Abstract
Purpose
Accurate component placement and restoration of patient anatomy are critical in total hip arthroplasty (THA) surgery. Although intraoperative radiographs are sometimes utilized, it is unclear whether this practice can improve accuracy.
Materials and Methods
This study evaluated acetabular cup abduction, anteversion, leg length, and offset among 100 posterior approach THAs performed without imaging (No X-ray group) and compared them to a subsequent series of 100 THAs where an intraoperative radiograph was taken with the trial components in place (X-ray group). THAs were performed using a posterior approach by a single, experienced surgeon whose goal was to place the cup at 45° of abduction and 30° of anteversion. Supine anteroposterior pelvic digital radiographs taken at the first (nominal 4-week) postoperative visit were used for measurements.
Results
Slight differences in cup abduction (47°±6° vs 44°±6°, respectively, P=0.003) and anteversion angle (35°±6° vs 31°±6°, respectively, P<0.001) were observed between the X-ray and No X-ray groups; however, a similar proportion of cups within 10° of the target angles was observed (76% vs 83%, respectively, P=0.22). No difference in offset measurements (1.1±6.6 mm vs 0.3±6.9 mm, respectively, P=0.42) or leg lengths (0.3±3.8 mm vs 0.3±4.8 mm, respectively, P=0.94) was observed between the X-ray and No X-ray groups; however, the X-ray group showed less leg length variation (P=0.05).
Conclusion
In this study, the routine use of intraoperative radiographs was not associated with improved implant positioning for uncomplicated primary THA.
Keywords: Arthroplasty, Replacement, Hip, X-rays
INTRODUCTION
Optimal component placement, equalization of leg lengths, and recreation of offset are important surgical objectives during total hip arthroplasty (THA). Although the ideal cup position has not been established and may depend on intraoperative considerations as well as patient-specific factors, most surgeons have a desired cup orientation1,2,3,4). Historically, many surgeons have aimed for the Lewinnek “safe zone”, which is defined as 30°−50° of abduction and 5°−25° of anteversion5). However, recent literature has questioned the clinical efficacy of this “safe zone” and some surgeons using the posterior approach aim for more anteversion1,3,6). Methods for optimizing cup position include anatomic landmarks, room landmarks, acetabular cup coverage, and ischial or pubis palpation7,8). Intraoperative estimation of leg lengths can be performed by gross measurement with the legs held side by side, calibrating the neck cut to a predetermined level, or using intraoperative measurements such as anchoring a pin in the ilium and measuring to a fixed point on the femur. Accurate preoperative templating can be helpful in these processes. Computer navigation and robotics are additional options9,10).
Interest in intraoperative imaging using radiographs or fluoroscopy to assist with component positioning has shown a recent increase6,11,12,13,14,15,16,17,18,19,20,21). For anterior approach THA, a variety of intraoperative measurements can be made by supine positioning of patients, which facilitates obtaining an anteroposterior (AP) pelvic view. The use of a fracture table makes it challenging to directly assess stability based on intraoperative range of motion testing; therefore, these measurements are particularly important. To assist the surgeon, there are a number of techniques and software products for measurement of intraoperative radiographs. Some of these techniques have been adapted for use with the posterior approach while the patient is in the lateral decubitus position9). The purpose of this study was to determine whether the use of an intraoperative radiograph improved the postoperative acetabular cup abduction and anteversion angles, leg length, and offset for posterior approach THAs performed by a single, experienced surgeon.
MATERIALS AND METHODS
This study was approved by the Ethical Committee of the Inova Mount Vernon Hospital (No. 15-2125). This was a retrospective radiographic review and therefore informed consent was waived.
The cohort for this study included 200 primary THAs comprised of 100 cases performed without an intraoperative radiograph (No X-ray group) and a subsequent series of 100 cases performed with an intraoperative radiograph (X-ray group). All 200 THAs were performed using the posterior approach by a single, experienced, arthroplasty surgeon at Inova Mount Vernon Hospital. The 100 THAs performed prior to the use of intraoperative radiographs were performed between April and December of 2014. The 100 THAs performed using intraoperative radiographs to assist with positioning of the implant components were performed between March and December of 2016. During the period between the two cohorts the surgeon intermittently used intraoperative radiographs while optimizing his radiographic technique.
The No X-ray group included 82 Summit stems (DePuy, Warsaw, IN, USA), 16 AML (DePuy), one Accolade II (Stryker, Mahwah, NJ, USA), and one Wagner Cone (Zimmer, Warsaw, IN, USA). The X-ray group included 93 Summit stems, four cemented C-Stems (DePuy), two AMLs, and one S-ROM (DePuy). All cups had a hemispheric geometry with a porous surface for cementless implant fixation. Pinnacle cups (DePuy) were used in all 100 cases in the No X-ray group, and there were 98 Pinnacle cups (DePuy) and two Tritanium (Stryker) cups in the the X-ray group. The target cup position was 45° of abduction and 30° of anteversion with equal radiographic leg lengths and offset in all cases. Determination of cup position was based on anatomic landmarks prior to the use of an intraoperative radiograph. Particular attention was paid to the transverse acetabular ligament, amount of superior and posterior cup exposed, and the position of the cup with regard to the overall orientation of the pelvis. For assessment of leg length, a pin was inserted in the iliac crest, which was used to establish a reference point on the femur22). With the introduction of intraoperative imaging, a pin in the iliac crest was no longer used and a digital radiograph was obtained with the trial acetabular and femoral components in place while the patient remained in the lateral decubitus position. Patient positioning was adjusted with the goal of obtaining an AP pelvic radiograph with the beam centered on the pubic symphysis. Based on this radiograph, the surgeon adjusted the position of his final components as needed. The anteversion or inclination of the cup was changed when the final implant was impacted based on the discrepancy of the trial position from the desired position. Incorrect leg length or offset was adjusted with neck offset, stem position, or head length to obtain equal radiographic offset and leg length.
Supine AP pelvic digital radiographs taken at the first (nominal 4-week) postoperative visit were used for measurements of cup abduction, anteversion, leg length difference, and offset. Measurements of cup abduction and anteversion were performed using Martell's Hip Analysis Suite software (ver. 8.0.4.1; University of Chicago, Chicago, IL, USA). The individuals who performed measurements were not involved with the primary THAs included in the study population. Leg length measurement was based on the relative distance from the lesser trochanters to the transischial line while offset was measured from the pubic symphysis to an equivalent point on the lesser trochanters (Fig. 1). Radiographs were calibrated based on the known size of the femoral head on the postoperative radiograph. Cases were excluded if the 4-week radiographs were not adequate to make a measurement due to poor contrast, symphysis rotation more than 1 cm from the center of the sacrum, pelvic obliquity more than 10°, or severe preoperative bony deformity. Thirty six patients were excluded from the study population, including five cases where the edges of the acetabular component were not well-defined, 20 cases in which the pelvic radiograph was overly angulated or rotated, and 11 cases with bony deformity on either the surgical or contralateral hip that made equal leg lengths inappropriate (including four hips with femoral deformity, three posttraumatic, two with dysplasia, one with head collapse, and one with a periprosthetic fracture). Among the cohorts, the number of exclusions was similar, with 19 in the No X-ray group and 17 in the X-ray group (P=0.76). Accounting for these exclusions, the 100 hips in the No X-ray group were derived from a consecutive series of 119 primary THAs and the 100 hips in the X-ray group were derived from a consecutive series of 117 primary THAs. Patients included in each group were similar with regard to age, sex, and body mass index (Table 1).
Fig. 1. The 4-week postoperative radiograph from a left total hip arthroplasty performed without an intraoperative radiograph (No X-ray group) shows the cup at 44° of abduction (yellow line) and 30° of anteversion (yellow ellipse). The left leg is 6.5 mm (8.3–1.8 mm) shorter than the right leg and has 5.9 mm (100.6–94.7 mm) less offset.
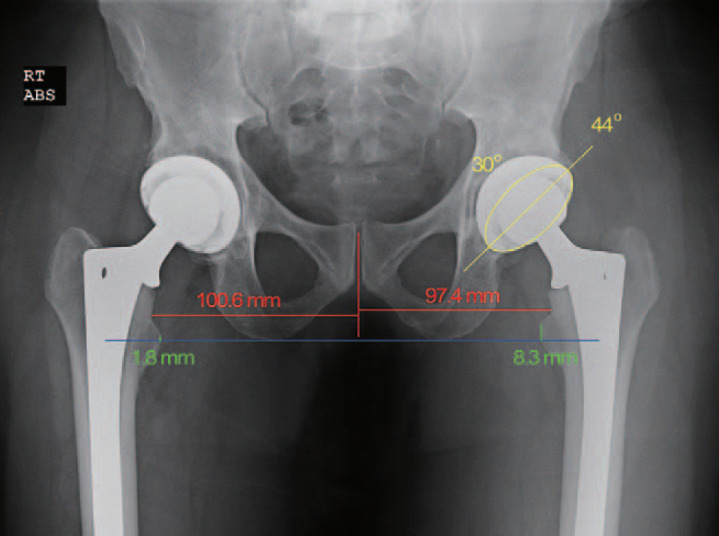
Table 1. Summary of Demographic Information for Both Cohorts.
| Parameter | No X-ray group | X-ray group | P-value |
|---|---|---|---|
| No. of THAs | 100 | 100 | N/A |
| Age at surgery (yr) | 64±10 (31–86) | 65±10 (37–89) | 0.69 |
| Female | 48% | 54% | 0.40 |
| Body mass index (kg/m2) | 29.1±5.4 (19.4–47.6) | 28.5±5.1 (18.4–44.1) | 0.41 |
Values are presented as number only, mean±standard deviation (range), or % only.
THA: total hip arthroplasty, N/A: not applicable.
For statistical analyses, categorical variables are summarized using percentages based on frequencies and continuous variables are reported using means, standard deviations, medians, and ranges. Comparisons between the groups were performed using parametric (independent samples t-test) or nonparametric (Mann—Whitney U) tests based on the nature of the data under consideration. Differences in variances among groups were assessed using Levene's homogeneity of variance test. Comparisons of binary categorical data were evaluated using Pearson's chi-square test. A P-value of 0.05 was defined as the threshold for statistical significance. Based on the 100 hips included in each group, this study had a power of 80% to detect a 15% difference (75% vs 90%) in cup placement accuracy (defined as cups within 10 degrees of the target anteversion and inclination angles) based on a two-tailed test using a criterion for significance (alpha) equal to 0.05. Statistical analyses were performed using IBM SPSS Statistics (ver. 27.0.1.0; IBM, Armonk, NY, USA).
RESULTS
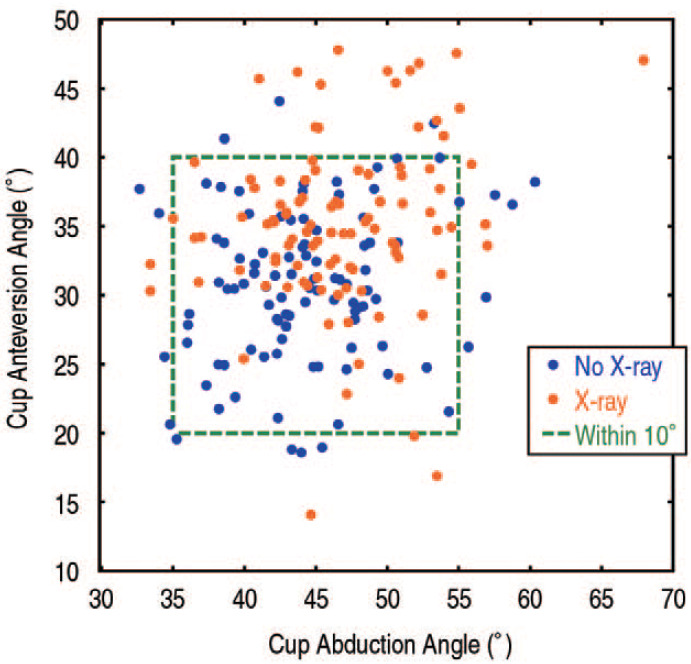
The mean cup abduction angle was 44° for the No X-ray group (Fig. 1) and 47° for the X-ray group (Fig. 2). In the No X-ray group, 90% of cups were within 10° of the 45° abduction target compared to 93% in the X-ray group (P=0.45; Table 2). Anteversion angles within 10° of the 30° target were achieved in 93% of cups in the No X-ray group and 81% of cups in the X-ray group (P=0.01). Considering both abduction and anteversion, a similar distribution of the cases around the target angles was observed in both study groups. In the No X-ray group 83% of cups and in the X-ray group 76% of cups had measurements within 10° of the target values (P=0.22; Fig. 3).
Fig. 2. The 4-week postoperative radiograph from a right total hip arthroplasty performed using an intraoperative radiograph (X-ray group) shows the cup at 47° of abduction (yellow line) and 35° of anteversion (yellow ellipse). The right leg is 6.6 mm (25.8–19.2 mm) longer than the left leg and has 1.5 mm (116.7–115.2 mm) more offset.
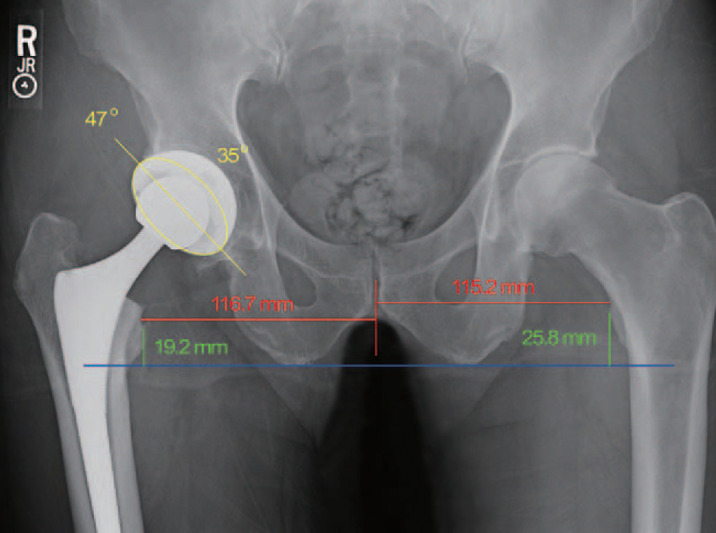
Table 2. Outcome Data.
| Parameter | No X-ray group | X-ray group | P-value |
|---|---|---|---|
| No. of THAs | 100 | 100 | N/A |
| Cup abduction angle (°) | 44±6 (33–60) | 47±6 (33–68) | 0.003 |
| Cup abduction angle within 10° of 45° target | 90% | 93% | 0.45 |
| Cup anteversion angle (°) | 31±6 (19–44) | 35±6 (14–48) | <0.001 |
| Cup anteversion angle within 10° of 30° target | 93% | 81% | 0.01 |
| Abduction and anteversion angles both within 10° of target | 83% | 76% | 0.22 |
| Leg length difference (mm) | 0.3±4.8 (−15.4 to 16.1) | 0.3±3.8 (−9.4 to 10.0) | 0.94 |
| Leg length difference within 5 mm | 73% | 82% | 0.13 |
| Offset difference (mm) | 0.3±6.9 (−14 to 17) | 1.1±6.6 (−15 to 17) | 0.42 |
| Offset difference within 5 mm | 48% | 56% | 0.26 |
Values are presented as number only, mean±standard deviation (range), or % only. Leg length and offset differences are calculated as the study hip measurement minus the contralateral side measurement.
THA: total hip arthroplasty, N/A: not applicable.
Fig. 3. Cup orientation data for total hip arthroplasties (THAs) performed with (X-ray group) and without an intraoperative radiograph (No X-ray group) illustrate similar percentages within 10° (green dashed box) of the 45° abduction and 30° anteversion target angles. However, cases performed without an intraoperative radiograph tended to be closer to the 30° anteversion target.
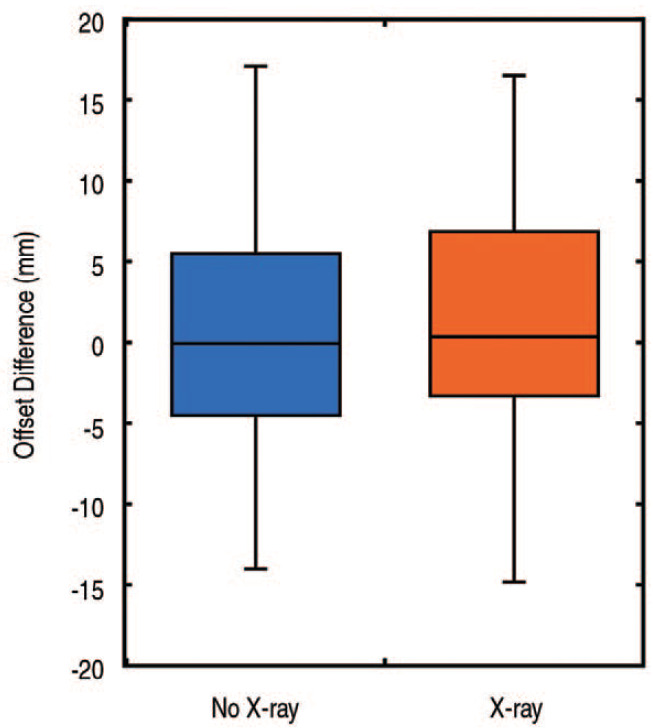
Radiographic leg lengths were within 5 mm of the contralateral hip for 73% of patients in the No X-ray group and 82% of patients in the X-ray group (P=0.13; Table 2). The mean difference in leg lengths between the operative and contralateral side was nearly identical for the No X-ray and X-ray groups (0.3±4.8 mm vs 0.3±3.8 mm, respectively, P=0.94). However, the X-ray group showed less variation (P=0.05; Fig. 4). No differences with regard to offset were observed between the No X-ray and X-ray groups (0.3±6.9 mm vs 1.1±6.6 mm, respectively, P=0.42; Fig. 5).
Fig. 4. A box and whiskers plot of the leg length differences shows similar mean values, but the cases performed with an intraoperative radiograph show less variation (P=0.05).
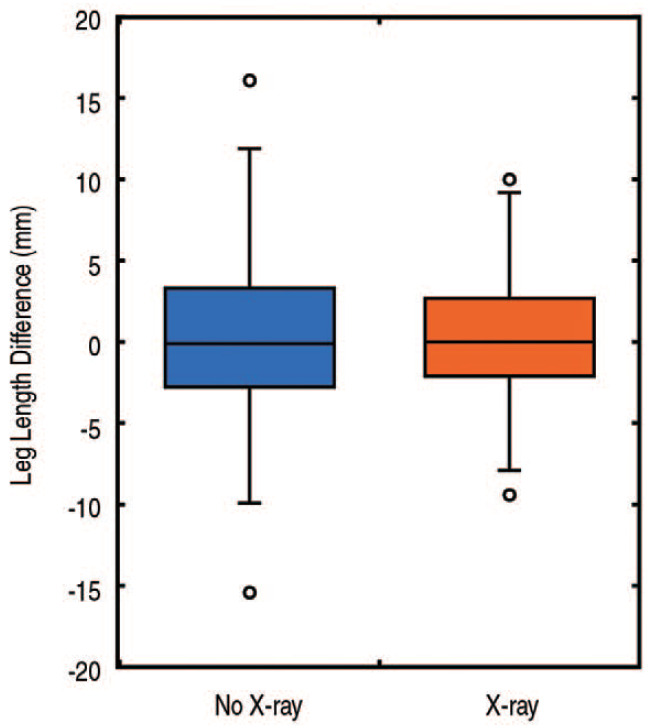
Fig. 5. A box and whiskers plot of the offset differences illustrates similar values for the cases done with and without an intraoperative radiograph.
DISCUSSION
This retrospective review of 200 routine THAs performed by an experienced arthroplasty surgeon using a posterior approach showed similar results for component placement with regard to cup abduction, leg length, anteversion, and offset regardless of whether an intraoperative radiograph was used to assist with component positioning. While prior studies have examined the use of intraoperative imaging and other techniques for various aspects of component positioning (Table 3)6,11,12,13,14,15,16,17,18,19,20,21,23,24,25,26,27), this study provides a comprehensive assessment of component positioning by reporting cup abduction, anteversion, leg length, and offset for primary THAs performed using the posterior approach.
Table 3. Component Positioning Data.
| Study | Design | Surgical approach | Component positioning technique | No. of cases | Cup abduction angle (°) | Cup anteversion angle (°) | Criteria for acceptable cup placement | Cases meeting criteria (%) | Leg length difference (mm) | Offset (mm) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abduction | Anteversion | Both | ||||||||||
| Matta et al.23) | Retrospective | Direct anterior | Fluoroscopy | 494 | 42±4 | 19.4±5.2 | Abduction: 35°–50° |
96 | 93 | NR | 3±2 | NR |
| Anteversion: 10°–25° | ||||||||||||
| Rathod et al.11) | Retrospective | Posterior | Freehand | 293 | 41.2±7.0 | 24.0±8.7 | Abduction: 30°–50° |
86 | 77 | NR | NR | NR |
| Anteversion: 10°–30° | ||||||||||||
| Direct anterior learning curve | Fluoroscopy | 96 | 39.3±5.1 | 20.2±6.3 | 95 | 91 | NR | NR | NR | |||
| Direct anterior | Fluoroscopy | 286 | 40.4±4.4 | 13.3±4.0 | 98 | 97 | NR | NR | NR | |||
| Ezzet and McCauley12) | Retrospective | Posterior | X-ray | 200 | 43.7±5.5 | NR | Surgeon-specific abduction angles ranging from 35°–55° |
86 | NR | NR | 1.5±5.6 | NR |
| Leucht et al.13) | Retrospective | Posterior | Freehand | 100 | 40.8±5.0 | 35.3±7.1 | Abduction: 30°–50° |
96 | 58 | 57 | 2.7±5.2 | NR |
| Anteversion: 15°–35° | ||||||||||||
| Direct anterior | Fluoroscopy | 100 | 43.4±5.6 | 25.9±8.2 | 89 | 80 | 73 | 0.7±3.7 | NR | |||
| Hamilton et al.14) | Retrospective | Posterior | Freehand | 100 | 44.3±6.5 | 22.6±6.2 | Abduction: 30°–50° |
79 | 64 | NR | NR | NR |
| Anteversion: 5°–25° | ||||||||||||
| Direct anterior | Fluoroscopy | 100 | 44.2±5.0 | 17.6±4.5 | 90 | 92 | NR | NR | NR | |||
| Goodman et al.15) | Retrospective | Direct anterior | Freehand | 100 | 37.5±7.4 | 24.9±5.5 | Abduction: 30°–50° |
76 | 79 | NR | NR | NR |
| Anteversion: 10°–30° | ||||||||||||
| Fluoroscopy | 100 | 43.2±4.5 | 21.8±4.6 | 94 | 93 | NR | NR | NR | ||||
| Lin et al.16) | Retrospective | Posterior | Freehand | 86 | NR | 18.9 | Abduction: 30°–50° |
85 | 72 | NR | 64% <5 mm difference |
68% <5 mm difference† |
| Anteversion: 5°–25° | ||||||||||||
| Direct anterior | Fluoroscopy | 108 | NR | 22.7 | 96 | 66 | NR | 60% <5 mm difference |
75% <5 mm difference† |
|||
| Penenberg et al.6) | Retrospective | Posterior | X-ray | 369 | 39.5±4.6 | 26.6±4.7 | Abduction: 30°–50° |
97.8 | 97.6 | NR | NR | NR |
| Anteversion: 15°–35° | ||||||||||||
| Bingham et al.17) | Retrospective | Direct anterior | Freehand | 140 | 39.9 | 31.1 | Abduction: 30°–50° |
NR | NR | NR | 0.8 | NR |
| Anteversion: 15°–35° | ||||||||||||
| Fluoroscopy | 125 | 39.4 | 30.2 | NR | NR | NR | 1.1 | NR | ||||
| Gililland et al.18) | Retrospective Comparative | Direct anterior | Fluoroscopy | 60 | NR | NR | Abduction: 30°–50° |
83 | NR | NR | 88% within 10 mm | 67% within 10 mm* |
| Fluoroscopy with grid | 39 | NR | NR | 97 | NR | NR | 100% within 10 mm | 85% within 10 mm* | ||||
| Sariali et al.24) | Prospective RCT | Direct anterior | Freehand | 28 | 43.8±7.7 | 24.8±6.9 | Abduction: 30°–50° |
86 | 61 | 54 | NR | NR |
| Anteversion: 5°–25° | ||||||||||||
| CT-based 3D planning | 28 | 37.6±6.1 | 12.6±6.2 | 86 | 93 | 79 | NR | NR | ||||
| Lass et al.25) | Prospective RCT | Modified transgluteal in supine position | Freehand | 63 | 37.7±5.2 | 17.3±10.4 | Abduction: 30°–50° |
92 | 63 | 56 | 4.4±5.6 | NR |
| Anteversion: 5°–25° | ||||||||||||
| Imageless navigation | 62 | 38.6±3.6 | 19.5±4.6 | 100 | 90 | 90 | 2.7±3.8 | NR | ||||
| Weber et al.19) | Prospective RCT | Anterolateral in lateral decubitus position | Fluoroscopy | 61 | NR | NR | NR | NR | NR | NR | 0.6±4.1 | −0.4±3.9*, 2.1±3.9† |
| Imageless navigation | 55 | NR | NR | NR | NR | NR | 0.4±2.2 | −0.6±1.9*, 0.4±2.7† |
||||
| Redmond et al.26) | Retrospective | Posterior | Robotic-assisted (Mako) | 105 | 40.1±3.4 | 20.9±3.6 | NR | NR | NR | NR | 1.9±2.8 | 0.1±4.4* |
| Direct anterior | 41 | 41.5±5.9 | 22.3±4.8 | NR | NR | NR | 2.3±3.7 | −2.4±4.2* | ||||
| Kalteis et al.27) | Prospective RCT | Modified transgluteal approach in supine position | Freehand | 30 | 43.7±7.3 | 22.2±14.2 | Abduction: 30°–50° |
63 | 70 | 47 | NR | NR |
| Anteversion: 5°–25° | ||||||||||||
| CT-based navigation (VectorVision Hip 3.0) | 30 | 41.6±4.0 | 10.7±5.3 | 100 | 83 | 83 | NR | NR | ||||
| Imageless navigation (VectorVision Hip 3.0 landmark-based module) | 30 | 43.2±4.0 | 15.2±5.5 | 97 | 93 | 93 | NR | NR | ||||
| Domb et al.20) | Retrospective | Posterior | Freehand with alignment guide | 708 | 41.7±5.3 | 21.8±6.1 | Abduction: 30°–50° |
NR | NR | 69 | 3.4±3.0 | 4.7±3.8 |
| Anteversion: 5°–25° | ||||||||||||
| X-ray | 59 | 41.9±7.3 | 19.6±9.0 | NR | NR | 64 | 3.7±2.9 | 3.9±3.3 | ||||
| Robotic-assisted (Mako) | 135 | 40.1±3.3 | 16.9±3.9 | NR | NR | 98 | 3.3±2.5 | 4.3±3.5 | ||||
| Direct anterior | Fluoroscopy | 942 | 42.0±5.1 | 20.4±7.2 | NR | NR | 73 | 2.6±2.5 | 3.5±3.0 | |||
| CT-based navigation (VectorVision Hip 3.0) | 43 | 44.7±2.9 | 14.8±5.1 | NR | NR | 91 | 1.8±1.2 | 3.1±2.6 | ||||
| Robotic-assisted (Mako) | 93 | 40.8±4.9 | 19.4±4.8 | NR | NR | 87 | 3.0±2.6 | 3.9±3.1 | ||||
| Hamilton et al.21) | Prospective RCT | Direct anterior | Fluoroscopy | 100 | 42.3±4.1 | 21.8±3.6 | Abduction: 30°–50° |
98 | 99 | 97 | NR | NR |
| Anteversion: 10°–30° | ||||||||||||
| Fluoroscopy with positioning software | 100 | 40.4±3.5 | 20.8±3.0 | 100 | 99 | 99 | NR | NR | ||||
| Current study | Retrospective | Posterior | Freehand | 100 | 44±6 | 31±6 | Abduction: 35°–55° |
90 | 93 | 83 | 0.3±4.8 | 0.3±6.9 |
| Abduction: 20°–40° | ||||||||||||
| X-ray | 100 | 47±6 | 35±6 | 93 | 81 | 76 | 0.3±3.8 | 1.1±6.6 | ||||
Values are presented as number only, mean±standard deviation, or % only.
NR: not reported, RCT: randomized controlled trial, CT: computed tomography.
*Global offset, †Femoral offset.
Similar to our results, Domb et al.20), who compared freehand placement using an alignment guide to the use of an intraoperative radiograph, found no improvements in component position for the posterior approach. In addition, Bingham et al.17) found no clinical or statistically-significant difference in cup positioning or leg length discrepancy in a comparison of THAs performed using intraoperative fluoroscopy to those performed without imaging among 298 patients undergoing supine anterior approach THA performed by two experienced surgeons. They concluded that equivalent radiographic results and leg length differences are achievable without the use of intraoperative imaging. In contrast, prior studies have reported the utility of intraoperative radiographs based on the frequency of intraoperative component repositioning. Penenberg et al.6) reported that among 369 consecutive patients undergoing THA via the posterior approach, 28% of cups were repositioned on the basis of intraoperative radiographic measurements, and abduction angles within 30°–50° and anteversion within 15°–35° was achieved for over 97% of cases. Ezzet and McCauley12), who also examined the use of intraoperative radiographs using the posterior approach found that 50% of the component positions were changed based on the imaging, with acceptable cup abduction angles achieved in 86% of cases. Although these studies demonstrate excellent final component position, they lack a comparison to a control group where intraoperative radiographs were not used.
Several studies have demonstrated improvements in cup position using intraoperative imaging with the anterior approach compared to the posterior approach without intraoperative imaging11,13,14,16). However, use of different surgical approaches confounds the ability to assess the utility of intraoperative imaging. Other options for optimizing intraoperative component positioning include computer navigation or robot-assisted surgery. Despite the high accuracy reported with use of these techniques (Table 3)9,10), they also have challenges, which may include increased operative time, additional costs, and not all surgeons have access to them.
There are several reasons why intraoperative radiographs may not have been beneficial in this study. One, obtaining a reliable AP radiograph of the pelvis intraoperatively is difficult with the patient in the lateral decubitus position and multiple studies have shown that cup position measurements are influenced by pelvic positioning28,29,30,31). Second, the intraoperative radiograph was obtained during trialing, and therefore it is possible that the final component position differed from that of the trials. Finally, even if an acceptable AP radiograph was obtained intraoperatively, the orientation of the pelvis may differ from the 4-week postoperative AP supine film despite attempts to obtain similar radiographs.
This study has limitations that should be considered when interpreting our results. The first is the retrospective nature of our analyses. Although the groups were not randomized, there were no differences in patient demographics and the surgeon's practice did not change over the interval used for this study. The 200 THAs included in this study do not represent a consecutive series. A gap between the No X-ray and X-ray groups was intentionally incorporated to exclude any potential learning curve as the surgeon optimized his intraoperative radiographic technique. Within each group, the 100 THAs are also not a consecutive series. However, exclusions were made with similar frequencies in the No X-ray and X-ray groups (16% vs 14.5%, respectively) and based on quality of postoperative radiographic images or patient anatomic deformities with no intention to omit THAs with poor component placement. While a single implant design was used for 87.5% (175/200) of the stems and 99% (198/200) of the cups, possible confounding factors might be the use of different implants and instrumentation systems. Although we measured cup abduction, anteversion, leg length, and offset, we did not quantify femoral anteversion, which contributes to combined anteversion and can influence component stability. In addition, we do not routinely obtain full length standing films at our institution. As a consequence, we used the distance from the lesser trochanter to the trans-ischial line on the 4-week followup radiograph as a proxy for leg length. Although not a true leg length, this measurement is commonly employed in the joint replacement community16,17,18,19,20,21,22,23,24,25,26,27,32). The data used for this study also represent the experience of a single surgeon who has performed more than 4,000 hip replacements over almost three decades and may not be generalizable to what could be expected for other surgeons, particularly those with less experience. Intraoperative measurements performed during surgery were not recorded. As a consequence, we cannot evaluate the differences between the trial components based on the intraoperative radiograph and the final components as measured on the 4-week follow-up radiograph. We also did not record how often the components were repositioned after the intraoperative radiograph was evaluated and a radiograph with the final components in place was not obtained.
CONCLUSION
The use of an intraoperative radiograph was not associated with clinically important improvements in final component positioning for an experienced surgeon. While an intraoperative radiograph could be useful for challenging cases or altered anatomy, this study did not find utility in its routine use for posterior approach primary THA.
Footnotes
CONFLICT OF INTEREST: The authors declare that there is no potential conflict of interest relevant to this article. The Inova Health System provided financial support that was used to undertake this research but did not restrict or define the scope of this study.
References
- 1.Abdel MP, von Roth P, Jennings MT, Hanssen AD, Pagnano MW. What safe zone? The vast majority of dislocated THAs are within the Lewinnek safe zone for acetabular component position. Clin Orthop Relat Res. 2016;474:386–391. doi: 10.1007/s11999-015-4432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadhu A, Nam D, Coobs BR, Barrack TN, Nunley RM, Barrack RL. Acetabular component position and the risk of dislocation following primary and revision total hip arthroplasty: a matched cohort analysis. J Arthroplasty. 2017;32:987–991. doi: 10.1016/j.arth.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Danoff JR, Bobman JT, Cunn G, et al. Redefining the acetabular component safe zone for posterior approach total hip arthroplasty. J Arthroplasty. 2016;31:506–511. doi: 10.1016/j.arth.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 4.DelSole EM, Vigdorchik JM, Schwarzkopf R, Errico TJ, Buckland AJ. Total hip arthroplasty in the spinal deformity population: does degree of sagittal deformity affect rates of safe zone placement, instability, or revision? J Arthroplasty. 2017;32:1910–1917. doi: 10.1016/j.arth.2016.12.039. [DOI] [PubMed] [Google Scholar]
- 5.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 6.Penenberg BL, Samagh SP, Rajaee SS, Woehnl A, Brien WW. Digital radiography in total hip arthroplasty: technique and radiographic results. J Bone Joint Surg Am. 2018;100:226–235. doi: 10.2106/JBJS.16.01501. [DOI] [PubMed] [Google Scholar]
- 7.Merle C, Grammatopoulos G, Waldstein W, et al. Comparison of native anatomy with recommended safe component orientation in total hip arthroplasty for primary osteoarthritis. J Bone Joint Surg Am. 2013;95:e172. doi: 10.2106/JBJS.L.01014. [DOI] [PubMed] [Google Scholar]
- 8.Kalteis T, Sendtner E, Beverland D, et al. The role of the transverse acetabular ligament for acetabular component orientation in total hip replacement: an analysis of acetabular component position and range of movement using navigation software. J Bone Joint Surg Br. 2011;93:1021–1026. doi: 10.1302/0301-620X.93B8.25720. [DOI] [PubMed] [Google Scholar]
- 9.Domb BG, El Bitar YF, Sadik AY, Stake CE, Botser IB. Comparison of robotic-assisted and conventional acetabular cup placement in THA: a matched-pair controlled study. Clin Orthop Relat Res. 2014;472:329–336. doi: 10.1007/s11999-013-3253-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu K, Li YM, Zhang HF, Wang CG, Xu YQ, Li ZJ. Computer navigation in total hip arthroplasty: a meta-analysis of randomized controlled trials. Int J Surg. 2014;12:528–533. doi: 10.1016/j.ijsu.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 11.Rathod PA, Bhalla S, Deshmukh AJ, Rodriguez JA. Does fluoroscopy with anterior hip arthroplasty decrease acetabular cup variability compared with a nonguided posterior approach? Clin Orthop Relat Res. 2014;472:1877–1885. doi: 10.1007/s11999-014-3512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezzet KA, McCauley JC. Use of intraoperative X-rays to optimize component position and leg length during total hip arthroplasty. J Arthroplasty. 2014;29:580–585. doi: 10.1016/j.arth.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Leucht P, Huddleston HG, Bellino MJ, Huddleston JI. Does intraoperative fluoroscopy optimize limb length and the precision of acetabular positioning in primary THA? Orthopedics. 2015;38:e380–e386. doi: 10.3928/01477447-20150504-54. [DOI] [PubMed] [Google Scholar]
- 14.Hamilton WG, Parks NL, Huynh C. Comparison of cup alignment, jump distance, and complications in consecutive series of anterior approach and posterior approach total hip arthroplasty. J Arthroplasty. 2015;30:1959–1962. doi: 10.1016/j.arth.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Goodman GP, Goyal N, Parks NL, Hopper RH, Jr, Hamilton WG. Intraoperative fluoroscopy with a direct anterior approach reduces variation in acetabular cup abduction angle. Hip Int. 2017;27:573–577. doi: 10.5301/hipint.5000507. [DOI] [PubMed] [Google Scholar]
- 16.Lin TJ, Bendich I, Ha AS, Keeney BJ, Moschetti WE, Tomek IM. A comparison of radiographic outcomes after total hip arthroplasty between the posterior approach and direct anterior approach with intraoperative fluoroscopy. J Arthroplasty. 2017;32:616–623. doi: 10.1016/j.arth.2016.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bingham JS, Spangehl MJ, Hines JT, Taunton MJ, Schwartz AJ. Does intraoperative fluoroscopy improve limb-length discrepancy and acetabular component positioning during direct anterior total hip arthroplasty? J Arthroplasty. 2018;33:2927–2931. doi: 10.1016/j.arth.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Gililland JM, Anderson LA, Boffeli SL, Pelt CE, Peters CL, Kubiak EN. A fluoroscopic grid in supine total hip arthroplasty: improving cup position, limb length, and hip offset. J Arthroplasty. 2012;27(8 Suppl):111–116. doi: 10.1016/j.arth.2012.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Weber M, Woerner M, Springorum R, et al. Fluoroscopy and imageless navigation enable an equivalent reconstruction of leg length and global and femoral offset in THA. Clin Orthop Relat Res. 2014;472:3150–3158. doi: 10.1007/s11999-014-3740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domb BG, Redmond JM, Louis SS, et al. Accuracy of component positioning in 1980 total hip arthroplasties: a comparative analysis by surgical technique and mode of guidance. J Arthroplasty. 2015;30:2208–2218. doi: 10.1016/j.arth.2015.06.059. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton WG, Parks NL, McDonald JF, 3rd, Pfefferle KJ. A prospective, randomized study of surgical positioning software shows improved cup placement in total hip arthroplasty. Orthopedics. 2019;42:42–47. doi: 10.3928/01477447-20190103-02. [DOI] [PubMed] [Google Scholar]
- 22.McGee HM, Scott JH. A simple method of obtaining equal leg length in total hip arthroplasty. Clin Orthop Relat Res. 1985;(194):269–270. [PubMed] [Google Scholar]
- 23.Matta JM, Shahrdar C, Ferguson T. Single-incision anterior approach for total hip arthroplasty on an orthopaedic table. Clin Orthop Relat Res. 2005;441:115–124. doi: 10.1097/01.blo.0000194309.70518.cb. [DOI] [PubMed] [Google Scholar]
- 24.Sariali E, Boukhelifa N, Catonne Y, Pascal Moussellard H. Comparison of three-dimensional planning-assisted and conventional acetabular cup positioning in total hip arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2016;98:108–116. doi: 10.2106/JBJS.N.00753. [DOI] [PubMed] [Google Scholar]
- 25.Lass R, Kubista B, Olischar B, Frantal S, Windhager R, Giurea A. Total hip arthroplasty using imageless computer-assisted hip navigation: a prospective randomized study. J Arthroplasty. 2014;29:786–791. doi: 10.1016/j.arth.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 26.Redmond JM, Gupta A, Hammarstedt JE, Petrakos A, Stake CE, Domb BG. Accuracy of component placement in robotic-assisted total hip arthroplasty. Orthopedics. 2016;39:193–199. doi: 10.3928/01477447-20160404-06. [DOI] [PubMed] [Google Scholar]
- 27.Kalteis T, Handel M, Bathis H, Perlick L, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty: is it as accurate as CT-based navigation? J Bone Joint Surg Br. 2006;88:163–167. doi: 10.1302/0301-620X.88B2.17163. [DOI] [PubMed] [Google Scholar]
- 28.Chen E, Goertz W, Lill CA. Implant position calculation for acetabular cup placement considering pelvic lateral tilt and inclination. Comput Aided Surg. 2006;11:309–316. doi: 10.3109/10929080601090516. [DOI] [PubMed] [Google Scholar]
- 29.Kanawade V, Dorr LD, Wan Z. Predictability of acetabular component angular change with postural shift from standing to sitting position. J Bone Joint Surg Am. 2014;96:978–986. doi: 10.2106/JBJS.M.00765. [DOI] [PubMed] [Google Scholar]
- 30.Kanazawa M, Nakashima Y, Ohishi M, et al. Pelvic tilt and movement during total hip arthroplasty in the lateral decubitus position. Mod Rheumatol. 2016;26:435–440. doi: 10.3109/14397595.2015.1092914. [DOI] [PubMed] [Google Scholar]
- 31.Mellano CR, Spitzer AI. How does pelvic rotation or tilt affect radiographic measurement of acetabular component inclination angle during THA? J Orthop. 2015;12:222–227. doi: 10.1016/j.jor.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herisson O, Felden A, Hamadouche M, Anract P, Biau DJ. Validity and reliability of intraoperative radiographs to assess leg length during total hip arthroplasty: correlation and reproducibility of anatomic distances. J Arthroplasty. 2016;31:2784–2788. doi: 10.1016/j.arth.2016.05.004. [DOI] [PubMed] [Google Scholar]


