Abstract
Background
The association between body mass index (BMI) and clinical outcomes following an acute myocardial infarction (AMI) remains controversial. Our objective was to investigate the relationship between BMI and AMI presentation, in-hospital clinical course and mortality in the contemporary era of AMI management.
Methods
Patients, hospitalized for an AMI between October 2015 and December 2016, were identified in the National Inpatient Sample (NIS) database. Socio-demographic and clinical data, including BMI, were collected and outcomes, including length of stay and mortality, were analyzed. Patients were divided into 6 BMI (kg/m2) subgroups; under-weight (≤19), normal-weight (20–25), over-weight (26–30), obese I (31–35), obese II (36–39) and extremely obese (≥40). Multivariable logistic regression model was used to identify predictors of in-hospital mortality. Linear regression model was used to identify predictors of length of stay (LOS).
Results
An estimated total of 125,405 hospitalizations for an AMI across the US were analyzed. Compared to the other BMI subgroups, the under-weight, normal-weight and extremely obese groups presented with a non-ST segment elevation AMI (NSTEMI) more frequently and were less likely to undergo coronary revascularization. The data show a J-shaped relationship between BMI and study outcomes with lower mortality in patients with BMI over 25 compared to normal- and low-weight patients. In the multivariate regression model, BMI group was found to be an independent predictor of mortality.
Conclusion
J-shaped relationship between BMI and mortality was documented in patients hospitalized for an AMI in the recent years. These findings confirm that the “obesity paradox” persists during the contemporary era of an AMI management.
Keywords: body mass index, BMI, acute myocardial infarction, obesity paradox
Introduction
The body mass index (BMI) is the metric currently in use to define anthropometric height/weight characteristics, by dividing weight in kilogram by height in meters squared, kg/m2.1 World Health Organization (WHO) classification categorizes patient’s weight as under-weight; BMI <18.5 kg/m2, normal-weight; BMI 18.5–24.9 kg/m2, over-weight; BMI 25–29.9 kg/m2, obese class I; BMI 30–34.9 kg/m2, obese class II; BMI 35–39.9 kg/m2, and extremely obese; BMI ≥ 40 kg/m2.2 BMI is widely used for routine characterization of weight status in epidemiology, clinical nutrition, and research. In population-based studies, higher BMI was associated with increased incidence and severity of major cardiovascular risk factors.3,4 BMI is proven to be an independent risk factor for various cardiovascular (CV) conditions such as congestive heart failure, atrial and ventricular arrhythmia, sudden cardiac death, stroke and acute coronary syndrome (ACS).1,4
However, the association between specific BMI range and major adverse outcomes in patients, hospitalized for acute myocardial infarction (AMI), remains controversial. Some studies showed lower risk of mortality in AMI patients with a BMI of 20–25 kg/m2, and increased mortality in patients below and above this BMI range.5–7 At the same time, other studies showed an unexpected “protective effect” of higher BMI values noted as the “obesity paradox”.8–12 Several studies suggest a U-shaped or J-shapes relationship between BMI and mortality in AMI patients, whereby overweight and obese patients exhibit the most favorable outcomes.10,11,13–15 Vast-majority of these studies were performed before or in the early days of drug eluting stents utilization and before the era of the potent antiplatelet agents, Prasugrel and Ticagrelor. We aimed at describing the BMI distribution as well as baseline characteristics, treatment strategies and outcomes in the different BMI subgroups patients admitted with an AMI in the recent years.
Methods
Data Source
Detailed information on the methods were provided in our prior study by Elbaz et al.16 Briefly, the data were drawn from the National Inpatient Sample (NIS), the Healthcare Cost and Utilization Project (HCUP), and Agency for Healthcare Research and Quality (AHRQ).17,18
The NIS database includes only de-identified data; therefore, this study was deemed exempt from institutional review by the Human Research Committee of Poriya Medical Center.
The NIS is the largest collection of all-payer data on inpatient hospitalizations in the United States (US). The dataset represents an approximate 20% stratified sample of all inpatient discharges from US hospitals.19 This information includes patient-level as demographic characteristics, primary and secondary diagnoses and procedures, comorbidities, length of stay (LOS) and hospital-level factors such as patient as hospital region, teaching status, bed size, and cost of hospitalization. National estimates can be calculated using the patient-level and hospital-level sampling weights that are provided by the HCUP.
For the purpose of this study, we obtained data for the years 2015 (last quarter) and 2016. International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) was used from the last quarter of 2015 and thereafter for reporting diagnoses and procedures in the NIS database during the study period. For each index hospitalization, the database provides a principal discharge diagnosis and a maximum of 14 or 24 additional diagnoses (depending on the year), in addition to a maximum of 15 procedures. The reason we only included the data coded with ICD-10 codes is that the ICD-10 system includes individual codes for BMI values and ranges.
Study Population and Variables
We identified patients 18 years of age or older with a primary diagnosis of AMI based on ICD-10-CM code starting with I21.xx or I22.xx, who have one of the Z68.x codes as I10-Dx1 to I10-Dx30. These codes represent the six subgroups in our study; Z68.1; BMI equal or below 19; under-weight group, Z68.20–25; BMI 20–25; normal-weight group, Z68.26–30; BMI 26–30; over-weight group, Z68.31–35; BMI 31–35; obese I group, Z68.36–39; BMI 36–39; obese II group and Z68.4; BMI equal or above 40; extremely obese group.
The following patient demographics were collected from the database; age, sex, and race. Prior comorbidities were identified from the documentation of the corresponding ICD-10 codes during the index hospitalization. For the purposes of calculating Deyo-Charlson Comorbidity Index (Deyo-CCI), additional co-morbidities were identified from the database using ICD-10-CM codes. Deyo-CCI is a modification of the Charlson Comorbidity Index, containing 17 comorbidities conditions with differential weights, with a total score ranging from 0 to 33 (Detailed information on Deyo-CCI provided in the Appendix Table 1). Higher Deyo-CCI scores indicates to greater burden of comorbid diseases and is associated with mortality one year after admission.20 The index has been used extensively in studies from administrative databases, with proved validity in predicting short- and long-term outcomes.21,22
Our primary outcome in this study was in-hospital mortality. Length of stay was the secondary outcome we analyzed.
Statistical Analysis
The chi-square (χ2) test and Wilcoxon Rank Sum test were used to compare categorical variables and continuous variables, respectively. The NIS provides discharge sample weights that are calculated within each sampling stratum as the ratio of discharges in the universe to discharges in the sample.23 We generated a weighted logistic regression model to identify independent predictors of in-hospital mortality. Candidate variables included patient-level characteristics, Deyo-CCI and hospital-level factors. We retained all predictor variables that were associated with our primary and secondary outcome with p < 0.05 in our final multivariable regression model. Furthermore, analysis was performed using linear regression with length of stay as a dependent variable.
For all analyses, we used SAS® software version 9.4 (SAS Institute Inc., Cary, NC.) A p-value <0.05 was considered statistically significant.
Results
Study Cohort
A total of 24,181 AMI hospitalizations across the US during 2015 (last quarter) and 2016 were included in the analysis. After implementing the weighting method, these represented an estimated total of 125,405 hospitalizations for AMI, in patients who had BMI information during the index hospitalization. The majority of patients (56.9%) were male and the mean age of the cohort was 63±29.3 years.
The data reveals that 75.6% of all hospitalizations included patients, presented with non-ST-segment elevation myocardial infarction (NSTEMI) while 24.4% were hospitalized for suspected ST-segment elevation myocardial infarction (STEMI). As shown in Table 1, 71.7% of the patients received coronary intervention during the index hospitalization.
Table 1.
Frequency Distribution of Baseline Characteristics by BMI Group in AMI Patients
| BMI Groups Kg/m2 | ≤19 Under-Weight | 20–25 Normal-Weight | 26–30 Over-Weight | 31–35 Obese I Group | 36–39 Obese II Group | ≥ 40 Extremely Obese | Total | P-value |
|---|---|---|---|---|---|---|---|---|
| BMI kg/m2, n | ||||||||
| Unweighteda | 1519 | 1137 | 3347 | 6883 | 4596 | 7599 | 25,081 | |
| Weightedb | 7595 | 5685 | 16,735 | 34,415 | 22,980 | 37,995 | 125,405 | |
| Age Group, % | <0.001 | |||||||
| 18–44 yrs | 1.3 | 2.4 | 5.0 | 7.6 | 9.9 | 11.6 | 8.3 | |
| 45–59 yrs | 9.0 | 14.7 | 27.4 | 33.5 | 34.1 | 35.7 | 31.1 | |
| 60–74 yrs | 28.6 | 30.0 | 42.2 | 41.7 | 41.9 | 42.8 | 40.6 | |
| 75 yrs or older | 61.2 | 52.9 | 25.5 | 17.3 | 14.0 | 10.7 | 20.1 | |
| Gender, % | <0.001 | |||||||
| Female | 61.3 | 42.6 | 37.4 | 36.5 | 39.4 | 50.1 | 43.0 | |
| Male | 38.7 | 57.3 | 62.6 | 63.5 | 60.5 | 49.9 | 56.9 | |
| Race, % | <0.001 | |||||||
| White | 72.1 | 66.4 | 70.1 | 70.1 | 71.7 | 68.8 | 70.0 | |
| Non-white | 22.8 | 27.1 | 23.2 | 22.7 | 21.3 | 24.6 | 23.3 | |
| Comorbidity, % | ||||||||
| HTN | 43.4 | 45.3 | 57.2 | 59.0 | 59.8 | 55.9 | 56.4 | <0.001 |
| CHF | 21.5 | 19.3 | 14.5 | 13.5 | 15.1 | 17.6 | 15.9 | <0.001 |
| DM | 16.1 | 29.1 | 37.4 | 40.9 | 42.8 | 47.2 | 40.7 | <0.001 |
| CRD | 27.6 | 33.7 | 24.1 | 23.2 | 23.7 | 28.1 | 25.6 | <0.001 |
| COPD | 42.3 | 27.5 | 19.7 | 21.3 | 23.2 | 29.3 | 25.4 | <0.001 |
| PVD | 20.7 | 14.5 | 13.3 | 12.2 | 12.1 | 10.5 | 12.4 | <0.001 |
| Atrial Fibrillation/Flutter | 28.9 | 27.7 | 15.8 | 15.0 | 15.7 | 18.5 | 17.7 | <0.001 |
| Prior MI | 14.8 | 15.0 | 16.2 | 15.6 | 16.3 | 15.0 | 15.6 | <0.001 |
| Deyo-CCI, % | <0.001 | |||||||
| 1 | 7.6 | 11.6 | 19.4 | 19.7 | 19.3 | 14.6 | 16.9 | |
| 2 or higher | 92.4 | 88.4 | 80.6 | 80.3 | 80.7 | 85.4 | 83.1 |
Notes: aRepresents the number of observations in the NIS database. bRepresents total national estimates after applying sampling weights.
Abbreviations: CHF, congestive heart failure; COPD, chronic obstructive lung disease; CRD, chronic renal disease; Deyo-CCI, Deyo-Charlson Comorbidity Index; DM, diabetes mellitus; HTN, hypertension; MI, myocardial infarction; PVD, peripheral vascular disease.
Patients Characteristics, AMI Presentation and Treatment Approach by BMI Group
Study population baseline characteristics, the AMI type and the treatment approach are presented in detail in Tables 1 and 2. Female predominance and higher prevalence of comorbidities including congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), chronic renal disease (CRD), atrial fibrillation/flutter and higher Deyo-CCI scores were documented in the under-weight, normal-weight and in the extremely obese groups (Table 1). These patients presented more frequently with NSTEMI and were less likely to undergo invasive revascularization (PCI and CABG) during the index hospitalization. On the contrary, male sex was predominant in the over-weight, obese I and obese II patient groups, who had presented more frequently with STEMI and underwent more revascularization procedures (Table 2).
Table 2.
Frequency Distribution of Clinical Course and Outcomes by BMI Group
| ≤19 Under-Weight | 20–25 Normal-Weight | 26–30 Over-Weight | 31–35 Obese I Group | 36–39 Obese II Group | ≥ 40 Extremely Obese | Total | P-value | |
|---|---|---|---|---|---|---|---|---|
| NSTEMI, % | 82.1 | 79.1 | 71.3 | 72.9 | 73.8 | 79.0 | 75.6 | <0.001 |
| STEMI, % | 18.2 | 21.2 | 29.2 | 27.7 | 26.5 | 21.4 | 24.4 | <0.001 |
| VT/VF | 7.2 | 6.9 | 7.9 | 8.0 | 8.1 | 7.6 | 7.8 | 0.007 |
| Coronary Angiography, % | 38.1 | 49.9 | 75.0 | 77.0 | 77.2 | 72.1 | 71.7 | <0.001 |
| PCI, % | 21.5 | 31.1 | 55.7 | 58.6 | 56.9 | 49.5 | 51.6 | <0.001 |
| CABG, % | 1.1 | 2.1 | 2.7 | 2.7 | 2.6 | 1.8 | 2.3 | <0.001 |
| Thrombolytic Therapy % | 0.9 | 0.4 | 1.3 | 1.0 | 1.1 | 1.1 | 1.1 | <0.001 |
| Mortality, % | 9.2 | 6.4 | 3.3 | 2.7 | 2.6 | 3.6 | 3.6 | <0.001 |
| LOS (days), Mean ± SEM | 6.11 ± 0.17 | 6.16 ± 0.21 | 4.14 ± 0.08 | 3.71 ± 0.05 | 3.78 ± 0.06 | 4.42 ± 0.06 | 4.25 ± 0.04 | <0.001 |
Abbreviations: BMI, body mass index Kg/m2; CABG, coronary bypass graft; LOS, length of stay; NSTEMI, non-ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST segment myocardial infarction; VF/VT, ventricular flutter/ventricular fibrillation.
Length of Stay and Mortality by BMI Groups
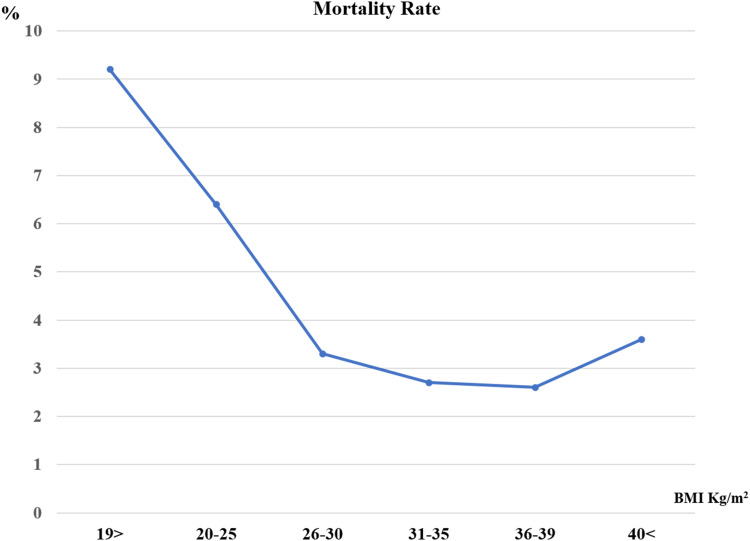
J-shaped relationship between the BMI and the study outcomes was documented, while the over-weight, obese I and obese II patient’s subgroups (BMI 26–39) exhibited lower total mortality and shorter LOS, with higher mortality and longer LOS below and above this range. The overall rate of total mortality during the study period was 3.6% with a significantly higher mortality rate in under-weight (9.2%), normal-weight (6.2%) patient’s population, P<0.001 (Figure 1).
Figure 1.
Mortality rate via BMI groups.
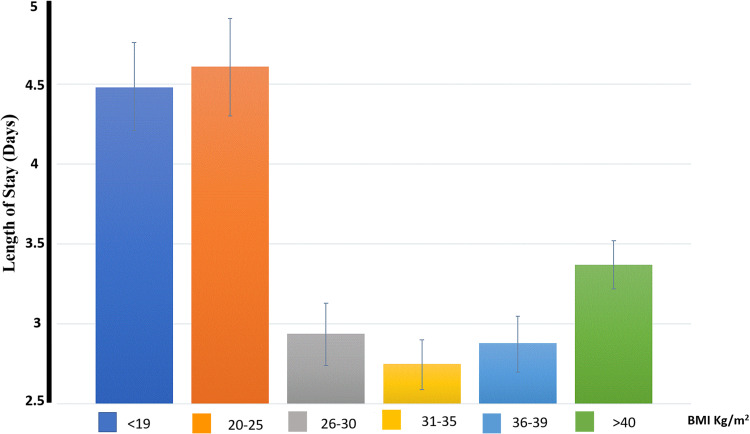
Longer LOS was documented in the under-weight, normal-weight and extremely obese patients (6.11±0.17, 6.16±0.21, 4.42±0.06 respectively) compared to the over-weight, obese I, obese II patients (4.14±0.08, 3.71±0.05, 3.78±0.06 respectively), P<0.001. The same findings were documented in the linear regression model (Appendix Tables 2 and 3). Longer LOS was documented in those three groups included BMI <19, 20–25 and >40 (Figure 2).
Figure 2.
Length of stay via BMI groups.
Predictors of in-Hospital Mortality
In an unadjusted analysis, we found that BMI below or equal to 19kg/m2, older age, increasing Deyo-CCI score, chronic renal failure, atrial fibrillation/flutter, congestive heart failure, peripheral vascular disease, female sex and chronic obstructive pulmonary disease, all increased the odds of in-hospital mortality (p < 0.001) (Table 3A). After adjusting for potential confounders, BMI below 19 kg/m2 remained an independent predictor of worse outcome and in-hospital mortality in a multivariate analysis (Table 3B). At the same time, BMI >25 was found to be an independent predictor of lower mortality among the study population (Table 3B).
Table 3.
Univariate (A) and Multivariate (B) Analysis for Predictors of in-Hospital Mortality, 2015–2016
| Predictor | Odds Ratio (95% CI) | P-value |
|---|---|---|
| 3A. Univariate Analysis | ||
| BMI kg/m2 Group | <0.001 | |
| ≤ 19 | 1.47 (1.29–1.67) | <0.001 |
| 20–25 | 1.00 (reference) | N/A |
| 26–30 | 0.50 (0.44–0.57) | <0.001 |
| 31–35 | 0.40 (0.36–0.46) | <0.001 |
| 36–39 | 0.39 (0.34–0.44) | <0.001 |
| ≥40 | 0.54 (0.48–0.61) | <0.001 |
| Age Group, years | <0.001 | |
| 18–44 | 1.00 (reference) | N/A |
| 45–59 | 2.43 (1.94–3.04) | <0.001 |
| 60–74 | 4.68 (3.77–5.83) | <0.001 |
| ≥75 | 9.00 (7.23–11.20) | <0.001 |
| Deyo-CCI | <0.001 | |
| 1 | 1.00 (reference) | N/A |
| 2 or higher | 3.42 (3.02–3.88) | <0.001 |
| Gender | 0.007 | |
| Male | 1.00 (reference) | N/A |
| Female | 1.09 (1.02–1.15) | 0.007 |
| Race | 0.048 | |
| Non-white | 1.00 (reference) | N/A |
| White | 1.08 (1.00–1.16) | 0.048 |
| Comorbidities | ||
| Smoking Status | ||
| Non-Smokers | 1.00 (reference) | N/A |
| Past-Smokers | 1.66 (1.61–1.72) | <0.001 |
| Current-Smokers | 1.57 (1.52–1.61) | <0.001 |
| Atrial Fibrillation/Flutter | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 2.26 (2.11–2.41) | <0.001 |
| COPD | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 1.20 (1.12–1.28) | <0.001 |
| CHF | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 1.86 (1.74–2.00) | <0.001 |
| DM | 0.357 | |
| No | 1.00 (reference) | N/A |
| Yes | 0.97 (0.91–1.03) | 0.357 |
| HTN | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 0.40 (0.38–0.43) | <0.001 |
| PVD | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 1.58 (1.46–1.70) | <0.001 |
| CRF | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 2.31 (2.17–2.45) | <0.001 |
| Revascularization | 0.51 (0.48–0.54) | <0.001 |
| 3B. Multivariate Analysis | ||
| BMI kg/m2 Group | <0.001 | |
| ≤19 | 1.34 (1.17–1.54) | <0.001 |
| 20–25 | 1.00 (reference) | N/A |
| 26–30 | 0.61 (0.52–0.70) | <0.001 |
| 31–35 | 0.56 (0.49–0.64) | <0.001 |
| 36–39 | 0.56 (0.48–0.64) | <0.001 |
| ≥ 40 | 0.83 (0.73–0.94) | 0.005 |
| Age Group, years | <0.001 | |
| 18–44 yrs | 1.00 (reference) | N/A |
| 45–59 yrs | 2.36 (1.86–3.00) | <0.001 |
| 60–74 yrs | 4.26 (3.38–5.39) | <0.001 |
| 75 yrs or older | 6.94 (5.47–8.81) | <0.001 |
| Gender | <0.001 | |
| Male | 1.00 (reference) | N/A |
| Female | 0.84 (0.79–0.90) | <0.001 |
| Race | 0.531 | |
| Non-white | 1.00 (reference) | N/A |
| White | 1.02 (0.95–1.11) | 0.531 |
| Deyo-CCI | <0.001 | |
| 1 | 1.00 (reference) | N/A |
| 2 or higher | 2.42 (2.12–2.77) | <0.001 |
| Comorbidities | ||
| Smoking Status | ||
| Non-Smokers | 1.00 (reference) | N/A |
| Past-Smokers | 1.63 (1.59–1.69) | <0.001 |
| Current-Smokers | 1.78 (1.71–1.85) | <0.001 |
| Atrial Fibrillation/Flutter | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 1.60 (1.49–1.71) | <0.001 |
| CHF | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 1.46 (1.35–1.57) | <0.001 |
| COPD | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 0.86 (0.80–0.93) | <0.001 |
| DM | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 0.81 (0.76–0.87) | <0.001 |
| HTN | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 0.52 (0.48–0.55) | <0.001 |
| PVD | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 1.18 (1.09–1.28) | <0.001 |
| Renal failure | <0.001 | |
| No | 1.00 (reference) | N/A |
| Yes | 1.74 (1.63–1.86) | <0.001 |
| Revascularization | 0.69 (0.65–0.74) | <0.001 |
Abbreviations: BMI, body mass index kg/m2; CHF, congestive heart failure; COPD, chronic obstructive lung disease; CRD, chronic renal disease; Deyo-CCI, Deyo-Charlson Comorbidity Index; DM, diabetes mellitus; HTN, hypertension; PVD, peripheral vascular disease.
Discussion
Utilizing data from the NIS, the largest all-payer inpatient database in the US, we identified a weighted total of 125,405 patients to investigate the relationship between BMI and in-hospital outcomes among patients hospitalized for an AMI event. To our knowledge, this is the single largest study, analyzing the relationship between BMI on an AMI presentation and outcomes. This nationwide data analysis reveals a J-shaped relationship between the BMI and in-hospital mortality during hospitalization for an AMI in the US during the study period. BMI above the normal range (>25) was an independent predictor of lower mortality and shorter length of stay in patients hospitalized for an AMI in the US during the study period.
These results are consistent with prior reports, including studies that followed patients after the discharge and showed that overweight and moderate obesity were associated with lower mortality after an ACS.24,25 Angeras et al showed U-shaped relationship between the BMI and mortality in 64,436 patients who underwent coronary angiography in the context of an ACS in Sweden.24 Similarly to the findings in our study, Angeras et al report a nadir in mortality among overweight or obese (<BMI of 35) patients, while underweight and normal weight patients had the highest risk for mortality during follow up.24 Of notice, their study enrolled patients between 2005 and 2008, the early days of drug eluting stents and before the era of the potent antiplatelet agents, Prasugrel and Ticagrelor, routinely used nowadays.
On the other hand, some studies did not support the obesity paradox and showed increased mortality in patients above 40kg/m2.5–7 Das et al found that the risk-adjusted of in-hospital mortality rates were significantly higher for Class III obesity who admitted with ACS (≥40Kg/m2).7 Similar results of lower survival rate showed by Lazzeri et al that found that lean and overweight patients had higher mortality rate compared to normal-weight patients.26
Our study population represents the entire, nationwide population of patients admitted for an AMI in the US between October 2015 and December 2016, hence eliminating the “selection bias” in some of the prior studies in AMI patients. As shown in Table 1, the overweight and obese patients in our study were younger, finding that could have contributed to their improved survival. This observation was described previously as well, while 20 out of 26 reports included in a 2014 meta-analysis of the “obesity paradox” studies by Niedziela et al, documented younger patients in the overweight and obese patients.27 Not surprisingly, patients in the higher BMI groups suffered from increased prevalence of cardiovascular risk factors such as DM, HTN. Niedziela et al showed similar U-shaped relation between the BMI and mortality in AMI patients. Interestingly, many of the studies that were included in the meta-analysis lacked the “under-weight” and “severe obesity” patient populations. In addition, as mentioned before, the vast majority of patients, enrolled into the different trials included in this meta-analysis, were treated in a different era of AMI management, before DES became routinely implanted in AMI and patients received more potent antiplatelet agents than Clopidogrel, some even before the routine PCI and dual antiplatelet therapy use. The findings in our report show that the “obesity paradox” persists during the contemporary AMI management era.27
We report lower proportion of women in the obese patients’ groups, consistent with some prior reports, while other studies document female predominance among their obese patients.14,28–31 Female sex was shown to be a predictor of improved outcome in a multivariate regression analysis, therefore, the lower mortality rate in the obese patients in our study cannot be attributed to patients’ sex.
There is a paucity of publications studying mechanisms to explain this lower post-ACS survival rate of patients with normal and low BMI status. One possible explanation is that high BMI may confer survival benefits by providing nutritional and caloric reserves in severely and critically ill patients. This is supported by previous studies in other chronic, debilitating CV and non-CV conditions, in which an under-weight and normal weight BMIs were associated with a higher mortality rate compared to higher-BMIs groups.4,32–34 Other studies supported that severe cardiovascular disease, such in heart failure patients, results in tissue hypoperfusion and cardiac cachexia. The hypothesis is that this state results from a heightened metabolic or increased catabolic state, associated with worse prognosis.35,36 On the other hand, higher BMI state might indicate a better metabolic reserve and tolerance to metabolic stress and consequently to a better prognosis.37 We assume that a similar mechanism may play a role in patients who are suddenly exposed to an intense inflammatory and catabolic stress associated with the AMI event.
Our study should be interpreted in the contexts of several limitations. The NIS database is a retrospective administrative database that contains discharge-level records and as such is susceptible to coding errors. This is an observational, non-controlled cohort study, and no conclusions on causality can be drawn from these results. Another limitation is that we could not verify whether the patient’s BMI documentation represents an actual measurement performed during the index admission or documentation of the patient’s weight per prior report. These limitations are counterbalanced by the real world, nationwide nature of the data, as well as mitigation of reporting bias introduced by selective publication of results from specialized centers. In addition, the lack of patient identifiers in the NIS precluded us from using other outcome variables and mortality measures such as at 30-day. We could only capture events that occurred only during the index hospitalization.
In Conclusion, J-shaped relationship between BMI and mortality was documented in patients hospitalized for an AMI in the recent years. These findings confirm that the “obesity paradox” persists during the contemporary era of an AMI management.
Acknowledgments
The corresponding author affirms that he has listed everyone who contributed significantly to the work. The corresponding author had access to all the study data, take responsibility for the accuracy of the analysis, and had authority over manuscript preparation and the decision to submit the manuscript for publication. The corresponding author confirms that all authors read and approve the manuscript. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, agreed to the submitted journal, and agree to be accountable for all aspects of the work.
Funding Statement
There is no funding to report.
Disclosures
The authors report no conflicts of interest in this work.
References
- 1.Muller MJ, Braun W, Enderle J, Bosy-Westphal A. Beyond BMI: conceptual issues related to overweight and obese patients. Obes Facts. 2016;9:193–205. doi: 10.1159/000445380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Body mass index - BMI. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi. Accessed July 2020.
- 3.Khan SS, Ning H, Wilkins JT, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3:280–287. doi: 10.1001/jamacardio.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53:1925–1932. doi: 10.1016/j.jacc.2008.12.068 [DOI] [PubMed] [Google Scholar]
- 5.Das SR, Alexander KP, Chen AY. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58:2642–2650. doi: 10.1016/j.jacc.2011.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global BMIMC, Di Angelantonio E, Bhupathiraju SN, Wormser D, Gao P, Kaptoge S. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. doi: 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neeland IJ, Das SR, Simon DN, Diercks DB, Alexander KP. The obesity paradox, extreme obesity, and long-term outcomes in older adults with ST-segment elevation myocardial infarction: results from the NCDR. Eur Heart J Qual Care Clin Outcomes. 2017;3:183–191. doi: 10.1093/ehjqcco/qcx010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kadakia MB, Fox CS, Scirica BM, Murphy SA. Central obesity and cardiovascular outcomes in patients with acute coronary syndrome: observations from the MERLIN-TIMI 36 trial. Heart. 2011;97:1782–1787. doi: 10.1136/heartjnl-2011-300231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buettner HJ, Mueller C, Gick M, et al. The impact of obesity on mortality in UA/non-ST-segment elevation myocardial infarction. Eur Heart J. 2007;28:1694–1701. doi: 10.1093/eurheartj/ehm220 [DOI] [PubMed] [Google Scholar]
- 10.Diercks DB, Roe MT, Mulgund J. The obesity paradox in non-ST-segment elevation acute coronary syndromes: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the American College of Cardiology/American Heart Association Guidelines Quality Improvement Initiative. Am Heart J. 2006;152:140–148. [DOI] [PubMed] [Google Scholar]
- 11.Mahaffey KW, Tonev ST, Spinler SA, et al. Obesity in patients with non-ST-segment elevation acute coronary syndromes: results from the SYNERGY trial. Int J Cardiol. 2010;139:123–133. doi: 10.1016/j.ijcard.2008.10.008 [DOI] [PubMed] [Google Scholar]
- 12.Uretsky S, Messerli FH, Bangalore S, Champion A, Cooper-Dehoff RM. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120:863–870. doi: 10.1016/j.amjmed.2007.05.011 [DOI] [PubMed] [Google Scholar]
- 13.Nigam A, Wright RS, Allison TG, Williams BA, Kopecky SL, Reeder GS. Excess weight at time of presentation of myocardial infarction is associated with lower initial mortality risks but higher long-term risks including recurrent re-infarction and cardiac death. Int J Cardiol. 2006;110:153–159. doi: 10.1016/j.ijcard.2005.06.040 [DOI] [PubMed] [Google Scholar]
- 14.Eisenstein EL, McGuire DK, Bhapkar MV, et al. Elevated body mass index and intermediate-term clinical outcomes after acute coronary syndromes. Am J Med. 2005;118:981–990. doi: 10.1016/j.amjmed.2005.02.017 [DOI] [PubMed] [Google Scholar]
- 15.Lamelas P, Schwalm JD, Quazi I, Mehta S, Devereaux PJ. Effect of body mass index on clinical events after acute coronary syndromes. Am J Cardiol. 2017;120:1453–1459. doi: 10.1016/j.amjcard.2017.07.043 [DOI] [PubMed] [Google Scholar]
- 16.Elbaz-Greener G, Rozen G, Kusniec F, et al. Trends in utilization and safety of in-hospital coronary artery bypass grafting during a non-ST-segment elevation myocardial infarction. Am J Cardiol. 2020;134:32–40. doi: 10.1016/j.amjcard.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 17.Healthcare Cost and Utilization Project (HCUP). 2000–2011. Agency for Healthcare Research and Quality R, MD. Available from:www.hcup-us.ahrq.gov/nisoverview.jsp. AccessedJuly2020. [PubMed] [Google Scholar]
- 18.HCUP National Inpatient Sample (NIS). Healthcare Cost and Utilization Project (HCUP). 2012–2013. Agency for Healthcare Research and Quality R, MD. Available from:www.hcup-us.ahrq.gov/nisoverview.jsp. AccessedJuly2020. [Google Scholar]
- 19.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract. 2002;5:143–151. [PubMed] [Google Scholar]
- 20.Deyo RA, Cherkin DC, Ciol Ma. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 21.Chu YT, Ng YY, Wu SC. Comparison of different comorbidity measures for use with administrative data in predicting short- and long-term mortality. BMC Health Serv Res. 2010;10:140. doi: 10.1186/1472-6963-10-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radovanovic D, Seifert B, Urban P, et al. Validity of Charlson Comorbidity Index in patients hospitalised with acute coronary syndrome. Insights from the nationwide AMIS Plus registry 2002–2012. Heart. 2014;100:288–294. doi: 10.1136/heartjnl-2013-304588 [DOI] [PubMed] [Google Scholar]
- 23.Hosseini SM, Moazzami K, Rozen G, Vaid J, Saleh A, Heist KE. Utilization and in-hospital complications of cardiac resynchronization therapy: trends in the United States from 2003 to 2013. Eur Heart J. 2017;38:2122–2128. doi: 10.1093/eurheartj/ehx100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angeras O, Albertsson P, Karason K, et al. Evidence for obesity paradox in patients with acute coronary syndromes: a report from the Swedish Coronary Angiography and Angioplasty Registry. Eur Heart J. 2013;34:345–353. doi: 10.1093/eurheartj/ehs217 [DOI] [PubMed] [Google Scholar]
- 25.Kragelund C, Hassager C, Hildebrandt P, Torp-Pedersen C. Impact of obesity on long-term prognosis following acute myocardial infarction. Int J Cardiol. 2005;98:123–131. doi: 10.1016/j.ijcard.2004.03.042 [DOI] [PubMed] [Google Scholar]
- 26.Lazzeri C, Valente S, Chiostri M, et al. Impact of age on the prognostic value of body mass index in ST-Elevation myocardial infarction. NMCD. 2012;23:205–211. [DOI] [PubMed] [Google Scholar]
- 27.Niedziela J, Hudzik B, Niedziela N, et al. The obesity paradox in acute coronary syndrome: a meta-analysis. Eur J Epidemiol. 2014;29(11):801–812. doi: 10.1007/s10654-014-9961-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aronson D, Nassar M, Goldberg T, Kapeliovich M. The impact of body mass index on clinical outcomes after acute myocardial infarction. Int J Cardiol. 2010;145:476–480. doi: 10.1016/j.ijcard.2009.12.029 [DOI] [PubMed] [Google Scholar]
- 29.Lopez-Jimenez F, Jacobsen SJ, Reeder GS, Weston SA. Prevalence and secular trends of excess body weight and impact on outcomes after myocardial infarction in the community. Chest. 2004;125:1205–1212. doi: 10.1378/chest.125.4.1205 [DOI] [PubMed] [Google Scholar]
- 30.Mehta L, Devlin W, McCullough PA, O’Neill WW, Skelding KA, Stone GW. Impact of body mass index on outcomes after percutaneous coronary intervention in patients with acute myocardial infarction. Am J Cardiol. 2007;99:906–910. doi: 10.1016/j.amjcard.2006.11.038 [DOI] [PubMed] [Google Scholar]
- 31.Mehta RH, Gitt AK, Junger C, et al. Body mass index and effectiveness of reperfusion strategies: implications for the management of patients with ST-elevation myocardial infarction. J Interv Cardiol. 2008;21:8–14. doi: 10.1111/j.1540-8183.2007.00311.x [DOI] [PubMed] [Google Scholar]
- 32.Oliveros H, Villamor E. Obesity and mortality in critically ill adults: a systematic review and meta-analysis. Obesity (Silver Spring). 2008;16:515–521. doi: 10.1038/oby.2007.102 [DOI] [PubMed] [Google Scholar]
- 33.Kalantar-Zadeh K, Horwich TB, Oreopoulos A, Kovesdy CP, Younessi H. Risk factor paradox in wasting diseases. Curr Opin Clin Nutr Metab Care. 2007;10:433–442. doi: 10.1097/MCO.0b013e3281a30594 [DOI] [PubMed] [Google Scholar]
- 34.Habbu A, Lakkis NM, Dokainish H. The obesity paradox: fact or fiction? Am J Cardiol. 2006;98:944–948. doi: 10.1016/j.amjcard.2006.04.039 [DOI] [PubMed] [Google Scholar]
- 35.Kenchaiah S, Pocock SJ, Wang D, et al. Body mass index and prognosis in patients with chronic heart failure insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Circ. 2007;116:627–636. doi: 10.1161/CIRCULATIONAHA.106.679779 [DOI] [PubMed] [Google Scholar]
- 36.Berry C, Clark AL. Catabolism in chronic heart failure. Eur Heart J. 2000;21:521–532. doi: 10.1053/euhj.1999.1882 [DOI] [PubMed] [Google Scholar]
- 37.Coats A, Anker SD, Roecker EB, et al. Prevention and reversal of cardiac cachexia in patients with severe heart failure by carvedilol: results of the COPERNICUS study. Circulation. 2001;10:4II–437. [Google Scholar]