtronic and Abbott St Jude Medical. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Dr Nissen has received funding from Finnish Association for the Study of Pain and travel funding from Boston Scientific.
Contributor Information
Tomas Hoikkanen, Neurosurgery of KUH NeuroCenter, Kuopio University Hospital, Kuopio, Finland.
Mette Nissen, Neurosurgery of KUH NeuroCenter, Kuopio University Hospital, Kuopio, Finland; Faculty of Health Sciences, School of Medicine, Institute of Clinical Medicine, University of Eastern Finland, Kuopio, Finland.
Tiina-Mari Ikäheimo, Neurosurgery of KUH NeuroCenter, Kuopio University Hospital, Kuopio, Finland.
Henna-Kaisa Jyrkkänen, Neurosurgery of KUH NeuroCenter, Kuopio University Hospital, Kuopio, Finland.
Jukka Huttunen, Neurosurgery of KUH NeuroCenter, Kuopio University Hospital, Kuopio, Finland.
Mikael von und zu Fraunberg, Neurosurgery of KUH NeuroCenter, Kuopio University Hospital, Kuopio, Finland; Faculty of Health Sciences, School of Medicine, Institute of Clinical Medicine, University of Eastern Finland, Kuopio, Finland.
REFERENCES
- 1.Birklein F, Schlereth T.. Complex regional pain syndrome-significant progress in understanding. Pain. 2015;156 (suppl 1):S94-S103. [DOI] [PubMed] [Google Scholar]
- 2.de Mos M, de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BHC, Sturkenboom MCJM. The incidence of complex regional pain syndrome: a population-based study. Pain. 2007;129(1):12-20. [DOI] [PubMed] [Google Scholar]
- 3.Walker SM, Cousins MJ.. Complex regional pain syndromes: including “reflex sympathetic dystrophy” and “causalgia”. Anaesth Intensive Care. 1997;25(2):113-125. [DOI] [PubMed] [Google Scholar]
- 4.Stanton-Hicks MD, Burton AW, Bruehl SPet al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Pract. 2002;2(1):1-16. [DOI] [PubMed] [Google Scholar]
- 5.Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342(8878):1012-1016. [DOI] [PubMed] [Google Scholar]
- 6.Harden RN, Bruehl S, Perez RSGMet al. Validation of proposed diagnostic criteria (the “Budapest criteria”) for complex regional pain syndrome. Pain. 2010;150(2):268-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsamadicy AA, Yang S, Sergesketter ARet al. Prevalence and cost analysis of complex regional pain syndrome (CRPS): a role for neuromodulation. Neuromodulation. 2018;21(5):423-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper M, Clark V.. Neuroinflammation, neuroautoimmunity, and the co-morbidities of complex regional pain syndrome. J Neuroimmune Pharmacol. 2013;8(3):452-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Course of symptoms and quality of life measurement in complex regional pain syndrome: a pilot survey. J Pain Symptom Manage. 2000;20(4):286-292. [DOI] [PubMed] [Google Scholar]
- 10.Cossins L, Okell RW, Cameron H, Simpson B, Poole HM, Goebel A.. Treatment of complex regional pain syndrome in adults: a systematic review of randomized controlled trials published from June 2000 to February 2012. Eur J Pain. 2013;17(2):158-173. [DOI] [PubMed] [Google Scholar]
- 11.Chang C, McDonnell P, Gershwin ME.. Complex regional pain syndrome—false hopes and miscommunications. Autoimmun Rev. 2019;18(3):270-278. [DOI] [PubMed] [Google Scholar]
- 12.Grigoras CA, Karanika S, Velmahos Eet al. Correlation of opioid mortality with prescriptions and social determinants: a cross-sectional study of Medicare enrollees. Drugs. 2018;78(1):111-121. [DOI] [PubMed] [Google Scholar]
- 13.Kemler MA, Furnee CA.. Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology. 2002;59(8):1203-1209. [DOI] [PubMed] [Google Scholar]
- 14.Calvillo O, Racz G, Didie J, Smith K.. Neuroaugmentation in the treatment of complex regional pain syndrome of the upper extremity. Acta Orthop Belg. 2002;68:127-133. [PubMed] [Google Scholar]
- 15.Oakley JC, Weiner RL. Spinal cord stimulation for complex regional pain syndrome: a prospective study of 19 patients at two centers. Neuromodulation. 1999;2(1):47-50. [DOI] [PubMed] [Google Scholar]
- 16.Bennett DS, Alo KM, Oakley J, Feler CA.. Spinal cord stimulation for complex regional pain syndrome I [RSD]: a retrospective multicenter experience from 1995 to 1998 of 101 patients. Neuromodulation. 1999;2(3):202-210. [DOI] [PubMed] [Google Scholar]
- 17.Kemler MA, Barendse GA, Van Kleef M, Van Den Wildenberg F A, Weber WE.. Electrical spinal cord stimulation in reflex sympathetic dystrophy: retrospective analysis of 23 patients. J Neurosurg. 1999;90(1 suppl):79-83. [DOI] [PubMed] [Google Scholar]
- 18.Forouzanfar T, Kemler MA, Weber WE, Kessels AG, van Kleef M.. Spinal cord stimulation in complex regional pain syndrome: cervical and lumbar devices are comparably effective. Br J Anaesth. 2004;92(3):348-353. [DOI] [PubMed] [Google Scholar]
- 19.Kumar K, Hunter G, Demeria D.. Spinal cord stimulation in treatment of chronic benign pain: challenges in treatment planning and present status, a 22-year experience. Neurosurgery. 2006;58(3):481-496. [DOI] [PubMed] [Google Scholar]
- 20.Kemler MA, Barendse GA, van Kleef Met al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000;343(9):618-624. [DOI] [PubMed] [Google Scholar]
- 21.Kemler MA, De Vet HC, Barendse GA, Van Den Wildenberg FA, Van Kleef M.. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years' follow-up of the randomized controlled trial. Ann Neurol. 2004;55(1):13-18. [DOI] [PubMed] [Google Scholar]
- 22.Reig E, Abejon D.. Spinal cord stimulation: a 20-year retrospective analysis in 260 patients. Neuromodulation. 2009;12(3):232-239. [DOI] [PubMed] [Google Scholar]
- 23.Chivukula S, Tempel ZJ, Weiner GMet al. Cervical and cervicomedullary spinal cord stimulation for chronic pain: efficacy and outcomes. Clin Neurol Neurosurg. 2014;127:33-41. [DOI] [PubMed] [Google Scholar]
- 24.Hayek SM, Veizi E, Hanes M.. Treatment-limiting complications of percutaneous spinal cord stimulator implants: a review of eight years of experience from an academic center database. Neuromodulation. 2015;18(7):603-609. [DOI] [PubMed] [Google Scholar]
- 25.Risson EG, Serpa AP, Berger JJ, Koerbel RFH, Koerbel A.. Spinal cord stimulation in the treatment of complex regional pain syndrome type 1: is trial truly required? Clin Neurol Neurosurg. 2018;171:156-162. [DOI] [PubMed] [Google Scholar]
- 26.Mekhail N, Costandi S, Mehanny DSet al. The impact of tobacco smoking on spinal cord stimulation effectiveness in complex regional pain syndrome patients. Neuromodulation. 2020;23(1):133-139. [DOI] [PubMed] [Google Scholar]
- 27.Harke H, Gretenkort P, Ladleif HU, Rahman S.. Spinal cord stimulation in sympathetically maintained complex regional pain syndrome type I with severe disability. A prospective clinical study. Eur J Pain. 2005;9(4):363-363. [DOI] [PubMed] [Google Scholar]
- 28.Sanders RA, Moeschler SM, Gazelka HMet al. Patient outcomes and spinal cord stimulation: a retrospective case series evaluating patient satisfaction, pain scores, and opioid requirements. Pain Pract. 2016;16(7):899-904. [DOI] [PubMed] [Google Scholar]
- 29.Kumar K, Rizvi S, Bnurs SB.. Spinal cord stimulation is effective in management of complex regional pain syndrome I: fact or fiction. Neurosurgery. 2011;69(3):566-580. [DOI] [PubMed] [Google Scholar]
- 30.Mekhail NA, Mathews M, Nageeb F, Guirguis M, Mekhail MN, Cheng J.. Retrospective review of 707 cases of spinal cord stimulation: indications and complications. Pain practice. 2011;11(2):148-153. [DOI] [PubMed] [Google Scholar]
- 31.Levy RM, Mekhail N, Kramer Jet al. Therapy habituation at 12 months: spinal cord stimulation versus dorsal root ganglion stimulation for complex regional pain syndrome type I and II. J Pain. 2020;21(3-4):399-408. [DOI] [PubMed] [Google Scholar]
- 32.Kemler MA, de Vet HC, Barendse GA, van den Wildenberg FA, van Kleef M.. Effect of spinal cord stimulation for chronic complex regional pain syndrome type I: five-year final follow-up of patients in a randomized controlled trial. J Neurosurg. 2008;108(2):292-298. [DOI] [PubMed] [Google Scholar]
- 33.Geurts JW, Smits H, Kemler MA, Brunner F, Kessels AG, van Kleef M.. Spinal cord stimulation for complex regional pain syndrome type I: a prospective cohort study with long-term follow-up. Neuromodulation. 2013;16(6):523-529; discussion 529. [DOI] [PubMed] [Google Scholar]
- 34.Kemler MA, Raphael JH, Bentley A, Taylor RS. The cost-effectiveness of spinal cord stimulation for complex regional pain syndrome. Value Health. 2010;13(6):735-742. [DOI] [PubMed] [Google Scholar]
- 35.O’Connell NE, Wand BM, McAuley J, Marston L, Moseley GL.. Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database Syst Rev. 2013;2013(4):CD009416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verrills P, Sinclair C, Barnard A.. A review of spinal cord stimulation systems for chronic pain. J Pain Res. 2016;9:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nissen M, Ikäheimo T, Huttunen J, Leinonen V, von und zu Fraunberg Mikael.. Long-term outcome of spinal cord stimulation in failed back surgery syndrome: 20 years of experience with 224 consecutive patients. Neurosurgery. 2019;84(5):1011-1018. [DOI] [PubMed] [Google Scholar]
- 38.Kumar K, Nath RK, Toth C.. Spinal cord stimulation is effective in the management of reflex sympathetic dystrophy. Neurosurgery. 1997;40(3):503-509. [DOI] [PubMed] [Google Scholar]
- 39.Gopal H, Fitzgerald J, McCrory C.. Spinal cord stimulation for FBSS and CRPS: a review of 80 cases with on-table trial of stimulation. J Back Musculoskelet Rehabil. 2016;29(1):7-13. [DOI] [PubMed] [Google Scholar]
- 40.Maher DP, Martins YC, Doshi Tet al. Neuropathic pain medication use does not alter outcomes of spinal cord stimulation for lower extremity pain. Neuromodulation. 2018;21(1):106-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kriek N, Groeneweg JG, Stronks DL, de Ridder D, Huygen FJ.. Preferred frequencies and waveforms for spinal cord stimulation in patients with complex regional pain syndrome: a multicentre, double-blind, randomized and placebo-controlled crossover trial. Eur J Pain. 2017;21(3):507-519. [DOI] [PubMed] [Google Scholar]
- 42.Zucco F, Ciampichini R, Lavano Aet al. Cost-effectiveness and cost-utility analysis of spinal cord stimulation in patients with failed back surgery syndrome: results from the PRECISE study. Neuromodulation. 2015;18(4):266-276; discussion 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farber SH, Han JL, Elsamadicy AAet al. Long-term cost utility of spinal cord stimulation in patients with failed back surgery syndrome. Pain Physician. 2017;20(6):E797-E805. [PMC free article] [PubMed] [Google Scholar]