Abstract
BACKGROUND
Gross total resection (GTR) of contrast-enhancing tumor is associated with increased survival in primary glioblastoma. Recently, there has been increasing interest in performing supratotal resections (SpTRs) for glioblastoma.
OBJECTIVE
To address the published results, which have varied in part due to lack of consensus on the definition and appropriate use of SpTR.
METHODS
A crowdsourcing approach was used to survey 21 neurosurgical oncologists representing 14 health systems nationwide. Participants were presented with 11 definitions of SpTR and asked to rate the appropriateness of each definition. Participants reviewed T1-weighed postcontrast and fluid-attenuated inversion-recovery magnetic resonance imaging for 22 anatomically distinct glioblastomas. Participants were asked to assess the tumor location's eloquence, the perceived equipoise of enrolling patients in a randomized trial comparing gross total to SpTR, and their personal treatment plans.
RESULTS
Most neurosurgeons surveyed (n = 18, 85.7%) agree that GTR plus resection of some noncontrast enhancement is an appropriate definition for SpTR. Overall, moderate inter-rater agreement existed regarding eloquence, equipoise, and personal treatment plans. The 4 neurosurgeons who had performed >10 SpTRs for glioblastomas in the past year were more likely to recommend it as their treatment plan (P < .005). Cases were divided into 3 anatomically distinct groups based upon perceived eloquence. Anterior temporal and right frontal glioblastomas were considered the best randomization candidates.
CONCLUSION
We established a consensus definition for SpTR of glioblastoma and identified anatomically distinct locations deemed most amenable to SpTR. These results may be used to plan prospective trials investigating the potential clinical utility of SpTR for glioblastoma.
Keywords: Glioblastoma, Neuro-oncology, Supratotal resection, Crowdsourcing
ABBREVIATIONS
- 5-ALA
5-aminolevulinic acid
- AI
aggressiveness index
- GTR
gross total resection
- OS
overall survival
- RI
resectability index
- SpTR
supratotal resection
- STR
subtotal resection
Glioblastoma (GBM) is the most aggressive adult primary central nervous system malignancy.1,2 GBM is an invasive tumor that infiltrates surrounding brain parenchyma beyond contrast-enhancing regions on magnetic resonance imaging (MRI).2,3 This contributes to low survival rates; median survival in patients following standard of care is only 15 mo.2,4 Moreover, almost every GBM patient experiences disease progression ∼7 to 10 mo after initial treatment.5
Studies have established that gadolinium-enhancing tumor gross total resection (GTR) correlates with increased survival and decreased disease progression compared to subtotal resection (STR) or biopsy due to cytoreductive effects.6-9 Even with GTR, tumor recurrence is inevitable at/near the primary resection site.2,3,10,11
This finding, along with work in low-grade gliomas demonstrating a relationship between supratotal resection (SpTR) and longer overall survival (OS) without new, postoperative deficits, encouraged applying this technique to GBM.12,13-16 Systematic reviews demonstrate a positive correlation between SpTR and OS in GBM.12,17,18 However, authors observed heterogeneity between the results of these studies, attributable to differences in the definition and application of SpTR. This supports the establishment of a single definition of SpTR for GBM.
The use of SpTR has been hindered by concerns that it may impair function of adjacent tissue and cause new, postoperative deficits that lower quality of life (QOL) and decrease OS.19,20 These findings encourage neurosurgeons to balance benefits of extensive resection with deficits, and lead some to pursue SpTR for GBMs within relatively noneloquent areas (ie, right frontal or anterior temporal lobes).21,22 Because meta-analyses have not included anatomic location as a variable, effect of tumor location on OS after SpTR has not been established.
We used crowdsourcing, recruiting experienced neurosurgical oncologist to problem solve through collective intelligence, to establish a definition for SpTR in GBM by majority agreement, and to determine consensus on the appropriate clinical use of SpTR in GBM.23
METHODS
Survey Participants
A 133-question online survey (eAppendix) was sent to 34 well-established neurosurgical oncology faculty, selected in part based upon publication record on GBM surgical outcomes, practice in high-volume academic medical centers, and willingness to complete this survey.23 Demographics included years in practice, neuro-oncology fellowship training, adjunct methods routinely used during GBM surgery, and operative volume.
Definitions of GBM SpTR
A comprehensive literature search identified 11 unique definitions for SpTR of GBM (Supplement).18,24-34 Respondents were asked whether they agreed, disagreed, or strongly agreed/disagreed that each definition was appropriate to use in a potential future clinical trial assessing SpTR for GBM.
Radiographic Data
To investigate anatomic locations where SpTR was considered appropriate treatment for GBM, preoperative MRI data were sourced from 22 adult (≥18 yr) GBM patients (Supplement). This study was exempt from obtaining patient consent; all radiographic data were de-identified as required by HIPAA regulations and Institutional Review Board (IRB) protocol (IRB00196609). For each case, participants assessed eloquence of the tumor's location, perceived equipoise of enrollment in a randomized clinical trial comparing GTR to SpTR, and their personal surgical plan. Consensus on each case's eloquence, equipoise, or plan was defined as >70% agreement among experts.35
Software and Data Collection
The survey and responses were created, distributed, and stored using Qualtrics XM (Provo, Utah). We found no applicable reporting guidelines that would apply to this article. By following the EQUATOR reporting guidelines decision tree (http://www.equatornetwork.org/wp-content/uploads/2013/11/20160226-RG-decision-tree-for-Wizard-CC-BY-26-February-2016.pdf), we found that none of the most popular checklists are appropriate for our study design.
Statistical Analysis
Statistical analyses were performed with SPSS Statistics software 25 (IBM) and R version 4.0.1. P-values <.05 were considered statistically significant (Supplement). A “resectability index” (RI) quantified perceived level of resectability for each case; an “aggressiveness index” (AI) was calculated for each surgeon (Supplement).23
RESULTS
Definition of SpTR for GBM
Twenty-one neurosurgeons from 14 health systems completed this survey, representing 62% of the 34 invited to participate (Supplement). Most surveyed (n = 18, 85.7%) agree/strongly agree GTR plus resection of any noncontrast-enhanced disease is an appropriate definition for SpTR (Table 1). In bivariate unadjusted analysis, neurosurgeons in practice >10 yr were more likely to endorse this definition (n = 10, 100%) than counterparts (n = 8, 73%, P = .031). Resection 1 to 2 cm beyond contrast-enhancing disease was the second most endorsed definition with 14 (66.6%) neurosurgeons agreeing/strongly agreeing this was a fitting definition. Neurosurgeons performing >50 GBM SpTRs over their careers were more likely to agree/strongly agree this was a good definition (n = 7, 100%, P = .011) (Supplement).
TABLE 1.
Agreement and Disagreement Among 21 Neurosurgical Oncologists Regarding 11 Published Definitions of SpTR for GBM
| Definition | Disagree or strongly disagree, N (%) | Agree or strongly agree, N (%) |
|---|---|---|
| Extent of T2 FLAIR resection | ||
| Any decrease in post-op FLAIR volume30,31 | 12 (57.1) | 9 (42.9) |
| GTR + >25% of FLAIR abnormality region34 | 14 (66.7) | 7 (33.3) |
| GTR + >45-50% of FLAIR abnormality region25 | 16 (76.2) | 5 (23.8) |
| GTR + >54% of FLAIR abnormality region33 | 15 (71.4) | 6 (28.6) |
| GTR + >75% of FLAIR abnormality region26 | 14 (66.7) | 7 (33.3) |
| GTR + 100% of FLAIR abnormality region24,25 | 9 (42.8) | 12 (57.1) |
| Other extent of resection definitions | ||
| Any resection beyond GTR32 | 11 (52.4) | 10 (47.6) |
| GTR + resection of edematous tissue involved radiographically normal gyrus27 | 10 (47.6) | 11 (52.4) |
| GTR + any resection of noncontrast-enhanced disease33 | 3 (14.3) | 18 (85.7) |
| Resection 1 to 2 cm beyond contrast enhancement28,29 | 7 (33.3) | 14 (66.6) |
| GTR + resection of surrounding noneloquent, radiographically normal cortex and white matter26 | 11 (52.3) | 10 (47.6) |
Agreement Among Surgeons on Eloquence of GBM Location and Treatment Plan
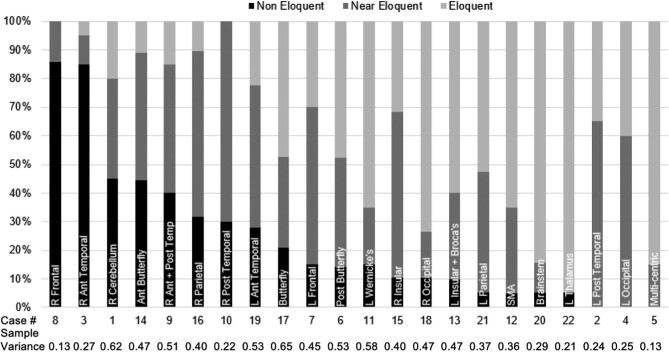
Consensus on noneloquence of GBM location was reached for tumors in the right frontal (85.7%) and right anterior temporal lobe (85.0%) (Figure 1). Sample variances were among the lowest of the 22 cases—0.13 and 0.27, respectively—indicating a high level of agreement between respondents (Supplement).
FIGURE 1.
The percentage of neurosurgical oncologists who rated each GBM’s location as noneloquent, near eloquent, or eloquent. R: right; L: left; Ant: anterior; Post: posterior.
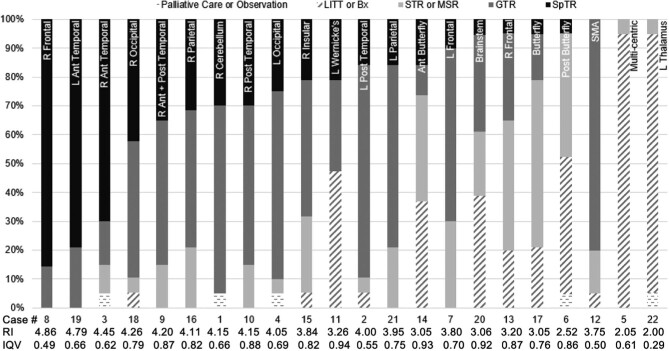
Figure 2 details neurosurgeons’ most likely treatment plan for each case. Right frontal and both anterior temporal lobes were considered most amenable to SpTR (Supplement).
FIGURE 2.
The percentage of neurosurgical oncologists who selected palliative care or observation; laser interstitial thermal therapy (LITT), stereotactic biopsy, or excisional biopsy (Bx); subtotal resection (STR) or maximal safe resection (MSR); gross total resection (GTR); or supratotal resection (SpTR) for each case. The resectability index (RI) and interquartile variance (IQV) for each case are listed below. A higher RI value represents a GBM that is considered on average to be amenable to more aggressive treatment, such as SpTR; a higher IQV represents less agreement between surgeons on proposed treatment plan. Differences between cases’ RIs are statistically significant as determined by ANOVA (P < .0005). R: right; L: left; Ant: anterior; Post: posterior.
Plans varied by individual surgeons’ tendencies, quantified using AI. Scores ranged from 3.05 to 4.34 (higher score indicating surgeon chose more aggressive surgical goal, like SpTR, over conservative strategy, like biopsy, with greater frequency relative to peers) (Figure 3). Differences in AI scores between surgeons were deemed statistically significant (P = .001, Supplement).
FIGURE 3.
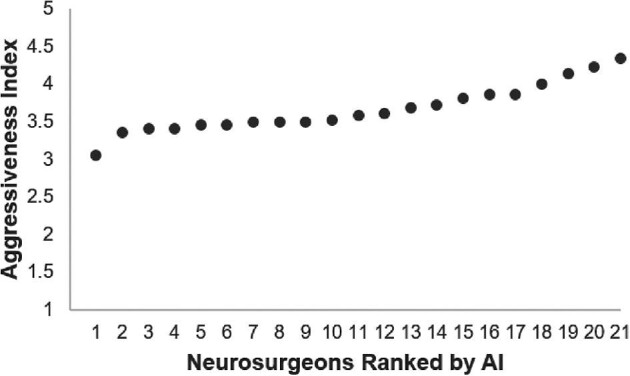
Scatter plot representing the aggressiveness index (AI) for each neurosurgeon. Possible scores range from 1 to 5, with a higher AI value signifying a neurosurgeon who has more aggressive surgical goals, as calculated by averaging the numerical value representing the selected treatment plans over the 22 sample GBM cases relative to peers. Differences between neurosurgeons’ AIs are statistically significant as determined by ANOVA (P = .001).
Neurosurgeons performing >10 SpTRs in the past year (n = 4) were more likely to recommend SpTR relative to peers in unadjusted bivariate analysis (n = 17, P < .005). This association was seen specifically for GBMs in the left posterior temporal (P = .004), left occipital (P = .001), left frontal (P = .032), right posterior temporal (P = .003), right parietal (P = .018), right occipital (P = .018), and left parietal lobes (P = .004).
Agreement Among Surgeons Regarding Randomizing to SpTR vs GTR
When asked whether cases could be reasonably randomized in a clinical trial, consensus was reached for GBMs located in right frontal, left anterior temporal, and right anterior temporal lobes (Table 2). Left posterior temporal, butterfly, multicentric, brainstem, SMA, left insular region extending into Broca's area, and left thalamic GBMs were felt to not be reasonable candidates for randomization by >70% of respondents. The most controversial cases, in which consensus was not reached and sample variance was greatest, included GBMs in the right occipital lobe (2.44) and a large lesion involving nearly the entirety of the right anterior and posterior temporal lobe (2.12).
TABLE 2.
A Crowdsourcing Approach Revealed Consensus Among Neurosurgeons Regarding the Equipoise of GTR or SpTR for Anatomically Distinct GBMs
| Case # | Tumor location | Probably or definitely no, N (%) | Maybe, N (%) | Probably or definitely yes, N (%) | Sample variance |
|---|---|---|---|---|---|
| Group A | |||||
| 8 | R Frontal | 2 (9.5) | 0 (0.0) | 19 (90.5) | 1.13 |
| 19 | L Ant Temporal | 3 (15.8) | 0 (0.0) | 16 (84.2) | 1.33 |
| 3 | R Ant Temporal | 4 (21.0) | 1 (5.3) | 14 (73.7) | 2.11 |
| Group B | |||||
| 1 | R Cerebellum | 7 (36.9) | 2 (10.5) | 10 (52.6) | 1.92 |
| 16 | R Parietal | 4 (21.0) | 5 (26.3) | 10 (52.6) | 1.59 |
| 18 | R Occipital | 9 (47.4) | 2 (10.5) | 8 (42.1) | 2.44 |
| 9 | R Ant + Post-temporal | 11 (55.0) | 2 (10.0) | 7 (35.0) | 2.12 |
| 10 | R Post-temporal | 9 (45.0) | 4 (20.0) | 7 (35.0) | 1.92 |
| 11 | L Wernicke's | 10 (50.0) | 3 (15.0) | 7 (35.0) | 1.91 |
| 4 | L Occipital | 10 (52.6) | 3 (15.8) | 6 (31.6) | 1.84 |
| 15 | R Insular | 12 (63.1) | 2 (10.5) | 5 (26.3) | 1.82 |
| 21 | L Parietal | 13 (68.4) | 2 (10.5) | 4 (21.1) | 1.54 |
| 7 | L Frontal | 13 (65.0) | 4 (20.0) | 3 (15.0) | 1.19 |
| Group C | |||||
| 2 | L Post-temporal | 15 (75.0) | 0 (0.0) | 5 (25.0) | 2.03 |
| 17 | Butterfly | 17 (89.5) | 0 (0.0) | 2 (10.5) | 1.25 |
| 14 | Anterior Butterfly | 16 (84.2) | 1 (5.3) | 2 (10.5) | 1.04 |
| 6 | Posterior Butterfly | 19 (90.5) | 0 (0.0) | 2 (9.5) | 1.16 |
| 5 | Multicentric | 18 (94.8) | 0 (0.0) | 1 (5.3) | 0.87 |
| 20 | Brainstem | 18 (94.8) | 0 (0.0) | 1 (5.3) | 0.58 |
| 12 | SMA | 16 (80.0) | 3 (15.0) | 1 (5.0) | 0.77 |
| 13 | L Insular + Broca's | 19 (95.0) | 0 (0.0) | 1 (5.0) | 0.88 |
| 22 | L Thalamus | 19 (100.0) | 0 (0.0) | 0 (0.0) | 0.05 |
Group A: >70% strong consensus to randomize, group B: no overwhelming consensus, and group C: >70% strong consensus to not randomize.
R: right; L: left; Ant: anterior; Post: posterior.
DISCUSSION
The results of this survey not only demonstrate the potential of crowdsourcing to address complex neurosurgical questions but also capture the current beliefs of 21 members of the neurosurgical oncologist community regarding the definition of SpTR and its potential use in GBM management. As quantified by the AI, neurosurgeons vary in the aggressiveness of their surgical goals, likely due in part to their experience with performing SpTRs for GBMs Nevertheless, we demonstrate that consensus can be reached in a large group of neurosurgical oncologists presented with a variety of GBMs in specific anatomic locations. These findings, build upon prior work in related fields, suggest that there may be utility in surveying groups of knowledgeable experts to help make critical decisions in the treatment of patients, especially when the optimal treatment is unknown.23,35
The abundance of definitions for SpTR has complicated research into its utility in GBM treatment.12,17 One recent systematic review by Jackson et al18 included 11 studies and 810 patients who underwent SpTR of their GBMs In their meta-analysis, they divided the studies into 3 subgroups based on the definition of SpTR that was used and then assessed OS after SpTR. The first group included studies in which SpTR was defined based on the amount of T2-fluid-attenuated inversion-recovery (FLAIR), enhancing tissue resected as determined on postoperative imaging. Studies in the second group defined SpTR as resection of 5-aminolevulinic acid (5-ALA) fluorescing tissue outside of contrast-enhancing regions on MRI. Studies in the third group, in which SpTR was defined as extended anatomic resection of the surrounding gyrus and normal white matter, demonstrated the greatest OS advantage after SpTR. These results encourage the selection of a single, anatomically based definition of SpTR.
The definition of SpTR that was endorsed as the most appropriate in our survey—“GTR plus any resection of noncontrast-enhanced disease”—is unique compared to the other choices because it specifically stipulates the resection of disease outside the contrast-enhanced region, instead of resection of FLAIR abnormality. This distinction is important because the T2 FLAIR region seen on imaging in GBM patients may not only represent nonenhancing tumor cells but also cerebral edema or demyelination.20,36 The preference of our survey respondents for a definition with this specificity alludes to the importance of being able to safely distinguish GBM cells outside traditionally contrast-enhanced regions intraoperatively. This goal may be achieved through the use of intraoperative adjuvants like 5-ALA fluorescence or intraoperative MRI to help maximize resection while maintaining surgical safety.37-39
A primary goal of this work was to provide a unifying definition of SpTR in GBM that could be used a priori to standardize clinical research involving SpTR; the most commonly endorsed definition does not satisfy this condition. Similarly, we recognize that definitions of SpTR dependent upon postoperative calculations of FLAIR extent of resection have provided interesting clinic insights in prior retrospective studies. Nevertheless, such calculation estimates are not easily performed a priori, and thus, they hold less clinical utility in the design of future prospective studies on SpTR.26 In contrast, two-thirds of the cohort and all of the surgeons who have performed more than 50 SpTRs over their careers agreed or strongly agreed that “resection 1 to 2 cm beyond contrast enhancement” was a suitable definition for SpTR of GBM. This more uniform definition provides the neurosurgeon a definitive a priori goal for extent of resection. In addition, it sets a quantifiable benchmark for what can and cannot be classified as SpTR and enables the neurosurgeon to determine when SpTR has been achieved during surgery using intraoperative navigation.
In addition, a number of studies have demonstrated that GBM recurs within 2 cm of the original contrast-enhancing tumor in 80% to 95% of recurrences.28,40-49 Choucair et al,40 for example, identified that of 405 patients with GBM, over 90% died from a recurrence around the site of the original tumor. He used these data to justify including a 2-cm margin around the tumor bed in the radiation therapy field. Gaspar et al42 observed that in 51 patients with recurrent supratentorial malignant glioma, 49 (96%) recurrences were within a 2 cm margin of the tumor bed. In his systematic review, Wilson47 rationalized this previously established 80% to 90% local recurrence statistic with his own findings from examinations of postmortem and biopsy samples from GBM patients. He found that 98% of GBM tumor cells preoperatively are concentrated either within the contrast-enhancing tumor or 2 cm from its edge. After resection of the tumor, 77% of the residual cell mass is still located within 2 cm of the resection cavity. This finding has been corroborated by recent single-cell RNA sequencing analyses of GBM cells at the tumor's periphery and additionally suggests that these cells drive recurrence.48,49 Finally, De Bonis et al28 demonstrated that resecting 1 to 2 cm beyond tumor borders may translate to lower recurrence rates. Although 18 of 36 (50%) SpTR patients had local GBM recurrence, 42 of 52 (81%) GTR patients’ tumors recurred locally, and progression-free survival (PFS) between the 2 cohorts trended toward being higher for SpTR patients (SpTR: median 12 mo; 95% CI 7-17; GTR: median 9 mo, 95% CI 7-13; P = .09). Given this rationale and the relatively high level of acceptance of this definition within our cohort, we recommend that the definition of SpTR as 1 to 2 cm beyond contrast enhancement be considered for standard use in clinical practice and in the design of future prospective studies.
As expected from results of previous research, GBMs in locations classified as noneloquent by consensus, including the right frontal and right anterior temporal lobes, had the highest RIs and were considered by consensus most amenable to enrollment in an RCT. GBMs classified as involving eloquent structures had the lowest RIs and by near-total consensus were classified as not fit to randomize. However, GBMs in certain regions that were considered eloquent or near eloquent, such as the right and left occipital lobes, were still chosen to be treated by most respondents with either GTR or SpTR. Although aggressively treating GBMs in these locations may cause new postoperative visual deficits and affect the patient's QOL, these specific deficits have not been associated with decreased survival time like surgically acquired motor or language deficits have.19,20 These results demonstrate that neurosurgical oncologists consider the anatomic location of a GBM and potential postoperative deficits that may result from its resection as essential variables that help determine if SpTR is a reasonable option.
Currently, 2 clinical trials (NCT02676687 and NCT04243005) are being conducted to assess the efficacy of SpTR upon survival for gliomas, including GBM. The first clinical trial, “Supratotal Resection for Gliomas Within Noneloquent Areas,” started in February 2016 at Southwest Hospital in China and includes patients with both high- and low-grade gliomas in noneloquent areas. The study aims to compare patients’ PFS rates after SpTR, defined as removal of brain parenchyma at least 1 cm beyond the radiographic margins of tumor as seen on contrast-enhancement MRI. The second clinical trial, a European study titled “Supramarginal Resection in Glioblastoma,” began in January 2020 and includes patients with GBM in any supratentorial location. It compares OS after GTR to supramarginal resection, defined as removal of at least 1 cm of T2 FLAIR abnormality around contrast-enhancing tumor. Given the results of our survey, we propose that the utility of SpTR to treat GBM should be investigated by a clinical trial with stricter stipulations than the aforementioned studies. We further propose that only patients with GBMs in locations that a panel of experts considered amenable to randomization, namely the right frontal and bilateral anterior temporal lobes, should be included in such a future study after available evidence has demonstrated that equipoise for outcomes and complications exists between GTR and SpTR. Our study clearly demonstrates that SpTR is not considered feasible in every GBM patient, and this finding should be considered during the design of future clinical trials and prospective studies.
Limitations
In the commercial sector where it was developed, crowdsourcing typically involved the input of hundreds of people to solve a problem. In this study, a smaller population—21 neurosurgeons in the United States—was surveyed. If these 21 neurosurgeons chose to participate in our survey because they hold strong opinions about SpTR, a response bias may have been introduced into our results that influences internal validity. Additionally, survey invitees were chosen in part based on their familiarity with GBM and its treatment. This selection bias may have influenced the external validity of our results. To address this, our group is now conducting a similar survey among a larger group of American and European neurosurgeons to assess whether a larger, more heterogeneous group of neurosurgeons will reach similar agreement on the definition and application of SpTR for GBM.
In this survey, participants were asked to make decisions about the treatment of GBM patients using only anatomic information provided by postcontrast and FLAIR MRI. However, when making decisions about surgical treatment of GBM, clinical data are taken into account. In addition, surgeons often make decisions about extent of resection intraoperatively using 5-ALA fluorescence or other adjuncts. Because this information was not provided on the survey, it was possible to more directly investigate the relationship between proposed treatment plans and anatomy alone; however, this constraint also limited the generalizability of the results.
CONCLUSION
Resection 1 to 2 cm beyond contrast enhancement may be adopted as a more standardized definition for future clinical work and research studies investigating SpTR of GBM. Furthermore, we have identified that GBMs involving the right frontal and bilateral anterior temporal lobes are considered most amenable to SpTR by a group of neurosurgical oncologists. Ongoing surveys will determine whether the broader neurosurgical community agrees with this consensus definition and application of SpTR. Nevertheless, these preliminary results support planning prospective studies, including future clinical trials, to further investigate the potential clinical utility of SpTR for GBMs in these locations.
Funding
The authors acknowledge assistance for clinical data coordination and retrieval from the Core for Clinical Research Data Acquisition, supported in part by the Johns Hopkins Institute for Clinical and Translational Research (UL1TR001079). This research was also supported in part by the Intramural Research Program of the NIH, NINDS.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
The authors would like to thank Erinma Elibe, MPH, for her contributions toward creating the online survey, and they would like to acknowledge members of the Johns Hopkins Neuro-Oncology Surgical Outcomes Lab for their valuable input that has helped inform this project.
Notes
The abstract of this manuscript was presented as a poster at the 2020 Society for Neuro-Oncology's (SNO) Annual Scientific Meeting and Education Day held virtually on November 19, 2020.
Contributor Information
Adham M Khalafallah, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Maureen Rakovec, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Chetan Bettegowda, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Christopher M Jackson, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Gary L Gallia, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Jon D Weingart, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Michael Lim, Department of Neurosurgery, Stanford University School of Medicine, Stanford, California, USA.
Yoshua Esquenazi, Vivian L. Smith Department of Neurosurgery, University of Texas Health Science Center, Houston, Texas, USA.
Brad E Zacharia, Department of Neurosurgery, Penn State Milton S. Hershey Medical Center, Pennsylvania State University College of Medicine, Hershey, Pennsylvania, USA.
Ezequiel Goldschmidt, Department of Neurological Surgery, University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, USA.
Mateo Ziu, Inova Neuroscience and Spine Institute, University of Virginia Medical School—Inova Campus, Falls Church, Virginia, USA.
Michael E Ivan, Sylvester Comprehensive Cancer Center, Department of Neurological Surgery, University of Miami Miller School of Medicine, Miami, Florida, USA.
Andrew S Venteicher, Center for Skull Base and Pituitary Surgery, Department of Neurosurgery, University of Minnesota, Minneapolis, Minnesota, USA.
Edjah K Nduom, Surgical Neurology Branch, National Institute of Neurological Disorders and Stroke, National Institutes of Health (NIH), Bethesda, Maryland, USA.
Adam N Mamelak, Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, California, USA.
Ray M Chu, Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, California, USA.
John S Yu, Department of Neurosurgery, Cedars-Sinai Medical Center, Los Angeles, California, USA.
Jason P Sheehan, Department of Neurological Surgery, University of Virginia Health System, Charlottesville, Virginia, USA.
Brian V Nahed, Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Bob S Carter, Department of Neurosurgery, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA.
Mitchel S Berger, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Raymond Sawaya, Division of Surgery, Department of Neurosurgery, University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Debraj Mukherjee, Department of Neurosurgery, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, USA.
eAppendix. Complete survey.
Supplement. eMethods 1: Definitions of GBM SpTR. eMethods 2: Radiographic data. eMethods 3: Statistical analysis. eResults 1: Neurosurgical oncologist cohort. eResults 2: Bivariate analysis of neurosurgeon practice characteristics and definitions of SpTR of GBM. eResults 3: Agreement among surgeons on eloquence of GBM location and treatment plan. eResults 4: Bivariate analysis of neurosurgeon practice characteristics and AI scores. eFigure 1: Institutions represented by survey respondents. eFigure 2: Practice characteristics of survey respondents. eFigure 3: Scatter plots of AI scores.
REFERENCES
- 1.Wirshing HG, Galanis E, Weller M.. Glioblastoma. Handb Clin Neurol. 2016;134:381-397. [DOI] [PubMed] [Google Scholar]
- 2.Omuro A, DeAngelis LM.. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842-1850. [DOI] [PubMed] [Google Scholar]
- 3.Price SJ, Gillard JH.. Imaging biomarkers of brain tumor margin and tumor invasion. Br J Radiol. 2011;84(2):S159-S167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stupp R, Mason WP, van den Bent MJet al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-996. [DOI] [PubMed] [Google Scholar]
- 5.Omuro AM, Faivre S, Raymond E. Lessons learned in the development of targeted therapy for malignant gliomas. Mol Cancer Ther. 2007;6(7):1909-1919. [DOI] [PubMed] [Google Scholar]
- 6.Simpson JR, Horton J, Scott Cet al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26(2):239-244. [DOI] [PubMed] [Google Scholar]
- 7.Jeremic B, Grujicic D, Antunovic Vet al. Influence of extent of surgery and tumor location on treatment outcome of patients with glioblastoma multiforme treated with combined modality approach. J Neurooncol. 1994;21(2):177-185. [DOI] [PubMed] [Google Scholar]
- 8.Ushio Y, Kochi M, Hamada Jet al. Effect of surgical removal on survival and quality of life in patients with supratentorial glioblastoma. Neurol Med Chir. 2005;45(9):454-461. [DOI] [PubMed] [Google Scholar]
- 9.Stummer W, Reulen HJ, Meinel Tet al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62(3):564-576. [DOI] [PubMed] [Google Scholar]
- 10.Davis ME.Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20(5):S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson TA, Karajannis MA, Harter DH.. Glioblastoma multiforme: state of the art and future therapeutics. Surg Neurol Int. 2014;5:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Leeuw CN, Vogelbaum MA.. Supratotal resection in glioma: a systematic review. Neuro Oncol. 2019;21(2):179-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffau H.Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochir. 2012;154(4):575-584. [DOI] [PubMed] [Google Scholar]
- 14.Duffau H.Surgery for diffuse low-grade gliomas (DLGG) oncological considerations. In: Duffau H, ed. Diffuse Low-Grade Gliomas in Adults. London, UK: Springer-Verlag; 2013:359-374. [Google Scholar]
- 15.Lima GLO, Duffau H.. Is there a risk of seizures in “preventive” awake surgery for incidental diffuse low-grade gliomas? J Neurosurg. 2015;122(6):1397-1405. [DOI] [PubMed] [Google Scholar]
- 16.Lima GLO, Dezamis E, Corns Ret al. Surgical resection of incidental diffuse gliomas involving eloquent brain areas. Rationale, functional, epileptological, and oncological outcomes. Neurochirurgie. 2017;63(3):250-258. [DOI] [PubMed] [Google Scholar]
- 17.Incekara F, Koene S, Vincent Aet al. Association between supratotal glioblastoma resection and patient survival: a systematic review and meta-analysis. World Neurosurg. 2019;127:617-624. [DOI] [PubMed] [Google Scholar]
- 18.Jackson C, Choi J, Khalafallah AMet al. A systematic review and meta-analysis of supratotal versus gross total resection for glioblastoma. J Neurooncol 2020;148(3):419-431. [DOI] [PubMed] [Google Scholar]
- 19.McGirt MJ, Mukherjee D, Chaichana KLet al. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65(3):463-470. [DOI] [PubMed] [Google Scholar]
- 20.Rahman M, Abbatematteo J, De Leo EKet al. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg. 2017;127(1):123-131. [DOI] [PubMed] [Google Scholar]
- 21.Schneider M, Potthoff AL, Keil VCet al. Surgery for temporal glioblastoma: lobectomy outranks oncosurgical-based gross-total resection. J Neurooncol. 2019;145(1):143-150. [DOI] [PubMed] [Google Scholar]
- 22.Roh TH, Kang SG, Moon JHet al. Survival benefit of lobectomy over gross-total resection without lobectomy in cases of glioblastoma in the noneloquent area: a retrospective study. J Neurosurg. 2019;132(3):895-901. [DOI] [PubMed] [Google Scholar]
- 23.Sonabend A, Zacharia BE, Cloney MBet al. Defining gioblastoma resectability through the wisdom of the crowd: a proof-of-principle study. Neurosurgery. 2017;80(4):590-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitabayashi T, Nakada M, Futura Tet al. NC-09: the impact of supratotal resection for glioblastoma. Neuro Oncol. 2014;16(Suppl 5):v136. [Google Scholar]
- 25.Molinaro AM, Hervey-Jumper S, Morshed RAet al. Association of maximal extent of resection of contrast-enhanced and non-contrast tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6(4):495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li YM, Suki D, Hess K, Sawaya R.. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016;124(4):977-988. [DOI] [PubMed] [Google Scholar]
- 27.Mampre D, Ehresman J, Pinilla-Monsalve Get al. Extending the resetion beyond the contrast-enhancement for glioblastoma: feasibility, efficacy, and outcomes. Br J Neurosurg. 2018;32(5):528-535. [DOI] [PubMed] [Google Scholar]
- 28.De Bonis P, Anile C, Pompucci Aet al. The influence of surgery on recurrence pattern of glioblastoma. Clin Neurol Neurosurg. 2013;155(1):37-43. [DOI] [PubMed] [Google Scholar]
- 29.Glenn CA, Baker CM, Conner AKet al. An examination of the role of supramaximal resection of temporal lobe glioblastoma multiforme. World Neurosurg. 2018;114:e747-e755. [DOI] [PubMed] [Google Scholar]
- 30.Esquenazi Y, Friedman E, Liu Zet al. The survival advantage of “supratotal” resection of glioblastoma using selective cortical mapping and the subpial technique. Neurosurgery. 2017;81(2):275-288. [DOI] [PubMed] [Google Scholar]
- 31.Hamada SM, Abou-Zeid AH.. Anatomical resection in glioblastoma: extent of resection and its impact on duration of survival. Egypt J Neurol Psychiatry Neurosurg. 2016;53(3):135-145. [Google Scholar]
- 32.Lu M, Fu ZH, He XJet al. T2 fluid-attenuated inversion recovery resection for glioblastoma involving eloquent brain areas facilitated through awake craniotomy and clinical outcome. World Neurosurg. 2020;135:e738-e747. [DOI] [PubMed] [Google Scholar]
- 33.Pessina F, Navarria P, Cozzi Let al. Maximize surgical resection beyond contrast-enhancing boundaries in newly diagnosed glioblastoma multiforme: is it useful and safe? A single institution retrospective experience. J Neurooncol. 2017;135(1):129-139. [DOI] [PubMed] [Google Scholar]
- 34.Aldave G, Tejada S, Pay Eet al. Prognostic value of residual fluoreescent tissue in glioblastoma patients after gross total resection in 5-aminolevulinic acid-guided surgery. Neurosurgery. 2013;72(6):915-921. [DOI] [PubMed] [Google Scholar]
- 35.Ghogawala Z, Schwartz JS, Benzel ECet al. Increased patient enrollment to a randomized surgical trial through equipoise polling of an expert surgeon panel. Ann Surg 2016;264(1):81-86. [DOI] [PubMed] [Google Scholar]
- 36.Shukla G, Alexander GS, Bakas Set al. Advanced magnetic resonance imaging in glioblastoma: a review. Chin Clin Oncol. 2017;6(4):40. [DOI] [PubMed] [Google Scholar]
- 37.Lasocki A, Gaillard F.. Non-Contrast-Enhancing tumor: a new frontier in glioblastoma research. Am J Neuroradiol. 2019;40(5):758-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muoio B, Giovanella L, Treglia G.. Recent developments of 18F-FET PET in neuro-oncology. Curr Med Chem. 2018;25(26):3061-3073. [DOI] [PubMed] [Google Scholar]
- 39.Certo F, Stummer W, Farah JOet al. Supramarginal resection of glioblastoma: 5-ALA fluorescence, combined intraoperative strategies, and correlation with survival. J Neurosurg Sci. 2019;63(6):625-632. [DOI] [PubMed] [Google Scholar]
- 40.Choucair AK, Levin VA, Gutin PHet al. Development of multiple lesions during radiation therapy and chemotherapy in patients with gliomas. J Neurosurg. 1986;65(5):654-658. [DOI] [PubMed] [Google Scholar]
- 41.Hou LC, Veeravagu A, Hsu AR, Tse VCK.. Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20(4):E5. [DOI] [PubMed] [Google Scholar]
- 42.Gaspar LE, Fisher BJ, Macdonald DRet al. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24(1):55-57. [DOI] [PubMed] [Google Scholar]
- 43.Burger PC, Dubois PJ, Schold SC Jret al. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg. 1983;58(2):159-169. [DOI] [PubMed] [Google Scholar]
- 44.Petrecca K, Guiot MC, Panet-Raymond V, Souhami L.. Failute pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. J Neurooncol. 2013;111(1):19-23. [DOI] [PubMed] [Google Scholar]
- 45.Hochberg FH, Pruitt A.. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907. [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Jiang T.. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331(2):139-146. [DOI] [PubMed] [Google Scholar]
- 47.Wilson CB.Glioblastoma: the past, the present, and the future. Clin Neurosurg. 1992;38:32-48. [PubMed] [Google Scholar]
- 48.Darmanis S, Sloan SA, Croote Det al. Single-cell RNA-seq analysis of infiltrating neoplastic cells at the migrating front of human glioblastoma. Cell Rep. 2017;21(5):1399-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khalafallah AM, Huq S, Jimenez AE, Serra R, Bettegowda C, Mukherjee D.. “Zooming in” on glioblastoma: understanding tumor heterogeneity and its clinical implications in the era of single-cell ribonucleic acid sequencing. Neurosurgery. 2021;88(3):477-486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.