Abstract
BACKGROUND
Accurate stereotactic biopsies of brain tumors are imperative for diagnosis and tailoring of the therapy. Repetitive needle insertions enhance risks of brain lesioning, hemorrhage, and complications due to prolonged procedure.
OBJECTIVE
To investigate clinical benefits of a combined 5-aminolaevulinic acid (5-ALA) fluorescence and laser Doppler flowmetry system for the detection of malignant brain tumor and blood vessels in stereotactic biopsies.
METHODS
Planning of targets and trajectories was followed by optical measurements in 20 patients, using the Leksell Stereotactic System and a manual insertion device. Fluorescence spectra, microvascular blood flow, and tissue grayness were recorded each millimeter along the paths. Biopsies were taken at preplanned positions. The diagnoses were compared with the fluorescence signals. The recordings were plotted against measurement positions and compared. Sites indicating a risk of hemorrhage were counted as well as the time for the procedures.
RESULTS
Signals were recorded along 28 trajectories, and 78 biopsies were collected. The final diagnosis showed 17 glioblastomas, 2 lymphomas, and 1 astrocytoma grade III. Fluorescence was seen along 23 of the paths with 4 having the peak of 5-ALA fluorescence 3 mm or more from the precalculated target. There was increased microcirculation in 40 of 905 measured positions. The measurement time for each trajectory was 5 to 10 min.
CONCLUSION
The probe provided direct feedback of increased blood flow along the trajectory and of malignant tissue in the vicinity of the target. The method can increase the precision and the safety of the biopsy procedure and reduce time.
Keywords: Biopsy, Optical, Tumor, Fluorescence, Spectroscopy, Stereotactic navigation, 5-ALA
ABBREVIATIONS
- 5-ALA
5-aminolaevulinic acid
- D
diagnostic
- HGT
high-grade tumor
- LDF
laser Doppler flowmetry
- LSS
Leksell Stereotactic System
- ND
nondiagnostic
- Neg
negative
- NM
not measurable
- OR
operating room
- Pos
positive
- PpIX
protoporphyrin IX
- TC
tumor cell
- TLI
total light intensity
Accurate stereotactic biopsies of brain tumors are imperative for diagnosis and tailoring of the therapy.1 Despite accurate planning, the intracranial conditions can change, eg, by brain shift, conceivably causing misdiagnosis. Diffuse limitations of the tumor do not always correspond to contrast enhancing magnetic resonance imaging (MRI).2 Neuronavigation systems3,4 can visualize the needle trajectory based on the preoperative MRI but cannot give direct feedback on the tissue at the target. These are typical reasons for repetitive needle insertions, enhancing risks of brain lesioning, hemorrhage,5 and complications due to prolonged procedure. There is therefore a need to improve the precision and reduce the risks of stereotactic biopsies.6-8
Experience in 5-aminolaevulinic acid (5-ALA) fluorescence guidance for brain tumor resection may be applied to biopsies. Hefti et al9 presented the use of fluorescence microscopy for direct investigation of tissue samples in the operating room (OR). Widhalm and colleagues10 applied the blue-light neurosurgical microscope to examine stereotactically acquired tissue samples. Fluorescence microscopy demonstrated potential clinical benefits by the reduced number of biopsy samples and shorter surgical time. The grading was done visually, however, thus subjectively. Also, it was done extracranially, as with all current methods. The concept of utilizing 5-ALA fluorescence for biopsies is, however, not entirely new. A fluorescence system with dual laser sources and an optical fiber was evaluated at Linköping University Hospital during stereotactic biopsies in the beginning of the millennium.11 The idea was reinvented as a hand-held probe for fluorescence recording during resection12 and further modified for stereotactic systems.13 Optical fibers were added to also include microcirculation recordings by laser Doppler flowmetry (LDF).14 In contrast to other techniques,15-18 LDF presents blood flow as real-time curves, a method originally designed for stereotactic deep brain stimulation (DBS) implantations.19,20 These combined features enable the forward-looking probe to act as a “vessel alarm” along the trajectory of the needle and at the same time as a tumor detector.
The aim of the study was to investigate the workability and the safety of the use of the combined 5-ALA fluorescence and LDF probe for possible clinical benefits in patients undergoing stereotactic brain tumor biopsies. Emphasis was set on efficacy of real-time malignant tissue identification and safety by detecting increased blood flow along the trajectory and overall efficiency of the intervention.
METHODS
Patients
Twenty consecutive patients with a suspected brain tumor, not eligible for resection, were referred for biopsy and included in the study. The study was approved by the local ethics board (M 129-07, 2015/138-32), and all patients gave informed written consent. The patients were given 5-ALA orally (20 mg/kg, Gliolan®, Medac GmbH, Wedel, Germany)21 2 to 3 h prior to surgery.
Imaging and Trajectory Planning
Stereotactic T1-weighted MRI (Ingenia 3T, Philips Healthcare, Eindhoven, The Netherlands) and/or computed tomography (GE Lightspeed Ultra, GE Healthcare, Amersham, United Kingdom) with contrast medium were performed on the day of surgery. Up to 3 trajectories were planned with Surgiplan® (Elekta Instrument AB, Stockholm, Sweden) in each case. Biopsy positions were defined at –6 or –5 mm, –3 mm, 0 (target), and +3 and +5 or +6 mm. The biopsy procedure and anesthesia were conducted according to the clinical routine with the addition that the optical probe was used to create the trajectory for the biopsy needle.
Optical Systems and Probe
A forward-looking optical probe combining 5-ALA-induced fluorescence and LDF measurements was used. The probe is compatible with the Leksell Stereotactic System (LSS, G-frame, Elekta Instrument AB, Stockholm, Sweden) at a length of 190 mm and a diameter of 2.2 mm. Blue laser light (wavelength 405 nm) interacts with the tissue and emits a spectrum with autofluorescence, and in the presence of protoporphyrin IX (PpIX), a peak at wavelength 635 nm (red color).12,22 The LDF records the tissue's microcirculation (perfusion, range 0-1000 arbitrary units, a.u.), and the gray-white variations (total light intensity [TLI] range 0-10 a.u.).14 Prior to sterilization with Sterrad®, the probe was tested in a standard microsphere solution (PF1001 Motility, Perimed AB, Järfälla, Sweden). This ascertained comparable LDF signal levels.13
Stereotactic Biopsy Procedure Using Optical Guidance
To create the trajectory for the biopsy needle and to detect increased blood flow indicative of vessels across the passage and thus to ultimately detect tumor tissue, the probe was conveyed forward from the cerebral cortex toward the planned target. A hand-driven insertion device positioned on the LSS ensured the controlled movement of the probe in 1-mm steps.14 This method was used in 18 of the surgeries. Frameless navigation (Stealth, S8, Medtronic Inc, Minneapolis, Minnesota) was implemented in combination with the LSS in 1 procedure and as a stand-alone tool in 2 procedures. At each tissue position, perfusion, and TLI were recorded over 5 to 10 s. This was followed by 3 fluorescence spectra (3 s). Immediate feedback on PpIX fluorescence, perfusion, and TLI was displayed on a monitor in the OR. In case of elevated perfusion (>100 a.u.),14,15 the measurement was extended.
When the planned positions were reached and the measurements finalized, the probe was removed and replaced with the side-cutting biopsy needle (diameter 2.1 mm, Backlund Catheter Insertion Needle Kit, Elekta Instruments AB, Stockholm, Sweden). Tissue samples were harvested clockwise in 4 quadrants. According to the clinical routine, the samples were sent for immediate smear-based section examination by a neuropathologist. The remaining samples were marked and used for detailed postoperative analysis.
Data Analysis and Statistics
The definitive diagnosis of the biopsies was based on the WHO grading,23 approximately 2 wk after surgery by a neuropathologist. All biopsies were re-examined by a second, senior neuropathologist (M.H.). The pathological examination is described in earlier publications.12,13 Using the LDF movement artifacts between positions as landmarks, the average perfusion, TLI, and PpIX fluorescence were calculated postoperatively for each position. The ratio between PpIX at 635 nm and autofluorescence maximum in the same spectra was calculated according to previous definitions.22 The number of elevated perfusion values was based on defined intervals (A-peak: 101-250, B-peak 251-500, C-peak > 00 a.u.).14 C-peaks indicate the highest risk of hemorrhaging.
Curves representing the PpIX fluorescence ratio, perfusion, and TLI were plotted against measurement positions along the trajectories. The total trajectory length and length to the first PpIX peak were measured. Times for the measurements, surgery, and the waiting for the pathological investigation were summarized.
RESULTS
A total of 14 males and 6 females, with median age 64 (range 41-84 yr), were included in the study. The combined probe was successfully implemented during all stereotactic brain biopsies. Fluorescence spectra and LDF signals were recorded from 808 and 905 sites, respectively, along 28 trajectories. Biopsies were taken and analyzed using frozen sectioning during the operation. Additional biopsies were collected from a total of 78 sites. PpIX fluorescence was available along 23/28 trajectories. In 2 trajectories, the signal was blocked by blood at the probe tip, and along 3 trajectories, PpIX fluorescence was absent (Table).
TABLE.
Fluorescence Response Along the Trajectory Paths, the Intraoperative Pathology Diagnosis, and the Final Diagnosis
| Patient no. | No. of paths | Fluorescence along paths | Intraop diagnosis | Final diagnosis | 0 mm fluorescence | 0 mm diagnosis |
|---|---|---|---|---|---|---|
| 1 | 1 (+1) | Pos | ND | Glioblastoma | Pos | HGT |
| 2 | 2 | Pos | – | Glioblastoma | Pos | HGT |
| Pos | D | Neg | Gliosis + TC | |||
| 3 | 1 | Pos | D | Glioblastoma | Pos | HGT |
| 4 | 1 | Pos | D | Glioblastoma | Pos | Gliosis + TC |
| 5 | 1 | Pos | D | Glioblastoma | Pos | HGT |
| 6 | 2 | Neg | – | Glioblastoma | Neg | |
| Pos | D | Pos | HGT | |||
| 7 | 1 | Pos | D | Glioblastoma | Pos | HGT |
| 8 | 2 | Pos | ND | Glioblastoma | Neg | HGT |
| Pos | – | Pos | HGT | |||
| 9 | 3 | NM | D | Lymphoma | No signal | HGT |
| Pos | – | Neg | HGT | |||
| Pos | – | Pos | HGT | |||
| 10 | 1 | Pos | ND | Glioblastoma | Pos | HGT |
| 11 | 1 | Pos | D | Glioblastoma | Neg | Necrosis |
| 12 | 1 | Pos | D | Lymphoma | Pos | Gliosis + TC |
| 13 | 1 | Pos | D | Glioblastoma | Pos | HGT |
| 14 | 1 | Pos | D | Glioblastoma | Pos | HGT |
| 15 | 2 | NM | D | Glioblastoma | No signal | HGT |
| Pos | – | Pos | HGT | |||
| 16 | 1 | Pos | D | Glioblastoma | Pos | HGT |
| 17 | 1 | Pos | D | Glioblastoma | Pos | HGT |
| 18 | 3 | Neg | – | Glioblastoma | Neg | – |
| Neg | – | Neg | – | |||
| Pos | D | Pos | HGT | |||
| 19 | 1 | Pos | D | Astrocytoma | Pos | HGT |
| 20 | 1 | Pos | D | Glioblastoma | Pos | HGT |
ND: nondiagnostic; D: diagnostic; Pos: positive; Neg: negative; TC: tumor cell; HGT: high-grade tumor; NM: not measurable. Dash (–) is assigned to trajectories where no biopsies were taken.
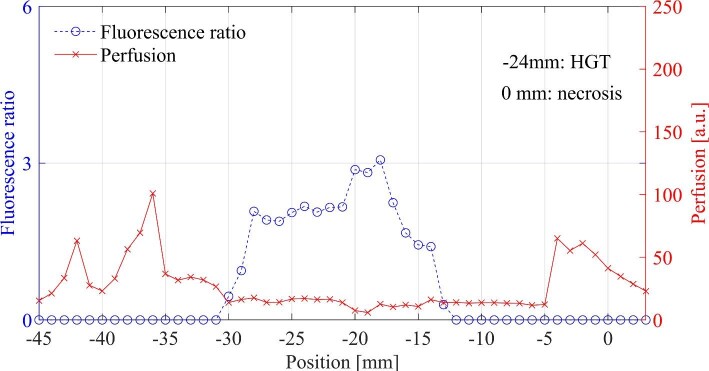
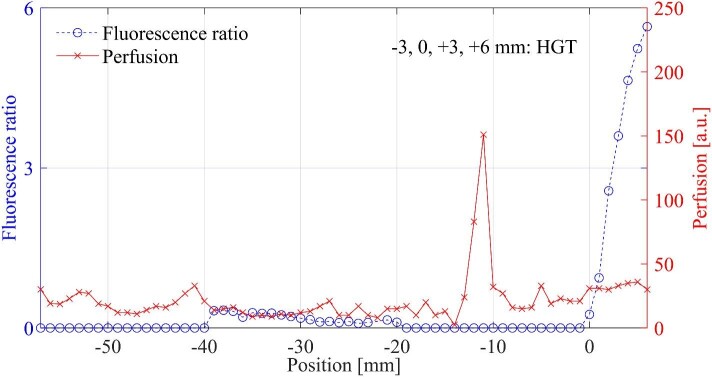
The fluorescence signature usually started with a small peak, and increased when reaching and passing through the tumor, corresponding to the contrast enhancement in the MRI. A decrease of the peaks beyond the tumor could be observed in a few cases. A schematic setting and typical recordings from various locations are presented in Figure 1. The PpIX signals are plotted vs positions along the trajectory and compared to the MRI. In 4/23 trajectories, no PpIX was measured at the precalculated target. In 3 of these trajectories, PpIX fluorescence appeared within 3 mm from the target, and in 1 case, the fluorescence was visible already in the interval –30 to –13 mm (Figure 2) ahead of the intended target. Corresponding MRI with trajectories is presented in Figure 3. In another patient, weak PpIX fluorescence was visible –40 to –20 mm before the precalculated target and strong PpIX fluorescence appeared 0 to +5 mm beyond (Figures 4 and 5). In this patient, increased blood flow (A-peak) was found –11 to –12 mm from the target.
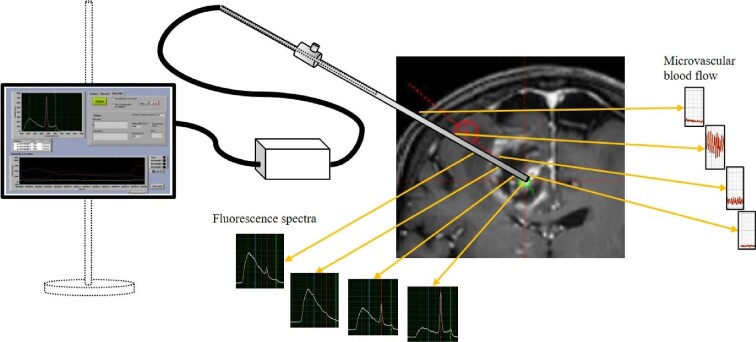
FIGURE 1.
Schematic setup of a measurement procedure. The microvascular blood flow was measured along the trajectory (right) from the cortical insertion to the target point inside the tumor. The fluorescence signals increase from the marginal zone (left) to the contrast enhancing parts of the tumor, and they are low in the necrotic parts.
FIGURE 2.
Fluorescence ratio and perfusion, in which PpIX was detected with strong intensity –13 to –30 mm earlier than the planned target. The histopathological examination of selected biopsy points is noted in the graphs.
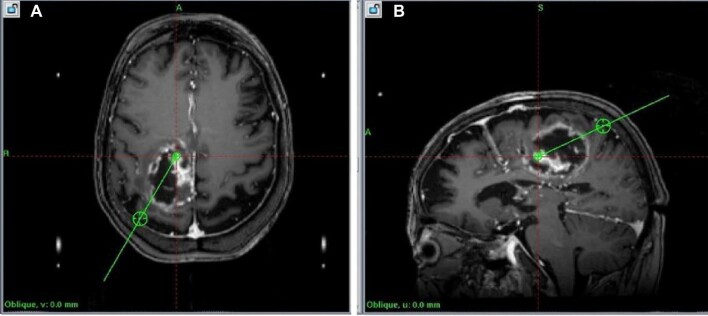
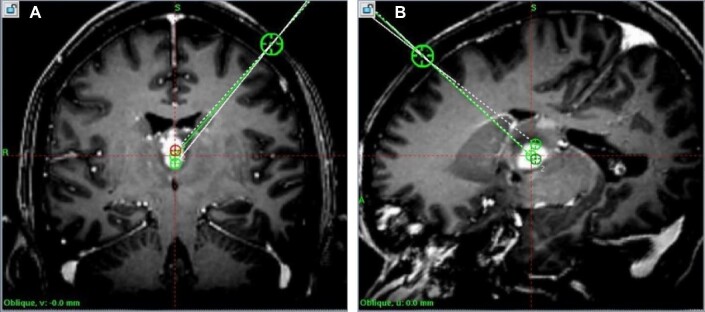
FIGURE 3.
A, Planned trajectories, axial view, corresponding to Figure 2. B, Planned trajectories, sagittal view, corresponding to Figure 2.
FIGURE 4.
Fluorescence ratio and perfusion (blood flow), in which PpIX was detected with a weak intensity –40 to –20 mm. A perfusion A-peak was present –11 to –12 mm from the precalculated biopsy point. The histopathologic examination of selected biopsy points is noted in the graphs.
FIGURE 5.
A, Planned trajectories, coronal view, corresponding to Figure 4. B, Planned trajectories, sagittal view, corresponding to Figure 4.
The intraoperative pathology diagnosis was reported within 48 ± 14 min (range 18-90 min, n = 18) from the time of biopsy sampling, including transport and pathology examination. The duration for the pathology examination was 29 ± 10 min (range 15-53 min). For comparison, the optical measurement time varied between 5 and 10 min and depended on the length of the trajectory. The overall diagnostic yield for the intraoperative samples using frozen sectioning, without the aid of fluorescence measurements, was 85% (17 out of 20 patients). In comparison, PpIX fluorescence could be measured along at least 1 trajectory in all cases (Table). The final diagnoses were glioblastoma (n = 17), lymphomas (n = 2), and astrocytoma grade III (n = 1).
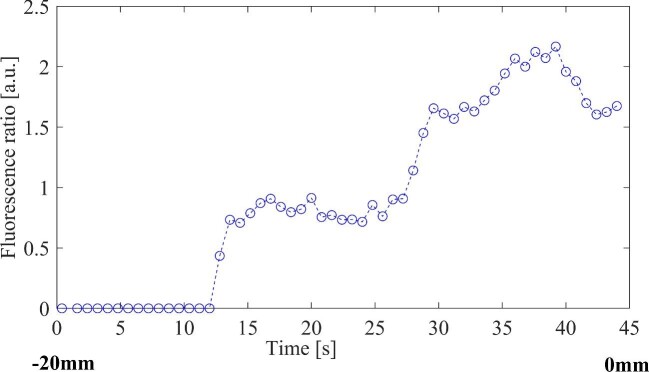
The setup with both the LSS and the Stealth system is presented in Figure 6. The PpIX peak is presented on the monitor in the background. One of these measurements was performed continuously and without stopping at each millimeter. PpIX ratio values are plotted against the measurement positions and visualized in Figure 7.
FIGURE 6.
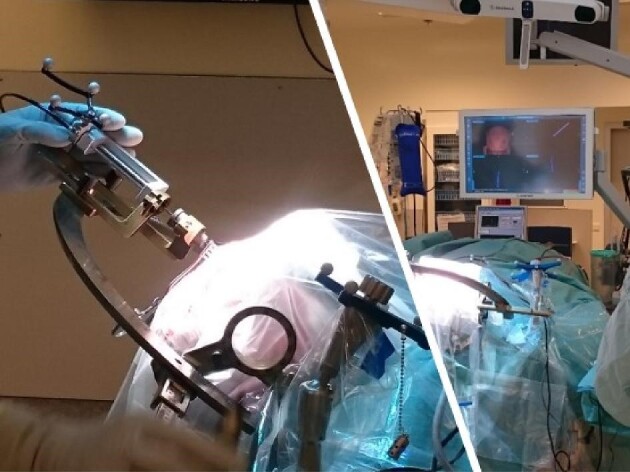
The Leksell Stereotactic System and the mechanical insertion device used together with the Stealth Navigation System.
FIGURE 7.
Example of PpIX peaks from a continuous measurement, in which the mechanical insertion device was moved without stopping. Biopsy from ±0 mm showed a mixture of HGT and gliosis and the total measurement time was 45 s. HGT, high-grade tumor.
No hemorrhage was detected with LDF along any of the measured trajectories, although in one case, a bleeding was detected inside the tumor necrosis. In general, the blood flow was low along the trajectories. There were 34 A-peaks, 5 B-peaks, and 1 C-peak in 905 measurements. The TLI signals were below the measurable perfusion threshold in 73 sites. In Figure 4, an example of an A-peak is presented along the trajectory at –11 to –12 mm.
DISCUSSION
A forward-looking probe for 5-ALA fluorescence and blood flow detection during stereotactic biopsy was evaluated. The probe gave direct feedback of a malignant tumor and increased blood flow, as a warning of blood vessel presence. Both features may help increase the accuracy of tissue sampling as well as reduce the risks associated with repeated insertion of the biopsy needle.
By combining the LSS system and the mechanical insertion device,14 it was possible to compare the fluorescence spectra, blood flow, and gray-white reflectance with millimeter precision to the biopsy samples. For all surgeries, high PpIX fluorescence was visible along at least one of the planned trajectories. Not all samples taken at the planned target point were diagnostic, but where the fluorescence peak was high, the samples were diagnostic. This suggests that fluorescence may be used to increase the diagnostic yield of stereotactic biopsies.
In the first measurement session, clear PpIX peaks were found along the first trajectory. The first report on the intraoperative histopathology could not confirm the hypothesized diagnosis. For that reason, a second trajectory became necessary, however, without access to the optical equipment. The final histopathology diagnosis confirmed the initial measurement of PpIX fluorescence as a high-grade tumor (Table, case 1). In the following biopsies, optical measurements were made for all trajectories. In another case, a discrepant finding in comparison to the contrast enhancing volume on the MRI was seen. Distinct fluorescence peaks were found along the trajectory before the planned main target point was reached (at –30 to –13 mm; Figures 2 and 3A and 3B). Biopsies at these levels confirmed high-grade tumor both with the intraoperative and postoperative histology. Despite the small number of patients, the findings demonstrate potential clinical benefit by reducing the number of trajectories and biopsies. In addition to the 18 high-grade gliomas, 2 of the patients with fluorescence peaks were diagnosed with lymphoma. Previous investigations with the blue-light microscope have confirmed the applicability for lymphoma in 5-ALA fluorescence guidance.10,24,25
When the system was used with frameless navigation, the probe could be calibrated and used in a similar fashion as previously described with ultrasound navigation.26 Data were collected in real time in a similar way as with the LSS. However, it was difficult to link the exact measurement position along the trajectory without the mechanical drive. The use of the LSS together with the mechanical insertion device made it possible to compare optical data with the histopathological results of the biopsies with a precision of 1 to 2 mm. This procedure, however, is only necessary when investigating the correlation between different modalities in order to demonstrate the accuracy of this novel concept. In clinical practice, it can be omitted. For optimal use with frameless navigation, a future redesign of the probe is necessary. This may be preferable, as modern frameless navigation systems allow for swifter handling in the OR with sufficient accuracy, without the inconveniences associated with the application of the conventional stereotactic frames. However, the conventional stereotactic systems are still widespread in use. The new design of the probe will be compatible with these systems as well.
In stereotactic brain biopsies, every new trajectory poses a separate risk of lesioning cerebral tissue or causing hemorrhage. Bleeding caused by the actual tissue collection, due to pathological vessel structures in the tumorous tissue, is thus not always possible to avoid. Some localizations do present higher risks of lesioning vessels, eg, around the insula or the thalamus. If possible, these risks are minimized by meticulous preoperative planning, where a trajectory is directed to avoid radiologically visible blood vessels on T1 MRI with contrast media. Nonetheless, the smallest vessels do not always appear on MRI. Also, brain shift may put the most thorough planning of the trajectory at risk. To detect vessels along the trajectory, a forward-looking device is necessary. The LDF component provides this function, as has been documented in more than 130 DBS implantations.14,15,20
The current probe design does not allow for simultaneous measurement at the exact position of the biopsy as suggested by other groups.16,19 Considering that the biopsy region already has been monitored with the forward-looking probe when passing the intended biopsy point by 2 to 6 mm, potential high blood flow spots would have been found with this approach, and the biopsy samples are taken at the highest fluorescence peak. A probe with side-looking windows has previously been presented by us27 in which LDF was combined with a lesioning and impedance measurement. That design is complex to implement in the clinical setting, and it does not provide the safety aspects as intended by our forward-looking probe. Inside the tumor, pathological vessels may still cause hemorrhages, as their structures and patterns are unphysiological and ubiquitous, but these bleeds are usually minor; thus, the effects are not comparable to a lesion of a branch of cerebral arteries. In total, 4.4% of all LDF recorded locations showed increased blood flow. None were considered as high risk. This corresponds to data found during DBS surgery.14,15 Trajectories along high-risk biopsy regions such as the insula would probably increase the number of high blood flow indications from the LDF system.
The total time for controlled optical recording with the LSS and the mechanical device is dependent on the trajectory length. To capture a reliable LDF signal representing perfusion without interference from movement, the probe must be kept still. This is an inherent drawback with LDF, as the actual movements of the red blood cells in the microvasculature generate the Doppler shift, which is further processed to the LDF signal. Because of a thorough fixation of the probe to the LSS by the mechanical insertion device, no interference from external movements appears during a tissue recording. This only appears when the probe is moved from one site to the next. A typical recording time is 5 to 10 s when held still at the actual site. If the signal is high, the measurement can be prolonged in order to ascertain that there is no vasomotion or a rapid increase or decrease of the blood flow, both of which could indicate a vessel structure. In a clinical routine implementation, the measurement time for a full-length trajectory of 50 to 60 mm can be reduced to approximately 5 min.
We anticipate that fluorescence-guided biopsies may reduce the number of trajectories required to indicate the optimal biopsy site. In addition, the method may minimize the overall time for surgery because no waiting time for the preliminary histopathological diagnosis is necessary. Further studies are required to extend the findings of the current study.
CONCLUSION
A system for direct optical brain tumor detection and warning for possible blood vessels along the trajectory has been evaluated in 20 patients undergoing stereotactic biopsy. When fully deployed in clinical routine, the real-time feedback in the OR is expected to shorten the time for the procedure and increase the precision of the actual biopsy sites. This should improve diagnostic yield and reduce the risk of bleeding complications.
Funding
The study was financially supported by the LiU Cancer Strategic Research Grant, The Swedish Childhood Cancer Foundation (grant no. MT 2013-0043), ALF Grants Region Östergötland (grant no. LIO-599651), and Swedish Foundation for Strategic Research (RMX18-0056).
Disclosures
Drs Richter, Haj-Hosseini, and Wårdell are inventors of a related patent. The other authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article. Drs Richter and Wårdell have shares in FluoLink AB, Linköping, Sweden.
Acknowledgments
The authors are grateful to the staff at the Department of Neurosurgery and the Department of Pathology at Linköping University Hospital for clinical measurements and tissue preparation.
Contributor Information
Johan Richter, Department of Biomedical Engineering, Linköping University, Linköping, Sweden; Department of Neurosurgery, Linköping University, Linköping, Sweden; Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden.
Neda Haj-Hosseini, Department of Biomedical Engineering, Linköping University, Linköping, Sweden.
Peter Milos, Department of Neurosurgery, Linköping University, Linköping, Sweden; Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden.
Martin Hallbeck, Department of Biomedical and Clinical Sciences, Linköping University, Linköping, Sweden; Department of Clinical Pathology, Linköping University, Linköping, Sweden.
Karin Wårdell, Department of Biomedical Engineering, Linköping University, Linköping, Sweden.
REFERENCES
- 1. Sciortino T, Fernandes B, Conti Nibali Met al. Frameless stereotactic biopsy for precision neurosurgery: diagnostic value, safety, and accuracy. Acta Neurochir. 2019;161(5):967-974. [DOI] [PubMed] [Google Scholar]
- 2. Blystad I, Warntjes JBM, Smedby O, Lundberg P, Larsson EM, Tisell A. Quantitative MRI for analysis of peritumoral edema in malignant gliomas. PLoS One. 2017;12(5):e0177135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gempt J, Buchmann N, Ryang YMet al. Frameless image-guided stereotaxy with real-time visual feedback for brain biopsy. Acta Neurochir. 2012;154(9):1663-1667. [DOI] [PubMed] [Google Scholar]
- 4. AEbelo AM, Noer VR, Schulz MK, Kristensen BW, Pedersen CB, Poulsen FR. Frameless stereotactic neuronavigated biopsy: a retrospective study of morbidity, diagnostic yield, and the potential of fluorescence: a single-center clinical investigation. Clin Neurol Neurosurg. 2019;181:28-32. [DOI] [PubMed] [Google Scholar]
- 5. Kongkham PN, Knifed E, Tamber MS, Bernstein M. Complications in 622 cases of frame-based stereotactic biopsy, a decreasing procedure. Can J Neurol Sci. 2008;35(1):79-84. [DOI] [PubMed] [Google Scholar]
- 6. Akshulakov SK, Kerimbayev TT, Biryuchkov MY, Urunbayev YA, Farhadi DS, Byvaltsev VA. Current trends for improving safety of stereotactic brain biopsies: advanced optical methods for vessel avoidance and tumor detection. Front Oncol. 2019;9:947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waters JD, Gonda DD, Reddy H, Kasper EM, Warnke PC, Chen CC. Diagnostic yield of stereotactic needle-biopsies of sub-cubic centimeter intracranial lesions. Surg Neurol Int. 2013;4(Suppl 3):S176-S181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bonnan M. Towards real-time optical brain biopsies? Clin Neurol Neurosurg. 2015;134:4-6. [DOI] [PubMed] [Google Scholar]
- 9. Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H. 5-Aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institution. Swiss Med Wkly. 2008;138(11-12):180-185. [DOI] [PubMed] [Google Scholar]
- 10. Widhalm G, Minchev G, Woehrer Aet al. Strong 5-aminolevulinic acid-induced fluorescence is a novel intraoperative marker for representative tissue samples in stereotactic brain tumor biopsies. Neurosurg Rev. 2012;35(3):381-391; discussion 391. [DOI] [PubMed] [Google Scholar]
- 11. Backlund EO, Pålsson S, Sturnegk P, Eriksson O, Wårdell K, Andersson-Engels S. Exploration of tissue fluorescence in primary brain tumors during stereotactic biopsy—a pilot study. Paper presented at:European Society for Stereotactic and Functional Neurosurgery; October 9-12, 2002; Toulouse, France. [Google Scholar]
- 12. Richter JCO, Haj-Hosseini N, Hallbeck M, Wårdell K. Combination of hand-held probe and microscopy for fluorescence guided surgery in the brain tumor marginal zone. Photodiagn Photodyn Ther. 2017;18:185-192. [DOI] [PubMed] [Google Scholar]
- 13. Haj-Hosseini N, Richter J, Milos P, Hallbeck M, Wårdell K. 5-ALA fluorescence and laser Doppler flowmetry for guidance in a stereotactic brain tumor biopsy. Biomedical Optics Express. 2018;9(5):2284-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wårdell K, Zsigmond P, Richter J, Hemm S.. Relationship between laser Doppler signals and anatomy during deep brain stimulation electrode implantation toward the ventral intermediate nucleus and subthalamic nucleus. Neurosurgery. 2013;72(2 Suppl Operative):ons127-ons140. [DOI] [PubMed] [Google Scholar]
- 15. Picot F, Goyette A, Obaid Set al. Interstitial imaging with multiple diffusive reflectance spectroscopy projections for in vivo blood vessels detection during brain needle biopsy procedures. Neurophotonics. 2019;6(2):025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thien A, Rao JP, Ng WH, King NK.. The Fluoropen: a simple low-cost device to detect intraoperative fluorescein fluorescence in stereotactic needle biopsy of brain tumors. Acta Neurochir. 2017;159(2):371-375. [DOI] [PubMed] [Google Scholar]
- 17. Markwardt NA, Stepp H, Franz Get al. Remission spectrometry for blood vessel detection during stereotactic biopsy of brain tumors. J Biophotonics. 2017;10(8):1080-1094. [DOI] [PubMed] [Google Scholar]
- 18. Ramakonar H, Quirk BC, Kirk RWet al. Intraoperative detection of blood vessels with an imaging needle during neurosurgery in humans. Sci Adv. 2018;4(12):eaav4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zsigmond P, Hemm-Ode S, Wårdell K. Optical measurements during deep brain stimulation lead implantation: safety aspects. Stereotact Funct Neurosurg. 2017;95(6):392-399. [DOI] [PubMed] [Google Scholar]
- 20. Wårdell K, Blomstedt P, Richter Jet al. Intracerebral microvascular measurements during deep brain stimulation implantation using laser Doppler perfusion monitoring. Stereotact Funct Neurosurg. 2007;85(6):279-286. [DOI] [PubMed] [Google Scholar]
- 21. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392-401. [DOI] [PubMed] [Google Scholar]
- 22. Haj-Hosseini N, Richter J, Andersson-Engels S, Wårdell K. Optical touch pointer for fluorescence guided glioblastoma resection using 5-aminolevulinic acid. Lasers Surg Med. 2010;42(1):9-14. [DOI] [PubMed] [Google Scholar]
- 23. Louis DN, Perry A, Reifenberger Get al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803-820. [DOI] [PubMed] [Google Scholar]
- 24. Kiesel B, Millesi M, Woehrer Aet al. 5-ALA-induced fluorescence as a marker for diagnostic tissue in stereotactic biopsies of intracranial lymphomas: experience in 41 patients. Neurosurg Focus. 2018;44(6):E7. [DOI] [PubMed] [Google Scholar]
- 25. Millesi M, Kiesel B, Wohrer Aet al. Is intraoperative pathology needed if 5-aminolevulinic-acid-induced tissue fluorescence is found in stereotactic brain tumor biopsy? Neurosurgery. 2020;86(3):366-373. [DOI] [PubMed] [Google Scholar]
- 26. Richter JC, Haj-Hosseini N, Andersson-Engel S, Wårdell K. Fluorescence spectroscopy measurements in ultrasonic navigated resection of malignant brain tumors. Lasers Surg Med. 2011;43(1):8-14. [DOI] [PubMed] [Google Scholar]
- 27. Antonsson J, Eriksson O, Lundberg P, Wårdell K. Optical measurements during experimental stereotactic radiofrequency lesioning. Stereotact Funct Neurosurg. 2006;84(2-3):118-124. [DOI] [PubMed] [Google Scholar]