Abstract
BACKGROUND
Core Outcome Sets (COSs) are necessary to standardize reporting in research studies. This is urgently required in the field of chronic subdural hematoma (CSDH), one of the most common disease entities managed in neurosurgery and the topic of several recent trials. To complement the development of a COS, a standardized definition and baseline Data Elements (DEs) to be collected in CSDH patients, would further improve study quality and comparability in this heterogeneous population.
OBJECTIVE
To, first, define a standardized COS for reporting in all future CSDH studies; and, second, to identify a unified CSDH Definition and set of DEs for reporting in future CSDH studies.
METHODS
The overall study design includes a Delphi survey process among 150 respondents from 2 main stakeholder groups: healthcare professionals or researchers (HCPRs) and Patients or carers. HCPR, patients and carers will all be invited to complete the survey on the COS, only the HCPR survey will include questions on definition and DE.
EXPECTED OUTCOMES
It is expected that the COS, definition, and DE will be developed through this Delphi survey and that these can be applied in future CSDH studies. This is necessary to help align future research studies on CSDH and to understand the effects of different treatments on patient function and recovery.
DISCUSSION
This Delphi survey should result in consensus on a COS and a standardized CSDH Definition and DEs to be used in future CSDH studies.
Keywords: Chronic subdural hematoma, Core Outcomes and Data Elements, Delphi
ABBREVIATIONS
- CODEs
Core Outcomes and Data Elements
- COS
Core Outcome Set
- COS-STAD
Core Outcome Set-Standards for Development
- CSDH
chronic subdural hematoma
- DEs
Data Elements
- HCPRs
healthcare professionals or researchers
GENERAL INFORMATION
Protocol title: Defining Core Outcomes and Data Elements (CODEs) in chronic subdural hematoma (CSDH), CODE-CSDH, 2021.
This protocol was approved by the University Hospitals Plymouth NHS Trust, Derriford Hospital, Plymouth, PL6 8DH, UK (Reference CA_2020-21-303) and registered at clinicaltrials.gov on April 14, 2021 with Identifier NCT04850612. This study is supported by The Netherlands Organisation for Health Research and Development (ZonMw project number 843002824, 2017), Postbus 93 245, 2509 AE The Hague, The Netherlands.
RATIONALE AND BACAKGROUND INFORMATION
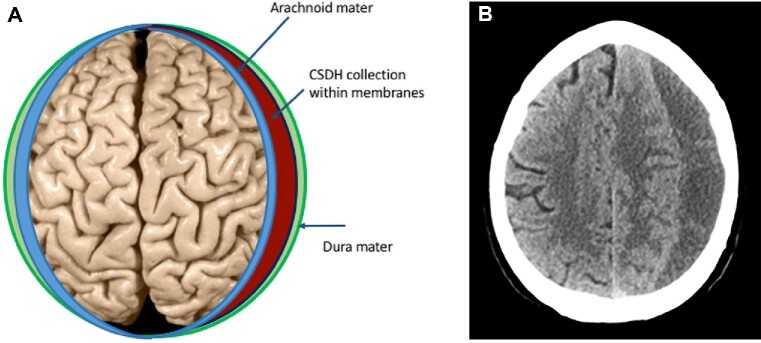
CSDH is a collection of blood and fluid surrounded by membranes that accumulates on the brain surface over weeks to months (Figure 1A). It predominately affects older people and often has a delayed association with a preceding head trauma. Patients commonly present with symptoms such as confusion, unbalanced walking, limb weakness, and headaches. This might differentially lead to suspicion of stroke, although the onset is more gradual, and computed tomography (CT) imaging of the head is diagnostic (Figure 1B).
FIGURE 1.
A, Anatomic location of CSDH: graphic highlighting layers overlying the brain involved in CSDH. B, Anatomic location of CSDH: corresponding CT image of CSDH showing area of low density (dark gray) on the right side of the image.
The incidence of CSDH has been increasing rapidly from 1.7 to 31 per 100 000 persons per year up to 2000, to around 20 to 80 per 100 000 persons per year from 2000 to 2019.1-4 The projected increase in incidence implies that CSDH may become the most common neurosurgical condition treated in adults.5 The mainstay of treatment is surgical drainage of the collection and patients spend an average of 7 d in the neurosurgery unit.6
There has been significant growth in the number of CSDH publications in recent years mirroring progress in potential management options and outcomes.7 Interest in alternatives to surgery including drug therapies and radiological occlusion of blood vessels is growing, but the efficacy of these alternatives is yet to be established.8-11 Currently, there is wide variation in how and when patient outcome is measured among studies,12 making it difficult to conclusively compare between studies or generalize the data to a wider population. Much could be gained by agreeing a minimum set of standardized outcomes that should be measured and reported in all CSDH studies, known as a Core Outcome Set (COS).13,14 A COS will help align future research studies and is necessary to understand the effect of different treatments on patient function and recovery. To ensure study results are relevant, the COS needs to be informed by what is important to patients as well as to clinicians and researchers. In addition to outcomes, the type of patient included in CSDH studies and baseline data reported on them varies greatly.15 A standardized definition of CSDH and set of baseline Data Elements (DEs) would help clarify the populations being studied. This is important as outcomes in CSDH patients can be influenced by different patterns on imaging,16 operative techniques employed,17 and patient baseline characteristics.18
An established process for development of a COS and how this should be reported is already defined by the COS-STAP (Core Outcome Set-Standardized Protocol items) group and is adhered to in the design of this protocol.19 We have followed a design already reported by the CORMAC (Core Outcome Research Measures in Anal Cancer) and OMERACT (Outcome MEasures in Rheumatoid Arthritis Clinical Trials) groups, whereby a Delphi survey is used to gain agreement among healthcare professionals and patients on what is important when measuring outcome.20,21 This is described in further detail in the methodology section. To help overcome potential barriers of implementing a COS, we will seek to include international perspectives from healthcare professionals and researchers already involved in the field of CSDH such that the COS will be endorsed by those conducting the research.
STUDY GOALS AND OBJECTIVES
The scope of this study is determined as per the COS-Standards for Development (COS-STAD) recommendations.22 The COS is to be applied to any research studies or trials that include adult patients diagnosed with CSDH. The COS will cover all interventions including surgical, radiological, medical, and observation. The primary objective is to define a standardized COS for reporting in all future CSDH studies through a Delphi survey process including all relevant stakeholders. The secondary objective is to identify a unified CSDH definition and set of DEs for reporting in future CSDH studies, through a Delphi survey process including healthcare professional or researcher (HCPR) stakeholders.
STUDY DESIGN
The overall study design includes a Delphi survey process.23 This is a process whereby all outcomes and DEs from the CSDH literature and expert opinion are presented to a large group of patients, carers, and HCPR via a survey. Each survey participant has the opportunity to rank the elements and outcomes in levels of importance to them, and the survey is then repeated including presentation of the results from the first round, in order to attempt to gain agreement between participants on what is important. All elements are then reviewed at a final “consensus” meeting.
METHODOLOGY
Participant Eligibility for Delphi Survey
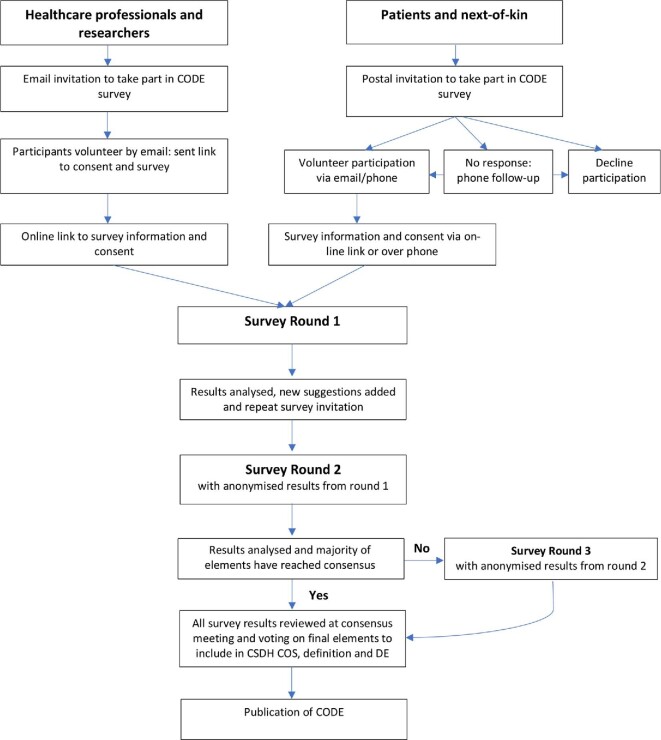
In line with COS-STAD, 2 main stakeholder groups will be included to complete the survey; HCPRs and Patients or carers. See Figure 2 for the study flow chart.
FIGURE 2.
Study flowchart.
HCPR will include those who conduct research in CSDH and those with experience in managing different aspects of patient care for CSDH, including but not limited to neurosurgeons, neurologists, anesthetists, neuroradiologists, elderly care physicians, neurorehabilitation specialists, general practitioners, CSDH researchers, and clinical nurse specialists.
Patients will be eligible for participation if they are adults (≥18 yr of age) and if they were admitted to a neurosurgical unit with a diagnosis of CSDH within the last 5 yr. In the cases where a patient lacks capacity to give consent, a carer will be invited to take part on their behalf. It is critical to include the carers, as a considerable number of patients with CSDH have cognitive deficits prior to surgery, which will preclude them from having capacity to take part. These patients have different outcomes and care needs, which are important to consider in COSs and, therefore, we are grateful to carers who can advocate for them.
Number of Participants
Previous COS development studies have reported 140 to 150 respondents.20,21 To reach a similar number of stakeholders, it is estimated that 50 to 60 neurosurgeons will participate, as this is the primary group involved in CSDH management. The remaining HCPR stakeholder groups should contribute a further 50 to 60 responses combined and patient/carer stakeholders approximately 20 to 30 responses. No new participants will be invited after completion of round 1 and it will be stressed that participation includes taking part in2 rounds with the aim to minimize attrition.
Participant Recruitment
To incorporate all the appropriate HCPRs, the main sources of invitation will include colleagues and research collaborators of the study investigators, Society of British Neurological Surgeons (SBNS), Dutch Subdural Hematoma Research (DSHR) group, Nederlandse Vereniging voor Neurochirurgie (NVvN), Canadian Neurosurgical Society—iCORIC (international COllaborative Research Initiative in CSDH), and a group of international clinical researchers with specific experience specific to CSDH. The survey will also be advertised on social media platforms accessed by relevant HCPR with contact details available to receive full information and access to the survey. Those who agree to take part will be asked to invite other relevant colleagues or societies thus increasing the uptake through the “snowball” effect.
Patients (or their carer), who were admitted with a diagnosis of CSDH in the participating neurosurgical unit (University Hospitals Plymouth NHS Trust) will be sent information by post to either decline or accept an interest in taking part. A follow-up phone call will take place from a trained clinical member of the study team if no response is received. Patients who indicate an interest in participation will receive further information via email, phone, or post depending on their preference.
Survey Development
This is a process of iterative surveys, with completion by all stakeholders over2 rounds. The online survey will be delivered using a data management open-source software program (LimeSurvey, version 2.06LTS) in both rounds.
For the development of this study, the investigators will review the data collected from three sources: (1) systematic reviews on CODEs,12,15 (2) data reported in recent systematic review of on-going CSDH trials,7 which enables inclusion of any more recent data that may not have been captured in the earlier published CODE reviews, and (3) expert opinion from the study investigators regarding any additional elements not already included.
For HCPR, these data will be amalgamated into 2 Delphi surveys: a COS survey and a definition and DEs survey. The COS-survey summarizes all potential outcomes in CSDH, which will be categorized into the following key domains: mortality, recurrence, complications, functional outcome, and radiological outcome. In addition to ranking the importance of each domain we will aim to identify key components of each domain (eg, measure of functional outcome) and the time points at which they should be reported. The definition and DE survey summarizes key elements relating to the definition of CSDH and baseline data on patients diagnosed with CSDH.
The patient/carer survey will be adapted to contain simplified language appropriate to a non-HCPR audience and will be reviewed by the patient representative. It will contain the same core outcomes as the HCPR survey, but may be worded differently and each term will be explained in full. The definition and DE survey will not be included for patients as this refers to the diagnosis and assessment of CSDH, which is only relevant to clinicians.
The surveys will only be available in the English language.
Survey Process
The target is for all participants to partake in both rounds of the survey. Each survey will be circulated with an email (or phone consultation) explaining its purpose and a reminder email will be sent to encourage a high response rate. At the start of each survey, HCPR participants will be asked to identify their specialty, their level of training, and the country they practice in. The latter to ensure we have good geographical spread of representation. Patients/carer will be asked to identify whether they are a patient or carer and their age. These basic demographics will maintain anonymity but enable us to understand the spread of people taking part.
In round 1, HCPR participants will be asked to rank all options in the survey on a 9-point Likert scale, from very unimportant (1) to very important (9). A score of 7 to 9 will be evaluated as being critically important and 1 to 3 as limited importance. Patients/carer participants will be asked to rank all options in the survey on a 5-point Likert scale, from very unimportant (1) to very important (5). A score of 4 to 5 will be evaluated as being critically important and 1 to 2 as limited importance.
In round 2, the anonymized summary results from round 1 will be presented, categorized by stakeholders (HCPR or patients/carers). Participants will be asked to rank the outcomes again with this knowledge in mind. All outcomes from round 1 will be included in round 2 and with any suggested new additions made in round 1.
The aim is to formulate a list of outcomes that the majority of all stakeholders agree are “critically important” for inclusion in the final COS. Agreement on a CSDH definition and DE will also be sought. If consensus cannot be reached in the majority of outcomes, then a third round may be conducted, repeating the process from round 2, before a final consensus meeting is held.
Consensus Meeting
After survey completion the study, investigators will review the results for inclusion in the final proposed COS, definition, and DE and assign each outcome to one of three categories:
An element will be included if 70% or more of respondents rate the element as critically important (7-9) AND 15% or fewer rate it as limited importance (1-3).
The element will be excluded if 70% or more respondents rate the element as limited important (1-3) AND 15% or fewer rate it as critically important (7-9).
When neither of the above criteria is met, this requires a more elaborate discussion at the consensus meeting.
These 3 categories will be discussed during the final (virtual) consensus meeting. In addition to the study investigators, a selection of iCORIC members from different international sites who have specialized experience in CSDH trials will be invited. The elements in category 1 and 2 will be presented first. If participants have a fundamental reason to include or exclude a certain element, this will be discussed. The attendees will take an electronic poll to confirm that >70% of those in attendance agree on inclusion or exclusion of the selected elements in the COS, definition, and DE. The elements from category 3 will be discussed and voted on, using the 9-point Likert scale. If consensus cannot be met by >70% of participants, these items will be reviewed together. Individual contrasting views will be evaluated and all participants will have an equal opportunity to explain their point of view before reiteration of the voting. Outcomes meeting the criteria for category 1 will then be included in the COS; all other items will be disregarded.
DISCUSSION
CSDH is increasing in incidence and a growing research focus. There is no international consensus on a standardized COS in CSDH, which is necessary to help align future research studies and understand the effect of new treatment strategies on patient function and recovery. This will be complemented by developing a clear definition of CSDH and a set of baseline DEs reportable in studies. It is expected that consensus on a CSDH COS, definition, and baseline data element set will be reached through this Delphi survey involving relevant patient, carer, and healthcare professional and researcher stakeholders.
TRIAL STATUS
This study commenced on May 3 2021. The first round of this Delphi process was completed on July 12 2021.
SAFETY CONSIDERATIONS
Due to the voluntary nature of the survey and ability to withdraw participation at any time point there are no perceived risks to participants.
FOLLOW-UP
Follow-up after completion of the Delphi survey is not applicable.
DATA MANAGEMENT AND STATISTICAL ANALYSIS
Descriptive statistics showing the distribution of responses from each round of the survey will be displayed in the subsequent round. Highlighting the dropout rate between rounds, with an aim to have a minimum of 70% of participants from round 1 taking part in round 2.
QUALITY ASSURANCE
All data will be stored securely and all individuals accessing any of the data will comply with the requirements of the Data Protection Act 2018 and the General Data Protection Regulation (GDPR) and its statements regarding the collection, storage, processing, and disclosure of any personal information.
EXPECTED OUTCOMES OF THE STUDY
It is expected that a COS, definition, and DE will be developed through this Delphi and that these will be used in future CSDH studies. This is necessary to help align future research studies on CSDH and to understand the effects of different treatments on patient function and recovery.
DURATION OF THE PROJECT
Rounds 1 and 2 are expected both to be completed within 6 wk. Evaluation of results after both rounds will demand 4 wk. The estimated total duration is a maximum of 6 mo.
PROJECT MANAGEMENT
The survey will be distributed among HCPR by authors DCH, CI-M, and EE. Patients/carers will be contacted by EE at the University Hospitals Plymouth NHS Trust.
ETHICS
Informed consent shall be requested through the first page of the online survey and for patients/carers a verbal or email consent will be recorded. The study protocol, consent forms, and survey were approved by University Hospitals Plymouth NHS trust and there were no ethical concerns.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
CODE-CSDH collaborators: Prof Louise Allan, Professor of Geriatric Medicine; Laura Burring, General Practitioner; Ian Fletcher, Patient Representative; Suzy Hope, Elderly Care Clinical Lecturer; Hester Lingsma, Associate Professor Medical Decision Making; Prof. Aad van der Lugt, Neuroradiologist; Harry Mee, Neurorehabilitation Specialist; Stephen Mullins, Clinical Lecturer in Neurology; Daniel Stubbs, Neuroanesthetic Trainee; Silvia Tarantino, Trauma Research Nurse Specialist; Dagmar Verbaan, Associate Professor Evidence-based Neurosurgery; and Jess Welbourne, Neuroanesthetic Consultant.
Contributor Information
Dana C Holl, Department of Neurosurgery, Erasmus Medical Centre, Erasmus MC Stroke Centre, Rotterdam, the Netherlands.
Aswin Chari, Developmental Neurosciences, Great Ormond Street Institute of Child Health, University College London, London, UK; Department of Neurosurgery, Great Ormond Street Hospital, London, UK.
Christian Iorio-Morin, Division of Neurosurgery, Université de Sherbrooke, Sherbrooke, Canada.
Ruben Dammers, Department of Neurosurgery, Erasmus Medical Centre, Erasmus MC Stroke Centre, Rotterdam, the Netherlands.
Niels A van der Gaag, University Neurosurgical Centre Holland (UNCH), Leiden University Medical Centre, Haaglanden Medical Centre, Haga Teaching Hospital, the Netherlands.
Angelos G Kolias, Division of Neurosurgery, Addenbrooke's Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; NIHR Global Health Research Group on Neurotrauma, University of Cambridge, Cambridge, UK.
Peter J Hutchinson, Division of Neurosurgery, Addenbrooke's Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK; NIHR Global Health Research Group on Neurotrauma, University of Cambridge, Cambridge, UK.
Ellie Edlmann, Department of Neurosurgery, South West Neurosurgical Centre, University Hospitals Plymouth NHS Trust, Plymouth, UK; Faculty of Health, Peninsula Medical School, University of Plymouth, Plymouth, UK.
REFERENCES
- 1.Foelholm R, Waltimo O. Epidemiology of chronic subdural haematoma. Acta Neurochir (Wien). 1975;32(3-4):247-250. [DOI] [PubMed] [Google Scholar]
- 2.Rauhala M, Luoto TM, Huhtala Het al. The incidence of chronic subdural hematomas from 1990 to 2015 in a defined Finnish population. J Neurosurg. 2019;132(4):1147-1157. [DOI] [PubMed] [Google Scholar]
- 3.Kudo H, Kuwamura K, Izawa I, Sawa H, Tamaki N. Chronic subdural hematoma in elderly people: present status on Awaji Island and epidemiological prospect. Neurol Med Chir (Tokyo). 1992;32(4):207-209. [DOI] [PubMed] [Google Scholar]
- 4.Balser D, Farooq S, Mehmood T, Reyes M, Samadani U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J Neurosurg. 2015;123(5):1209-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adhiyaman V, Chattopadhyay I, Irshad F, Curran D, Abraham S. Increasing incidence of chronic subdural haematoma in the elderly. QJM. 2017;110(6):375-378. [DOI] [PubMed] [Google Scholar]
- 6.Brennan PM, Kolias AG, Joannides AJet al. The management and outcome for patients with chronic subdural hematoma: a prospective, multicenter, observational cohort study in the United Kingdom. J Neurosurg. 2017;127(4):732-739. [DOI] [PubMed] [Google Scholar]
- 7.Edlmann E, Holl DC, Lingsma HFet al. Systematic review of current randomised control trials in chronic subdural haematoma and proposal for an international collaborative approach. Acta Neurochir (Wien). 2020;162(4):763-776. [DOI] [PubMed] [Google Scholar]
- 8.Kolias AG, Edlmann E, Thelin EPet al. Dexamethasone for adult patients with a symptomatic chronic subdural haematoma (Dex-CSDH) trial: study protocol for a randomised controlled trial. Trials. 2018;19(1):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miah IP, Holl DC, Peul WCet al. Dexamethasone therapy versus surgery for chronic subdural haematoma (DECSA trial): study protocol for a randomised controlled trial. Trials. 2018;19(1):575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iorio-Morin C, Blanchard J, Richer M, Mathieu D. Tranexamic Acid in Chronic Subdural Hematomas (TRACS): study protocol for a randomized controlled trial. Trials. 2016;17(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio-Morin C, Touchette C, Levesque M, Effendi K, Fortin D, Mathieu D. Chronic subdural hematoma: toward a new management paradigm for an increasingly complex population. J Neurotrauma. 2018;35(16):1882-1885. [DOI] [PubMed] [Google Scholar]
- 12.Chari A, Hocking KC, Broughton Eet al. Core outcomes and common data elements in chronic subdural hematoma: a systematic review of the literature focusing on reported outcomes. J Neurotrauma. 2016;33(13):1212-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson PR, Altman DG, Blazeby JMet al. Developing core outcome sets for clinical trials: issues to consider. Trials. 2012;13(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williamson PR, Altman DG, Blazeby JM, Clarke M, Gargon E. The COMET (Core Outcome Measures in Effectiveness Trials) initiative. Trials. 2011;12(S1):A70. [Google Scholar]
- 15.Chari A, Hocking KC, Edlmann Eet al. Core outcomes and common data elements in chronic subdural hematoma: a systematic review of the literature focusing on baseline and peri-operative care data elements. J Neurotrauma. 2016;33(17):1569-1575. [DOI] [PubMed] [Google Scholar]
- 16.Nakaguchi H, Tanishima T, Yoshimasu N. Factors in the natural history of chronic subdural hematomas that influence their postoperative recurrence. J Neurosurg. 2001;95(2):256-262. [DOI] [PubMed] [Google Scholar]
- 17.Santarius T, Kirkpatrick PJ, Ganesan Det al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009;374(9695):1067-1073. [DOI] [PubMed] [Google Scholar]
- 18.Christopher E, Poon MTC, Glancz LJet al. Outcomes following surgery in subgroups of comatose and very elderly patients with chronic subdural hematoma. Neurosurg Rev. 2019;42(2):427-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkham JJ, Gorst S, Altman DGet al. Core Outcome Set-STAndardised Protocol Items: the COS-STAP Statement. Trials. 2019;20(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fish R, Sanders C, Williamson PR, Renehan AG. Core Outcome Research Measures in Anal Cancer (CORMAC): protocol for systematic review, qualitative interviews and Delphi survey to develop a core outcome set in anal cancer. BMJ Open. 2017;7(11):e018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hatemi G, Meara A, Ozguler Yet al. Developing a core set of outcome measures for Behçet disease: report from OMERACT 2016. J Rheumatol. 2017;44(11):1750-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirkham JJ, Davis K, Altman DGet al. Core Outcome Set-STAndards for Development: the COS-STAD recommendations. PLoS Med. 2017;14(11):e1002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linstone HA, Turoff M. The Delphi Method. Addison-Wesley; 1975. [Google Scholar]