Abstract
Background
Health services have traditionally been developed to focus on specific diseases or medical specialties. Involving consumers as partners in planning, delivering and evaluating health services may lead to services that are person‐centred and so better able to meet the needs of and provide care for individuals. Globally, governments recommend consumer involvement in healthcare decision‐making at the systems level, as a strategy for promoting person‐centred health services. However, the effects of this 'working in partnership' approach to healthcare decision‐making are unclear. Working in partnership is defined here as collaborative relationships between at least one consumer and health provider, meeting jointly and regularly in formal group formats, to equally contribute to and collaborate on health service‐related decision‐making in real time. In this review, the terms 'consumer' and 'health provider' refer to partnership participants, and 'health service user' and 'health service provider' refer to trial participants.
This review of effects of partnership interventions was undertaken concurrently with a Cochrane Qualitative Evidence Synthesis (QES) entitled Consumers and health providers working in partnership for the promotion of person‐centred health services: a co‐produced qualitative evidence synthesis.
Objectives
To assess the effects of consumers and health providers working in partnership, as an intervention to promote person‐centred health services.
Search methods
We searched the CENTRAL, MEDLINE, Embase, PsycINFO and CINAHL databases from 2000 to April 2019; PROQUEST Dissertations and Theses Global from 2016 to April 2019; and grey literature and online trial registries from 2000 until September 2019.
Selection criteria
We included randomised controlled trials (RCTs), quasi‐RCTs, and cluster‐RCTs of ‘working in partnership’ interventions meeting these three criteria: both consumer and provider participants meet; they meet jointly and regularly in formal group formats; and they make actual decisions that relate to the person‐centredness of health service(s).
Data collection and analysis
Two review authors independently screened most titles and abstracts. One review author screened a subset of titles and abstracts (i.e. those identified through clinical trials registries searches, those classified by the Cochrane RCT Classifier as unlikely to be an RCT, and those identified through other sources). Two review authors independently screened all full texts of potentially eligible articles for inclusion. In case of disagreement, they consulted a third review author to reach consensus. One review author extracted data and assessed risk of bias for all included studies and a second review author independently cross‐checked all data and assessments. Any discrepancies were resolved by discussion, or by consulting a third review author to reach consensus. Meta‐analysis was not possible due to the small number of included trials and their heterogeneity; we synthesised results descriptively by comparison and outcome. We reported the following outcomes in GRADE ‘Summary of findings’ tables: health service alterations; the degree to which changed service reflects health service user priorities; health service users' ratings of health service performance; health service users' health service utilisation patterns; resources associated with the decision‐making process; resources associated with implementing decisions; and adverse events.
Main results
We included five trials (one RCT and four cluster‐RCTs), with 16,257 health service users and more than 469 health service providers as trial participants. For two trials, the aims of the partnerships were to directly improve the person‐centredness of health services (via health service planning, and discharge co‐ordination). In the remaining trials, the aims were indirect (training first‐year medical doctors on patient safety) or broader in focus (which could include person‐centredness of health services that targeted the public/community, households or health service delivery to improve maternal and neonatal mortality). Three trials were conducted in high income‐countries, one was in a middle‐income country and one was in a low‐income country. Two studies evaluated working in partnership interventions, compared to usual practice without partnership (Comparison 1); and three studies evaluated working in partnership as part of a multi‐component intervention, compared to the same intervention without partnership (Comparison 2). No studies evaluated one form of working in partnership compared to another (Comparison 3).
The effects of consumers and health providers working in partnership compared to usual practice without partnership are uncertain: only one of the two studies that assessed this comparison measured health service alteration outcomes, and data were not usable, as only intervention group data were reported. Additionally, none of the included studies evaluating this comparison measured the other primary or secondary outcomes we sought for the 'Summary of findings' table.
We are also unsure about the effects of consumers and health providers working in partnership as part of a multi‐component intervention compared to the same intervention without partnership. Very low‐certainty evidence indicated there may be little or no difference on health service alterations or health service user health service performance ratings (two studies); or on health service user health service utilisation patterns and adverse events (one study each). No studies evaluating this comparison reported the degree to which health service alterations reflect health service user priorities, or resource use.
Overall, our confidence in the findings about the effects of working in partnership interventions was very low due to indirectness, imprecision and publication bias, and serious concerns about risk of selection bias; performance bias, detection bias and reporting bias in most studies.
Authors' conclusions
The effects of consumers and providers working in partnership as an intervention, or as part of a multi‐component intervention, are uncertain, due to a lack of high‐quality evidence and/or due to a lack of studies. Further well‐designed RCTs with a clear focus on assessing outcomes directly related to partnerships for patient‐centred health services are needed in this area, which may also benefit from mixed‐methods and qualitative research to build the evidence base.
Keywords: Humans; Infant, Newborn; Delivery of Health Care; Family; Health Services; Infant Mortality; Patient Safety
Plain language summary
When healthcare consumers (patients, carers and family members) and healthcare providers work together as partners to plan, deliver and evaluate health services, what effects does this have?
What are person‐centred health services?
Traditionally, health services have been developed by healthcare providers and focus on specific diseases or medical specialties. Involving consumers as partners in planning, delivering and evaluating health services may lead to services that are better able to meet the needs of and provide care for individuals.
Why we did this Cochrane review
Governments worldwide recommend that healthcare providers work with consumers to promote person‐centred health services. However, the effects of healthcare providers and consumers working together are unclear.
We reviewed the evidence from research studies to find out about the effects of healthcare providers and consumers working together to plan, deliver and evaluate health services.
Specifically, we wanted to know if consumers and healthcare providers working together in partnership – in the form of regular meetings in which consumers and providers were invited to contribute as equals to decisions about health services – had an impact on:
‐ changes to health services;
‐ the extent to which changes to health services reflected service users’ priorities;
‐ users’ ratings of health services;
‐ health service use; and
‐ time and money needed to make or act on decisions about health services.
We also wanted to find out if there were any unwanted (adverse) effects.
What did we do?
First, we searched the medical literature for studies that compared:
‐ consumers and healthcare providers working in partnership against usual practice or other strategies with no partnership; or
‐ different ways of working in partnership (for example, with fewer or more consumers, or with online or face‐to‐face meetings).
We then compared the results, and summarised the evidence from all the studies. Finally, we rated our confidence in the evidence, based on factors such as study methods and sizes, and the consistency of findings across studies.
What did we find?
We found five studies that involved a total of 16,257 health service users and more than 469 health service providers. Three studies took place in high income‐countries and one each in middle‐ and low‐income countries.
The studies compared:
‐ working in partnership against usual practice without partnership working (2 studies); and
‐ working in partnership as part of a wider strategy to promote person‐centred health services, against the same wider strategy without partnership working (3 studies).
No studies evaluated one form of working in partnership compared to another.
What are the main results of our review?
The studies provided insufficient evidence to determine if working in partnership had any effects compared to usual practice or wider strategies with no working in partnership.
No studies investigated:
‐ impacts on the extent to which changes to health services reflected service users’ priorities, or
‐ the resources needed to make or act on decisions about health services.
Few studies investigated:
‐ impacts on changes to health services;
‐ users’ ratings of health services;
‐ health service use; and
‐ adverse events.
The few studies that did investigate these outcomes either did not report usable information or produced findings in which we have very little confidence. These studies were small, used methods likely to introduce errors in their results and focused on specific settings or populations. Their results are unlikely to reflect the results of all the studies that have been conducted in this area, some of which have not made their results public yet.
What does this mean?
There is not enough robust evidence to determine the effects of consumers and providers working in partnership to plan, deliver or evaluate health services.
This review highlights the need for well‐designed studies with a clear focus on evaluating the effects of partnerships for promoting person‐centred care in health services. This area of research may also benefit from studies that investigate why certain partnerships between consumers and healthcare providers may be more successful than others, and an accompanying qualitative evidence synthesis addressing this aspect is forthcoming.
How up‐to‐date is this review?
The evidence in this Cochrane Review is current to April 2019.
Summary of findings
Summary of findings 1. Consumers and providers working in partnership compared with usual practice.
| Comparison 1 | |||
|
Patients or population: consumer and provider partnership participants or trial participants Settings: community, policy, teaching or health care setting Intervention: working in partnership Comparison: usual practice | |||
| Outcomes | Impacts | No. of studies | Certainty of the evidence (GRADE) |
| Health service alterations (changes to services resulting from decisions) |
No studies that comparatively evaluated this outcome were found1. | ‐ | ‐ |
| Degree to which health service alterations reflect health service user (trial participant) priorities (demand responsiveness) | No studies that evaluated this outcome were found. | ‐ | ‐ |
| Health service user (trial participant) health service performance ratings (local accountability) | No studies that evaluated this outcome were found. | ‐ | ‐ |
| Health service user (trial participant) health service utilisation patterns | No studies that evaluated this outcome were found. | ‐ | ‐ |
| Resources associated with decision‐making process | No studies that evaluated this outcome were found. | ‐ | ‐ |
| Resources associated with implementing decisions (e.g. changed services) | No studies that evaluated this outcome were found. | ‐ | ‐ |
| Adverse events | No studies that evaluated this outcome were found. | ‐ | ‐ |
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||
1One study (Persson 2013) identified problems and actions taken to address these in the intervention group only.
Summary of findings 2. Multi‐component intervention with consumers and providers working in partnership compared to the same intervention without partnership.
| Comparison 2 | |||
|
Patients or population: consumer and provider partnership participants or trial participants Settings: community, policy, teaching or health care setting Intervention: multi‐component intervention that includes working in partnership Comparison: same intervention without partnership | |||
| Outcomes | Impacts | No. of studies | Certainty of the evidence (GRADE) |
| Health service alterations (changes to services resulting from decisions) Follow‐up: 12 months (Wu 2019) to 21 months (O'Connor 2019) |
We are uncertain about the effects of multi‐component interventions on this outcome. Two studies (862 participants) were identified, both reporting little to no difference between groups, but results could not be pooled in meta‐analysis due to differences in outcome measures. |
2 | +OOO VERY LOW a,b,c,d |
| Degree to which health service alterations reflect health service user (trial participant) priorities (demand responsiveness) | No studies that evaluated this outcome were found. | ‐ | ‐ |
| Health service user (trial participant) health service performance ratings (local accountability) Follow‐up: 12 months (Greco 2006) to 21 months (O'Connor 2019) |
We are uncertain about the effects of multi‐component interventions on this outcome. Two studies (one randomised 792 participants, the other randomised 26 clusters with 8967 participants) were identified, both reporting little to no difference between groups, but result could not be pooled in meta‐analysis due to differences in outcome measures. |
2 | +OOO VERY LOW a,b,d |
| Health service user (trial participant) health service utilisation patterns Follow‐up: 12 months (Wu 2019) |
We are uncertain about the effects of multi‐component interventions on this outcome. One study (384 participants) reported little to no difference between groups. |
1 | +OOO VERY LOW a,b,d,e |
| Resources associated with decision‐making process | No studies that evaluated this outcome were found. | ‐ | ‐ |
| Resources associated with implementing decisions (e.g. changed services) | No studies that evaluated this outcome were found. | ‐ | ‐ |
| Adverse events Follow‐up: 12 months (Wu 2019) |
We are uncertain about the effects of multi‐component interventions on this outcome. One study (384 participants) reported that no harms were observed in either group. |
1 | +OOO VERY LOW a,b,d,e |
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||
aDowngraded by one level for crucial risk of bias for multiple criteria: high or unclear for methods of sequence generation (O'Connor 2019; Wu 2019); allocation concealment (Greco 2006; O'Connor 2019; Wu 2019); blinding (unclear for participants/providers/outcome assessors (Greco 2006; O'Connor 2019); providers (Wu 2019); loss to follow‐up (Greco 2006; O'Connor 2019); selective outcome reporting/analyses (Greco 2006; O'Connor 2019; Wu 2019); and other sources of bias (Greco 2006; O'Connor 2019).
bDowngraded by one level for some indirectness: compared to the review question one or more studies are restricted in setting and population (O'Connor 2019; Wu 2019).
cDowngraded by one level for some imprecision: although results were based on studies (Greco 2006; O'Connor 2019) with a total number of events >300; effect sizes are small and suggest little to no effect with the intervention but include benefit or harm. One or more studies didn't explicitly define the MID for this outcome or present CIs.
dDowngraded by one level as publication bias is strongly suspected: results come from studies unlikely to be representative of the studies that have been conducted, as protocols exist for completed trials not yet published.
eDowngraded by one level for some imprecision as the total number of events is less than 300 and the optimal sample size is not clear (Wu 2019).
Background
This review assessed the effects of consumers and health providers working in partnership, as an intervention, on health services planning, delivery and evaluation. Such partnerships may lead to more person‐centred health services. In this review, we use the term 'consumer' to mean patients, their carers and family members, in recognition of the roles that different people may undertake in health care and health care planning. We define ‘working in partnership’ as consumers and health providers making decisions together, in formal group formats (such as committees, councils, boards, or steering groups), about aspects of health service planning, delivery, or evaluation (or a combination), with the aim of making health services person‐centred (see Glossary of key terms in Appendix 1). This review was conducted concurrently with a Cochrane Qualitative Evidence Synthesis (QES) entitled Consumers and health providers working in partnership for the promotion of person‐centred health services: a co‐produced qualitative evidence synthesis (Merner 2019; Figure 1).
1.
Modified infographic comparing the Intervention effects review process (on the left) and the Qualitative evidence synthesis review process (on the right (Kaufman 2011))
Description of the condition
Historical and theoretical context of working in partnership for the promotion of person‐centred care
The concept of consumers and providers working in partnerships in healthcare decision‐making is based on paradigms of recovery, empowerment, and human, democratic, or consumer rights. The mental health consumer recovery and empowerment movement explicitly utilises consumer experiential knowledge through working in partnership, to transform and innovate services and policies (Pelletier 2011). In some countries, the impetus for working in partnership in decision‐making at the health service level, in addition to the point of care level (whether consultation or encounter), has been driven by healthcare safety and quality standards and rights. For example, the Australian National Safety and Quality Health Service Standards mandate that health service organisations partner with consumers in health governance, policy, and planning to design, deliver, and evaluate healthcare systems and services (ACSQHC 2017). The Australian Charter of Healthcare Rights states that people using the Australian healthcare system have the right to participate in decision‐making and choices about their own care, and about health service planning and policies (ACSQHC 2008). Partnership with consumers at the governance level is also becoming more common internationally (National Patient Safety Foundation 2014).
Person‐centred care definition and features
Worldwide, healthcare sectors are adopting person‐centred principles to enhance quality of care, and empower consumers to participate in their care (Byrne 2020; Delaney 2018; Mockford 2012; Stone 2008; Tritter 2003). There are various definitions of person‐centred care, but there is no single, universally accepted definition (Byrne 2020), and terms such as individualised or personalised, and patient‐, family‐, or user‐centred care are conceptually similar (Greene 2012). Common to these terms and definitions is the provision of health care that emphasises personhood and partnership (Edgman‐Levitan 2013; Hubbard 2007). This review adopts the following definition of person‐centred care: ‘planning, delivery, and evaluation of health care that is grounded in mutually beneficial partnerships among healthcare providers, patients, and families’ (IPFCC 2012).
Person‐centred care is an overarching concept or ethos, which has often been implemented at the health service level. Its implementation may also affect interactions at the point of care in many different ways. For instance, the Picker Institute identifies the following principles for person‐centred care, which underpin interventions at point of care: respecting consumer preferences and values; providing emotional support, physical comfort, information, communication, and education; continuity and transitions, co‐ordination of, and access to care; and involvement of the family and friends (Picker Institute 1987). Person‐centred care contrasts with systems‐ or provider‐centred care, which has been criticised for being paternalistic, medically dominated, and illness‐oriented (Bardes 2012; Berwick 2009).
Working in partnership for the promotion of person‐centred health services
Working in partnership may be a key intervention for the promotion of person‐centred health services. It is the focus of this review, but it is also important to note that this is only one of several elements of person‐centred care and that other elements exist outside this review's focus. Working in partnership may impact organisational leadership, strategic vision, consumer involvement, measurement and feedback of consumer experience, staff capacity building, incentives, accountability, and a culture supportive of learning and change (Edgman‐Levitan 2013; Luxford 2011). Qualitative research has identified that factors embedded within the broader health service(s) and health system, and policies are important to facilitate person‐centred care in the consultation process (i.e. at the point of care delivery (Batalden 2016; Leyshon 2015; Ogden 2017)).
At point of care, person‐centred consultations typically have three main features: eliciting and skilfully listening to the consumer’s personal narrative; encouraging the consumer’s active participation in goal setting; and documenting goals (Moore 2017). Interventions that support one or more of these features include shared decision‐making (Legaré 2014), decision aids (Stacey 2017), personalised care planning (Coulter 2011), family‐centred care (Shields 2012), or family‐initiated care escalation interventions (Mackintosh 2020). These interventions promote person‐centred care by focusing on consumer involvement in the clinical consultation process, which influences the responsiveness of care delivery at the level of individual consumers. Interpersonal and communication skills training of providers also helps to promote person‐centred care in the consultation process (Dwamena 2012; Gilligan 2021; Repper 2007).
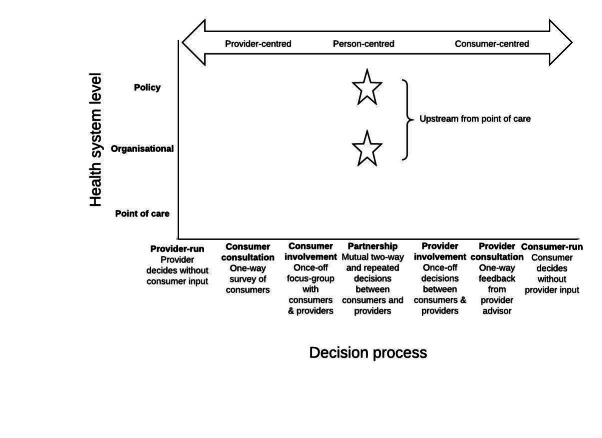
In contrast, the current review focuses on the involvement of consumers in partnership with health providers as one of the key ways in which person‐centred care can be promoted at the health service level i.e. upstream, at a higher level than the point of care (Figure 2).
2.
Decision‐making at different levels of the health system influences the person‐centeredness of health services
Description of the intervention
Defining working in partnership as an intervention
The Australian Commission for Safety and Quality in Health Care defines partnerships as "healthcare organisations, healthcare providers, and policy‐makers actively working with people who use the healthcare system, to ensure that health information and services meet people’s needs" (ACSQHC 2018). The World Health Organization (WHO) further defines partnership "as a collaborative relationship between two or more parties, based on trust, equality, and mutual understanding, for the achievement of a specified goal. Partnerships involve risks as well as benefits, making shared accountability critical” (WHO 2009). The WHO definition identifies partnerships as a form of collaboration. While ‘collaborate’ features on the participation spectrum (Arnstein 1969), and partnerships are considered an emergent process (Wildridge 2004; Wolf 2017), working in partnership is a distinct type of collaboration that occurs over a sustained time span to allow for the ongoing process of developing constructive relationships (Ocloo 2021). Hence, one‐off consumer participation in collaborations, even when they are intended to promote person‐centred care at the health service level, do not fit within the parameters of this review (Armstrong 2018; Fucile 2017; McKenzie 2017).
We included trials that evaluate the effects of working in partnership (i.e. collaborative relationships between at least one consumer and health provider, meeting jointly and regularly in formal group formats, to equally contribute to and collaborate in real‐time), on decisions intended to promote person‐centred care in one or more areas of a health service or services. These formal group formats could include committees, councils, boards, or steering groups, which meet more than once (either for an ongoing or time‐limited duration) in real‐time (face‐to‐face or virtually).
Purpose(s) of working in partnership
Promoting person‐centred care at the health service level may be achieved by working in partnership to set priorities, identify problems, design solutions, or implement initiatives that reorient the responsiveness of health services towards the information and service delivery needs and experiences of consumers (Figure 3). Partnership approaches to develop policies or identify and monitor performance indicators may also influence person‐centred care at the health service level. Working in partnership on such decisions may improve health service performance ratings of affordability, physical accessibility, acceptability, safety, quality, service availability, and accountability. Working in partnership may improve the responsiveness of health services to the consumers who use them (ACSQHC 2011; Doyle 2013; Edgman‐Levitan 2013; National Patient Safety Foundation 2014; Rathert 2012). Working in partnership on these decisional activities may result in changes that promote person‐centred health services.
3.

How working in partnership may influence person‐centred care outcomes at the health service level
In research, numerous terms connote working in partnership at the health service level. Working in partnership underpins participatory action research, co‐production, user‐centred design, experience‐based design, and co‐design (Batalden 2016; Cooke 2016; Jun 2018; Sanders 2008). Common to these collaborative decision‐making approaches is that they empower consumers at the health service level (Sanders 2008) and may reorient health services from a ‘provider‐focus’ to a ‘patient‐focus’ (Luxford 2011). Partnership approaches to decision‐making are frequently illustrated by the maxim ‘nothing about me, without me’ (Berwick 2009; Coulter 2011; Delbanco 2001; Nelson 1998) and a move from the clinical paradigm of 'what is the matter?' to 'what matters to you?' (Edgman‐Levitan 2013).
Optimising partnership working
Ottmann and colleagues caution that engagement and participation of consumers alone does not suffice to enable this shift. They argue that to ensure truly collaborative decision‐making, the contribution of stakeholder voices requires monitoring and amplification where necessary, in order to account for intrinsic power imbalances (Ottmann 2011). For example, in their research, administrative and operational ‘imperatives' dominated consumers’ voices; to address this power imbalance, the researchers adopted the role of consumer advocate (Ottmann 2011). We planned to conduct a subgroup analysis that focused on the effects of attempts to address intrinsic power imbalances in preparation for partnerships, for example, by providing a salary or financial reimbursement, orientation, training, coaching, or support (via an advocate, facilitator, moderator, or mentor). However, there were insufficient trials to do so.
How consumers are selected can also contribute to power imbalances, for example, by handpicking or inviting ‘appropriate’ or ‘acquiescent’ representatives, or by overlooking class or ethnic groups from whom comments are seldom heard (Ocloo 2016). Another power imbalance to be considered is whether the partnership is professionally dominated (Ocloo 2016). Therefore, in subgroup analysis, we planned to consider the methods of recruitment, whether the researchers ensured the inclusion of a diverse consumer or provider participant group (e.g. caregivers, vulnerable people, range of health providers) and the ratio of consumers to providers (e.g. consumer majority, provider majority, or equal). Due to too few included trials, we were unable to conduct planned subgroup analyses.
How the intervention might work
Working in partnership interventions might work by strengthening the demand responsiveness and local accountability of health services, by including consumers in health service planning and policy decision‐making (Björkman Nyqvist 2017). Responsiveness and accountability require information about (1) the needs, preferences, experiences, and priorities of consumers of the service, as well as (2) ratings of health service(s), such as performance indicators. Consumers and health providers working in partnership, means that both consumer and provider perspectives are available, and feed into health service decision‐making (Edgman‐Levitan 2013).
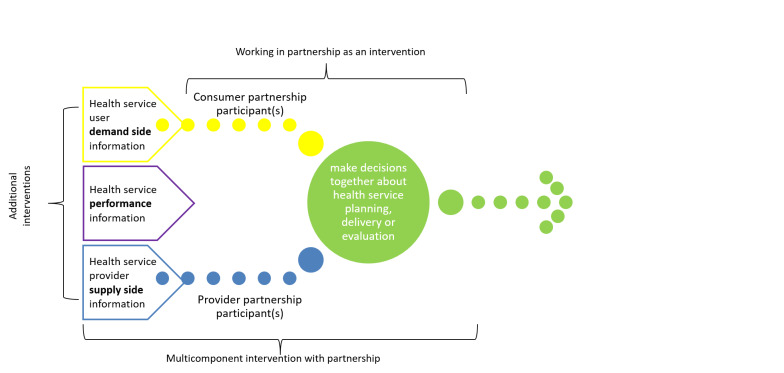
Recent trials focusing on working in partnership vary in the frame of reference for partnership decision‐making. In some trials, the consumers and health providers directly involved in the partnership (the partnership participants) approach decision‐making using their own experience as a point of reference (Björkman Nyqvist 2017; Ong 2017; Palmer 2015; Palmer 2016). In other trials, the partnership participants are explicitly required to incorporate additional information that has been gathered systematically, as part of the trial, into their decision‐making (Björkman 2009; Greco 2006; Gullo 2017; Waiswa 2016). This additional information may include the broader health service user perspectives (demand side), the broader provider perspectives (supply side), or health performance information (see Figure 4).
4.
‘Working in partnership' interventions alone and as a component of multi‐component interventions
We differentiated between working in partnership as an intervention on its own, which only incorporates the viewpoints of the consumer and provider partnership participants into their decision‐making; and working in partnership as part of a multi‐component intervention, in which partnership participants consider additional information (e.g. demand side, supply side, or performance information) that has been gathered systematically, as part of the trial, into their decision‐making.
Why it is important to do this review
The primary objective of this review is to identify whether a common type of partnering with consumers (meeting together in formal group formats) is effective in achieving person‐centred health services. This review parallels a Cochrane QES that explores consumers' and health providers' experiences of the same format (Merner 2019). We anticipate that combining the results of both reviews will provide guidance for consumers, health providers and policymakers about this form of partnership and together form a comprehensive and cohesive assessment of the evidence on partnering.
Trials evaluating the effects of upstream interventions of consumer involvement in developing health care policy, research, and services have been synthesised in other systematic reviews (Hubbard 2007; Nilsen 2006). Nilsen and colleagues focus on all forms of consumer engagement (i.e. consult, involve, collaborate and empower) (Nilsen 2006). We limit our focus to partnership approaches (i.e. collaborate but with an ongoing or time‐limited duration, excluding one‐off collaborations). We also limit our focus to decisional activities intended to promote person‐centred care in one or more areas of a health service(s), whereas Nilsen 2006 and Hubbard 2007 both focus on broader types of activities in all areas of research, policy, and healthcare services, with consumers broadly (Nilsen 2006), and people affected by cancer (Hubbard 2007). An overview of reviews of the theory, barriers and enablers for consumer and public involvement across health, social care and consumer safety has also recently been published (Ocloo 2021). However, no reviews have specifically evaluated the effects of consumers working in partnership, as an intervention to promote person‐centred health services, which is the focus here.
An earlier review in this area explored the effects of involving consumers in the planning and development of health care, but at that time, there were no comparative or experimental studies available (Crawford 2002). Crawford and colleagues identified that involving consumers contributed to changes to services. However, they also noted that the effects of involvement on quality of care (accessibility and acceptability of services) or impact on consumers' satisfaction, health, or quality of life, had not been examined (Crawford 2002). In the absence of trial evaluations, reviews based on research in this upstream context have focused on consumer participation and involvement predominantly as an agenda or aspiration, with guidance based on case studies of one‐off collaboration examples. Sharma and colleagues identified that engaging people in partnerships, shared decision‐making, and meaningful participation in health system improvement, all promoted person‐centred care (Sharma 2015).
Given recently conducted or planned trials in the area (Greco 2006; Palmer 2015; Palmer 2016), a systematic review is timely. The Cochrane Consumers and Communication Group has also identified the promotion and implementation of person‐centred care as a priority review topic (Synnot 2018; Synnot 2019).
By focusing on partnership activities, our review contributes to Cochrane's growing evidence base for interventions to promote person‐centred care, which currently has an exclusive focus on consumer participation in interventions occurring at the point of care (Coulter 2015; Dwamena 2012; Legaré 2014; Mackintosh 2020; Shields 2012; Stacey 2017).
Objectives
To assess the effects of consumers and health providers working in partnership, as an intervention to promote person‐centred health services.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), cluster‐RCTs, and quasi‐RCTs (a trial in which randomisation is attempted, but subject to potential manipulation, such as allocating participants by day of the week, date of birth, or sequence of entry into the trial), as we anticipated that few, properly conducted RCTs will have focused on consumers and health providers working in partnership.
Types of participants
We included trials in which the following groups were participants.
Consumer partnership participants. Consumer partnership participants refers to people who are fulfilling an advisory or representative role within the partnership. These roles might include a consumer or patient representative; consumer consultant; consumer with acute or chronic condition(s), their caregiver or family member; community members, general public or citizens; representatives, consultants, or members of consumer organisations.
Health provider partnership participants. Health provider partnership participants refers to people who are fulfilling an advisory or representative role within the partnership. These roles might include, for example: a clinician (such as doctor, nurse, allied health, or community health worker from any discipline), health service manager, supervisor or administrator (including quality coordinators, chief executives, etc.), health policy‐maker, or consumer liaison officer. As we are interested in partnerships between consumers and health providers, we will exclude partnerships in which health providers take on the role of consumer, or partnerships between consumers and providers who are primarily health researchers or academics.
Health service users and health service providers. Health service users and health service providers refers to the consumers and providers who are not directly involved in the partnership intervention, but are participants in trials that evaluate the effects of the partnership intervention.
Partnership groups could include multiple stakeholders, as long as the goal was to make decisions to promote person‐centred care. Partnerships could be committees that developed in‐service training or vocational education curriculum directed towards post‐registration or post‐graduate level students, as long as they included at least one consumer.
Types of interventions
We included trials evaluating the effects of consumers and health providers working in partnership as an intervention, to make decisions with the aim of promoting person‐centred care in one or more areas of a health service or services. We included trials of working in partnership in formal groups that meet face‐to‐face or virtually, more than once.
We defined person‐centred care as “planning, delivery, and evaluation of health care that is grounded in mutually beneficial partnerships among healthcare providers, patients, and families” (IPFCC 2012). Examples of person‐centred care decisions at the health service level included: identify appropriate and responsive healthcare indicators; improve continuity or follow‐up of care; service (re)development, (re)design of physical spaces, or improve coordination of care across providers and settings (or a combination).
We included trials evaluating the effects of consumers and providers working in partnership in formal groups or committees to develop in‐service training or vocational education curriculum directed towards post‐registration or post‐graduate level students (i.e. student cohort likely to be existing providers, and therefore partnership intervention may influence person‐centredness of health service).
We defined health services as public or privately funded services that provide direct care to consumers in primary (e.g. community health centres, general practitioner practices, private practices, dispensaries), secondary (e.g. specialist outpatient clinics), or tertiary settings (e.g. hospitals). We included home and residential services only when they primarily provide health or nursing care (e.g. home‐based nursing services, nursing homes, residential rehabilitation services, or hospices).
Working in partnership has three key components (see Table 3): (1) both consumer and provider participants meet (2) jointly (e.g. face‐to‐face, online, phone) in a formal group format regularly (e.g. over time, more than once), to (3) to consider or make an actual decision that relates to the person‐centredness of health service(s).
1. Key components of working in partnership versus usual practice.
| Key components of working in partnership | (1) Partnership participant types | (2) Joint formal group format, meets over time | (3) Decision relates to person‐centeredness of health service | |||
| Working in partnership as an intervention | At least one consumer | At least one heath service provider | Opportunity to influence deliberation and decision‐making by meeting jointly (e.g. f2f, online, phone) |
Formal group format (e.g. board, committee, council, steering or work group) |
Meets more than once (e.g. time‐limited or ongoing) |
Joint decisions about health service planning, delivery, or evaluation |
| Usual practice – may contain some, but not all, key components of working in partnership | e.g. no consumer participant, or consumer(s) involved, but not in decision‐making | (or) no health service provider participant, or provider(s) involved, but not in decision‐making | (or) group does not meet jointly e.g. independent deliberation and decision‐making |
(or) group is informal or ad‐hoc | (or) group meets only once | (or) either the consumer or health service provider participant provides feedback, or acts in an advisory or consultative capacity, rather than decision‐making, for health service planning, delivery, or evaluation |
We assess three comparisons in this review. Comparisons 1 and 2 assess the effects of partnership versus no partnership (with no other differences between groups), while Comparison 3 compares the effects of different versions of partnership.
Comparison 1. Consumers and health providers working in partnership compared to usual practice without partnership (i.e. usual ways of decision‐making may contain some but not all key components of working in partnership).
Studies for this comparison answer the question: ‘compared to usual practice without partnership, what is the effectiveness of consumers and health providers working in partnership, as an intervention?’. For example, ‘what is the effect of facilitated partnership (intervention) compared to no partnership (usual practice)?’ illustrates this comparison (Palmer 2015).
Examples of usual practice may include:
decision‐making involves some consumer input, but decisions are not made jointly;
providers independently make decisions;
decision‐making meets some key components, but group format is informal, meets once‐off, or does not meet together in real‐time.
Comparison 2. Consumers and health providers working in partnership, as part of a multi‐component intervention, compared to the same multi‐component intervention without consumers and health providers working in partnership.
Studies for this comparison answer the question: ‘what is the effectiveness of a multi‐component intervention that includes consumers and health providers working in partnership, compared to the same multi‐component intervention without partnership?’. For example, ‘what is the effect of facilitated partnership plus health service consumer (demand side) information (multi‐component intervention with partnership) compared to health service consumer (demand side) information without partnership (same multi‐component intervention without partnership)?’ serves to illustrate this comparison (Greco 2006).
For example, a multi‐component intervention may include co‐interventions, such as health service consumer or provider information (demand and supply), or health service performance information (or both). Working in partnership would be part of one multi‐component intervention arm, but not the other.
Comparison 3. One form of consumers and health providers working in partnership, compared to another form of consumers and health providers working in partnership.
Studies for this comparison answer the question: ‘what is the effectiveness of one form, versus another, of consumers and health providers working in partnership, as an intervention?’. For example, both groups have working in partnership interventions that fulfil all key components, but the intervention and comparator groups differ in the nature of a key feature, such as partnership participant composition (ratio of consumers and providers), or frequency, or format (online format versus face‐to‐face) of the meetings.
Excluded interventions
We excluded trials where the comparison did not enable us to isolate the effects of consumers and providers working in partnership. This included:
consumers and health providers working in partnership as part of a multi‐component intervention compared to usual practice; or
consumers and health providers working in partnership, compared to an active control that does not include working in partnership (i.e. comparator is a different intervention).
In these cases, the comparisons will not allow us to evaluate the effect of working in partnership as an intervention, as the intervention and comparator groups differ on more than just the partnership component. An example illustrative of an excluded comparison is, ‘what is the effect of health service consumer (demand side) information plus partnership (multi‐component intervention with partnership) compared to no health service consumer (demand side) information and no partnership (usual practice)?’ (Boivin 2014).
As working in partnership is a distinct type of collaboration that occurs over time, we excluded one‐off collaborations involving consumers in group formats, even when they are intended to promote person‐centred care. We excluded studies that involve partnering with consumers for decision‐making about an individual's care or treatment. We also excluded studies about partnering with consumers for health services research (planning, undertaking, or disseminating research), including a health service’s management of research (research funding panels, setting research priorities, research ethics and research governance (Gray‐Burrows 2018)).
We excluded trials that examine committees that develop educational programmes or training for pre‐registration or undergraduate students, as we are interested in working in partnership as a strategy to promote person‐centred health services. Undergraduate students may not yet be employed as providers, and therefore less able to either directly or indirectly influence the person‐centredness of the health service as part of the intervention (Klein 1999).
As we are interested in consumer‐provider partnerships, we excluded studies of researchers or academics working in partnership with consumers if providers were not also partnership participants. Similarly, we excluded studies in which researchers or academics were working in partnership with health providers, if consumers were not partnership participants.
Types of outcome measures
This is the first Cochrane Review on this topic and so included a wide range of outcomes to inform future conceptual development and research. We did not identify from trials any additional outcomes that we did not anticipate at the protocol stage and that we considered important to consumers or health providers making decisions.
Health service alterations (changes to services resulting from decisions)
Addition, rationalisation, substitution, expansion, or revision of health services (e.g. changes to policies, performance indicators, resources, processes or systems, programmes, settings (e.g. relocating a stroke rehabilitation service from the hospital to the community), education, information, physical structures, or culture or values of services)
Degree to which health service alterations reflect health service user (trial participant) priorities (demand responsiveness)
Comparability of partnership decision(s) with health service user preference(s) or priorities
Health service user (trial participant) health service performance ratings (local accountability)
Physical accessibility, e.g. simplified appointment procedures, extended opening times, transport to unit, parking, signage, security
Affordability
Acceptability e.g. satisfaction, retention or disengagement of existing consumers, attracting new consumers, appointment attendance or nonattendance
Safety
Quality
Accountability
Health service user (trial participant) ratings of health service utilisation patterns
Uptake of altered services or changes in coverage
Health service provider (trial participant) outcomes
Satisfaction, staff engagement, retention or turnover, well‐being
Adverse events
Measures of complaints, harms, litigation, damage to health service reputation, staff disengagement or turnover, increased rate of consumer failure to attend appointments, etc.
Resource use
Cost (time, money) associated with decision‐making process (e.g. cost of organising and running meetings, training (providers and consumers), remuneration, coordination, or meeting space)
Cost (time, money) associated with implementing new or changes in service
Consumer (partnership participant) outcomes*
Attendance and retention rates in formal group formats
Preparedness to participate (e.g. feeling informed, motivation or empowerment to be involved, attitudes towards partnership, etc.)
Experiences of participation (e.g. satisfaction, preferences, knowledge, well‐being, involvement, etc.)
Adverse outcomes and experiences (e.g. isolation, exploitation, uncertainty, conflict, decreased well‐being, disengagement from health service)
Provider (partnership participant) outcomes*
Attendance and retention rates in formal group formats
Preparedness to participate (e.g. feeling informed, confidence, attitudes towards partnership, etc.)
Experiences of participation (e.g. satisfaction, preferences, job satisfaction, well‐being, etc.)
Adverse outcomes and experiences (e.g. dissatisfaction, worsening attitudes towards consumers, emotional exhaustion, work overload, decreased well‐being, disengagement or resigning from employment, and conflict)
Measures of partnership among provider and consumer partnership participants*
Degree of shared decision‐making involvement, capacity building, trust, etc
We expected that outcomes denoted above with a star (*) would likely be measured for both the intervention and control groups only in Comparison 3 (e.g. in head‐to‐head comparison of partnership interventions).
We did not exclude studies based on the presence or absence of outcomes reported.
Two review authors independently assigned the outcomes reported in each included study to the review’s outcome categories, and resolved any differences in categorisation by involving a third review author.
Where more than one outcome measure was available in one trial for the same outcome we planned to:
select the primary outcome that has been identified by the study authors;
where no primary outcome was identified, we planned to select the one specified in the sample size calculation;
if there were no sample size calculations, we planned to rank the effect estimates (i.e. listed them in order from largest to smallest) and select the median effect estimate;
where there were an even number of outcomes, we planned to select the outcome whose effect estimate is ranked n/2, where n is the number of outcomes.
We planned to use the selection steps above to inform the statistical analysis (i.e. pooling, synthesis). However, as there were insufficient trials for statistical analysis, we collected data on more than one outcome measure per category per trial to inform descriptive findings. Where a study reported multiple outcome measures for the same outcome, we extracted all. Review authors then met to discuss and reach consensus on the most relevant outcome measure for evaluating partnering with consumers (whether objective or subjective). This outcome was selected to take forward for the analysis of intervention effectiveness.
Timing of outcome assessment
We grouped time points into short‐, medium‐, and long‐term time points. For the purpose of meta‐analysis, we planned to select one time point for each outcome from each study. However, as we did not have sufficient numbers of trials measuring the outcomes to be able to conduct meta‐analyses, we chose to report descriptively the longest‐term time point because this was most likely to be relevant to consumers and decision makers.
Main outcomes for ‘Summary of findings’ tables
We reported the following outcomes in the ‘Summary of findings’ tables.
Health service alterations (changes to services resulting from decisions).
Degree to which changed service reflects health service user priorities (demand responsiveness).
Health service user (trial participant) ratings of health service performance (local accountability).
Health service user (trial participant) health service utilisation patterns.
Resources associated with decision‐making process.
Resources associated with implementing decisions (e.g. changed services).
Adverse events.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases (searches were initially conducted in April 2019 and then updated on 23 February 2021):
the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, to 8 April 2019);
MEDLINE Ovid* (1946 to 8 April 2019);
Embase Ovid* (1947 to 8 April 2019);
PsycINFO Ovid* (1806 to April Week 1 2019);
CINAHL EBSCO Host* (1937 to 8 April 2019);
PROQUEST Dissertations and Theses Global* (2016 to 8 April 2019).
Studies identified as potentially relevant from the updated searches run in February 2021 are listed as Studies awaiting classification, to be considered in future updates to the review.
The outputs of databases denoted above with a star (*) were sorted by the Cochrane RCT Classifier. The RCT Classifier assigned a probability (from 0 to 100) to each citation for being a true randomised trial. The titles and abstracts of any records determined by RCT Classifier to be unlikely to be an RCT (or quasi‐RCT) with the classifier scores of nine or less were screened by one review author for potential inclusion. Two authors independently screened the citations classified as likely to be an RCT. All records determined to be relevant in terms of scope at title and abstract screening stage were then screened in full text by two review authors.
We searched online trial registers including ClinicalTrials.gov at the US National Institutes of Health (from 2000 to 23 February 2021), and the WHO International Clinical Trials Registry Platform (ICTRP) (from 2000 to September 2019). For the updated search in February 2021, it was assumed that both registries were covered by updated searches of other databases, as registry records have been made available via CENTRAL since April 2019).
We present the strategy for MEDLINE Ovid in Appendix 2. We tailored this strategy to other databases and report them in Appendix 3, Appendix 4, Appendix 5, Appendix 6, Appendix 7 and Appendix 8.
We also searched Web of Science (2000 to 8 April 2019) using the ‘All databases’ option to search forward on citations of 14 selected references, used to validate the search strategy; see Appendix 9.
We restricted the search period, as the qualitative review scoping searches of this topic showed a proliferation of studies about partnering with consumers published after 2000. Additionally, the definition of person‐centred care has developed considerably over the past decades to include aspects broader than partnering with individuals during consultations. Our assessment shows that a consistent and recognisable definition of working in partnership to promote person‐centred health services has been used most often since 2000. We aimed to assess and build the evidence on what is currently accepted as partnering in the context of person‐centred health services. Therefore, in this review, we searched from 2000 onwards to exclude older, conceptually inconsistent studies. We excluded publications in languages other than English.
Searching other resources
We searched relevant grey literature sources, such as websites (e.g. the WHO, Health Quality Improvement Partnership UK, Involve UK, Health Foundation UK, Beryl Institute, James Lind Alliance, International Association for Public Participation, Institute for Patient‐ and Family‐Centered Care (formerly Picker Institute Europe), Health Issues Centre Australia, Planetree, The King's Fund, Consumer Health Organisation of Canada, Canadian Institutes of Health Research (CIHR), and the patient group ‐ One Voice Patient & Family Advisory Council, Mayo Clinic USA) during September 2019.
We attempted to contact experts in the field and authors of included studies to identify other potentially relevant studies. We searched reference lists of included studies and relevant systematic reviews.
Data collection and analysis
Selection of studies
To determine titles and abstracts that met the inclusion criteria, a subset of citations identified by searches were screened by one review author (records classified as unlikely to be RCTs by the RCT Classifier or identified through search of the clinical trials registries or other sources) and the remainder (records classified as likely to be RCTs by the RCT Classifier and those identified through the Cochrane Library search) were independently screened by at least two review authors, with consensus decisions made by a third review author. All the references identified at title and abstract stage and considered relevant by one or more review author were retrieved in full text. Two review authors independently screened all full‐text articles for inclusion or exclusion, with discrepancies resolved by discussion, and by consulting a third review author, if necessary, to reach consensus. We list all potentially relevant papers excluded from the review at this stage, with reasons provided in the ‘Characteristics of excluded studies’. We also provide citation details and any available information about ongoing studies, and collate and report details of duplicate publications, so that each study (rather than each report) is the unit of interest in the review. We report the screening and selection process in an adapted PRISMA flow chart in Figure 5 (Liberati 2009).
5.
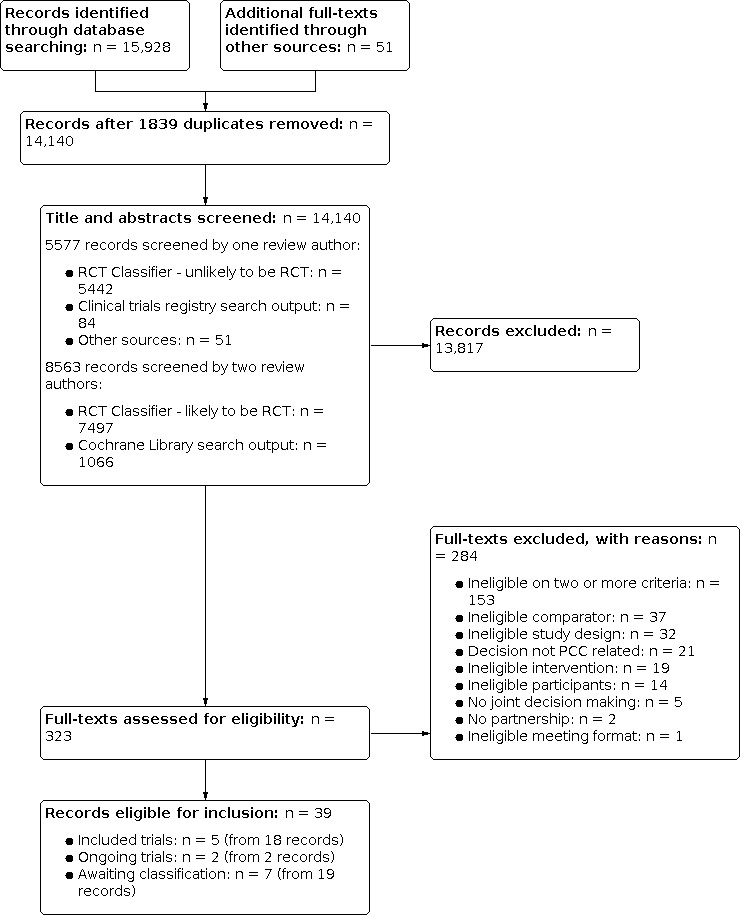
PRISMA diagram
Data extraction and management
One review author extracted data for all included studies and a second review author independently cross‐checked all data. Any discrepancies were resolved by discussion until consensus was reached. We developed and piloted a data extraction form, using Cochrane Consumers and Communication's data extraction template. We extracted data on the following items: details of the study; risk of bias items; criteria related to precision of the study (e.g. use of a power calculation); funding source and the declaration of interests for the primary investigators; details of consumers and providers; and setting. One review author entered all extracted data into Review Manager 5; a second review author independently checked entered data for accuracy against the data extraction sheets (Review Manager 2014).
Assessment of risk of bias in included studies
We assessed and report on the methodological risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions, and the Cochrane Consumers and Communication guidelines, which recommend the explicit reporting of the following individual elements for RCTs: random sequence generation; allocation sequence concealment; blinding (participants, personnel); blinding (outcome assessment); completeness of outcome data, selective outcome reporting (Higgins 2011; Ryan 2013).
For cluster‐RCTs, we also assessed and report the risk of bias associated with an additional domain, selective recruitment of cluster participants.
We planned to assess and report quasi‐RCTs as being at a high risk of bias for random sequence generation.
We considered blinding separately for different outcomes (for example, blinding may have the potential to differently affect subjective versus objective outcome measures). We judged each item as being at high, low, or unclear risk of bias as set out in the criteria provided by Higgins 2011, and provide a quote from the study report and a justification for our judgement for each item in the 'Risk of bias' table.
We deemed studies to be at the highest risk of bias if we scored them as at high or unclear risk of bias for either the sequence generation or allocation concealment domains, based on growing empirical evidence that these factors are particularly important potential sources of bias (Higgins 2011).
One review author assessed risk of bias for all included studies and a second review author independently cross‐checked all assessments, consensus was reached by resolving any disagreements through discussion. We attempted to contact study authors for additional information about the included studies, or for clarification of the study methods. We incorporated the results of the ‘Risk of bias' assessment into the review through standard tables, descriptive synthesis, and commentary about each of the elements. We provide an overall assessment of the risk of bias of included studies and a judgment about the internal validity of the review’s results.
Measures of treatment effect
For dichotomous outcomes, we planned to analyse data based on the number of events and the number of people assessed in the intervention and comparison groups. We planned to use these to calculate the risk ratio (RR) and 95% confidence interval (CI). For continuous measures, we planned to analyse data based on the mean, standard deviation (SD), and number of people assessed for both the intervention and comparison groups to calculate mean difference (MD) and 95% CI. If the MD was reported without individual group data, we used this to report the study results. If more than one study measured the same outcome using different tools, we planned to calculate the standardised mean difference (SMD) and 95% CI using the inverse variance method in Review Manager 5 (Review Manager 2014).
Unit of analysis issues
For any included cluster‐RCTs, we checked for unit‐of‐analysis errors. Where we found errors, and sufficient information was available, we planned to re‐analyse the data using the appropriate unit of analysis, by taking account of the intracluster correlation (ICC). We planned to obtain estimates of the ICC by contacting authors of included studies, or imputing them using estimates from external sources. It was not possible to obtain sufficient information to re‐analyse the data, so we reported effect estimates and annotated any unit‐of‐analysis errors.
Dealing with missing data
For participant data, where possible, we conducted analysis on an intention‐to‐treat basis; otherwise, data were analysed as reported. We reported on the levels of loss to follow‐up and reasons, and assessed this as a source of potential bias.
For missing outcome or summary data, we planned to impute missing data, and report any assumptions in the review. We planned to investigate, through sensitivity analyses, the effects of any imputed data on pooled effect estimates; however, there were too few studies to do so.
Assessment of heterogeneity
We planned to examine the heterogeneity across studies, to determine if there were considerable differences in settings, interventions, participants, and outcomes, and used this descriptive analysis to determine the most appropriate groupings of studies within each of the review's main comparisons. Where studies were considered similar enough (based on consideration of these factors) to allow pooling of data using meta‐analysis, we planned to assess the degree of heterogeneity by visual inspection of forest plots, and by examining the Chi² test for heterogeneity. Heterogeneity was to be quantified using the I² statistic. An I² value of 50% or more was to be considered to represent substantial levels of heterogeneity, and this value was to be interpreted in light of the size and direction of effects and the strength of the evidence for heterogeneity, based on the P value from the Chi² test (Higgins 2011). As there were insufficient studies pool effect estimates, we did not explore possible reasons for variability by conducting subgroup analysis.
Included studies were too dissimilar due to substantial clinical, methodological or statistical heterogeneity to pool statistically, therefore we did not report pooled results from meta‐analysis, but instead used a descriptive approach to data synthesis. There were too few studies to group studies with similar populations, intervention and methodological features to explore differences in intervention effects.
Assessment of reporting biases
We assessed reporting bias qualitatively, based on the characteristics of the included studies (e.g. to determine if only small studies that indicate positive findings are identified for inclusion).
We did not identify sufficient (i.e. 10 or more) studies for inclusion in the review, and therefore we did not construct a funnel plot to investigate small study effects, which may indicate the presence of publication bias. Therefore, we did not formally test for funnel plot asymmetry (Higgins 2011). Instead, publication bias was assessed by determining whether the included studies were likely to be representative of all relevant studies that have been conducted and where there were completed trials not yet published we considered this suggestive of publication bias.
Data synthesis
We could not conduct meta‐analyses as planned due to an insufficient number of studies identified within each comparison and each outcome. We decided not to meta‐analyse data as the included trials were not similar enough in terms of participants, settings, intervention, comparison, and outcome measures to ensure meaningful conclusions from a statistically pooled result.
As we were unable to pool the data statistically using meta‐analysis we conducted a descriptive synthesis of results. We present the major outcomes and results, organised by intervention categories according to the major types or aims (or both) of the identified interventions. Due to lack of trials, it was not possible to explore the possibility of organising the data by population, and explore heterogeneity in the results by investigating the subgroups identified below. Within the data categories, we explore the main comparisons of the review:
partnership intervention versus usual practice (without partnership);
multi‐component intervention with partnership versus multi‐component intervention without partnership;
one form of partnership intervention versus another.
If we had identified studies assessing the effects of more than one intervention, we would have compared each separately with usual practice, and with one another.
Using the synthesised quantitative findings to supplement the Cochrane qualitative evidence synthesis (QES)
The QES informed development of this review (Merner 2019). While we planned at the outset of these two pieces of work to integrate qualitative and quantitative findings from this review in the discussion (Harden 2018), the sparse data available and small number of trials included in this review did not lend itself to an in‐depth interpretation through a qualitative lens. Future updates of this review may consider looking at contextual and other information offered by the accompanying QES, but only if it is meaningful to do so.
Subgroup analysis and investigation of heterogeneity
A statistical subgroup analysis was not possible, nor was it possible to examine explanatory factors to explore the effects of interventions descriptively.
Sensitivity analysis
There were too few included studies to undertake planned sensitivity analyses.
Ensuring relevance to decisions in health care
Both the protocol for this review and the related QES protocol were co‐designed with a stakeholder advisory panel who were also to be directly involved in the production of the QES at the review stage (Merner 2019). A draft of the protocol for this review was shared with the stakeholder advisory panel prior to a stakeholder workshop day. During the workshop, the stakeholders provided the following feedback on the protocol.
Refine definitions to reflect practice e.g. consumers and health providers often ‘make decisions together’ rather than ‘sharing responsibility for decisions’.
Define terms in understandable, or lay language.
A diagram or infographic showing the differences between the effectiveness review and the QES is needed to help with clarity.
Explain the differences between point of care versus partnership in health service.
Clarify if Consumer Liaison Officer has a complaints role or advocate role.
Consider (in subgroup analyses) the role of power differentials, consumer representatives, whether led or chaired by consumers or professionals, who initiated the group, who leads partnerships, and hierarchies within service (e.g. palliative care – multidisciplinary care).
Relevant outcomes might include: participatory outcomes, such as cohesion or collaboration (perhaps in measures of increasing involvement or capacity building); consumer participation as stepping stone to higher‐level participation and involvement (i.e. capacity building, which benefits the individual consumer and the system); and personal well‐being. Decisions might result in: changes in systems or services; improved accessibility (of parking, signage, security and reduced theft); more dissemination of changed services and outcomes; rationalised services (i.e. increased focus on those that consumers want, on those that add value); growth in services (i.e. may demonstrate increased need); change of setting (e.g. hospital service to community, hospital to home setting); staff engagement, retention, etc; and financial cost savings (i.e. if experienced staff stay on, this may be more cost‐effective than adding new staff). Adverse events might include stakeholder disengagement, negative impacts on reputation, noncompliance, and failure to attend at point of care.
Relevant grey literature search sites might include: Beryl Institute, Health Foundation UK; work in Canada with First Nations (i.e. indigenous) people have led the way with community‐led engagement.
The stakeholder panel feedback resulted in the following changes to the protocol.
Changed ‘sharing responsibility for decisions’ to ‘make decisions together’ or alternatively ‘jointly make decisions’.
Glossary added to define terms (see Appendix 1).
Modified the infographic of the funnel diagram to outline the different steps in the qualitative and quantitative systematic review approaches (see Figure 1).
Developed figure to highlight the level of the health system where partnership‐based decision‐making might impact the person‐centeredness of health services (i.e. national, state, regional (policy) level, or local health service governance (organisational) level, as opposed to the direct care (point of care) level (see Figure 2)).
Removed the term 'Consumer Liaison Officer' as an example in the background, and referred instead to a consumer advocate role as a support component of facilitated partnerships.
Added to the methods our intent to consider the identified potential sub‐group analyses, if number of included trials allows.
Added the identified outcomes.
Added the grey literature resources.
A content expert provided feedback on the protocol and review, as part of Cochrane Consumers and Communication’s standard editorial process.
Summary of findings and assessment of the certainty of the evidence
Two review authors independently assessed the certainty of the evidence, using the GRADE criteria described in Schünemann 2011: methodological limitations, inconsistency, imprecision, indirectness and publication bias. We planned to prepare a ‘Summary of findings’ table for each of the three comparisons outlined above. However, we did not prepare a table for Comparison 3, as none of the included studies examined one form of partnership intervention versus another. We did not use GRADEpro software (GRADEpro GDT) to present the results of the meta‐analysis, as the findings were limited to descriptive synthesis. The seven key outcomes outlined in the Types of outcome measures section are presented in 'Summary of findings' tables for Comparison 1: Partnership intervention versus usual practice and Comparison 2: Multi‐component intervention with partnership versus multi‐component intervention without partnership.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, Characteristics of ongoing studies, and Characteristics of studies awaiting classification.
Results of the search
The combined database searches yielded 15,928 records. We obtained an additional 51 full‐text records through other sources. After removing duplicates (n = 1839), we screened 14,140 titles and abstracts. Of the title and abstracts screened, 8563 records were screened by two review authors (i.e. records classified as likely to be RCTs by RCT Classifier, n = 7497; and records obtained through Cochrane Library search, n = 1066). One review author screened 5577 other records (i.e. those classified as unlikely to be RCTs by the RCT Classifier, n = 5442; those identified by search of clinical trials registries, n = 84; and those identified through other sources, n = 51). Two review authors independently screened 323 full‐text articles. We excluded 284 full‐text records that did not meet the inclusion criteria and recorded our reasons for exclusion (see Characteristics of excluded studies).
We included five studies (reported in 18 records). One is an RCT (Jha 2015), and four are cluster‐RCTs (Greco 2006; O'Connor 2019; Persson 2013; Wu 2019). Two studies (Kjellström 2019; Sawtell 2018) are ongoing (see Characteristics of ongoing studies). Studies identified as potentially relevant from the updated searches run in February 2021 are listed as Studies awaiting classification. Seven studies (English 2018; Gai 2019; James 2013: Lindquist 2020; Morrison 2020; Palmer 2015; Shrestha 2011) reported in 19 records are awaiting classification (see Characteristics of studies awaiting classification). See Figure 5 for PRISMA diagram.
Included studies
Participants
A total of 16,257 health service users and at least 469 health service providers were trial participants in the five included studies (the number of included provider trial participants was unknown in Wu 2019). In two studies, the trial participants were health service users only (mothers with live births (Persson 2013) and pregnant women or mothers of children aged under five (O'Connor 2019)); in one study, the trial participants were health service providers only (first‐year medical trainee doctors employed in hospitals; Jha 2015); and in two studies, both health service users and providers were trial participants (Greco 2006; Wu 2019).
In two studies, all health service users were female (O'Connor 2019; Persson 2013), reflecting the trials' focus on maternal and child health; and in the other two studies the majority (63% to 68%) of health service users were female (Greco 2006; Wu 2019). None of the three studies including health service providers as trial participants provided demographic details (Greco 2006; Jha 2015; Wu 2019), although in Jha 2015 all participants were doctors in their first year after medical school and so were all considered to be at the same level. See Table 4 for more details of participants.
2. Study demographics.
| Study | Country; Degree of regional development; Healthcare setting | Partnership participants | Trial participants | Demographic details |
|
Jha 2015 RCT 283 first year trainee doctors randomised to partnership and usual practice |
Country: England Regional development: High‐income country; predominantly urban sites Healthcare setting: Post graduate medical schools at 5 hospital sites (unclear if public/privately funded) |
Consumer partners: 6 patients and 5 carers who had experienced harm or error during healthcare either to themselves or their families Provider partners: 8 clinicians involved in medical education of foundation year trainees |
Health service users: none Health service providers: 283 first year medical trainee doctors employed by 5 hospitals sites |
Consumer partners: None available (N/A) Provider partners: N/A Health service users: none; not relevant (N/R) Health service providers: N/A |
|
Persson 2013 Cluster‐RCT 90 communes* (a geopolitical unit) were randomised to partnership and usual practice *with 6306 births and neonatal mortality rate of 24/1000 live births in 2005. Communes with a lower mortality rate were excluded from the trial. |
Country: Vietnam Regional development: Middle‐income country; village sites Healthcare setting: Communities served by Commune Health Centres with 3‐6 staff members providing primary health care including reproductive and antenatal care to approximately 1000‐18,000 people. Delivery care is offered by Commune Health Centres, or by hospitals at district, province and regional levels. Each Commune Health Centre has a Village Health Worker who provides basic healthcare in the villages. |
Consumer partners: 11 members of the Women's Union recruited as lay women facilitators of Maternal and Newborn Health Groups (MNHG) Provider partners: Commune Health Centre staff (physician, midwife, nurse); a commune Village Health Worker, a population collaborator, the chairperson/vice chairperson of the commune; and two Women's Union representatives. Each of the 44 MNHGs had 8 members (352 partnership participants). |
Health service users: 1243 mothers randomly sampled from 22,377 live births located within 90 communes during first 3 years of trial July 2008‐June 2011 Health service providers: none |
Consumer partners: Age (mean): 32 years Sex: female only Education level: eligible to participate if completed secondary school. Provider partners: N/A Health service users: details for a random sample of mothers (398/7033) with live births during first year of trial: Age (%<20 years): intervention 8.9%; control 9.2% Sex: all female Education level (Lacks formal education): intervention 15%, control 21% SES (Poor household): intervention: 19%; control: 27%; Health service providers: N/R |
|
Greco 2006 Cluster‐RCT 28 health services randomised to multi‐component intervention with partnership and the same intervention without partnership |
Country: England Regional development: High‐income country; predominately coastal and rural sites Healthcare setting: Healthcare Community Practices (unclear if private or publicly funded) |
Consumer partners: 46 health service patients participated in Critical Friends Groups Provider partners: 57 health service staff members participated in Critical Friends Groups |
Health service users: 7537 patients completed baseline questionnaire and 8967 patients completed 12 month follow‐up Health service providers: 186 providers participated (109 participated both at baseline and 12 month follow‐up) |
Consumer partners: Age: 39.1% >65; 32.6% 40‐65; 28.3% <40 years; Sex: 65.2% female Provider partners: Sex: 78.9% female Health service users: Age (mean SD): Intervention: 51.47 (18.49); Control: 50.69 (18.84) Sex (female): Intervention: 68.5%; Control: 68.3% SES ‐ Jarman Material Deprivation Score (Mean range): ‐ Intervention: 2.97 (‐6.74 to 11.47); Control: 1.09 (‐9.76 to 13.3) Health service providers: N/A |
|
O'Connor 2019 Cluster‐RCT 10 communities* randomised to multi‐component intervention with partnership and the same intervention without partnership *with a maternal mortality rate of 136/10,000 live births and under‐5 mortality rate 114/1000 live births |
Country: Sierra Leone Regional development: Low‐income country; urban slum community sites Healthcare setting: communities served by Peripheral Health Units (Government primary health care facilities); communities already part of the Concern Worldwide Child Survival Project (Al Pikin Fo Liv: All Children Should Live) participated in the Operation Research Study |
Consumer partners: 49 Peer Supervisors of Community Health Workers (CHW) participated in Community Health Data Review (CHDR) meetings. Each community had 5‐12 Peer Supervisors. Provider partners: Peripheral Health Unit staff; and Health Management Committee and Ward Development Committee members participated in CHDR meetings. (30‐50 participants in each of the 10 CHDR meetings; includes Peer Supervisors) |
Health service users: 599 pregnant women or mothers of children under 5 years targeted by CHW (household respondents) at baseline and 792 household respondents at 21 months. Health service providers: none |
Consumer partners: details for CHWs overall (not just Peer Supervisors) Age (range): about two thirds 18‐34; 21% 35‐54 and 4% 55+ years Sex: 46% female Education: almost 60% completed some secondary school. Provider partners: N/A Health service users: all female but no other details Health service providers: N/R |
|
Wu 2019 Cluster‐RCT 22 community based organisations (CBOs) randomised to multi‐component intervention with partnership and the same intervention without partnership. |
Country: USA Degree of regional development: high‐income country; predominantly urban sites Healthcare setting: privately funded academic health care system comprising Johns Hopkins University School of Medicine; The Johns Hopkins Hospital; and Johns Hopkins Bayview Medical Centre |
Consumer partners: community leaders from not‐for‐profit CBOs that served adults and addressed one or more social determinants of health Provider partners: Johns Hopkins staff members as part of study team |
Health service uses: 5255 high‐risk outpatient patients (adults, at least one chronic condition, at least one visit to a Johns Hopkins site, high risk for future hospitalisation). Health service providers: Outpatient staff (case managers, community health workers, health educators and behavioural health specialists) and inpatient staff (social workers, case managers, hospitalists and nurse who help discharge patients to home) |
Consumer partners: N/A Provider partners: N/A Health service users: Age (mean): intervention: 62 years; control: 62 years Sex (female): intervention: 65%; control: 63% Education level: N/A SES (Insurance type): intervention: Medicare 64%; Priority Partners MCO 36%; control: Medicare 65%, Priority Partners MCO 35%; Health service providers: N/A |
Setting
Included studies were carried out in Sierra Leone (O'Connor 2019), UK (Greco 2006; Jha 2015), USA (Wu 2019) and Vietnam (Persson 2013). Studies included communities in high‐income settings (urban: Jha 2015; Wu 2019; coastal and rural: Greco 2006); middle‐income settings (village: Persson 2013); and low‐income settings (urban slum: O'Connor 2019). Three studies were based in primary care (Greco 2006; Jha 2015; Wu 2019) and two were based in communities, focusing on improving maternal and neonatal healthcare in communities with high neonatal, child and/or maternal mortality rates (O'Connor 2019; Persson 2013).
Interventions
To be eligible for inclusion, the intervention 'working in partnership' was required to have three key components: (1) both consumer and provider participants meet; (2) they meet jointly and regularly in formal group formats; (3) they make actual decisions that relate to the person‐centredness of health service(s). Although all included studies meet this definition, they differed greatly in terms of the participants involved, meeting purpose, formats, duration and decisions; see Table 5 for details.
3. Description of interventions.
| Study ID | Partnership intervention aim | Partnership intervention; Comparator; and Co‐interventions | Partnership format and location; Frequency and duration; Fidelity; and Tailored/modified | Decision‐making activity; Redressing power imbalances; and Training/support |
| Jha 2015 | Partnership intervention aim: to assess if facilitating trainee doctors to reflect on safety from the patient's perspectives and their experience of patient safety influences their beliefs, attitudes and intention of future behaviour. |
Partnership intervention: consumer and provider partners jointly co‐designed and co‐delivered patient safety curriculum. Comparator: usual practice, clinician‐led teaching sessions of standard patient safety curriculum (included regulatory and procedural, ethical and legal issues and communication with patients and record keeping handovers). Co‐interventions: common learning objectives derived from UK Foundation Program Curriculum adhered to throughout sessions and issues related to objectives discussed even if they did not naturally arise. |
Partnership format and location: face‐to‐face, 2x1 hour sessions of co‐developed patient safety curriculum presenting a patient narrative 15‐18 minutes in duration, followed by facilitated discussion delivered onsite in groups of 7‐10 trainee doctors. Frequency and duration: twice. time‐limited to the preparatory Patient Learning Journey workshops and in developing the training session, no other details Fidelity: aimed to standardise intervention across sites by using same co‐facilitators (patients and a trained independent chairperson) and by asking consumer partners to maintain consistent narratives. Tailored/modified: broad learning outcomes were standardized but key incidents shared in patient narratives varied. |
Decision‐making activity: partnership participants defined teaching session aims and objectives, decided on key narrative aspects, and facilitated discussion between patients and trainees. Redressing power imbalances: consumer partners provided with travel expenses and financial reimbursement for teaching and training attendance; and 2 consumer partners co‐delivered each session with a third consumer partner attending to observe and serve as reserve in case one consumer partner unable to attend. Training/Support: consumer partners provided with 4 preparatory Patient Learning Journey workshops facilitated by consumer and carer members from Patient Voice Group at University of Leeds; also given support and opportunity to debrief with a consumer (from Patient Voice Group) after co‐delivering training sessions. |
| Persson 2013 | Partnership intervention aim: to assess whether laywomen facilitation of Maternal and Newborn Health Groups (MNHGs) composed of stakeholders including health care staff, politicians and key persons in the communes can improve perinatal outcomes. |
Partnership intervention: partnership facilitators empowered and supported the MNHG members to identify local problems and actions in their communes about neonatal health, they collaborated to decide which problems to focus on and what actions to take directed towards pregnant women and their households, health services, or general public in order to address those problems. Comparator: usual practice: not described Co‐interventions: none |
Partnership format and location: face‐to‐face meetings, each lasting on average 2 hr (110 mins); meeting located at commune centres or health care centres. Frequency and duration: Monthly over a 3 year period (31 months) Fidelity: "Two research team members coordinated the facilitation process and acted as supervisors of the facilitators; i.e. field supervision and performing 2 day meetings with all facilitators once a month during the entire trial period. The intervention process was monitored continuously. Issues like MNHG's choice of improvement topics, activities for improving practice, the interaction between facilitators and group members, and progress of the facilitation process at all intervention sites were examined using several approaches, like interviews with facilitators and focus group discussions with MNHG members, analyses of facilitators diaries from MNHG meetings and the notes from the monthly meetings with the supervisors." (p 5, Eriksson 2016). See COI table for more details. Tailored/modified: Each MNHG was context‐specific and continuously negotiated and interpreted among stakeholders. |
Decision‐making activity: In MNHG Plan‐Do‐Study‐Act discussions centred on individual and common experiences in the local setting, the facilitator supported the group in critical reflection, problem identification, finding solutions and developing change strategies. The intervention strived to achieve local ownership and "bottom‐up" approach in empowering healthcare staff to improve practice. When appropriate, the facilitators would highlight recommendations in the National Guidelines. Redressing power imbalances: MNHG facilitators were paid on a full‐time basis for the 3 years of the intervention. Except for the Village Health Worker and the Women's Union employee (who were reimbursed travel expenses to and from the meetings), other members were neither paid nor received allowances ‐ as implementation work was assumed to be part of their normal duties. Training/support: 10 day training program for the facilitators "included theoretical sessions, group discussion, role‐plays, and field practice; covering topics such as group dynamics, quality improvement methods (e.g. brainstorming and the plan‐do‐study‐act cycle); and evidence based perinatal care. A facilitation manual and a specific diary were developed to guide the work of the facilitators. Two research team members coordinated the facilitation process and acted as supervisors of the facilitators, i.e. field supervision and performing 2 day meetings with all facilitators once a month during the entire trial period." (p3‐4, Persson 2013) |
| Greco 2006 | Partnership intervention aim: to assess whether Critical Friends Groups (CFGs) enable practices to interpret the results of systematic patient feedback (from the Improving Practice Questionnaire (IPQ)) and come up with an action plan that enables practices to make changes that are more patient focused. |
Partnership intervention: CFG meeting members jointly interpreted systematic IPQ patient feedback, agreed on and progressed improvement strategies. Comparator: control sites received IPQ results but no assistance (or encouragement) from researchers in setting up a CFG. Practice managers contacted at 12 months to find out about any patient involvement initiatives that had taken place during the time between their first and second IPQ. Co‐interventions: Control and intervention sites were given IPQ pilot study results |
Partnership format and location: face‐to‐face at participating practices; meeting length not described Frequency and duration: twice over a time‐limited period of approximately 12 weeks Fidelity: Not assessed. No control groups discussed IPQ results with patients during the research period. Tailored/modified: not described |
Decision‐making activity: in first CFG meeting members identified areas for attention and improvement within the practice based on IPQ results; agreed on 3‐5 improvement strategies for review at the next meeting, with meeting notes distributed to participants within a few days. In second CFG meeting members reviewed progress on improvement strategies identified earlier. Topics most frequently discussed at CFGs, after reviewing IPQ feedback, were: privacy at the reception desk (6/14 meetings) and waiting time to see doctor (12/14 meetings). Attempts to readdress intrinsic power imbalances: not described Training/Support: preliminary meetings facilitated by researchers held with patient‐only groups and with practice staff only groups at intervention sites that requested this. During preliminary meetings a researcher provided background information about the project, purpose of future meetings and the IPQ results. |
| O'Connor 2019 | Partnership intervention aim: “to 1) assess the extent to which the Participatory Community‐based Health Information System facilitated local community structures to use data to plan and implement actions for improving maternal, neonatal and child health and 2) assess the extent to which this contributed to improved community‐level maternal, neonatal and child health outcomes." (p 4, O'Connor 2019) |
Partnership intervention: Community Health Data Review (CHDR) meeting members jointly undertook a review of data collected by Community Health Workers and used the data to plan and implement actions for improving maternal, neonatal and child health practices (actions could target health services directly as well as households or communities) in addition to the Concern Worldwide Child Survival Project activities Comparator: usual practice in addition to Child Survival Project activities Co‐interventions: The Child Survival Project was implemented in all ten communities. In the broader Child Survival Project, 1325 volunteer CHWs were recruited and trained with the Ministry of Health and Sanitation 2012 National CHW Program training materials by Child Survival Project staff and the Western Area District Health Management Teams. CHWs were assigned 25 households to visit monthly and disseminate health messages, check for danger signs of illness and collect vital event and morbidity data using Ministry of Health and Sanitation registers. |
Partnership format and location: Face‐to‐face; no details on meeting length or location Frequency and duration: every two months over a 20 month period Fidelity: not described Tailored/modified: not described |
Decision‐making activity: data for the preceding 4‐6 months were reviewed in CHDR meetings. The Operation Research Study staff analysed CHW collected data with the Child Survival Project staff prior to the meeting and determined topics and data to present in CDHR meetings. "The Operation Research Study staff prepared simple data sheets to be used by participants, and participants used them to draw and interpret bar charts in front of the group. Records were kept of discussion topics." (p 5, O'Connor 2019); "Following the review of data, CHDR participants developed action points. Action points were documented during the meeting on flip chart paper which the HMC chairman kept after the meeting. Action points from previous meetings were reviewed in subsequent meetings and discussions held on the extent to which actions had been completed." (p6, O'Connor 2019) Redressing power imbalances: not described Training/support: Based on their performance during initial training, community leaders and Child Survival Project staff together selected 106 Peer Supervisors from the CHWs. Peer Supervisors received further training and were assigned 8‐12 CHWs to supervise. At least one Health Management and Ward Development Committee members from same zone as Peer Supervisor provided oversight and assistance. |
| Wu 2019 | Partnership intervention aim: to "enhance the capacity of both Community Based Organisation (CBO) staff and frontline hospital workers to address client needs by strengthening the bidirectional flow of information about health and social services and building networks that span both entities." (p e32, Wu 2019) |
Partnership intervention: In the Baltimore CONNECT meetings, consumers and providers jointly undertook a needs assessment and co‐developed an intervention (Healthify) to address clients’ needs by enhancing the health service (JHHS) and social service (CBO) co‐ordination. Comparator: usual practice‐ no other description Co‐interventions: Johns Hopkins Community Health Partnership (J‐CHiP) (not described) |
Partnership format and location: face‐to‐face meetings, 1.5 to 2 hours in duration; location rotated among the intervention CBOs (plus email and phone calls). Frequency and duration: monthly meetings over the 6 month co‐development period and the 12 month trial period Fidelity: Some contamination effects possible, as intervention and control CBOs may have shared some clients and provided services to the J‐CHiP cohort of patients. Healthify also listed both intervention and control CBOs. Tailored/modified: reverse innovation of the ACE framework by adapting the ACE approach to partner with intervention CBO leaders to co‐develop and implement a set of interventions, or toolkit. |
Decision‐making activity: "To begin, iCBO leaders completed a needs assessment to identify commonly faced challenges.The most salient issues identified were: referring clients to organisations for support, developing a stronger relationship with other CBOs to better serve clients, and interfacing with JHHS. The results of the needs assessment were directly linked to formation of strategies to enhance co‐ordination of health and social services." (p 302, Wu 2018) Redressing power imbalances: not described Training/support: no training described; each intervention site was assigned a student research assistant |
Consumer partnership participants included patients and carers with experience of harm or error during healthcare either to themselves or their families (Jha 2015); lay women members of a women’s union (Persson 2013); health service patients (Greco 2006); peer supervisors of community health workers (CHWs) (O'Connor 2019) and community leaders from not‐for‐profit community‐based organisations serving adults and addressing social determinants of health (Wu 2019).
Provider partnership participants included clinicians involved in education at graduate medical schools (Jha 2015); commune health centre staff (physician, midwife, nurse), village health workers, chairpersons/vice chairpersons and women's union representatives (village and commune levels) (Persson 2013); healthcare community practice staff (Greco 2006); peripheral health unit (government primary health care facility) staff; health management committee and ward development committee members (O'Connor 2019); and Johns Hopkins Health System (JHHS) staff members (Wu 2019).
Although meetings were all formalised, they varied in purpose. Partnership meetings included: curriculum co‐designed and delivered teaching sessions at five hospital sites (Jha 2015); maternal and newborn health group meetings in each of 44 communes (Persson 2013); 'Critical Friends Group' meetings in 28 health services (Greco 2006); community health data review meetings in ten communities (O'Connor 2019); Baltimore 'Community‐based Organizations Neighborhood Network: Enhancing Capacity Together' (CONNECT) participatory action research meetings with JHHS staff for 20 community‐based organisations (CBOs) (Wu 2019).
Decisions made in partnerships also differed in terms of directly or indirectly influencing the person‐centredness of health services. In the curriculum co‐designed and co‐delivered by consumers and providers, participants jointly developed and delivered the patient safety curriculum for first‐year postgraduate medical trainees, which influenced the person‐centredness of health service indirectly (Jha 2015). In the remaining trials, decisions and activities had a more direct impact. For instance, in maternal and newborn health group meetings, consumers and providers jointly identified local problems and agreed on actions to support the commune health centre staff and key commune stakeholders in improving perinatal health care practices. Actions were directed towards health services but also to pregnant women and their households, and to members of the general public (Persson 2013). Similarly, in 'Critical Friends Group' meetings, consumers and providers jointly interpreted systematic patient feedback and agreed on an action plan to enable practices to make changes that were more patient focused. This directly aimed to influence the person‐centredness of the health services (Greco 2006).
All partnership meetings took place face‐to‐face. Meeting length ranged from one to two hours (Jha 2015; Persson 2013; Wu 2019), and frequency and duration ranged from twice over 12 weeks (Greco 2006; Jha 2015) to monthly for three years (Persson 2013).
Training for partnership participants was reported in four studies (Greco 2006; Jha 2015; O'Connor 2019; Persson 2013). This took different forms, including a series of preparatory workshops facilitated by consumer and carer members from the Patient Voice Group at University of Leeds (Jha 2015); a facilitation manual and ten‐day training program for consumer partners (facilitators) delivered by researchers (Persson 2013); preliminary consumer‐only and provider‐only (where requested) group meetings facilitated by a researcher (Greco 2006); and training by project staff for consumer partners (peer supervisors) (O'Connor 2019).
Support for consumer partnership participants was also reported in four studies (Jha 2015; O'Connor 2019; Persson 2013; Wu 2019). This included opportunities to debrief with a consumer from the Patient Voice Group after co‐delivered teaching (Jha 2015); field supervision by two researchers and monthly two‐day meetings for consumer partners (facilitators) (Persson 2013); oversight and assistance from at least one local health management committee and ward development committee member (O'Connor 2019), and a student research assistant assigned to each intervention consumer site (CBOs) (Wu 2019).
Less common were explicit attempts to address intrinsic power imbalances (Jha 2015; Persson 2013). Where this occurred, strategies included providing travel expenses and financial reimbursement for attending teaching and training sessions, (Jha 2015) and payment on a full‐time basis for the three years of the intervention (Persson 2013).
Primary outcomes
Three trials measured health service alterations (changes to services resulting from decisions). In one trial this was in terms of problems identified and actions taken to address these (Persson 2013) but data were reported for the intervention arm only. Another study (O'Connor 2019) reported data from two outcome measures in this category: the number of mothers ever having had a visit from a CHW and number of mothers having had a home health visit from a CHW in the last year in which the CHW performed all roles. The third study (Wu 2019) measured CBO staff reports of the number of times they had received one or more referrals from healthcare staff.
No studies measured the degree to which health service alterations reflect health service user (trial participant) priorities (demand responsiveness).
Two studies measured outcomes related to health service user (trial participant) health service performance ratings (local accountability). O'Connor 2019 reported the number of mothers having had a CHW visit in the past year who found the visit helpful or somewhat helpful. In Greco 2006, overall satisfaction with this general practice (a subscale of the 'Improving Practice Questionnaire'; scale range zero to five, higher scores better) was reported.
One study (Wu 2019) identified the number of participants referred from the healthcare system to a CBO (via client report), relating to the category of health service user (trial participant) uptake of altered services or changes in coverage.
Three trials measured health service provider (trial participant) reported outcomes. Jha 2015 reported 'Attitudes to Patient Safety Questionnaire' scores from a 26‐item questionnaire with total scores ranging from 26 to 182 (higher scores indicating a more positive attitude). Persson 2013 measured healthcare staff knowledge on perinatal care, availability of equipment and drugs at health facilities, but did not report any data. The third trial (Wu 2019) reported several relevant outcomes (e.g. JHHS staff barriers to referring patients to CBOs, capacity for CBOs and healthcare organizations to work together) but data presented was not extractable for the comparison of intervention and control groups.
One study reported adverse events by descriptively reporting that no harms were observed in either group (Wu 2019).
Secondary outcomes
No studies measured resource use such as costs (time, money) associated with the decision‐making process (e.g. cost of organising and running meetings) or costs associated with implementing new or changes in services.
Consumer (partnership participant) reported outcomes were measured in three included trials (O'Connor 2019; Persson 2013; Wu 2019), but for one the data were not comparative (Persson 2013). O'Connor 2019 reported the ratio of peer supervisors reporting versus those trained which we identified as a proxy for consumer partnership participant retention rates. The third study (Wu 2019) reported the mean number of CBO staff rating that they worked together with health service moderately or extremely well.
Provider (partnership participant) outcomes were measured in two studies (O'Connor 2019; Persson 2013), but data for Persson 2013 was not comparative. O'Connor 2019 reported the health management committee's rating of their ability to fulfil their role of using health information in planning.
Two studies assessed measures of partnership among provider and consumer partnership participants. O'Connor 2019 reported on Health Management Committee (provider) rated ability to fulfil their role of reviewing and contributing to CHW activity plans. The other did not report comparative data but measured satisfaction with the partnership for consumer participants in the intervention group only (Wu 2019).
Excluded studies
We excluded 284 studies in total (see Characteristics of excluded studies). The most common reasons for exclusion were: ineligible on two or more criteria (n = 153); ineligible comparator (n = 37); ineligible study design (e.g. no attempt at randomisation) (n = 32) and decision not related to person‐centredness of health service (n = 21).
Risk of bias in included studies
See Characteristics of included studies, Figure 6 and Figure 7 for a summary assessment of the risk of bias in the included studies (Jha 2015 is not a cluster‐RCT and judgement on the domain 'selective recruitment of participants' is thus non‐applicable in Figure 6 and Figure 7). Overall, three of the five included studies were considered at highest risk of bias due to being rated at high or unclear risk of bias for the sequence generation and/or allocation concealment domains. Additionally, most of the studies had methodological limitations in the following domains: performance bias, detection bias, other sources of bias and selective recruitment of participants.
6.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
7.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Allocation
Risk of bias for random sequence generation was adequately described and rated as low in three studies (Greco 2006; Jha 2015; Persson 2013). It was unclear in one study (O'Connor 2019) as the process for the random allocation to the intervention and control group for the ten communities was not described, and high in another study (Wu 2019), as although a restricted randomisation process was conducted that constrained the allocation of organisations based on a range of factors (their ZIP code, client population size and the type of service offered) even if balance was managed on these factors there might be other potential confounders which were not considered. Allocation was adequately concealed in two studies (Jha 2015; Persson 2013), inadequate in one (Wu 2019) and measures taken were judged as unclear in two studies (Greco 2006; O'Connor 2019).
Selective recruitment of cluster participants was assessed for the four cluster‐RCTs. One was considered to be at low risk of bias, as there did not appear to be differential participant recruitment in clusters (Greco 2006), and at unclear risk of differential participant recruitment, as timing of recruitment was not mentioned (O'Connor 2019; Persson 2013; Wu 2019).
Blinding
Either participants (Jha 2015) or personnel (Persson 2013) were aware of their assignment in two studies, and as outcomes were subjective, this was judged as leading to a high risk of performance bias. For the remaining studies, blinding and potential effects on outcomes were rated as being at unclear risk of bias (Greco 2006; O'Connor 2019; Wu 2019).
One study stated that there was no blinding of outcome assessment (Jha 2015); we considered this study to have high risk of detection bias. Information provided by the trial authors for two further studies indicated that primary outcomes were assessed electronically, with statisticians analysing the data blind to group assignment (Wu 2019), or that data collectors were blind to group allocation and had no contact with those administering the intervention (Persson 2013). Both were therefore rated as at low risk of bias. We assessed the remaining two studies as having unclear risk of detection bias as there was no description of whether or not adequate steps were taken to blind outcome assessors (Greco 2006; O'Connor 2019).
Incomplete outcome data
One study was considered to have incomplete outcome data (due to 68% of participants missing from primary outcome (Attitudes to Patient Safety Questionnaire, APSQ) analysis at follow‐up; and because this was somewhat unbalanced between groups; 74% missing in the intervention and 62% in the control) and therefore at high risk of attrition bias (Jha 2015). One study was assessed as having unclear risk of attrition bias due to insufficient details on participant numbers over the course of the trial (O'Connor 2019). Three studies were considered to have low risk of attrition bias as there were no losses to follow‐up (Persson 2013); minimal incomplete outcome data (1.23% questionnaire responses excluded due to being incomplete; Greco 2006); or acceptable levels of attrition (7% both groups), for similar reasons across groups (Wu 2019).
Selective reporting
One study was considered to have high risk of reporting bias: it reported an additional outcome not mentioned in the protocol and did not report comparative data for the course evaluation mentioned in the study protocol (Jha 2015). Three studies were considered to be at unclear risk of reporting bias because although data were reported for all outcomes mentioned in the methods, we were not able to identify a trial protocol in two studies (Greco 2006; O'Connor 2019), and in the third, the protocol only listed the primary outcome measure; and there are additional outcomes in the publication (Wu 2019). The remaining trial reported all outcomes as planned, based on the protocol (Persson 2013), and was therefore assessed as having a low risk of selective reporting.
Other potential sources of bias
Three studies were considered to have unclear risk of other types of bias for the following reasons: a greater proportion of control group providers were the subject of the survey for their first time during the follow‐up, potentially explaining why overall mean scores were worse in the control group and better in the intervention group (Greco 2006); no characteristics were provided to assess baseline imbalance, participants with incomplete data for each time point were excluded from analysis, and there may have been communication between intervention and control participants (Jha 2015); there was substantial movement between communities (in and out) (O'Connor 2019). Two studies were considered to be at low risk of other potential sources of bias (Persson 2013; Wu 2019).
Effects of interventions
Comparison 1: consumers and health providers working in partnership compared to usual practice
Two included studies assessed this comparison (Jha 2015; Persson 2013). See Table 6 for extracted data and Table 1 for a summary of key findings.
4. Comparison 1 data.
| Health service alterations data (changes to services resulting from partnership decisions) | |||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group | Notes | |||||||||
| Events (n) | Total (N) | Events (n) | Total (N) | ||||||||||
| Persson 2013 | Problems identified and actions taken to address these | ‐ | ‐ | ‐ | ‐ | Intervention group data only | |||||||
| Degree to which health service alterations reflect health service user (trial participant) priorities (demand responsiveness). | |||||||||||||
| No studies assessed these outcomes | |||||||||||||
| Health service user (trial participant) health service performance ratings (local accountability) | |||||||||||||
| No studies assessed these outcomes | |||||||||||||
| Health service user (trial participant) health service utilisation patterns | |||||||||||||
| No studies assessed these outcomes | |||||||||||||
| Health service provider (trial participant) reported outcomes | |||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group | Notes | |||||||||
| Mean | 95% CI | N | Mean | 95% CI | N | ||||||||
| Jha 2015 | Overall attitudes in Attitudes to Patient Safety Questionnaire (26‐items measuring self‐reported opinion towards the causes, reporting and management of errors; overall range: 26‐182; higher scores better) (3‐6 weeks post‐training) | 134.16 | 131.01, 137.32 | 37 | 135.21 | 132.58, 137.84 | 53 | Mean of 1.05 lower in intervention group (4.20 lower, to 2.11 higher) | |||||
| Persson 2013 | Healthcare staff knowledge on perinatal care, availability of equipment and drugs at health facilities | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | No data reported | |||||
| Adverse events | |||||||||||||
| No study reported data that related adverse events for those who received partnership compared to usual practice | |||||||||||||
| Resource Use (Cost (time, money)) associated with decision‐making processor with implementing new/changes in service | |||||||||||||
| No studies reported outcomes such as resource use associated with decision‐making process (e.g. cost of organising and running meetings, training (providers/consumers), remuneration, coordination, or meeting space) or resource use associated with implementing new or changed services | |||||||||||||
| Consumer (partnership participant) reported behaviours/attitudes outcomes | |||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group | Notes | |||||||||
| Events | Total | Events | Total | ||||||||||
| Persson 2013 | Consumer partner attendance at meetings | ‐ | ‐ | ‐ | ‐ | Intervention group data only | |||||||
| Provider (partnership participant) reported behaviours/attitudes outcomes | |||||||||||||
|
Study |
Measure (Timing of assessment) | Intervention Group | Control group | Notes | |||||||||
| Events | Total | Events | Total | ||||||||||
| Persson 2013 | Provider partner attendance at meetings | ‐ | ‐ | ‐ | ‐ | Intervention group data only | |||||||
| Measures of partnership among provider and consumer partnership participants | |||||||||||||
| No trial reported these outcomes | |||||||||||||
No studies reported the following outcomes: degree to which health service alterations reflect health service user (trial participant) priorities (demand responsiveness); health service user (trial participant) health service performance ratings (local accountability); adverse events; resource use associated with decision‐making process, resource use associated with implementing new or changed services; or measures of partnership among provider and consumer partnership participants. Additionally, Persson 2013 measured the following outcomes relevant to this comparison but the data were not comparative: health service alterations (problems and actions taken to address these); consumer (partnership participant) reported outcomes (consumer partner attendance at meetings); and provider (partnership participant) reported outcomes (provider partner attendance at meetings). We are uncertain about the effects of the intervention on these outcomes, including those reported in Table 1, because of a lack of evidence.
Below we present findings organised by outcomes with comparative data.
Health service provider (trial participant) reported outcomes
Two trials assessed health service provider‐reported outcomes (Jha 2015; Persson 2013), but Persson 2013 did not present usable data; Jha 2015 reported little or no effect of the intervention on health service providers’ overall attitudes to patient safety (e.g. self‐reported opinion towards the causes, reporting and management of errors) at three to six weeks.
In summary, very low‐certainty evidence indicates that we are unsure about the effects of working in partnership compared to usual practice on health service provider reported outcomes. We had serious concerns about risk of bias for multiple domains (performance bias, detection bias, attrition bias, reporting bias, other sources of bias and selective recruitment of participants) as well as indirectness, imprecision and publication bias.
Comparison 2: multi‐component intervention with consumers and providers working in partnership compared to the same intervention without partnership
Three included studies assessed this comparison (Greco 2006; O'Connor 2019; Wu 2019). See Table 7 for extracted data and Table 2 for a summary of key findings. The timing of longest follow‐up ranged from 12 months (Greco 2006; Wu 2019) to 21 months (O'Connor 2019).
5. Comparison 2 data.
| Health service alterations data (changes to services resulting from partnership decisions) | |||||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group | Notes | |||||||||||
| Events | Total | Events | Total | ||||||||||||
| O'Connor 2019 | Number of mothers who have ever had a community health worker (CHW) visit (21 Months) |
257 |
379 | 271 | 413 | 2.2% increase in intervention group; p=0.954 | |||||||||
| O'Connor 2019 | Number of mothers who had a CHW home health visit in the last year in which the CHW performed all roles (21 months) | 79 | 379 | 87 | 413 | 0.3% decrease in intervention group; p=1.000 | |||||||||
| Wu 2019 | Community based organisation (CBO) staff report of receiving 1+ referral from healthcare staff (12 months) | 13 | 38 | 12 | 32 | 3.3% decrease in intervention group; p=0.48 (observed events calculated from % data) | |||||||||
| Degree to which health service alterations reflect health service user (trial participant) priorities (demand responsiveness). | |||||||||||||||
| No studies assessed these outcomes | |||||||||||||||
| Health service user (trial participant) health service performance ratings (local accountability) | |||||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group | Notes | |||||||||||
| Events | Total | Events | Total | ||||||||||||
| O'Connor 2019 | Number of mothers who had a CHW visit in the past year who found visit helpful or somewhat helpful (21 months) | 257 | 379 | 296 | 413 | 3.9% decrease in intervention group; p=0.246 | |||||||||
| Study | Measure (Timing of assessment) | Intervention Group | Control group | Notes | |||||||||||
| Mean | SD | N | Mean | SD | N | ||||||||||
| Greco 2006 | Overall client satisfaction with general practice (scale: 0‐5, higher scores better) (12 Months) | 4.17 | 0.19 | 14 sites (5000 respondents) | 4.23 | 0.19 | 12 sites (3967 respondents) | Mean of 0.06 lower in intervention group (no CIs reported) | |||||||
| Health service user (trial participant) health service utilisation patterns | |||||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group |
Notes |
|||||||||||
| Events | Total | Events | Total | ||||||||||||
| Wu 2019 | Referred from the healthcare system to a CBO (client report) (12 months) | 31 | 198 | 22 | 186 | 3.9% increase in intervention group; p=0.57 (observed events calculated from % data) | |||||||||
| Health service provider (trial participant) reported outcomes | |||||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group | Notes | |||||||||||
| Events | Total | Events | Total | ||||||||||||
| Wu 2019 | Provider barriers to referring patients to CBOs, capacity for CBOs and healthcare organizations to work together, confidence in knowledge about community resources, confidence in the capacity of CBOs to meet their clients’ needs, and number of referrals to CBOs (12 months) | ‐ | ‐ | ‐ | ‐ | Pre‐post data for inpatient and outpatient staff separately (but not comparatively for intervention and control group) | |||||||||
| Adverse events | |||||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group | Notes | |||||||||||
| Events | Total | Events | Total | ||||||||||||
| Wu 2019 | Harms (12 months) | ‐ | ‐ | ‐ | ‐ | No harms observed in either group | |||||||||
| Resource Use (Cost (time, money)) associated with decision‐making processor with implementing new/ changes in service | |||||||||||||||
| No studies reported outcomes such as resources use associated with decision‐making process (e.g. cost of organising and running meetings, training (providers and consumers), remuneration, coordination, or meeting space) or with implementing new or changes in services | |||||||||||||||
| Consumer (partnership participant) reported behaviours/attitudes outcomes | |||||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group |
Notes |
|||||||||||
| Events | Total | Events | Total | ||||||||||||
| O'Connor 2019 | Number of peer supervisors reporting/peer supervisors trained (21 months) | 39 | 49 | 42 | 57 | 5.9% increase in intervention group | |||||||||
| Wu 2019 | CBO staff ratings of working together with health service moderately or extremely well (12 months) | 22 | 38 | 14 | 32 | 14% increase in intervention group; p=0.24 (observed events calculated from % data) | |||||||||
| Provider (partnership participant) reported behaviours/attitudes outcomes | |||||||||||||||
|
Study |
Measure (Timing of assessment) | Intervention group | Control group |
Notes |
|||||||||||
| Events | Total | Events | Total | ||||||||||||
| O'Connor 2019 | Health management committee (HMC) use of health information in planning (21 months) | 40 | 75 | 38 | 75 | 2.7% increase in intervention group | |||||||||
| Measures of partnership among provider and consumer partnership participants | |||||||||||||||
| Study | Measure (Timing of assessment) | Intervention group | Control group |
Notes |
|||||||||||
| Events | Total | Events | Total | ||||||||||||
| O'Connor 2019 | HMC reviewed and contributed to CHW activity plans (21 months) | 31 | 75 | 35 | 75 | 5.3% decrease in intervention group | |||||||||
| Wu 2019 | iCBO partner satisfaction with the partnership self‐assessment tool (12 months) | ‐ | ‐ | ‐ | ‐ | Intervention group data only | |||||||||
CBO: COmmunity Based Organisations; CFG: Critical Friends Group; CHDR: Community Health Data Review; CHW: Community Health Workers; f2f: face‐to‐face; HMC: Health Management Committee; IPQ: Improving Practice Questionnaire; J‐CHiP: Johns Hopkins Community Health Partnership; JHHS: Johns Hopkins Health Service; MNHG: Maternal and Newborn Health Groups.
No studies reported the following outcomes: degree to which health service alterations reflected health service user preference(s) or priorities; resource use associated with decision‐making process or resource use associated with implementing new or changed services. One study (Wu 2019) measured outcomes reported by the health service providers (trial participants), but the data were not comparative; rather, data were reported in trial as pre‐post for inpatient staff and outpatient staff separately. Below we present findings, organised by outcomes with data.
Primary Outcomes
Health service alterations
Two studies (O'Connor 2019; Wu 2019) assessed health service alterations. These studies reported little or no effect of the intervention on the number of mothers who reported ever having a CHW visit; the number of mothers who reported having a home health CHW visit in the last year in which the CHW performed all roles; or the number of CBO staff who reported receiving one or more referral from healthcare staff.
In summary, very low‐certainty evidence indicates that we are unsure whether multi‐component interventions with partnership compared to without partnership result in health service alterations. We had serious concerns about risk of bias or lack of clarity across all domains (all items rated as at unclear or high risk of bias for at least one of the two studies). We also graded down for indirectness, imprecision and risk of publication bias.
Health service user (trial participant) health service performance ratings (local accountability)
Two studies (Greco 2006; O'Connor 2019) assessed the acceptability of health services to trial participants. Studies reported little or no effect of the intervention on the number of mothers who had a home health CHW visit in the past year who found the visit helpful or somewhat helpful; or health service user overall satisfaction with the general practice.
In summary, very low‐certainty evidence (due to serious concerns about risk of bias or lack of clarity across all domains (all items rated as at unclear or high risk of bias for at least one of the two studies), indirectness and publication bias) indicates that we are unsure about the effects of multi‐component interventions with partnership compared to without partnership on health service user (trial participant) health service performance ratings.
Health service user (trial participant) health service utilisation patterns
One study (Wu 2019) assessed trial participant health service utilisation patterns. There was little or no effect of the intervention on the number of clients who reported they were referred from the healthcare system to a CBO.
In summary, very low‐certainty evidence (due to serious concerns about methodological limitations (high risk of selection bias or lack of clarity across domains of performance bias, reporting bias and selective recruitment of participants), indirectness, imprecision and publication bias) indicates that we are unsure about the effects of multi‐component interventions with partnership compared to without partnership on trial participant health service utilization patterns.
Adverse events
One study (Wu 2019) descriptively reported that no harms were observed in either group.
Secondary outcomes
Consumer (partnership participant) reported outcomes
Two studies (O'Connor 2019; Wu 2019) assessed consumer partnership participant reported attitude or behaviour outcomes. There was little or no effect of the intervention on the ratio of peer supervisors reporting versus peer supervisors trained (a proxy measure for consumer partnership participant retention rates); or on community‐based organisation staff ratings of working together with health service moderately or extremely well.
In summary, very low‐certainty evidence (due to serious concerns about methodological limitations (risk of bias or lack of clarity across all domains, with all items rated as at unclear or high risk of bias for at least one of the two studies), indirectness, imprecision and publication bias) indicates that we are unsure about the effects of multi‐component interventions with partnership compared to without partnership on consumer partnership participant reported outcomes.
Provider (partnership participant) reported outcomes
One trial (O'Connor 2019) assessed provider partnership participant behaviour or attitude outcomes. There was little or no effect with the intervention on health management committee use of health information in planning.
In summary, very low‐certainty evidence indicates that we are unsure about the effects of multi‐component interventions with partnership compared to without partnership on provider partnership participant‐reported outcomes. We had serious concerns about risk of bias across all domains, as well as indirectness, imprecision and publication bias.
Measures of partnership among provider and consumer partnership participants
One trial (O'Connor 2019) assessed measures of partnership among provider and consumer partnership participants, reporting a small decrease with the intervention on the number of times health management committees reviewed and contributed to CHW activity plans. A second trial (Wu 2019) reported data about partner satisfaction with the partnership for consumer participants reported for the intervention group only.
In summary, very low‐certainty evidence indicates that we are unsure about the effects of multi‐component interventions with partnership compared to without partnership on measures of partnership among provider and consumer partnership participants. We had serious concerns about methodological limitations (all risk of bias domains items rated as at unclear or high risk of bias for at least one of the two studies), as well as indirectness, imprecision and publication bias.
Comparison 3: one form of consumers and health providers working in partnership, compared to another.
No included studies assessed this comparison.
Discussion
Summary of main results
We identified five studies; two that evaluated working in partnership interventions compared to usual practice without partnership (Comparison 1), and three that evaluated working in partnership as part of a multi‐component intervention, compared to the same intervention without partnership (Comparison 2). No studies evaluated one form of working in partnership compared to another (Comparison 3).
Our findings indicate that we are uncertain of the effects of working in partnership as an intervention to promote person‐centred health services. Primarily, this is because only a limited number of trials met our tightly defined inclusion criteria and those included were disparate in terms of settings, populations and context, outcomes measured, as well as the purpose and nature of the partnerships evaluated.
Predominantly, the evidence included in this review evaluated the effects of working in partnership as an intervention to promote person‐centred health services indirectly. For instance, only two studies examined ways to directly influence decisions about the person‐centredness of health services: Greco 2006 examined decision‐making to improve the person‐centredness of health service planning, while Wu 2019 assessed decisions about delivery related to discharge co‐ordination. In all other trials, the partnership decision‐making was either indirectly related to health service delivery or planning (e.g. via educating first‐year doctors on patient safety; Jha 2015); or the partnership aim was broader, with the health service only one of many avenues of decision‐making influence (e.g. public, households, community; O'Connor 2019 and Persson 2013).
Additionally, some studies were not primarily focused on partnership as a means of achieving person‐ centredness of health services but instead used partnering as a way of achieving clinical endpoints (such as maternal and neonatal mortality; O'Connor 2019 and Persson 2013). For this reason, many of the outcomes we sought related to person‐centredness of health service delivery were not measured.
While this review can only conclude that there are uncertain effects of partnership on a range of outcomes, there may be potential benefits or effects that are not yet adequately examined in trial‐based literature. Mixed‐methods or qualitative research methods may be particularly suited to better understanding elements of successful partnerships, and so to inform development of effective approaches. A qualitative evidence synthesis on this topic is currently underway (Merner 2019).
Overall completeness and applicability of evidence
Overall we identified a limited number of trials evaluating interventions with partnership compared to without partnership (Comparisons 1 and 2) and none for Comparison 3. Partnership decisions in the included studies influenced planning or delivery; none of the partnership decisions influenced evaluation of health services.
Many of the outcomes that we sought were not measured in the included studies. Often, this was because the partnerships evaluated had far broader aims than to improve person‐centredness of services. Some included trials aimed to improve clinical endpoints rather than examining the influence of partnering on the person‐ centredness of the health service. Even where relevant outcome measures were reported in such trials, these were most often examined in the intervention group only and therefore were not comparatively evaluated. Taken as a body of evidence, this review highlights the general lack of trials directly assessing the effects of partnership interventions and indicates that more research in the area is needed.
Although few studies were included, most were conducted very recently suggesting this is a burgeoning area of research. Additionally, we identified some protocols for trials that were very recently completed but not yet published, and some ongoing trials. Therefore, we know the evidence is growing and there is likely to be new emerging evidence that may help to build our understanding and certainty about the effects of these interventions. However, the effects of such interventions may also be usefully understood by alternative research approaches to trials: mixed‐methods and qualitative research may also help to unpack the complex effects of these complex interventions (Merner 2019).
It is also important to note that there are limitations inherent in conceptualising partnership as an intervention. This configures engagement and partnership as a process initiated by health services, decision makers and others, rather than a model arising from consumers and the community itself. Future research may wish to explore this in more depth, rather than necessarily adopting the definitions and inherent constraints of this current review related to this point.
Most included studies were from high‐income countries (Greco 2006; Jha 2015; Wu 2019), with two in middle‐income (Persson 2013) or low‐income countries (O'Connor 2019). This may also limit the applicability of the research. Further, none of the included studies measured resources associated with decision‐making process or with implementing decisions; and very few measured consumer‐ or provider‐partner reported outcomes or measures of partnership. These are important outcomes for health service decision‐making and may have profound impacts on the effects of these interventions.
Resources, including both time and money, required for the establishment of partnership interventions and their maintenance over time are also important considerations for this review. Neither were addressed by the included studies, but existing literature highlight the importance of both (Edgman‐Levitan 2013; National Patient Safety Foundation 2014). For instance, establishing respectful and trusting partnerships can take a long time, and therefore interventions built on such approaches are not quickly implemented (Boivin 2014). Such interventions, for instance aiming to identify health service priorities and to then inform implementation and evaluation on health and related outcomes, also imply an extended time course between conceptualisation and outcome. The trials included in this review were conducted, and outcomes measured, at relatively short time points and this may have contributed to the lack of effects seen across studies.
In terms of costs, engaging consumers in partnership activities to design and evaluate health services has the potential to save money by avoiding interventions that might be poorly designed, unnecessary or unacceptable to the people they are designed to help (Edgman‐Levitan 2013; National Patient Safety Foundation 2014). Future evaluations should include a cost analysis of these interventions to inform decision‐making about their usage, including costs to consumers and to organisations, particularly if interventions are intensive (e.g. requiring multiple face‐to‐face meetings) and/or conducted over long time periods (e.g. months to years). Considerations of costs and time required for partnership are important as there is a wide range of approaches through which consumer feedback might be incorporated into the design and evaluation of health services. This includes mechanisms such as consumer surveys to feed into system redesign, or consumer review of patient portals and education materials. Such flexible, relatively quick approaches may be easier to implement, and may also offer the advantage of potentially allowing a wider, more diverse range of consumers to participate and engage with these mechanisms to shape systems responses than might be possible through a structured series of face to face meetings like partnership.
This current review cannot answer all of these questions, and given the narrowness of the selection criteria the trials identified here represent only a sliver of existing consumer and provider partnership intervention evaluations, and this in turn represents only a small piece of the wider consumer engagement research available to inform systems’ move towards delivery of person‐centred care. Other trials exist that are beyond the focus of this review, such as one‐off partnerships as an intervention to improve person‐centredness of health services and partnerships in research and/or at the point of care contexts or more pragmatic trials where partnership is a component of the intervention but the effect of partnership as an intervention on its own is not able to be determined. As research in this area continues to develop, it will be critical for studies to adequately consider the changing landscape (Byrne 2020). As an example of this, with the shift towards collaborative models of different people working together (variously called co‐design or co‐production) there is growing emphasis on the need for all parties (both consumers and health providers, as well as decision‐makers) to receive training and support so that they have adequate skills to undertake these roles (Edgman‐Levitan 2013). This contrasts with earlier models of consumer engagement, where the focus was on upskilling or informing consumer partners in order to address power imbalances. With recognition of the desirability of truly collaborative models of engagement has come recognition of the different challenges such models of working together entail for the different parties involved (Boivin 2018) – both consumers and others.
Quality of the evidence
We assessed risk of bias using the Cochrane 'Risk of bias' tool, and certainty of evidence using the GRADE approach. The certainty of evidence was downgraded to very low for all outcomes. These judgements were based primarily on methodological limitations identified in relation to one or more risk of bias criteria (risk of selection bias, performance bias, detection bias and reporting bias), and due to concerns over imprecision (where studies did not explicitly define the optimal sample size or minimally important difference, or did not report confidence intervals). Publication bias was also strongly suspected, as results are from studies unlikely to be representative of all relevant studies that have been conducted; we are also aware of completed trials not yet published. Finally, compared to the review question, several studies were restricted in setting and population, leading to downgrading over concerns about indirectness.
Potential biases in the review process
Where multiple outcomes were measured within an outcome category, we selected the outcome measure we judged to be most directly related to the partnership decision made. For example, for the outcome category 'Health service user (trial participant) health service utilisation patterns', we selected 'Client‐reported referral from the healthcare system' over "CBO client healthcare utilisation (health system billing claims) data" (Wu 2019). This was because the decision made in partnership between health services and community‐based organisation leaders was designed to influence the co‐ordination between health and social services (CBOs) at discharge. Although these judgements were made through discussion and consensus between at least two review authors, it is possible that others may reach different decisions about the reporting and relative importance of such outcomes.
Our searches were comprehensive and systematic, but given the complexity of the intervention and the variety of terms in use, we may have missed potentially eligible studies. Additionally, as we limited our search to publications since 2000, there may be a small number of earlier trials that we have missed. Finally, we excluded studies published in languages other than English, and so may have not included some relevant papers. The typically low level of certainty of evidence from trials in this area suggests that any such trials are unlikely to change the conclusions of the review; however, we cannot discount this as a potential source of bias, and this decision may be revised in future updates of the review.
Agreements and disagreements with other studies or reviews
While there are several systematic reviews on relevant topics, e.g. public involvement in health care policy (Conklin 2015); healthcare planning, development and redesign (Crawford 2002; Dalton 2016; Haldane 2019; Milton 2012; Mockford 2012; Nilsen 2006; Tempfer 2011); and safety and quality of care delivery (Bombard 2018; McMillan 2013; Ocloo 2021), this is the first review to examine ‘working in partnership’ as an intervention to promote person‐centred health services. One of the earlier reviews in this area explored the effects of involving consumers in the planning and development of health care (not specifically working in partnership as defined here) and identified no comparative or experimental studies (Crawford 2002). We have identified five included trials and nine ongoing or recently completed trials.
These reviews support our conclusion that there is currently a lack of robust evidence to determine the effects of consumers and providers working in partnership as an intervention to promote person‐centred health services. However, consumer involvement is underpinned by moral and political imperatives (Esmail 2015), including democratic principles and patient rights to be informed, active partners in the health care system (Conklin 2015; Hart 2004).
Authors' conclusions
Implications for practice.
This review indicates there is uncertainty about the effects of consumers and providers working in partnership as an intervention to promote person‐centred health services. However, the lack of large, well‐designed trials with a primary focus on this intervention and purpose should not be interpreted as implying that these approaches have no value. Although effectiveness remains uncertain, partnership approaches are already warranted on political and moral grounds. The benefit of building the evidence base for effectiveness in the future is that it may help to provide guidance to health services about the best partnership approaches to adopt.
Implications for research.
In future, research might further investigate whether partnership approaches influence the person‐centredness of health services, and under what conditions. Such studies could be designed with a closer focus on the aim of partnership interventions and directly relevant outcomes, particularly interim outcomes of partnership interventions, resource use and measures of participation in decision‐making.
Future studies might meaningfully contribute to this area in many ways, and some of the questions that most need to be answered may not be best addressed in randomised trials. A role for pragmatic, mixed‐methods research in this area, to identify the best approaches, and to build on current understanding of enablers and barriers to the development and implementation of person‐centred health services, are needed. To ensure that data from comparative studies is most useful and meaningful, outcomes should be collected from both intervention and control groups.
To be most meaningful, future studies might consider a range of outcomes relevant to health services and to consumers, including both potential positive and negative (adverse) outcomes. A scan of the literature and stakeholder advice used to inform outcome selection for this review identified that such partnership evaluations could include measures of satisfaction with decisional process; attendance at meetings; costs associated with decision‐making and implementing new or changed services (for health care organisations and for consumers); degree to which health service alterations reflect service user priorities (demand responsiveness) and capacity to work together. Potential cost savings of partnership and consumer engagement approaches by ensuring that interventions and health services are appropriately designed to meet people's needs might also be usefully assessed in future research.
History
Protocol first published: Issue 7, 2019
Notes
This review is based on standard text and guidance provided by Cochrane Consumers and Communication (Ryan 2016).
Acknowledgements
Cochrane Consumers and Communication supported the authors in the development of the protocol and review. Cochrane Central Editorial Service (https://community.cochrane.org/review-production/production-resources/cochranes-central-editorial-service) conducted the editorial and peer‐review process for this review. Contributors to the editorial process were: Sign‐off Editor (final editorial decision): Lisa Bero, Senior Editor, Research Integrity and Cochrane Public Health and Health Systems; Handling Editor (selecting peer reviewers, collating peer‐reviewer comments, providing editorial guidance to authors, editing): Joey Kwong, Cochrane Central Editorial Service; Editorial Assistant (editorial policy checks and supporting editorial team): Leticia Rodrigues, Cochrane Central Editorial Service; Copy Editor (copy‐editing and production): Hacsi Horvath, Cochrane Copy Edit Support; Peer‐reviewers (providing comments and a recommendation): Antoine Boivin, Canada Research Chair in Partnership with Patients and Communities, University of Montreal, Canada (clinical/content); Amy‐Louise Byrne, Lecturer in Nursing, School of Nursing, Midwifery and Social Sciences, Central Queensland University (clinical/content); Susan Edgman‐Levitan, Executive Director, MGH Stoeckle Center for Primary Care Innovation; Lecturer in Medicine, Harvard Medical School (clinical/content); Vijayluxmi Bose, Health Communication Specialist, Independent Consultant (consumer); Jennifer Hilgart, Associate Editor, Cochrane Editorial & Methods Department (methods); Robin Featherstone, Information Specialist, Cochrane Editorial & Methods Department (search).
We thank Anne Parkhill for developing the search strategy. We also thank the authors of three of the included trials (Jha 2015, Persson 2013 and Wu 2019) for providing additional information about their studies upon request.
We gratefully acknowledge the input and guidance of the Stakeholder Advisory Panel formed for the QES (Merner 2019). The Australian‐based Stakeholder Advisory Panel consists of consumers, healthcare professionals, and health policy‐makers. The members of the Panel are: Leslie Arnott (Consumer Representative, Victoria), Susan Biggar (Australian Health Practitioner Regulation Agency (AHPRA), Victoria, and Consumer Representative, Victoria), Noni Bourke (Chief of Organisational Support & Development, Bass Coast Health, Victoria), Paul Bryden (Consumer Representative, Queensland), Renee Chmielewski (Manager, Planning and Patient Experience, The Royal Victorian Eye and Ear Hospital, Victoria), Leia Earnshaw (Consumer Representative, Australian Capital Territory), Marie Gill (Consultant, Gill and Wilcox Consultancy, Victoria), Fiona Martin (Clinical Practice Lead/Health Psychologist, Catholic Care, Victoria), Nathalie Martinek (Practitioner Trauma & Fatigue: Prevention & Recovery Professional Mentor & Mediator for Physicians and Other Health Professionals (until October 2018)), Louise McKinlay (Director, Patient Experience & Partnerships, Safer Care Victoria), David Menzies (Manager, Chronic Disease Programs, South Eastern Melbourne Primary Health Network, Victoria), Nancy Messino (Clinical Trials Quality Officer, Victorian Comprehensive Cancer Centre, Victoria), Anne Mussared (Consumer Representative, South Australia), Naomi Poole (Program Manager, Partnering with Consumers, Australian Commission on Safety and Quality in Health Care, New South Wales), Nora Refahi (Consumer Representative, Victoria), Lorraine Smith (School of Pharmacy, Faculty of Medicine and Health, University of Sydney, Camperdown, New South Wales), Roshni Sonawane (Consultant Paediatrician, Rockingham General Hospital and Wexford Specialist clinics, Murdoch, Western Australia), and Cheryl Wardrope (Patient Safety and Quality Service, Children’s Health Queensland Hospital and Health Service, South Brisbane, Queensland).
Appendices
Appendix 1. Glossary of key terms
Consumer partnership participant(s): refers to people who are fulfilling an advisory or representative role within the partnership. These roles might include a consumer or patient representative; consumer consultant; consumer with acute or chronic condition(s), their carer, or family member; community members, general public, or citizens; representatives, consultants, or members of consumer organisations.
Facilitated partnership: assistance is provided (e.g. by researchers, consumer advocates, or others) to help partnership participants to work in partnership (e.g. provide training or support before, or moderate or advocate during meetings).
Formal group format: refers to an organised group, such as a committee, council, board, or steering group.
Health services: defined as public or privately funded services that provide direct care to consumers in primary (e.g. community health centres, general practitioner practices, private practices, dispensaries), secondary (e.g. specialist outpatient clinics), or tertiary settings (e.g. hospitals). We will include home and residential services only when they primarily provide health or nursing care (e.g. home‐based nursing services, nursing homes, residential rehabilitation services, or hospices).
Health service performance information: (as an added intervention): data are collected about the performance of health service beyond that experienced by partnership participants (i.e. could be information about a measure of service quality at baseline, or performance in relation to other services), and provided to partnership participants for consideration during decision‐making. Alternatively, consumer and provider partnership participants independently generate health service performance indicator ratings before meeting as a group.
Health service user or provider (demand or supply side) information (as an added intervention): data are collected about the needs, preferences, experiences, or priorities of the people who use (demand side), or who provide (supply side) the health service, beyond those who are partnership participants (i.e. additional information is gathered systematically from health service users and providers as part of the trial), and provided to partnership participants for consideration during decision‐making.
Intervention effects review: in a systematic review of intervention effects, the researchers aim to locate, assess the risk of bias, and synthesise all of the available evidence related to a specific research question about the effects of an intervention. In this case, the question is ‘what are the effects of consumers and providers working in partnership to promote person‐centred health services'?
Partnership at health service level (i.e. upstream, at a higher level than the point of care): consumer and health providers jointly plan, develop, and monitor health services at the national, state, or regional policy or organisational governance level.
Parternship at point of care: refers the clinical consultation (or encounter) level during which individual health practitioner(s) interact with individual patient(s) to jointly plan and manage their own health care, sometimes called the direct care level (can include more than one consumer and provider interacting in self‐management groups). Partnerships at the point of care level are excluded from this review.
Provider partnership participant(s): refers to people who are fulfilling an advisory or representative role within the partnership. These roles might include, for example: a clinician (such as a doctor, nurse, allied health, or community health worker from any discipline), health service manager, supervisor, or administrator (including quality co‐ordinators, chief executives, etc.), health policy maker, or consumer liaison officer. Health provider participants do not include people who are primarily health researchers or academics.
Qualitative evidence synthesis: in a systematic review of qualitative evidence, the researchers aim to locate, assess the methodological quality, and synthesise evidence related to a specific research question about the experience of a phenomenon. When combined with an intervention effects review, the qualitative evidence synthesis aims to help understand how the intervention works, for whom, and in what context, and how best to implement it (Flemming 2019). In this case, the question is ‘what are the barriers, facilitators, and experiences of consumers and providers working in partnership to promote person‐centred health services'?
Working in partnership (as an intervention): defined as a joint meeting of at least one consumer and health provider, which occurs more than once, in a formal group format, to make decisions together, with the aim of promoting person‐centred care in one or more areas of a health service or services. The group is to meet face‐to‐face or virtually (i.e. meet in real‐time, on an ongoing or time‐limited basis).
Appendix 2. MEDLINE search strategy
exp Community Participation/
Stakeholder Participation/
Decision making/
exp Patient‐Centered Care/
((patient* or communit* or consumer* or user* or carer* or caregiver* or client* or famil* or lay*) adj3 (decid* or decision* or engag* or involv* or participat*)).ti,ab,kf.
or/1‐5
"Health Priorities"/
exp Patient Care Team/
exp Ambulatory Care Facilities/
*"Mental Health Services"/
*"Community Health Services"/
*"Health Services Administration"/
"Quality Improvement"/
*"Hospitals, Public"/
"Quality of Health Care"/
"Delivery of Health Care"/
"Delivery of Health Care, Integrated"/
or/7‐17
"Community‐Institutional Relations"/
"Advisory Committees"/og
(partner* or participat* or consult* or decision* or deliberation* or co#design* or involv* or contribut* or role* or empower* or engag* or collab* or advoca* or organi#ation* or respons*).ti,ab,kf.
(experience based adj2 design).ti,ab,kf.
or/19‐22
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/24‐31
Non‐Randomized Controlled Trials as Topic/
((cluster or quasi) adj3 trial*).tw.
or/33‐34
exp animals/ not humans.sh.
32 not 36
35 not 36
limit 37 to (english language and yr="2000 ‐Current")
limit 38 to (english language and yr="2000 ‐Current")
and/6,18,23,39
and/6,18,23,40
or/41‐42
Appendix 3. Cochrane Library search strategy
#1 MeSH descriptor: [Community Participation] explode all trees
#2 MeSH descriptor: [Stakeholder Engagement] this term only
#3 MeSH descriptor: [Decision Making] this term only
#4 MeSH descriptor: [Patient‐Centered Care] explode all trees
#5 (((patient* or communit* or consumer* or user* or carer* or caregiver* or client* or famil* or lay*) NEAR (decid* or decision* or engag* or involv* or participat*))):ti,ab,kw (Word variations have been searched)
#6 {OR #1‐#5}
#7 MeSH descriptor: [Health Priorities] this term only
#8 MeSH descriptor: [Patient Care Team] explode all trees
#9 MeSH descriptor: [Ambulatory Care Facilities] explode all trees
#10 MeSH descriptor: [Mental Health Services] this term only
#11 MeSH descriptor: [Community Health Services] this term only
#12 MeSH descriptor: [Health Services Administration] this term only
#13 MeSH descriptor: [Quality Improvement] this term only
#14 MeSH descriptor: [Hospitals, Public] this term only
#15 MeSH descriptor: [Quality of Health Care] this term only
#16 MeSH descriptor: [Delivery of Health Care] this term only
#17 MeSH descriptor: [Delivery of Health Care, Integrated] this term only
#18 {OR #7‐#17}
#19 MeSH descriptor: [Community‐Institutional Relations] this term only
#20 MeSH descriptor: [Advisory Committees] this term only and with qualifier(s): [organization & administration ‐ OG]
#21 ((partner* or participat* or consult* or decision* or deliberation* or co#design* or involv* or contribut* or role* or empower* or engag* or collab* or advoca* or organi#ation* or respons*)):ti,ab,kw (Word variations have been searched)
#22 ((experience based NEAR design)):ti,ab,kw (Word variations have been searched)
#23 {OR #19‐#22}
#24 #6 AND #18 AND #23
Appendix 4. CINAHL search strategy
| S31 | S20 AND S30 |
| S30 | S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 |
| S29 | TI (singl* or doubl* or tripl* or trebl*) and TI (blind* or mask*) |
| S28 | AB (singl* or doubl* or tripl* or trebl*) and AB (blind* or mask*) |
| S27 | AB (random* or trial or placebo*) or TI (random* or trial or placebo*) |
| S26 | MH Quantitative Studies |
| S25 | MH Placebos |
| S24 | MH Random Assignment |
| S23 | MH Clinical Trials+ |
| S22 | PT Clinical Trial |
| S21 | "randomi?ed controlled trial" or PT randomized controlled trial |
| S20 | S4 AND S8 AND S17 |
| S19 | S4 AND S8 AND S17 |
| S18 | S4 AND S8 AND S17 |
| S17 | S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 |
| S16 | MH "Program Evaluation" OR (AB (health service*)) |
| S15 | MH "Program Implementation" |
| S14 | MM "Quality Improvement" |
| S13 | MH "Decision Making" |
| S12 | MM "Community Mental Health Services" |
| S11 | MM "Decision Making, Patient" |
| S10 | MH "Community Health Services" |
| S9 | MM "Health Care Delivery" |
| S8 | S5 OR S6 OR S7 |
| S7 | TX (partner* or participat* or consult* or decision* or deliberation* or (co design*) or involv* or contribut* or role* or empower* or engag* or collab* or advoca* or organisation* or organization* or respons*) |
| S6 | TX (experience based N2 design) |
| S5 | (MH "Patient Centered Care") |
| S4 | S1 OR S2 OR S3 |
| S3 | MH "Professional‐Patient Relations" |
| S2 | (MH “Consumer Participation”) OR ( TI ( consumer N2 particip* OR client* N2 engage* OR stakeholder* N2 engage* OR communit* N2 particip* or patient* N2 particip* or client* N2 particip* or citizen* N2 particip* or consumer N2 involve* or patient* N2 involve* or client* N2 involve* or citizen* N2 involve* ) or AB ( consumer N2 particip* OR communit* N2 particip* or client* N2 engage* OR stakeholder* N2 engage* OR patient* N2 particip*or client* N2 particip* or citizen* N2 particip*) |
| S1 | (MH "Patient Care Conferences+") OR (MH "Consumer Attitudes") OR (stakeholder* N2 (participat* or engag* or involv* or satisf*)) OR (patient* N2 (participat* or engag* or involv* or satisf*)) |
Appendix 5. EMBASE search strategy
1. advisory committee/
2. ((patient* or communit* or consumer* or user* or carer* or caregiver* or client* or famil* or lay*) adj3 (partner* or consult* or decision* or deliberation* or contribut* or role* or empower* or collab* or advoca* or organi#ation* or respons*)).ti,ab,kw.
3. (co#design* or (experience based adj2 design)).ti,ab,kw.
4. (citizen$ adj (council? or jury or juries or panel?)).ti,ab,kw.
5. (public adj (meeting? or forum?)).ti,ab,kw.
6. participatory intervention?.ti,ab,kw.
7. community participation/
8. stakeholder engagement/
9. patient participation/
10. Patient‐Centered Care/
11. ((patient* or communit* or consumer* or user* or carer* or caregiver* or client* or famil* or lay*) adj3 (engag* or involv* or participat*)).ti,ab,kw.
12. or/7‐11
13. health care planning/
14. exp patient care/
15. *outpatient department/
16. *mental health service/
17. exp *"community care"/
18. exp *"health service"/
19. total quality management/
20. *"public hospital"/
21. exp health care quality/
22. exp health care delivery/
23. integrated health care system/
24. or/13‐23
25. public relations/
26. advisory committee/
27. ((patient* or communit* or consumer* or user* or carer* or caregiver* or client* or famil* or lay*) adj3 (partner* or consult* or decision* or deliberation* or contribut* or role* or empower* or collab* or advoca* or organi#ation* or respons* or author*)).ti,ab,kw.
28. (experience based adj2 design).ti,ab,kw.
29. (co‐design or codesign).ti,ab,kw.
30. (citizen$ adj (council? or jury or juries or panel?)).ti,ab,kw.
31. (public adj (meeting? or forum?)).ti,ab,kw.
32. participatory intervention?.ti,ab,kw.
33. governance.ti,ab,kw.
34. or/25‐33
35. randomized controlled trial/
36. controlled clinical trial/
37. single blind procedure/ or double blind procedure/
38. crossover procedure/
39. random*.tw.
40. placebo*.tw.
41. ((singl* or doubl*) adj (blind* or mask*)).tw.
42. (crossover or cross over or factorial* or latin square).tw.
43. (assign* or allocat* or volunteer*).tw.
44. or/35‐43
45. quasi experimental study/
46. ((cluster or quasi) adj3 trial*).tw.
47. or/45‐46
48. nonhuman/
49. 44 not 48
50. 47 not 48
51. and/12,24,34,49
52. and/12,24,34,50
53. limit 51 to (english language and yr="2000 ‐Current")
54. limit 52 to (english language and yr="2000 ‐Current")
55. or/53‐54
Appendix 6. PsycINFO search strategy
1. exp community involvement/
2. participation/ or client participation/ or involvement/
3. advocacy/
4. empowerment/
5. cooperation/ or collaboration/
6. or/1‐5
7. stakeholder/
8. clients/
9. patients/
10. or/7‐9
11. and/6,10
12. ((patient* or communit* or consumer* or user* or carer* or caregiver* or client* or famil* or lay*) adj3 (decid* or decision* or engag* or involv* or participat*)).ti,ab.
13. or/11‐12
14. exp Health Care Services/ or exp Health Care Delivery/
15. exp community involvement/
16. (partner* or participat* or consult* or decision* or deliberation* or co#design* or involv* or contribut* or role* or empower* or engag* or collab* or advoca* or organi#ation* or respons*).ti,ab.
17. (experience based adj2 design).ti,ab.
18. or/15‐17
19. and/13‐14,18
20. limit 19 to (english language and yr="2000 ‐Current")
21. random*.ti,ab,hw,id.
22. intervention.ti,ab,hw,id.
23. trial.ti,ab,hw,id.
24. placebo*.ti,ab,hw,id.
25. groups.ab.
26. ((singl* or doubl* or trebl* or tripl*) and (blind* or mask*)).ti,ab,hw,id.
27. (cross over or crossover).ti,ab,hw,id.
28. latin square.ti,ab,hw,id.
29. (assign* or allocat* or volunteer*).ti,ab,hw,id.
30. (control or controlled).ti,ab,hw,id.
31. treatment effectiveness evaluation/
32. mental health program evaluation/
33. exp experimental design/
34. "2100".md.
35. or/21‐34
36. animal.po.
37. 35 not 36
38. 20 and 37
Appendix 7. PROQUEST search strategy
PROQUEST Lowe_2021
noft(((health OR medical OR clinical) AND (service* OR hospital* OR care))) AND (noft((experience based design)) OR noft((partner* OR participat* OR consult* OR decision* OR deliberation* OR co#design* OR involv* OR contribut* OR role* OR empower* OR engag* OR collab* OR advoca* OR organi#ation* OR respons*))) AND noft(((patient* OR communit* OR consumer* OR user* OR carer* OR caregiver* OR client* OR famil* OR lay*) AND (decid* OR decision* OR engag* OR involv* OR participat*))) AND (noft(random*) OR noft(trial*))
Appendix 8. Clinical trials search strategy
CT GOV scanned from
citizen participation
citizen involvement
citizen engagement
Appendix 9. Web of Science search strategy
| STUDY author | TITLE | WoS references |
| Bjorkman | POWER TO THE PEOPLE | 215 |
| Boivin | What Are the Key Ingredients for Effective Public Involvement | |
| Boivin | Target for improvement | 8 |
| Boivin | Involving patients in setting priorities for healthcare improvement: a cluster randomized trial | 64 |
| English | A Community Engagement Method to Design Patient Engagement Materials | 4 |
| Greco | Impact of patient involvement | 1 |
| Gullo | Creating spaces for dialogue | 0 |
| Gullo | Effects of a social accountability approach, CARE's Community Score Card |
0 |
| Hanson | Expanded Quality Management Using Information Power | 2 |
| Nyqvist | Experimental Evidence on the Long‐Run Impact of Community‐Based Monitoring | 6 |
| Ong | A Community‐Partnered, Participatory, Cluster‐Randomized | 1 |
| Palmer | The CORE study protocol: a stepped wedge cluster randomised controlled trial | 15 |
| Palmer | Balancing opposing forces ‐ a nested process evaluation study protocol for a stepped wedge designed cluster randomized controlled | 0 |
| Waiswa | Community and District Empowerment for Scale‐up |
6 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Greco 2006.
| Study characteristics | ||
| Methods |
Study design: Cluster‐RCT; Unit of randomisation: health service practices.
Sample size/power calculation: Power calculations at the 5% significance level determined that 15 intervention and 15 control practices with at least 83 patient participants in each would provide 80% power to find a difference in Improving Practice Questionnaire "capacity" score of 2%. Country of study: North and East Devon; England Setting: Coastal and rural Healthcare Community Practices (unclear if publicly/privately funded) Study duration: Unclear Broader consumer involvement: none described |
|
| Participants |
Consumer partnership participants: (n = 46): health service patient members. Practices were encouraged to choose patients with differing patterns of attendance at surgery and to include patients from those attending branch surgeries as well as the main health centres (no other inclusion/exclusion criteria description)
Provider partnership participants: (n = 57): practice staff members.
Health service user trial participants: (n = unclear how many were eligible; 7537 patients completed baseline questionnaire and 8967 patients completed 12 month follow‐up)
Health service provider trial participants: (n = 279 providers eligible and 186 providers participated: 109 of these participated both at baseline and 12 month follow‐up).
|
|
| Interventions |
Randomised to intervention: 14 health practices; 4396 patients completed baseline questionnaire (analysed at 12 months: 14 practices with 5000 patients); a total of 107 providers (80 GPs, 20 nurses, 7 AHPs) participated and 66.4% (71/107) participated both at baseline and follow‐up. Nature of intervention: Critical Friends Group (CFG) meetings between patients and providers who in partnership interpret results of Improving Practice Questionnaire (IPQ: systematic patient feedback) and decide on an action plan that enables practices to make patient focused changes. Intervention aim: to assess whether CFG enables practices to interpret IPQ results and devise an action plan that enables practices to make changes that are more patient focused. Context of partnering: CFGs met twice combined (practice staff and patients) following separate preliminary meetings. During the first meeting, which took place 2 weeks following the initial preliminary meetings, group members exchanged information about themselves, their roles, and identified areas for attention and improvement within practice based on IPQ results. Meeting notes were distributed to participants a few days later. In the second meeting 8‐10 weeks following the preliminary meetings,improvement strategies were reviewed. Decision‐making activity: During the first CFG meeting, members interpreted the IPQ results and agreed on 3‐5 improvement strategies. During the second meeting, members reviewed progress on earlier identified improvement strategies. Topics most frequently discussed at CFGs, after reviewing baseline IPQ feedback were ‐ privacy at the reception desk (6/14 meetings) and waiting time to see the doctor (12/14 meetings). Meeting format, duration, frequency and location: face‐to‐face; two combined meetings; the preliminary meeting (in patient only and provider only groups) was facilitated by researchers, the nature of the two subsequent combined meetings was not described. Meetings were held at participating practices, duration unknown, no further description provided. Partnership duration: Time‐limited over approximately 12 weeks: preliminary meeting; two weeks later first combined meeting; 8‐10 weeks later second combined meeting Training/support: Preliminary meetings were held with patient‐only groups and with practice staff only groups at intervention sites that requested this. During preliminary meetings a researcher provided background information about the project, purpose of future meetings and IPQ results. Decision‐making process, attempts to resolve conflict: not described Diversity and ratio of consumer and provider participants: diversity not described; across all 14 CFGs the ratio of consumers to providers was 46:57 (ratio not provided for each CFG). Practices were advised to select equal numbers of practice staff and patients to participate within the CFG. Attempts to address intrinsic power imbalances: not described Theoretical basis for partnering: UK National Health Service (NHS) outlines ways to develop the role of patients within the new NHS as equal partners, whose voice and concerns can be heard, and British General Medical Services contract stipulates patient experience as a domain within the Quality and Outcomes Framework. The rationale for the intervention is to match the requirements of these governing bodies and find a way to move the patient involvement agenda towards engaging patients in the processes of change and improvement in healthcare. Tailoring/modification/adapting: Unclear Fidelity/integrity: Not assessed. No control groups discussed IPQ results with patients during the research period. Randomised to control: 14 health practice sites with 3141 patients completed baseline questionnaires (analysed at 12 months: 12 practices, and 3967); a total of 79 providers (55 GPs, 17 nurses and 7 AHPAs) participated and 48.1% (38/79) participated both at baseline and follow‐up. Nature of comparison: multifaceted intervention minus partnership: control sites received no assistance (or encouragement) from researchers in setting up a CFG. Practice managers were contacted at 12 months to find out about any patient involvement initiatives that had taken place since their first and second IPQ. Co‐intervention: Both intervention and control sites were given IPQ pilot study results. The initial pilot involved 42 GP practices (206 GPs and 8600 patients completed the IPQ) within the North and East Devon Healthcare Community. |
|
| Outcomes |
Outcomes measured at Baseline (T0) and follow‐up (12 months post‐intervention ‐ T1): Outcome measures relevant to the review (reporting available comparative data for longest time point measured):
Other study outcome measures (data extracted but not reported in review): Other subscales of service user experience via Improving Practice Questionnaire: 27 item survey. Items scored on a scale of 0 (poor) to 5 (excellent) |
|
| Notes |
Funding: NHS Modernisation Agency and the former North East Devon Regional Health Authority Conflict of interest: None disclosed (nor were affiliations disclosed) Other: Subscale was selected by review authors as the most relevant subscale from those reported of the IPQ. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Thirty practices were randomly selected from a pool of 42 practices (using a random number generator)... and were invited to participate in the study. If a practice declined to participate in the project another practice was randomly selected from the pool. Then, practices that agreed to participate were assigned randomly to either the treatment or control group by selecting a chip from an envelope. 15 chips in the envelopes were marked "T" for treatment group and the other 15 marked "C" for control". (p 488, Greco 2006) |
| Allocation concealment (selection bias) | Unclear risk | Unclear whether intervention allocations could have been foreseen during enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information provided for blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two (out of 14) control practice sites failed to carry out a follow‐up survey because they lacked the necessary time and resources. 208/8967 (1.23%) of questionnaire responses were excluded because less than half the questions on the questionnaire had been answered (p 495, Greco 2006). |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in study publication are reported however, no protocol has been identified for the trial. |
| Other bias | Unclear risk | No baseline imbalances. Of the 152 eligible providers 'in the intervention group, significantly more were involved in the IPQ survey both at baseline and at the 12 month follow‐up (66.4% (71/107)) than compared with the 127 (eligible) control group providers (48.1% (38/79)). Therefore in the control group, a greater proportion of providers were the subject of the survey for their first time during the follow‐up, potentially explaining why overall mean scores got worse in the control group and better in the intervention group'. (p 495, Greco 2006). This trial allocated clusters but analysed at individual level; does not seem to have been adjustment for clustering. |
| Selective recruitment of participants | Low risk | Does not appear to be differential participant recruitment in clusters for different interventions or baseline imbalances. |
Jha 2015.
| Study characteristics | ||
| Methods |
Study design: RCT; Unit of randomisation: all trainee doctors employed at 5 hospital sites in their first year following graduation
Sample size/power calculation: factoring in a clustering effect within each centre a sample size of 115 gave 80% power to detect an effect size of 0.53 Country of study: Yorkshire and Humer region, England, UK Setting: predominately urban postgraduate medical schools at Scarborough, Hull, York, Grimsby and Scunthorpe hospital sites, unclear if public/privately funded Study duration: 2011 ‐ 2012 Broader consumer involvement: "The patients … provide feedback on documents, e.g. our interview guide for trainee doctors and will take part in focus groups after the teaching sessions to refine the intervention" (p 4, Winterbottom 2010). Consumers were involved in training the patients and carers in a preparatory patient learning journey workshop. The facilitators of these preparatory workshops were members of the Patient Voice Group at the University of Leeds and patients and carers themselves. Patients and carers who co‐developed and co‐facilitated the training to the foundation year trainees were also provided with support and opportunity to debrief following training sessions with a consumer (member of the Patient Voice Group). Two consumers were involved in analysing the qualitative data and interpreted the lessons learned in light of the coding frame provided (Jha 2015). |
|
| Participants |
Consumer partnership participants: (n = 6 patients and 5 carers), people who had experienced harm or error during healthcare either to themselves or their families; recruited through the National Patient Safety Agency, Action for Victims of Medical Accidents and local press advertisements.
Provider partnership participants: (n = 8), clinicians involved in medical education of foundation year trainees (7 doctors, 1 pharmacist)
Health service user trial participants: (none) Health service provider trial participants: (n = 283), first year (postgraduate/foundation year) medical trainee doctors employed by 5 hospitals sites
|
|
| Interventions |
Randomised to intervention: 142 trainee doctors at 5 sites; (analysed: 126 trainee doctors; 16 excluded due to incomplete data) Nature of intervention: consumer and provider partners collaboratively co‐developed and co‐delivered patient safety curriculum to first year trainee medical graduates. Intervention aim: to assess whether facilitating trainees to reflect on safety from the patient's perspectives and their experience of patient safety influences their beliefs, attitudes and intention of future behaviour. Context of partnering: following consumer partner training in 4 preparatory Patient Learning Journey workshops facilitated by consumers and carers (from Patient Voice Group, University of Leeds), consumer and provider partners co‐developed and co‐delivered the patient safety curriculum in two sessions. Decision‐making activity: partnership participants defined teaching session aims and objectives, decided on key narrative aspects and facilitated a discussion between patients and trainees. Meeting format, frequency and location: face‐to‐face, 2 x 1 hour sessions of co‐developed patient safety curriculum presenting 1 patient narrative 15‐18 minutes in duration, followed by facilitated discussion delivered on site in groups of 7‐10 trainee doctors. Partnership duration: time‐limited to during the preparatory Patient Learning Journey workshops and in the development and delivery of the training session. Training/support: Consumer partners provided with 4 preparatory Patient Learning Journey workshops facilitated by consumer and carer members from Patient Voice Group, University of Leeds; also given support and opportunity to debrief with a consumer (from Patient Voice Group) following training sessions. Decision‐making process, attempts to resolve conflict: not described Diversity and ratio of consumer and provider participants: not described Attempts to address intrinsic power imbalances: consumer partners provided with travel expenses and financial reimbursement for teaching and training attendance and 2 consumer partners co‐delivered each teaching session with a third consumer partner attending to observe and serve as a reserve in case one consumer partner unable to attend. Theoretical basis for partnering: draws on the conceptual framework by Kumagai which is underpinned by theories of empathy and moral development and Mezirow's transformative learning theory. Transformative learning occurs when there is a process of effecting change in a frame of reference and other people's stories may help trainee doctors develop empathy and new understanding of the meaning of patient safety from the patient's perspective. Tailoring/modification/adapting: broad learning outcomes were standardized but examples of key incidents in narratives patients shared varied. Fidelity/integrity: aimed to standardise intervention across sites by using same co‐facilitators (patients and a trained independent chairperson) and by asking consumer partners to maintain consistent narratives. Randomised to control: 141 trainee doctors at 5 sites; (analysed: 110 trainee doctors; 31 excluded due to incomplete data) Nature of comparison: usual practice of standard patient safety curriculum in clinician‐led teaching sessions (included regulatory and procedural, ethical and legal issues and communication with patients and record keeping handovers). Co‐intervention: common learning objectives derived from UK Foundation Program Curriculum were adhered to during sessions and issues related to the objectives discussed even if they did not naturally arise. |
|
| Outcomes |
Outcomes measured at Baseline (T0); immediately post intervention (T1) and 4 to 6 weeks follow‐up (post‐intervention – T2): Outcome measures relevant to the review (reporting available comparative data for longest time point measured):
Other study outcome measures (data extracted but not reported in the review):
|
|
| Notes |
Funding: National Institute for Health Research Program Grant for Applied Research Reference Number RP‐PG‐0108‐10049 Conflict of interest: The authors declared they have no competing interests. Other: For outcomes not reported in this review, data for APSQ subscales was not reported for each subscale; nor for subscale groupings such as Knowledge of patient safety; or in extractable form for the PANAS data. Learning point takeaways was reported as qualitative data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Simple randomisation was carried out at an individual level on a 1:1 basis … this was done on‐site at the training day by an independent administrator using a randomisation sequence generated using randomly ordered envelopes containing allocations." (p 25‐6, Jha 2015). |
| Allocation concealment (selection bias) | Low risk | "The assignment in the first envelope was given to the first individual as defined by registration and so on." (p 26, Jha 2015). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of their assignment due the sessions having different formats (rated as high risk); blinding not described for personnel (rated unclear risk). Performance bias rated high risk overall. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No attempt to blind outcome assessors, and the outcomes were subjective (self‐reported); also for the qualitative analysis trainees were identifiable and so there was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For the primary outcome (APSQ) analysis was conducted on 122/283 participants immediately post intervention due to missing data: 56/142 in the intervention arm, and 66/141 in the control arm; and 90/283 participants at follow‐up of between 3‐6 weeks: 37/142 in the intervention and 53/141 in the control. While there were fewer participants missing data for the post intervention PANAS (138/142 in intervention and 125/141 in control) the data presented is not overall mean and SD, rather it is change in overall score from baseline; therefore it is not usable data for this review. Additionally the trial report mentions a secondary outcome of APSQ scored using subscales grouped by attitudes towards "patient safety training"; "confidence in reporting errors"; "working hours as a cause of error"; "error inevitability"; "professional incompetence as a cause of error"; "disclosure responsibility"; "team functioning"; "patient responsibility in reducing errors"; "importance of training" and "knowledge of patient safety". These were not reported. |
| Selective reporting (reporting bias) | High risk | The protocol does not mention PA&NA which is presented in the trial findings; additionally the protocol and trial mentions a course evaluation at the end of the teaching session to assess overall effectiveness for which data is not presented and a 7 item self rating of how knowledgeable participants feel about aspects of patient safety for which data is not provided in the trial report (it looks to be added as a subscale into the APSQ). |
| Other bias | Unclear risk | No characteristics of the participants randomised between groups were provided so not able assess baseline imbalance; the analysis presented is only for the participants with complete data for each time point (i.e. if missing data from baseline or post intervention or follow‐up the participants were excluded from analysis) this means that the data is largely not usable, additionally where change in overall score from baseline is reported it is not usable data for this review; there may have been communication between members of study groups post‐intervention, which may have lessened the likelihood of there being a difference between groups. |
O'Connor 2019.
| Study characteristics | ||
| Methods |
Study design: Cluster‐RCT; Unit of randomisation: community sites
Sample size/power calculation: "The sampling methodology provided a 95% confidence interval of +/‐8% or less for the prevalence of indicators measure in the population." (p 7, O'Connor 2019) "The number of CHWs trained was calculated using a Child Survival Project‐ and community‐led census and policy‐mandated population‐to‐CHW ratios." (p 4, O'Connor 2019) Country of study: Freetown, Western Urban District, Sierra Leone Setting: Low‐income Urban slum communities within Freetown Municipal area with the highest maternal mortality ratio in the world (136/10,000 live births) and under‐5 mortality rate was fourth highest in the world (114/1000 live births) (p 3, O'Connor 2019). Communities are served by government primary health care facilities (Peripheral Health Units) at the sub‐district level and these are supervised by District Health Management Team (consisting of 15 members) which is also responsible for coordinating public health interventions in the community, the Freetown City Council also supports the delivery of community health services. Each Peripheral Health Unit is supported by the Health Management Committee which liaises between the service and the community. Each community spans 1‐2 wards. Wards within Freetown Municipal area have an elected Ward Development Committee responsible for engaging community members on general development activities; the chair of the Ward Development Committee is also a Councillor on the Freetown City Council. Study duration: 2011 ‐ 2017 Broader consumer involvement: None described |
|
| Participants |
Consumer partnership participants: (n = 49), peer supervisors of CHWs participating in Community Health Data Review (CHDR) meetings, each community had 5‐12 Peer Supervisors. Peer Supervisors were selected by Child Survival Project staff together with community leaders, based on their performance during initial CHW training. CHW were selected by a community‐level process according to the National CHW Program selection criteria. Criteria included currently living in and have close connections to their geographical community; accepted by community members, and ability and motivation to serve their community. Note that the data presented here is overall for all CHW (not just Peer Supervisors)
Provider partnership participants: (n = unknown 30‐50 participants in each of the 10 CHDR meetings; includes Peer Supervisors), Peripheral Health Unit (government primary health care facility) staff; Health Management Committee members, and Ward Development Committee members participating in CHDR meetings.
Health service user trial participants: (n = 599 at baseline; 792 at 21 months), household respondents were pregnant women and mothers of children under 5 years targeted by CHW. Household respondents were selected randomly from 10 random households in each of the 60 clusters. Clusters were also randomly selected using a probability proportional to size methodology based on population projections from the most recent Government of Sierra Leone census.
Health service provider trial participants: none |
|
| Interventions |
Randomised to intervention: 5 communities. Baseline data were collected from: 299 household respondents; 509 Community Health Workers (CHW); 49 Peer Supervisors; 80 Ward Development Committees and 75 Health Management Committees. Follow‐up data were collected from: 379 household respondents; 509 CHW; 49 Peer Supervisors; 80 Ward Development Committees and 75 Health Management Committees. Nature of intervention: In the Operation Research Study the Participatory Community‐based Health Information System intervention consisted of Community Health Data Review meetings every two months to support Health Management Committees, Ward Development Committees and CHW Peer Supervisors to undertake two activities (1. review of data collected by CHWs and actions in response to this data and 2. after 6 months, verbal autopsies for deaths under 5 years) in addition to the Child Survival Project activities. Intervention aim: “to 1) assess the extent to which the Participatory Community‐based Health Information System facilitated local community structures to use data to plan and implement actions for improving maternal, neonatal and child health and 2) assess the extent to which this contributed to improved community‐level maternal, neonatal and child health outcomes." (p 4, O'Connor 2019) Context of partnering: Findings from monthly reports submitted by volunteer community health workers and verbal autopsy findings for deaths of children aged less than 5 years were processed and shared at Community Health Data Review (CHDR) meetings in each intervention community. These bimonthly meetings were attended by community leaders, including members of the Ward Development Committee and Health Management Committee, the Peer Supervisors, and representatives of the Peripheral Health Unit. Following a review of the information, attendees proposed actions to strengthen community‐based health services in their community. Decision‐making activity: Typically, data for the preceding 4‐6 months were reviewed in CHDR meeting. The Operation Research Study staff analysed CHW collected data with the Child Survival Project staff prior to the meeting and determined topics and data to present in CHDR meetings. "The Operation Research Study staff prepared simple data sheets to be used by participants, and participants used them to draw and interpret bar charts in front of the group. Records were kept of discussion topics." (p5, O'Connor 2019) "Following the review of data, CHDR participants developed action points. Action points were documented during the meeting on flip chart paper which the Health Management Committee chairman kept after the meeting. Action points from previous meetings were reviewed in subsequent meetings and discussions held on the extent to which actions had been completed." (p 6, O'Connor 2019) Meeting format, duration, frequency and location: face‐to‐face, duration unknown, every two months, location unclear Partnership duration: 20 months Training/support: Based on their performance during initial training, community leaders and Child Survival Project staff together selected 106 Peer supervisors from the CHWs receiving training. Peer supervisors were given additional training by project staff and were assigned 8‐12 CHWs to supervise. At least one Health Management Committee and Ward Development Committee member from the same zone as the Peer Supervisor provided oversight and assistance. Decision‐making process, attempts to resolve conflict: not described Diversity and ratio of consumer and provider participants: Each community had 5‐12 Peer Supervisors and there were 30‐50 participants in Community Health Data Review meetings. Generally, the same District Health Management Committee members, Ward Development Committee members and Peer Supervisors attended each meeting. Although Government primary health care facility (Peripheral Health Unit) In‐Charges rarely attended meetings, they generally sent the same representative to each meeting. At the beginning, Peripheral Health Unit staff attendance was not strong, but attendance improved after Health Management Committee members engaged Peripheral Health Unit staff. Ratio of consumer and provider participants not described. Attempts to address intrinsic power imbalances: not described Theoretical basis for partnering: Sierra Leone Government highlights the need for community engagement in policy document "Basic Package of Essential Health Services, 2015‐2020", it focus on CHWs to fulfil this role. The policy document also recognises Health Management Committees (that support each Peripheral Health Unit) and Ward Development Committees (that are responsible for engaging community members on general development activities and the chair is also a Councillor on the Freetown City Council) but does not outline roles or the ways in which they should fit into the health system. Tailoring/modification/adapting: not described Fidelity/integrity: not described Randomised to control: 5 communities. Baseline data were collected from: 300 household respondents, 710 CHW; 57 Peer Supervisors; 75 Ward Development Committees and 75 Health Management Committees. Follow‐up data were collected from: 413 household respondents, 710 CHW; 57 Peer Supervisors; 75 Ward Development Committees and 75 Health Management Committees Nature of comparison: multifaceted intervention minus partnership: control sites of the Operation Research Study consisted of usual practice in addition to Child Survival Project activities Co‐intervention: The Concern Worldwide Child Survival Project was implemented in all 10 communities. In the broader Child Survival Project, 1325 volunteer CHWs were recruited and trained with the Ministry of Health and Sanitation 2012 National CHW Program training materials by Child Survival Project staff and the Western Area District Health Management Teams. CHWs were assigned 25 households to visit monthly and disseminate health messages, check for danger signs of illness and collect vital event and morbidity data using Ministry of Health and Sanitation registers. |
|
| Outcomes |
Outcomes measured at Baseline (T0) and follow‐up (21 months post‐intervention ‐ T1): Outcome measures relevant to the review (reporting available comparative data for longest time point measured):
Other study outcome measures (data extracted but not reported in review):
|
|
| Notes |
Funding: United States Agency for International Development (USAID); Irish Aid and Concern Worldwide. Conflict of interest: "The authors completed the Unified Competing Interests Form … and declare no competing interests" Other: For other outcomes reported not reported further in this review, choice of outcomes was made jointly by review authors according to measure(s) most representative of the outcome concept(s) sought. Data for other outcomes was reported by the trial but not extracted and considered further for analysis in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The study was a cluster‐randomised controlled trial, with [10 previously selected] communities randomly selected [assigned] to either the intervention or comparison area. Within the 10 communities 30 clusters in the intervention and comparison areas each were selected at random using a probability proportional to size methodology based on population projections from the most recent Government of Sierra Leone census. Ten interviews were conducted in each cluster through a random selection of households and a random selection of the respondent within the household." (p 7, O'Connor 2019). |
| Allocation concealment (selection bias) | Unclear risk | Method use to conceal the allocation sequence not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on blinding of participants or personnel provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information about attrition or exclusions were reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol identified; all results for all outcomes mentioned in trial reported. |
| Other bias | Unclear risk | There was substantial movement between communities (in and out). |
| Selective recruitment of participants | Unclear risk | No information on recruitment bias (differential participant recruitment in clusters)/loss of clusters. |
Persson 2013.
| Study characteristics | ||
| Methods |
Study design: Cluster‐RCT; Unit of randomisation: commune (geopolitical sites)
Sample size/power calculation: "The sampling strategy was one‐stage cluster sampling with a probability proportional to size of the clusters. The Probability proportional to size, in this case number of births per year, was chosen to obtain similar distribution of sizes of clusters across intervention and control communes. Sampling was neither blocked nor paired. A sampling frame was established with a cumulative list of number of births in each of the communes in the 2005 survey. ... The design effect was arbitrarily estimated to be 1.5 [taking into account] the high number clusters and low average cluster size. A 3‐year sample would allow detection of 7/1000 neonatal mortality rate with 80% at 0.05 significance". (p 2‐3, Persson 2013) Country of study: Quang Ninh province, Vietnam (Middle‐income country) Setting: Commune Health Centres in with 3‐6 staff members provide primary health care including reproductive and antenatal care to approximately 1000‐18,000 people. Delivery care is offered by Commune Health Centres, or by hospital at district, province and regional levels. Each Commune Health Centre has a Village (community) Health Worker who provides basic healthcare in the villages. Private alternatives for antenatal care in addition to governmental health care system exist but in this province the private or non‐governmental sector plays a limited role in relation to delivery services. Study duration: 2008 ‐ 2011 Broader consumer involvement: Members from the Women's Union were also members of the intervention steering board (chaired by the hospital director of the regional Uong Bi hospital and the Provincial Health Bureau) |
|
| Participants |
Consumer partnership participants: (n = 11), Pairs of lay women were facilitators for the 44 Maternal and Newborn Health Groups (MNHGs). Each facilitator was responsible for 5‐8 MNHGs. Recruitment criteria included being an experienced Women's Union member, having completed secondary school and having children. Facilitators were recruited using local newspaper adverts and recommendations from communes. “Originally the Women's Union office in each of the 8 study districts selected two individuals among the applicants for further interview [by two researchers and the chairwomen of the Women's Union]… Out of the 16 applicants interviewed 8 were selected for further training" (p 5, Eriksson 2016). Three additional facilitators were recruited during the trial to replace facilitators leaving because of childbirth or new employment. *The Women's Union is an organisation with high national coverage, working on a range of welfare issues relevant for women in Vietnam, especially those related to healthcare. Data below relates to only the facilitators (not the Women's Union members of the MNHGs)
Provider partnership participants: (n = 352; each of the 44 MNHGs had 8 members), Commune Health Centre staff (physicians, midwifes, nurses); a commune Village Health Worker, a population collaborator, a chairperson/vice chairperson of the commune; and two Women's Union representatives
Health service user trial participants: (n = 1243 mothers randomly sampled from 22,377 live births located within 90 communes during first 3 years of trial July 2008‐June 2011). The demographic details below were from a random sample of mothers (398/7033) with live births during first year of trial. ("A 6% random sample of all registered live births, surviving the neonatal period, was continuously selected (each month) in order to represent the entire birth cohort") (p 4, Persson 2013). The demographic details below are for Intervention: n = 213; Control: n = 185 mothers
Health service provider trial participants: none |
|
| Interventions |
Randomised to intervention: 44 communes; analysed during years 1‐3: 656 mothers randomly sampled from 11,818 live births in 44 communes. Nature of intervention: laywomen facilitation of MNHGs during which members collaborated to decide which problems to focus on and what actions to take directed towards pregnant women and their households, health services, or general public in order to address those problems. Intervention aim: to reduce neonatal mortality and improve maternal delivery, and newborn care indicators. Stillbirth was defined as birth of a dead foetus after an estimated 28 weeks of gestation. Live birth was defined as birth of a foetus with any sign of viability. Neonatal death was defined as death of a live birth during the first 28 days of life. Context of partnering: "MNHGs were constituted in each intervention commune (by directives from the Provincial Health Bureau). ...The facilitators primarily used the plan‐do‐act cycle in mobilising the groups in identifying and prioritising local perinatal health problems and accomplishing improvement cycles that included concrete actions on prioritized problems directed towards pregnant women and their households, the health services, or the general public. Such improvement cycles on different problems were performed continuously over the intervention period in all MNHGs. Where possible one facilitator performed monthly meetings with the same MNHG over the 3 year period. Each facilitator was responsible for 5‐8 MNHGs. Twenty groups kept the same facilitator the whole period, 22 changed facilitator once, and two groups changed facilitator twice. When appropriate, facilitators were recommended to highlight recommendations provided by the Vietnamese National Standard and Guidelines on Reproductive Health Care Services." (p 4, Persson 2013) Decision‐making activity: In MNHGs Plan‐Do‐Study‐Act discussions centred on individual and common experiences in the local setting, the facilitator supported the group in critical reflection, problem identification, finding solutions and developing change strategies. The intervention aimed to achieve local ownership and "bottom‐up" approach in empowering healthcare staff to improve practice. When appropriate, the facilitators would highlight recommendations in the National Guidelines. "The groups identified 32 unique problems and implemented 39 unique actions. The identified problems targeted health issues concerning both women and neonates. Actions implemented were mainly communication activities." (p 1, Eriksson 2016) Meeting format, duration, frequency and location: Monthly face‐to‐face meetings lasting on average 2 hrs (110 mins), meetings located at commune centre or health care centre. Partnership duration: 3 years (31 months) Training/support: The facilitators were trained in problem solving, participatory and enabling approach in a 10 day training program which "included theoretical sessions, group discussion, role‐plays, and field practice. It covered topics such as group dynamics, quality improvement methods (e.g. brainstorming and the plan‐do‐study‐act cycle); and evidence based perinatal care. A facilitation manual and a specific diary were developed to guide the work of the facilitators. Two research team members coordinated the facilitation process and acted as supervisors of the facilitators, i.e. field supervision and performing 2 day meetings with all facilitators once a month during the entire trial period." (p 3‐4, Persson 2013) Decision‐making process, attempts to resolve conflict: facilitators were trained on brainstorming, the nominal group technique, the plan‐do‐study‐act cycle and the strengths‐weaknesses‐opportunities‐threats diagnostic tool. Diversity and ratio of consumer and provider participants: MNHGs consisted of 8 members: 3 Commune Health Centre staff (physician, midwife, nurse); a commune Village Health Worker, a population collaborator, the chairperson/vice chairperson of the commune; and two Women's Union representatives (from village and commune levels). Attempts to address intrinsic power imbalances: The MNHG facilitators were paid on a full‐time basis for the 3 years of the intervention. Except for the Village Health Worker and the Women's Union employee (who were reimbursed travel expenses to and from the meetings), other members were neither paid nor received allowances ‐ as implementation work was assumed to be part of their normal duties. Theoretical basis for partnering: study draws from “the Promoting Action on Research Implementation in Health Services Model. This middle range theory highlights three major ingredients for being successful in implementing research into practice: 1) the nature of the evidence being used, 2) the quality of the context in terms of coping with changes, and 3) the types of facilitation needed to ensure a successful change process. Implementation is conceived as a multifaceted intervention, rather than a more straightforward, linear process of translating knowledge from experts to the local level. In the trial the authors analysed the effect of facilitation of local stakeholder groups focusing maternal and neonatal health problems and actions." (p 7, Persson 2013) Tailoring/modification/adapting: Each MNHG was context‐specific and continuously negotiated and interpreted among stakeholders. Fidelity/integrity: "Two research team members coordinated the facilitation process and acted as supervisors of the facilitators; i.e. field supervision and performing 2 day meetings with all facilitators once a month during the entire trial period. The intervention process was monitored continuously. Issues like MNHG's choice of improvement topics, activities for improving practice, the interaction between facilitators and group members, and progress of the facilitation process at all intervention sites were examined using several approaches, like interviews with facilitators and focus group discussions with MNHG members, analyses of facilitators diaries from MNHG meetings and the notes from the monthly meetings with the supervisors." (p 5, Eriksson 2016); 1508 (out of 1584; 95%) of the planned MNHG meetings with a facilitator were completed; during the intervention a facilitator joined the MNHG activity in a commune 294 times; a facilitator supported a co‐facilitator at a MNHG meeting 122 (8%) of the time; a NeoKIP researcher supported a facilitator at a 95 MNHG meetings (6%); and 35 (97%) monthly meetings between supervisor and facilitators took place (p 12, Eriksson 2016). Overall attendance at the meetings was 86%; the Head of the Women's Union (village level) attended 97%; the Head of the Community Health Centre attended 95%; midwives attended 94%; Community (Village) Health Workers attended 90%; Nurse attended (88%); Population collaborator (87%); Chairwomen of the Women's Union (commune level) (87%); and Vice chairperson of peoples committee attended 61% (p 7, Eriksson 2016). "No deviations from the protocol were observed except from one out of 44 MNHGs that ended the facilitation intervention two‐thirds of the way into the trial. The facilitated intervention with MNHGs maintained a high activity with a large number of problems identified and actions taken, in spite of no extra financial benefits to the group members" (p 6, Eriksson 2016). "However, the NeoKIP intervention was a new approach for local stakeholders, who were not used to collaborate in this kind of group activity. This type of approach requires active and disciplined stakeholders who assess, discuss, and find ways to overcome contextual barriers that may impede the process of implementation." (p 8, Eriksson 2016) Randomised to control: 46 communes; analysed during years 1‐3: 587 mothers randomly sampled from 10,559 live births in 46 communes Nature of comparison: usual practice; baseline perinatal care comprised the following: 'At primary care level communal health centres (CHC) provide antenatal care (ANC), delivery services and postnatal care staffed by 3–6 persons (physician, nurses, midwife). The primary health care level is also supported by at least one part‐time village health worker (VHW) in each village, who mainly takes part in preventive services. Delivery services are also offered at hospitals at district, provincial and regional levels with more skilled staff and resources available at the higher levels' (p.1479, Nga 2010) Most women (76%) received 3+ ANC visits, in line with national guidelines. 92% of births occurred in health care facilities (regional, provincial or district hospitals, community health centres); 8% occurred at home (accounting for 20% of neonatal deaths). Co‐intervention: none |
|
| Outcomes |
Outcomes measured at Baseline (T0) and follow‐up (during first year of intervention ‐ T1; second year T2; third year T3; sixth year T4): Outcome measures relevant to the review (reporting available comparative data for longest time point measured):
Other study outcome measures (data extracted but not reported in review):
|
|
| Notes |
Funding: Swedish International Development Cooperation Agency (SIDA), Swedish Research Council and Uppsala University. Conflict of interest: Authors have declared that no competing interests exist. Other: For other outcomes reported by the trial but not reported in the review, these were judged, through author consensus, to be outside the scope of the selection criteria (e.g. neonatal mortality) and/or less relevant measures of an outcome for which multiple measures were reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random number list was used to subsequently allocate "intervention" or "control" to the list of communes, and 44 out of the 90 communes were allocated to intervention and 46 to control. The randomisation was preformed by one of the involved researchers at Uppsala University." (p 3, Persson 2013). |
| Allocation concealment (selection bias) | Low risk | "The sequence generation was concealed until the intervention was assigned; otherwise allocation was not masked." (p 3, Persson 2013) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unclear whether study participants were blind from knowledge of which intervention cluster they were assigned to; not able to blind personnel from knowledge of which intervention cluster they were assigned to. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No description of measures used, if any, to blind outcome assessors from knowledge of which intervention a participant received; The following relates to the outcome neonatal births/mortality "One (out 10) of the Village health Workers in the commune were involved in the Maternal and Newborn Health groups while all Village Health Workers were informants in the data collection system. The information on births and/or neonatal deaths provided by the village health workers who were involved in the intervention was most likely not biased since the updated list of pregnant women enabled a systematic enquiry on a monthly basis on pregnancy outcomes, as well as a triangulation of information sources and cross‐checking of data." (p 6, Perrson 2013) "Six data collectors were trained for 2 weeks. They attended monthly meetings at all commune health centres, all district hospitals, and the two provincial level hospitals in the area to collect data on live births and neonatal deaths. Further the data collectors met all Village Health Workers in the communes to collect data on live births and neonatal deaths in their villages. Triangulation was systematically performed of live births and neonatal deaths from all included sources (records and reports from the district, provincial and regional hospitals, commune health centres and village health workers) to ascertain that all data were registered and secure that no duplication of information occurred." (p 777, Eriksson 2018). Author contact confirms that data collectors were blinded to group allocation and had no contact with those involved in intervention delivery. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Zero losses to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Outcomes appear to be reported as planned based on those identified in protocol. |
| Other bias | Low risk | Groups were mostly balanced at baseline (except slightly more poor households in intervention group). Data collection was separated and verified by triangulation from different sources (and by analysts unaware of group assignment for at least some outcomes). |
| Selective recruitment of participants | Unclear risk | Not sure when participants were recruited to clusters. |
Wu 2019.
| Study characteristics | ||
| Methods |
Study design: Cluster‐RCT; Unit of randomisation: community based organisations (CBOs)
Sample size/power calculation: none identified Country of study: Baltimore, Marylands, US Setting: urban privately funded academic health care system (Johns Hopkins Health System (JHHS)). JHHS comprises Johns Hopkins University School of Medicine; The Johns Hopkins Hospital (1177 patient beds; 2230+ full‐time attending physicians) Johns Hopkins Bayview Medical Centre (448 patient beds; 710+ attending physicians). Study duration: 2014 ‐ 2016 Broader consumer involvement: two community leaders were paid co‐principal investigator and co‐investigator on the study team. Both reviewed and approved all stages of proposal and helped identify and recruit CBOs for participation, and recruited members to be interviewed. Both were involved in the partnership intervention co‐development process, implementation and evaluation. The community based co‐principal investigator led meetings with CBO leaders. Both contributed to paper writing and submission. |
|
| Participants |
Consumer partnership participants: (n = unknown), community leaders from not‐for‐profit CBOs that served adults and addressed one or more social determinants of health, located within the zip code of East or Southeast Baltimore
Provider partnership participants: (n = unknown), Johns Hopkins staff members as part of study team
Health service user trial participants: (n = 6491 patients assessed for eligibility; 5255 eligible patients), high‐risk Medicare and Medicaid Johns Hopkins Community Health Partnership (J‐CHiP) outpatient patients (adults, at least one chronic condition, at least one visit to J‐CHiP site, high risk for future hospitalisation). Patients with monthly healthcare utilization data, including emergency department visits and days hospitalised during both pre and post intervention and known home address were included.
Health service provider trial participants: (n = unknown), outpatient staff (case managers, community health workers, health educators and behavioural health specialists) and inpatient staff (social workers, case managers, hospitalists and nurse who help discharge patients to home) from JHHS.
|
|
| Interventions |
Randomised to intervention: 11 CBOs; 1997 patients (analysed: 10 CBOs; 1864 patients) Nature of intervention: After randomisation the partnership Baltimore CONNECT (Community‐based Organisations Neighbourhood Network: Enhancing Capacity Together) co‐developed an intervention/toolkit (Healthify). Intervention aim: to "enhance the capacity of both CBO staff and frontline hospital workers to address client needs by strengthening the bidirectional flow of information about health and social services and building networks that span both entities." (p e32, Wu 2019) Context of partnering: Partnership based on the African Partnerships for Patient Safety Engagement (ACE) Framework which is underpinned by Community Based Participatory Research and Participatory Action Research approaches. Decision‐making activity: "To begin, iCBO (intervention CBO) leaders completed a needs assessment to identify commonly faced challenges. The most salient issues identified were: referring clients to organisations for support, developing a stronger relationship with other CBOs to better serve clients, and interfacing with JHHS. Results of needs assessment were directly linked to formation of strategies to enhance co‐ordination of health and social services." (p 302, Wu 2018) Meeting format, duration, frequency and location: face‐to‐face (plus email and phone calls); each meeting 1.5 to 2 hours in duration; conducted monthly (for 6 months of co‐development and 12 months of the trial), with location rotated among iCBOs. Partnership duration: 6 months Training/support: no training described; each iCBO assigned a student research assistant Decision‐making process, attempts to resolve conflict: not described Diversity and ratio of consumer and provider participants: not described Attempts to address intrinsic power imbalances: not described Theoretical basis for partnering: Draws from the following 8 concepts of the ACE Framework: establish a community engagement advisory board; know the community; establish an enabling community engagement environment; raise patient quality and safety awareness locally and nationally; collect community knowledge and experiences; ensure robust communication mechanisms; feed into monitoring and evaluation; and develop a community ripple effect. Tailoring/modification/adapting: reverse innovation of the ACE Framework by adapting the ACE approach to partner with iCBO leaders to co‐develop and implement a set of interventions, or toolkit Fidelity/integrity: No assessment of fidelity, but there was survey data on trust, partnership etc (secondary outcomes). Some contamination effects possible, as intervention and control CBOs may have shared some clients and provided services to the J‐CHiP cohort of patients. Healthify also listed both intervention and control CBOs. Randomised to control: 11 CBOs, 3258 patients (analysed: 10 CBOs, 3053 patients) Nature of comparison: usual practice plus J‐CHIP ‐ no other description Co‐intervention: Johns Hopkins Community Health Partnership (not described) |
|
| Outcomes |
Outcomes measured at Baseline (T0) and follow‐up (12 months after intervention had ended ‐ T1): Outcome measures relevant to the review (reporting available comparative data for longest time point measured):
Other study outcome measures (extracted but not reported in review):
Following outcomes were not comparative (reported pooled data only):
Following outcomes measured at T1 for intervention arm only:
|
|
| Notes |
Funding: Patient‐Centred Outcomes Research Institute project grant (CD‐12‐11‐4948). Viva Dadwal supported by Fulbright Canada and the U.S Department of State's Fulbright Scholarship Programme. Conflict of interest: No financial disclosures reported by authors Other: *calculated standard deviation from standard error; ** calculated events from % data.For outcomes reported by the trial but not included in this review, authors identified through consensus that such outcomes were less relevant to the outcomes of the review (where multiple measures of an outcome were reported). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Cluster‐RCT "a restricted randomization process was conducted that constrained the allocation of organisations based on their ZIP code, client population (small, medium or large) and the type of service offered. There was purposeful balance in the number of churches, neighbourhood associations, and Hispanic serving organisation in each group as well as the primary type of services provided (e.g. food, housing, clothing)" (p e33, Wu 2019). Rated as at high risk ‐ even if balance was managed on these factors there might be other potential confounders which were not considered. |
| Allocation concealment (selection bias) | High risk | "The assignment of CBOs could not be blinded which introduced potential for bias." (p 305, Wu 2018) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | CBO clients were not likely to be aware of an individual CBO's group assignment; blinding of personnel not described. CBOs were randomised and therefore in the intervention group the leadership (at least) were aware of the intervention; control group may not have been aware of assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not described in manuscript. Author response indicates that primary outcomes were all electronic data, with statisticians blinded to assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 205 and 133 patients were excluded from the comparison and interventions arms respectively who did not have any utilisation data available either in the pre‐or post intervention period; these losses were about 7% in both groups; reasons were similar, and numbers remaining for analysis still large. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes measured reported in published methods are reported and trial protocol is registered NCT02222909 but protocol only lists primary outcomes measured i.e. Emergency Department visits + hospital days [Time Frame: Up to 1 year]; Number of Emergency Department Visits + Number of Hospital Days in the past 6 months for patients enrolled in the Johns Hopkins Community Partnership; and Change in number of clients seen per month [Time Frame: September 2014 ‐ September 2015] Change in median number of clients seen by Community Based Organizations per month; additional outcomes are reported in publications. |
| Other bias | Low risk | None. |
| Selective recruitment of participants | Unclear risk | "For allocation of J‐CHiP patients to a treatment arm, those who lived closer to an iCBO were allocated to the intervention group, and those who lived closer to a cCBO were allocated to the control group. Google application programming interfaces were used to determine the distance between each patient's residence and each of the participating CBOs." (p e33, Wu 2019); timing not mentioned. |
ACE: African Partnerships for Patient Safety Engagement Framework; ANC: Antenatal Care; APSQ: Attitudes to Patient Safety Questionnaire; CBO: COmmunity Based Organisation; CHC: Communal Health Centres; CFG: Critical Friends Group; CHDR: Community Health Data Review; CHW: Community Health Worker; CONNECT: Community‐based Organisations Neighbourhood Network: Enhancing Capacity Together; IPQ: Improving Practice Questionnaire; J‐CHiP: Johns Hopkins Community Health Partnership; JHHS: Johns Hopkins Health System; MNHG: Maternal and Newborn Health Groups; NMR: Neonatal Mortality Rate; VHW: Village Health Worker.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbey 2017 | Ineligible intervention (some other intervention evaluated) |
| Abelson 2003 | Ineligible on two or more criteria |
| Abelson 2016 | Ineligible study design (no attempt at randomisation) |
| Aboumatar 2017 | Ineligible intervention (some other intervention evaluated) |
| Acharya 2015 | Ineligible intervention (some other intervention evaluated) |
| Ackermann 2010 | Ineligible participants (no provider or no consumer) |
| Aggett 2009 | Ineligible on two or more criteria |
| Aibinuomo 2011 | Ineligible on two or more criteria |
| Aimola 2016 | Ineligible intervention (some other intervention evaluated) |
| Aldiss 2011 | Ineligible on two or more criteria |
| Alhassan 2015 | Ineligible on two or more criteria |
| Alhassan 2016 | Ineligible on two or more criteria |
| Alhassan 2016a | Ineligible on two or more criteria |
| Alhassan 2016b | Ineligible on two or more criteria |
| Alhassan 2017 | Ineligible on two or more criteria |
| Alhassan 2019 | Ineligible on two or more criteria |
| Aliyu 2013 | Ineligible on two or more criteria |
| Allen 2012 | Ineligible study design (no attempt at randomisation) |
| Allen 2013 | Ineligible on two or more criteria |
| Allen 2018 | Ineligible study design (no attempt at randomisation) |
| Allender 2016 | Ineligible on two or more criteria |
| Alvarado 1999 | Ineligible on two or more criteria |
| Andersson 2015 | Decision not PCC related |
| Andersson 2017 | Decision not PCC related (dengue control) |
| Angwenyi 2014 | Ineligible on two or more criteria |
| Anonymous 2005 | Ineligible participants (no provider or no consumer) (abstract of Manandhar 2004) |
| Armstrong 2018 | Ineligible meeting format (do not meet jointly or not formal group or once off) |
| Arunachalam 2012 | Decision not PCC related |
| Ayles 2013 | Ineligible on two or more criteria |
| Azad 2010 | Decision not PCC related (strengthen community) |
| Balcazar 2009 | Ineligible on two or more criteria |
| Balcazar 2010 | Ineligible on two or more criteria |
| Bashir 2019 | Ineligible on two or more criteria |
| Batterham 2014 | Ineligible on two or more criteria |
| Bedford 2014 | Ineligible on two or more criteria |
| Belin 2008 | Ineligible comparator |
| Beuermann 2018 | Decision not PCC related |
| Bitton 2018 | Ineligible study design (no attempt at randomisation) |
| Björkman 2009 | Ineligible comparator |
| Björkman 2010 | Ineligible on two or more criteria |
| Björkman Nyqvist 2017 | Ineligible study design |
| Blank Wilson 2014 | Ineligible on two or more criteria |
| Bloom 2014 | Ineligible study design (no attempt at randomisation) |
| Bogart 2009 | Ineligible on two or more criteria |
| Boivin 2011 | Ineligible on two or more criteria |
| Boivin 2014 | Ineligible comparator |
| Boivin 2014b | Ineligible comparator |
| Boivin 2018 | Ineligible on two or more criteria |
| Bos 2007 | Ineligible on two or more criteria |
| Bower 2015 | Ineligible participants (no provider or no consumer) |
| Bramble 2011 | Ineligible on two or more criteria |
| Brooker 2005 | Ineligible on two or more criteria |
| Brundage 2010 | Ineligible on two or more criteria |
| Burnell 2015 | Decision not PCC related |
| Cabassa 2014 | Ineligible on two or more criteria |
| Campbell‐Scherer 2014 | Ineligible on two or more criteria |
| Capara 2015 | Ineligible on two or more criteria |
| Cardoso 2017 | Ineligible intervention (some other intervention evaluated) |
| Carmen 2015 | No joint decision making |
| Castillo 2018 | Ineligible comparator |
| Castro 2012 | Ineligible on two or more criteria |
| Chaney 2011 | Ineligible on two or more criteria |
| Chang 2015 | Ineligible comparator |
| Cheever 2007 | Ineligible on two or more criteria |
| Chiew 2008 | Ineligible on two or more criteria |
| Chin 2007 | Ineligible on two or more criteria |
| Choi 2011 | Ineligible intervention |
| Choi 2016 | Ineligible intervention |
| Choi 2019 | Ineligible comparator |
| Chomat 2019 | Ineligible study design |
| Chumbley 2002 | No partnership |
| Chung 2010 | Ineligible comparator |
| Chung 2014 | Ineligible comparator |
| Chung 2015 | Ineligible comparator |
| Clarke 2014 | Ineligible on two or more criteria |
| Clausen 2018 | Ineligible study design (no attempt at randomisation) |
| Coker 2016 | Decision not PCC related |
| Coker 2019 | Ineligible intervention (some other intervention evaluated) |
| Colbourn 2013 | Decision not PCC related |
| Cooper 2016 | Ineligible study design (no attempt at randomisation) |
| Corrigan 2017 | No joint decision making |
| Decat 2013 | Ineligible on two or more criteria |
| de la Torre 2013 | Ineligible on two or more criteria |
| Donato 2019 | Ineligible on two or more criteria |
| Dowrick 2016 | Ineligible intervention (some other intervention evaluated) |
| Drummond 2014 | Ineligible on two or more criteria |
| El Ansari 2001 | Ineligible study design (no attempt at randomisation) |
| Elstad 2009 | Ineligible study design (no attempt at randomisation) |
| Erwin 2018 | Ineligible intervention |
| Esienumoh 2018 | Ineligible study design (no attempt at randomisation) |
| Farmer 2006 | Ineligible on two or more criteria |
| Fenenga 2015 | Ineligible on two or more criteria |
| Fottrell 2013 | Ineligible on two or more criteria |
| Fottrell 2016 | Ineligible on two or more criteria |
| Frank 2018 | Ineligible on two or more criteria |
| Fujimori 2014 | Ineligible on two or more criteria |
| Fulkerson 2021 | Ineligible study design |
| Galiatsatos 2017 | Ineligible study design (no attempt at randomisation) |
| Gellatly 2018 | Ineligible on two or more criteria |
| Gholipour 2016 | Ineligible on two or more criteria |
| Gitaka 2018 | Ineligible on two or more criteria |
| Gloppen 2012 | Ineligible on two or more criteria |
| Glynn 2015 | Ineligible on two or more criteria |
| Goldfinger 2012 | Ineligible on two or more criteria |
| Goodkind 2017 | Ineligible on two or more criteria |
| Gram 2018 | Ineligible on two or more criteria |
| Greenfield 2012 | Ineligible on two or more criteria |
| Gregory 2011 | Ineligible intervention (some other intervention evaluated) |
| Groene 2014 | Ineligible on two or more criteria |
| Grogan 2012 | Ineligible study design (no attempt at randomisation) |
| Gual 2012 | Ineligible on two or more criteria |
| Gullo 2017 | Ineligible comparator |
| Gullo 2018 | Ineligible comparator |
| Gummersbach 2013 | Ineligible on two or more criteria |
| Haines 2015 | Ineligible on two or more criteria |
| Hamilton 2017 | Ineligible on two or more criteria |
| Hanson 2014 | Ineligible study design (no attempt at randomisation) |
| Harding 2016 | Ineligible on two or more criteria |
| Harris 2013 | Ineligible on two or more criteria |
| Henderson 2017 | Ineligible intervention (some other intervention evaluated) |
| Hernandez 2013 | Ineligible on two or more criteria |
| Hobbs 2000 | Ineligible study design (no attempt at randomisation) |
| Hoffman 2018 | Ineligible on two or more criteria |
| Holt 2014 | Ineligible comparator |
| Hoos 2015 | Ineligible study design (no attempt at randomisation) |
| Houweling 2011 | Ineligible on two or more criteria |
| Houweling 2013 | Ineligible on two or more criteria |
| Huppelschoten 2015 | Ineligible comparator |
| Hwang 2012 | Ineligible comparator |
| Hynes 2017 | Ineligible study design (no attempt at randomisation) |
| Iezzoni 2018 | Ineligible comparator |
| Izquierdo 2016 | Ineligible comparator |
| Izquierdo 2018 | Ineligible comparator |
| Jha 2013 | Ineligible study design (no attempt at randomisation) |
| Källander 2015 | Ineligible comparator |
| Kang 2017 | Ineligible on two or more criteria |
| Kelly 2010 | Ineligible on two or more criteria |
| Khadyakov 2014 | Ineligible comparator |
| Khodyakov 2014 | Ineligible comparator |
| Khodyakov 2017 | Ineligible on two or more criteria |
| Khodyakov 2018 | Ineligible comparator |
| Kim 2014 | Ineligible on two or more criteria |
| Kim Yeary 2017 | Ineligible on two or more criteria |
| Kneipp 2011 | Decision not PCC related |
| Ko 2015 | Decision not PCC related |
| Ko 2016 | Decision not PCC related |
| Koerner 2014 | Ineligible on two or more criteria |
| Kogan 2017 | Ineligible comparator |
| Koniotou 2015 | Ineligible on two or more criteria |
| Krishnan 2017 | Ineligible intervention |
| Krist 2013 | Ineligible on two or more criteria |
| Lalonde 2011 | Ineligible on two or more criteria |
| Lam 2016 | Ineligible comparator |
| Lamb 2010 | Ineligible on two or more criteria |
| Lamontagne 2014 | No partnership |
| Landry 2017 | Ineligible comparator |
| Lawton 2016 | Ineligible on two or more criteria |
| Lawton 2017 | Ineligible on two or more criteria |
| Lewycka 2010 | Ineligible on two or more criteria |
| Lewycka 2013 | Ineligible on two or more criteria |
| Liddy 2011 | Ineligible on two or more criteria |
| Lippeveld 2017 | Ineligible on two or more criteria |
| Livingston 2013 | Ineligible on two or more criteria |
| Lodewijckx 2012 | Ineligible on two or more criteria |
| Loignon 2018 | Ineligible on two or more criteria |
| Lovell 2018 | Ineligible on two or more criteria |
| Luna 2015 | Ineligible study design (no attempt at randomisation) |
| Ma 2018 | Ineligible on two or more criteria |
| Machline‐Carrion 2019 | Ineligible participants |
| MacLeod 2012 | Ineligible on two or more criteria |
| Malfait 2018 | Ineligible study design (no attempt at randomisation) |
| Manandhar 2004 | Ineligible participants (no provider or no consumer) |
| Martinez Garcia 2013 | Ineligible on two or more criteria |
| Martínez‐Jaikel 2019 | Ineligible intervention |
| McCabe 2018 | Ineligible participants |
| McElfish 2017 | Decision not PCC related (research) |
| McKay 2011 | Ineligible intervention (some other intervention evaluated) |
| Mehta 2017 | Ineligible comparator |
| Mendel 2011 | Ineligible on two or more criteria |
| Mendel 2021 | Ineligible comparator |
| Mendenhall 2007 | Ineligible on two or more criteria |
| Meynard 2011 | Ineligible intervention (some other intervention evaluated) |
| Michaels 2017 | Ineligible participants (no provider or no consumer) |
| Miller 2017 | Ineligible on two or more criteria |
| Miller 2020 | Ineligible on two or more criteria |
| Mirza 2009 | Ineligible on two or more criteria |
| More 2008 | Ineligible intervention (some other intervention evaluated) |
| Morrison 2011 | Ineligible comparator |
| Mourad 2011 | Ineligible on two or more criteria |
| Nahar 2012 | Ineligible on two or more criteria |
| Nair 2015 | Ineligible comparator |
| Namazzi 2015 | Ineligible on two or more criteria |
| NCT02286193 | Ineligible on two or more criteria |
| NCT03035474 | Ineligible on two or more criteria |
| NCT03044145 | Ineligible on two or more criteria |
| NCT03222466 | Ineligible on two or more criteria |
| NCT04514133 | Ineligible on two or more criteria |
| Newman Owiredu 2017 | Ineligible on two or more criteria |
| Ngo 2016 | Ineligible comparator |
| Noel 2014 | Ineligible on two or more criteria |
| Ogbuoji Osondu 2018 | Ineligible participants |
| Ojerholm 2016 | Ineligible on two or more criteria |
| Oliver 2009 | Ineligible study design (no attempt at randomisation) |
| Ong 2013 | Ineligible participants (no provider or no consumer) |
| Ong 2017 | Ineligible comparator |
| Orozco‐Beltran 2013 | Ineligible on two or more criteria |
| Osrin 2003 | Ineligible on two or more criteria |
| Parchman 2013 | Ineligible on two or more criteria |
| Patel 2019a | Ineligible study design |
| Patel 2019b | Ineligible study design |
| Paton 2013 | Ineligible study design (no attempt at randomisation) |
| Patzer 2014 | Ineligible comparator |
| Peremans 2010 | Ineligible intervention |
| Peter 2015 | Ineligible on two or more criteria |
| Peterson 2018 | Ineligible on two or more criteria |
| Pramanik 2018 | Decision not PCC related |
| Prost 2018 | Ineligible on two or more criteria |
| Protik Ali 2018 | Decision not PCC related |
| Rai 2018 | Ineligible on two or more criteria |
| Raynes‐Greenow 2010 | Ineligible on two or more criteria |
| Reeves 2013 | Ineligible on two or more criteria |
| Reinhardt 2012 | Decision not PCC related |
| Rogers 2007 | Ineligible on two or more criteria |
| Sarmiento 2018 | Ineligible participants (no provider or no consumer) |
| Sauers‐Ford 2016 | Ineligible on two or more criteria |
| Scholl 2018 | Ineligible on two or more criteria |
| Schweitzer 2015 | Ineligible on two or more criteria |
| Segal 2010 | Decision not PCC related |
| Sepuchra 2003 | Ineligible on two or more criteria |
| Shagi 2008 | Ineligible on two or more criteria |
| Sherbourne 2017 | Ineligible comparator |
| Shukla 2018 | Ineligible study design (no attempt at randomisation) |
| Simonsen 2017 | Ineligible on two or more criteria |
| Singh 2020 | Ineligible study design |
| Sirilak 2013 | Ineligible on two or more criteria |
| Smiddy 2015 | Ineligible on two or more criteria |
| Smout 2016 | Ineligible on two or more criteria |
| Solomon 2017 | Ineligible on two or more criteria |
| South 2016 | Decision not PCC related |
| Spencer 2011 | Decision not PCC related |
| Springgate 2018 | Ineligible on two or more criteria |
| Springgate 2018b | Ineligible comparator |
| Stanhope 2013 | Ineligible on two or more criteria |
| Stephens 2018 | Ineligible on two or more criteria |
| Stockdale 2016 | Ineligible comparator |
| Storm 2011 | Ineligible study design (no attempt at randomisation) |
| Svetaz 2016 | Decision not PCC related |
| Taylor 2017 | Ineligible on two or more criteria |
| Thilly 2003 | Ineligible participants (no provider or no consumer) |
| Thornton 2003 | Ineligible on two or more criteria |
| Tondora 2010 | Ineligible on two or more criteria |
| Tran 2004 | Ineligible on two or more criteria |
| Tran 2018 | No joint decision making |
| Treloar 2015 | Ineligible on two or more criteria |
| Tripathy 2010 | Ineligible on two or more criteria |
| Tripathy 2011 | Ineligible on two or more criteria |
| Tripathy 2016 | Ineligible on two or more criteria |
| Tsianakas 2012 | Ineligible study design (no attempt at randomisation) |
| Tumiel‐Berhalter 2011 | Ineligible on two or more criteria |
| Uding 2007 | Ineligible on two or more criteria |
| Uding 2009 | Ineligible on two or more criteria |
| Vale 2018 | Decision not PCC related |
| Vanderboom 2014 | Ineligible participants (no provider or no consumer) |
| Van Malderen 2017 | No joint decision making |
| Van Malderen 2017a | No joint decision making |
| von dem Knesebeck 2002 | Ineligible on two or more criteria |
| Waiswa 2016 | Ineligible comparator |
| Wathne 2018 | Ineligible on two or more criteria |
| Watson 2001 | Ineligible on two or more criteria |
| Weinstein 2006 | Ineligible study design (no attempt at randomisation) |
| Wells 2013 | Ineligible comparator |
| Werner 2019 | Ineligible on two or more criteria |
| Wheatley 2002 | Ineligible on two or more criteria |
| Williams 2013 | Ineligible intervention (some other intervention evaluated) |
| Winters 2016 | Ineligible on two or more criteria |
| Wolf 2008 | Ineligible on two or more criteria |
| Wolfe 2019 | Ineligible participants |
| Wood Dauphinee 2011 | Ineligible on two or more criteria |
| Yano 2016 | Ineligible on two or more criteria |
| Younes 2012 | Ineligible on two or more criteria |
| Young 2005 | Ineligible study design (no attempt at randomisation) |
| Zhang 2020 | Ineligible participants |
| Zimmerman 2017 | Decision not PCC related |
| Zwar 2008 | Ineligible on two or more criteria |
PCC: person‐centred care.
Characteristics of studies awaiting classification [ordered by study ID]
English 2018.
| Methods | Cluster‐RCT; unit of randomisation ‐ geographic regions n = 26 |
| Participants | Inclusion Criteria: 18‐89 years, all sexes. Enrolled primary care staff (including physicians, nurse practitioners and physician assistants). Primary care practice must be family medicine or general internal medicine practices with a maximum of ten lead clinicians; and independent, or if in part of a larger organisation, must demonstrate on careful screening that they do not received significant quality improvement support from the larger organisation. Exclusion Criteria: Primary care practices with more than 10 lead clinicians; non‐independent primary care practices that receive significant quality improvement support from their system organisation. |
| Interventions | Multi‐component intervention with partnership (enhanced facilitation: standard facilitation plus community engagement in developing resources) compared with multi‐component intervention without partnership (standard facilitation intervention) |
| Outcomes | Primary outcomes: Timing: Baseline, 3, 6, 9, 12, and 15 months from baseline: Change in documentation of the following practice level indicators: aspirin therapy in patients with ischaemic vascular disease; blood pressure in patients with a diagnosis of hypertension; blood pressure in patients with adequately controlled blood pressure; fasting LDL in patients with a fasting LDL at or below the LDL goal; patients who had a fasting LDL test performed and prescribed a statin based on risk; and patients screened about tobacco use. Secondary outcomes: Timing: Baseline, 9 and 15 months from baseline: change of the documentation in primary care practices (based on practice‐level scores of change process capacity, adaptive reserve, clinician experience and implementation of patient‐centred medical home components). |
| Notes | trial registration #:NCT02515578 [new references Dickinson 2020 and Fernald 2020 identified 21/12/2020] |
Gai 2019.
| Methods | Cluster‐RCT; unit of randomisation: community clinic level Objectives: To evaluate the effects of a community‐based intervention on the utilisation of maternal and neonatal care provided by qualified facilities and skilled providers, in the context of the Safe Mothering Promotion Project (Phase II). 'The objective of SMPP Phase II was to improve maternal and neonatal health outcomes by: improving maternal and neonatal health (MNH) service delivery at health facilities; strengthening CSGs to implement community led actions for saving mother and newborn lives; involving the local government bodies to support MNH services; and empowering women, through awareness building, to obtain participation and accountability for overcoming barriers to access healthcare services' (p. 4). |
| Participants | Eligible: women having given birth in the year preceding data collection; residing in study areas (intervention or control areas) and able to give written informed consent. Location: Kalaroa Upazila of Satkhira District, Bangladesh Total n = 4675 women (intervention group: n = 2407 (1102 baseline, 1305 at follow up); control group: n = 2268 (1237 baseline, 1031 at follow up). |
| Interventions | Multicomponent intervention at healthcare facilities and community levels, involving Community Support Groups embedded within Community Clinics and serving to create demand and mobilise the community, working in collaboration with a Community Group (governing and management body, ensuring quality of care). Purpose is to promote better maternal and neonatal health outcomes; comparison with usual practice. |
| Outcomes | Primarily clinical and related outcomes, reflecting use of services for antenatal care, delivery,
postpartum care and neonatal care by pregnant and post‐partum women. 'The major indicators of the expected outcomes were: • Proportion of women received any and 4+ ANC from skilled health care providers; • Met need (proportion of women with complications received services from EmOC facilities) during pregnancy, childbirth and post‐partum period; • Delivery attended by skilled birth attendants; • Delivery conducted at health facilities; • Proportion of postpartum women received PNC from skilled providers within 42 days of delivery; • Proportion of sick newborns received services from skilled provider Information related to maternal and neonatal complications and care seeking were obtained from the respondents through face‐to‐face interview using a structured questionnaire' (p.9). |
| Notes | UMIN Clinical Trial Registry UMIN000031789. |
James 2013.
| Methods | Cluster‐RCT: unit of randomisation ‐ Community Health Centres, n = 16 (11 enrolled?) |
| Participants | Community Health Centres are eligible if they serve mostly Medicaid, uninsured, or lower‐income patients; be willing to be randomised to intervention or comparison, and willing to allow the research team access to Community Health Centres managers/directors, patients, and providers. Participants were eligible if they spoke English or Spanish and were aged 49 or older. |
| Interventions | Using a Community‐Based Participatory Research approach, collaborated with partners to implement and evaluate a systems‐level intervention for its effectiveness in increasing CRC screening rates vs usual practice |
| Outcomes | Timing: baseline, six months, and twelve months. Primary outcome: colorectal cancer screening by patient self‐report, with a chart‐audit in a sub‐sample of patients. Other outcomes: evaluated according to the Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance (RE‐AIM) conceptual framework. Six‐month and 12‐month surveys include self‐reported CRC screening, healthcare utilization, and awareness of screening or educational efforts |
| Notes | Trial registration #: NCT01299493 [new reference Muthukrishnan 2018 ‐ identified 21 December 2020] |
Lindquist 2020.
| Methods | Multi‐site RCT; n = 400 Objective: To assess the effectiveness of a long term support services (LTSS) planning tool for older adults and to involve community partners in disseminating information about the tool within the community. |
| Participants | Older adults and their family members; community partners (5 newly trained) Dissemination amongst diverse community groups in Chicago, Indiana and Hawaii |
| Interventions | LTSS planning tool, with community partner engagement to increase dissemination about the tool in patient partners. Train the trainer model. |
| Outcomes | Engagement of patient partners in dissemination activities. Dissemination activities (locations, dates; newly created accounts, web sessions and daily visitors to tool site). Assessed at 1 week and 1 month after dissemination activities. |
| Notes | Conference abstract only. Not clear if partnership is evaluated through randomised trial (may be in parent trial of tool effectiveness). |
Morrison 2020.
| Methods | Cluster‐randomised trial; 21 intervention Village Development Committee (VDCs) clusters and 22 control clusters; stratified (4 groups) based on previous women's group activity exposure. Objectives: To evaluate the impact of strengthened health management committees (HMCs) and community mobilisation through women’s groups on deliveries (institutional deliveries and those by trained health workers). |
| Participants | Eligible: women aged 12‐49 years, who had delivered a baby between 1 October 2010 and
30 September 2012. Makwanpur District, rural Nepal; containing 43 geopolitical VDCs. Complete data were recorded for 13,721 deliveries during the study period (intervention and control sites). |
| Interventions | Multicomponent intervention versus control. Strengthening health management committees (HMCs), via 4‐day workshop based on principles of Appreciative Inquiry. Women's group intervention: training of female community health volunteers in facilitation skills, participatory learning and action cycle process, and running meetings; provided with manual to guide discussions, and supervision. Women's groups run approximately monthly; discussions included those about barriers to institutional delivery and how to address these; followed by community and cluster meetings to garner support for implementation of strategies to address barriers; following implementation groups reflected on progress and planned and implemented further strategies or modified those already in place. |
| Outcomes | Primary outcomes: deliveries conducted by trained health workers; institutional deliveries Secondary outcomes: uptake of antenatal and postnatal care; live births; stillbirths |
| Notes | Current Controlled Trials ISRCTN99834806. Date of registration: 28 September 2010 |
Palmer 2015.
| Methods | Stepped wedge cluster‐RCT; unit of randomisation ‐ Community Support Services |
| Participants | Consumers: English speaking, aged 16 years or older, attends a program that is part of the Mental Health Community Support Service at the participating service provider; received a diagnosis of mental illness. Carers: English speaking, aged 18 years or older, supports someone who attends a program that is part of the Mental Health Community Support Service at the participating service provider. Staff: employed at the mental health service (any time fraction) and are involved in planning and/or the delivery of services related to the Mental Health Community Support Services. |
| Interventions | Mental Health Experience Co‐design (MH ECO) is a structured process for service users (consumers), carers and mental health staff to come together to identify improvements to service planning and delivery and co‐design the solutions to these improvements compared to clusters awaiting intervention as controls i.e. usual practice |
| Outcomes | Timing: 9, 18, and 27 months after intervention. Primary outcome: Psychosocial Recovery Assessment Scale ‐ Revised 24 Item RAS‐R; Secondary outcome: mental health and well‐being for consumers and carers (EUROHIS ‐ Quality of Life 8 Item Scale) and change in staff attitudes to recovery (RSA, Recovery Self Assessment Scale ‐ Provider Version and STARS Staff Attitudes to Recovery Scale) |
| Notes | The CORE study Prinical Investigator: A/Prof, Dr Victoria Jane Palmer, Department of General Practice, Melbourne Medical School. Trial registration #: ACTRN12614000457640 |
Shrestha 2011.
| Methods | Cluster‐RCT; unit of randomisation ‐ village development committee clusters, n = 60 |
| Participants | Main target population:Women of reproductive age; infants under a year of age and pregnancies in the district |
| Interventions | Two community‐based interventions involving Female Community Health Volunteers (1) MIRA Dhanusha community groups: a participatory intervention with women’s groups and (2) MIRA Dhanusha sepsis management: training of community volunteers in the recognition and management of neonatal sepsis |
| Outcomes | Primary outcome: neonatal mortality rates. Secondary outcomes: MIRA Dhanusha community group: stillbirth, infant and under‐two mortality rates, care practices and health care seeking behaviour, maternal diet, breastfeeding and complementary feeding practices, maternal and under‐2 anthropometric status. MIRA Dhanusha sepsis management: identification and treatment of neonatal sepsis by community health volunteers, infection‐specific neonatal mortality |
| Notes | ISRCTN: ISRCTN87820538; Principal investigator: Prof Anthony Costello, UCL Centre for International Health and Development |
CHC: Community Health Centre; CRC: Colorectal Cancer; EUROHIS‐QoL: shortened version of the WHO Quality of Life Instrument‐Abbreviated version; LDL: Low‐density lipoprotein; MH ECO: Mental Health Experience Co‐design; RSA: Recovery Self Assessment Scale; RAS‐R: Psychosocial Recovery Assessment Scale ‐ Revised; RE‐AIM: Reach, Efficacy/Effectiveness, Adoption, Implementation, and Maintenance; STARS: Staff Attitudes to Recovery Scale.
Characteristics of ongoing studies [ordered by study ID]
Kjellström 2019.
| Study name | ‘Samskapa’ research programme protocol |
| Methods | Various study designs; mixed‐method evaluations of at least nine case studies of coproduction |
| Participants | Various participants |
| Interventions | Coproduction in the health and social care sectors |
| Outcomes | Various studies measuring other outcomes |
| Starting date | Various start dates during a 5‐year period (2019–2024) |
| Contact information | Professor Sofia Kjellström; doctoral students taking part in the 9 studies: Andreas Gremyr (Jönköping University), Sarah McAllister (King’s College London), Sofia Persson (Jönköping University), Anne‐ Marie Suutari (Jönköping University), Mary Tanay (King’s College London) and Pontus Wallin (Jönköping University). |
| Notes |
Sawtell 2018.
| Study name | A pragmatic cluster population‐level randomised controlled trial of a community‐level intervention to increase early uptake of antenatal care (REACH Pregnancy Programme, Work Package 1) |
| Methods | Matched cluster‐RCT; unit of randomisation: electoral ward n = 10 |
| Participants | Wards: electoral wards, covered by maternity care providers enrolled on the study, where the proportion of women who have their first antenatal appointment by 12 weeks is below the NHS national target of 90% Participants: women in the selected electoral wards who give birth, at a hospital enrolled in the study, over a 12‐month period |
| Interventions | Using a co‐production process engages with local communities to identifying their perceptions/views on the issues and solutions to increase early booking for antenatal care; tailoring the design of the intervention and form and content of key intervention messages; and facilitating the communication of the intervention messages through community self‐help and local social networks compared with usual practice |
| Outcomes | Timing: baseline; first follow‐up (2‐7 months) and second follow‐up (8‐12 months): gestation at booking, antenatal admissions, emergency caesarean rates, gestation/weight at delivery, maternal/infant death, APGAR score, smoking, feeding; Other outcomes: feasibility, acceptability, fidelity and economics via interview/observations and reach, exposure, and acceptability via survey. |
| Starting date | 1 April 2015 to 1 April 2020; intention to publish date: 1 May 2021 |
| Contact information | Principal Investigator: Ms Mary Sawtell, University College London |
| Notes | ISRCTN63066975 |
Differences between protocol and review
We modified the review title from 'The effects of consumers and health providers working in partnership as an intervention for the promotion of person‐centred health services' based on peer referee feedback.
We added Networked Digital Library of Theses and Dissertations (during search update) and Web of Science (during original search) (both not identified in the protocol) to the search strategy so as to minimise potential for missed studies.
We used the RCT Classifier to sort the database search output into records likely to be RCTs and records unlikely to be RCTs. The title and abstract records that were identified as unlikely to be RCTs were screened by one review author in Covidence. Those identified as potentially relevant were then obtained in full‐text and screened by two review authors, with a process of resolving disagreements by consensus with a third review author. In addition to the records that were classified as unlikely to be RCT, only one author screened the Clinical Trials registries search outputs and conducted grey literature searching, snowballing and screening of review and included studies citations. Again all records identified as potentially relevant were screened in full‐text by two review authors with a process of achieving consensus by consulting a third review author. The remainder of the search output (those records identified as likely to be RCTs by the RCT Classifier and the Cochrane Library search outputs) were screened by two authors at title and abstract stage, however instead of retrieving in full text any papers identified as potentially relevant by at least one review author, records identified by two review authors as potentially relevant were obtained in full‐text; again where there was disagreement amongst review authors, a third review author independently provided a consensus decision. These screening decisions were made independently in Covidence. All references that were retrieved in full‐text were screened by two review authors, with a third review author providing a consensus decision.
Instead of two review authors independently extracting data and undertaking Risk of Bias assessments, one review author extracted all data and conducted all Risk of Bias assessments, the second review author independently cross‐checked all extracted data and assessments against the publications, consensus was reached by resolving any disagreements through discussion.
We could not conduct meta‐analyses, sensitivity analyses, subgroup analyses or investigate heterogeneity as planned due to an insufficient number of studies identified within each comparison and each outcome, instead results are synthesised descriptively by comparison and outcome, with data from the longest‐term time point. We planned the following sensitivity analyses to explore the impact of assumptions, imputed data, and the inclusion of studies at high risk of bias by:
comparing the results of studies at higher and lower risk of bias (remove from the analysis studies with a high or unclear rating on the sequence generation item of the ‘Risk of bias’ tool and see how robust the results are when based only on studies with low risk of bias);
comparing the results based on imputed data, e.g. when ICC values have been taken from external sources for cluster‐RCTs.
We planned subgroup analyses to examine trials that explicitly attempt to address intrinsic power imbalances in preparation for partnerships (e.g. provision of salary or financial reimbursement, orientation, training, coaching, or support (e.g. via an advocate, facilitator, moderator, mentor, or consumer liaison officer) versus trials that did not); the ratio of consumers to providers (consumer majority versus provider majority); and the partnership duration (e.g. ongoing versus time‐limited), but there were too few trials to do so.
As there were too few studies measuring similar outcomes to undertake statistical analyses, where a study did report multiple outcome measures for the same outcome, we extracted all, and review authors met to discuss and identify the outcome measure most relevant to person‐centred health care. Additionally, we did not use GRADEpro software to present the results of the meta‐analysis as the findings were limited to descriptive synthesis for each major comparisons of the review.
Contributions of authors
DL: conceptual development of the protocol and review, screening titles, abstracts and full‐text articles for inclusion and exclusions, extracting data for included trials and undertaking risk of bias and GRADE assessments, drafting of the protocol and review, organising and participating in the Stakeholder Panel workshop RR: conceptual development of the review, providing consensus decisions on eligibility of articles, extracting data for included trials and undertaking risk of bias and GRADE assessments, drafting of the review, screening updated search output LS: screening titles, abstracts and full‐text articles for inclusion and exclusions, cross‐checking data entered in RevMan with data extracted; providing feedback on drafts BM: conceptual development of the protocol, screening titles, abstracts and full‐text articles for inclusion and exclusions, providing feedback on drafts, organising and participating in Stakeholder Panel meetings and the workshop LW: conceptual development of the protocol, providing consensus decisions on eligibility of articles, providing feedback on drafts, organising and participating in the Stakeholder Panel workshop, screening updated search output LGW: conceptual development of the protocol, screening full‐text articles for inclusion and exclusions, providing feedback on drafts SH: conceptual development of the protocol and review, providing consensus decisions on eligibility of articles, drafting of the protocol and review, organising and participating in Stakeholder Panel meetings and the workshop
Sources of support
Internal sources
-
La Trobe University, Australia
BM receives funding from La Trobe University to undertake this review.
External sources
-
Safer Care Victoria and the National Health and Medical Research Centre, Australia
The work of the Cochrane Group is situated within the Centre for Health Communication and Participation, La Trobe University, and is supported, in part, by grants from Safer Care Victoria and the National Health and Medical Research Council.
Declarations of interest
DL is the Technical Editor for the Cochrane Consumers and Communication Group. RR is the Joint Co‐ordinating Editor of the Cochrane Consumers and Communication Group. LS: none known. BM is the Joint Managing Editor of the Cochrane Consumers and Communication Group. LW is the Joint Managing Editor of the Cochrane Consumers and Communication Group, and is a facilitator for the Australian Collaborative Pairs program, funded by the Consumers Health Forum of Australia. The Collaborative Pairs program aims to bring health service providers together with consumers to develop and strengthen their capacity to work together to transform the health system; participated in a podcast interview about the importance of consumer advocacy and involving consumers in the planning of health services; worked as a physiotherapist at Monash University. LGW: none known. SH is the Joint Co‐ordinating Editor of the Cochrane Consumers and Communication Group.
The work of the Cochrane Group is situated within the Centre for Health Communication and Participation, La Trobe University, and is supported, in part, by grants from Safer Care Victoria and the National Health and Medical Research Council Australia. SH, BM, DL, and LW were not involved in the editorial processes for this review. RR was the contact editor for the protocol but was not involved in editorial processes after protocol publication.
New
References
References to studies included in this review
Greco 2006 {published data only (unpublished sought but not used)}
- Greco M, Carter M, Powell R, Sweeney K, Jolliffe J, Stead J. Impact of patient involvement in general practice. Education for Primary Care 2006;17:486-96. [Google Scholar]
Jha 2015 {published and unpublished data}
- Jha V, Buckley H, Gabe R, Kanaan M, Lawton R, Melville C, et al. Patients as teachers: a randomised controlled trial on the use of personal stories of harm to raise awareness of patient safety for doctors in training. BMJ Quality & Safety 2015;24:21-30. [DOI: 10.1136/bmjqs-2014-002987] [DOI] [PubMed] [Google Scholar]
- Quinton N, Jha V, Symons J, Thompson Z. Clinical Education: enhancing practice through scholarship and research. Clinical Education Network Symposium, Leeds UK May 2018.
- Winterbottom AE, Jha V, Melville C, Corrado O, Symons J, Torgerson D, et al. A randomised controlled trial of patient led training in medical education: protocol. BMC Medical Education 2010;10:90. [DOI: 10.1186/1472-6920-10-90] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J, Lawton R, O’Hara J, Armitage G, Sheard L, Marsh C, et al. Improving patient safety through the involvement of patients: development and evaluation of novel interventions to engage patients in preventing patient safety incidents and protecting them against unintended harm. NIHR Journals Library.. Southampton, UK: NIHR Journals Library; 2016 Oct. (Programme Grants for Applied Research, No. 4.15), 2016. [DOI: https://www.ncbi.nlm.nih.gov/books/NBK390627/ doi: 10.3310/pgfar04150] [PubMed] [Google Scholar]
O'Connor 2019 {published data only (unpublished sought but not used)}
- O'Connor EC, Hutain J, Christensen M, Kamara Ms, Contech A, Sarriot E, et al. Piloting a participatory, community-based health information system for strengthening community based health services: findings of a cluster-randomized controlled trial in the slums of Freetown, Sierra Leone. Journal of Global Health 2019;9(1):1-15. [DOI: 10.7189/jogh.09.010418] [DOI] [PMC free article] [PubMed] [Google Scholar]
Persson 2013 {published and unpublished data}
- Eriksson L, Huy TQ, Duc DM, Selling KE, Hoa DP, Thuy NT. Process evaluation of a knowledge translation intervention using facilitation of local stakeholder groups to improve neonatal survival in the Quang Ninh province, Vietnam. Trials 2016;17(1):23. [DOI: 10.1186/s13063-015-1141-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson L, Nga NT, Hoa DTP, Duc DM, Bergström A, Wallin L, et al. Secular trend, seasonality and effects of a community-based intervention on neonatal mortality: follow-up of a cluster-randomised trial in Quang Ninh province, Vietnam. Journal of Epidemiology and Community Health 2018;72(9):776-82. [DOI: 10.1136/jech-2017-20925] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Målqvist M, Hoa DP, Persson LÅ, Ekholm Selling K. Effect of facilitation of local stakeholder groups on equity in neonatal survival; results from the NeoKIP trial in Northern Vietnam. PLoS One 2015;10(12):e0145510. [DOI: 10.1371/journal.pone.0145510] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nga NT, Hoa DT, Målqvist M, Persson LÅ, Ewald U. Causes of neonatal death: results from NeoKIP community-based trial in Quang Ninh province, Vietnam. Acta Paediatrica 2012;101:368-73. [DOI: 10.1111/j.1651-2227.2011.02513.x] [DOI] [PubMed] [Google Scholar]
- Nga NT, Målqvist M, Eriksson L, Hoa DP, Johansson A, Wallin L, et al. Perinatal services and outcomes in Quang Ninh province, Vietnam. Acta Paediatr 2010;99(10):1478-83. [DOI] [PubMed] [Google Scholar]
- Persson LÅ, Nga NT, Målqvist M, Thi Phuong Hoa D, Eriksson L, et al. Effect of facilitation of local maternal-and-newborn stakeholder groups on neonatal mortality: cluster-randomized controlled trial. PLOS Medicine 2013;10(5):e1001445. [DOI: 10.1371/journal.pmed.1001445] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin L, Målqvist M, Nga NT, Eriksson L, Persson LÅ, Hoa DP. Implementing knowledge into practice for improved neonatal survival; a cluster-randomised, community-based trial in Quang Ninh province, Vietnam. BMC Health Services Research 2011;11:239. [DOI: 10.1186/1472-6963-11-239] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2019 {published and unpublished data}
- Dadwal V, Basu L, Weston C M, Hwang S, Ibe C, Bone L, et al. How co-developed are community and academic partnerships? Progress in Community Health Partnerships 2017;11(4):387-95. [DOI: 10.1353/cpr.2017.0046] [DOI] [PubMed] [Google Scholar]
- Ibe CA, Basu L, Gooden R, Syed SB, Dadwal V, Bone LR, et al. From Kisiizi to Baltimore: cultivating knowledge brokers to support global innovation for community engagement in healthcare. Global Health 2018;14(1):19. [DOI: 10.1186/s12992-018-0339-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns Hopkins Bloomberg School of Public Health. Reverse innovation and patient engagement to improve quality of care and patient outcomes (CONNECT). ClinicalTrials.gov. [NCT02222909]
- Wu AW, Hwang S, Weston CM, Ibe C, Boonyasai RT, Bone L, et al. Baltimore CONNECT: a randomized trial to build partnership between community organizations and a local health system. Progress in Community Health Partnerships: Research, Education, and Action 2018;12(3):297-306. [DOI: 10.1353/cpr.2018.0054] [DOI] [PubMed] [Google Scholar]
- Wu AW, Weston CM, Ibe CA, Ruberman CF, Bone L, Romsai T. The Baltimore community-based Organizations Neighborhood Network: Enhancing Capacity Together (CONNECT) cluster RCT. American Journal of Preventive Medicine 2019;57(2):e31-41. [DOI: 10.1016/j.amepre.2019.03.013] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbey 2017 {published data only}
- Abbey M, Bartholomew LK, Chinbuah Margaret A, Gyapong M, Gyapong John O, den Borne B. Development of a theory and evidence-based program to promote community treatment of fevers in children under five in a rural district in Southern Ghana: an intervention mapping approach. BMC Public Health 2017;17(1):1-11. [DOI: 10.1186/s12889-016-3957-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Abelson 2003 {published data only}
- Abelson J, Eyles J, McLeod CB, Collins P, McMullan C, Forest PG. Does deliberation make a difference? Results from a citizens panel study of health goals priority setting. Health Policy 2003;66(1):95-106. [DOI: 10.1016/S0168-8510(03)00048-4] [DOI] [PubMed] [Google Scholar]
Abelson 2016 {published data only}
- Abelson J, Li K, Wilson G, Shields K, Schneider C, Boesveld S. Supporting quality public and patient engagement in health system organizations: development and usability testing of the Public and Patient Engagement Evaluation Tool. Health Expectations 2016;19(4):817-27. [DOI: 10.1111/hex.12378] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aboumatar 2017 {published data only}
- Aboumatar H, Naqibuddin M, Chung S, Adebowale H, Bone L, Brown T, et al. Better Respiratory Education and Treatment Help Empower (BREATHE) study: methodology and baseline characteristics of a randomized controlled trial testing a transitional care program to improve patient-centered care delivery among chronic obstructive pulmonary disease patients. Contemporary Clinical Trials 2017;62:159-67. [DOI: 10.1016/j.cct.2017.08.018] [DOI] [PubMed] [Google Scholar]
Acharya 2015 {published data only}
- Acharya A, Lalwani T, Dutta R, Rajaratnam Julie K, Ruducha J, Varkey Leila C, et al. Evaluating a large-scale community-based intervention to improve pregnancy and newborn health among the rural poor in India. American Journal of Public Health 2015;105(1):144-52. [DOI: 10.2105/AJPH.2014.302092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ackermann 2010 {published data only}
- Ackermann RT. Description of an integrated framework for building linkages among primary care clinics and community organizations for the prevention of type 2 diabetes: emerging themes from the CC-Link study. Chronic Illness 2010;6(2):89-100. [DOI: 10.1177/1742395310364857] [DOI] [PubMed] [Google Scholar]
Aggett 2009 {published data only}
- Aggett S, Charles M, Giri SR, Graves-Abe K, Mair R, Sherchan S. Increased access to trained health workers and improved AIDS care in far West Nepal as a result of mentoring and clinic optimization. HIV Medicine 2009;10(s2):202-3. [DOI: 10.1111/j.1468-1293.2009.00792.x] [DOI] [Google Scholar]
Aibinuomo 2011 {published data only}
- Aibinuomo, F. Primary Health Care (PHC) as the bedrock of the MDGs: community sensitization, mobilization and education on communication for positive health change. IFE PsychologIA 2011;19(1):259-42. [DOI: 10.4314/ifep.v19i1.64619] [DOI] [Google Scholar]
Aimola 2016 {published data only}
- Aimola L, Jasim S, Tripathi N, Tucker S, Worrall A, Quirk A, et al. Impact of peer-led quality improvement networks on quality of inpatient mental health care: study protocol for a cluster randomized controlled trial. BMC Psychiatry 2016;16:331. [DOI: 10.1186/s12888-016-1040-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aldiss 2011 {published data only}
- Aldiss S, Taylor R, Soanes L, Maguire R, Sage M, Kearney N, et al. Working in collaboration with young people and health professionals. A staged approach to the implementation of a randomised controlled trial. Journal of Research in Nursing 2011;16(6):561-76. [DOI: 10.1177/1744987110380803] [DOI] [Google Scholar]
Alhassan 2015 {published data only}
- Alhassan RK, Nketiah-Amponsah E, Spieker N, Arhinful DK, Ogink A, Ostenberg P, et al. Effect of community engagement interventions on patient safety and risk reduction efforts in primary health facilities: evidence from Ghana. PLoS One 2015;10(11):e0142389. [DOI: 10.1371/journal.pone.0142389] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhassan 2016 {published data only}
- Alhassan RK, Nketiah-Amponsah E, Spieker N, Arhinful DK, Rinke de Wit TF. Assessing the impact of community engagement interventions on health worker motivation and experiences with clients in primary health facilities in Ghana: a randomized cluster trial. PLoS One 2016;11(7):e0158541. [DOI: 10.1371/journal.pone.0158541] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhassan 2016a {published data only}
- Alhassan RK, Nketiah-Amponsah E, Arhinful DK. Design and implementation of community engagement interventions towards healthcare quality improvement in Ghana: a methodological approach. Health Economics Review 2016;6(1):49. [DOI: 10.1186/s13561-016-0128-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhassan 2016b {published data only}
- Alhassan RK, Nketiah-Amponsah E, Spieker N, Arhinful DK, Rinke de Wit TF. Perspectives of frontline health workers on Ghana’s National Health Insurance Scheme before and after community engagement interventions. BMC Health Services Research 2016;16:192. [DOI: 10.1186/s12913-016-1438-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alhassan 2017 {published data only}
- Alhassan RK. Thesis: Healthcare quality in Ghana: Improving healthcare quality and health worker motivation to promote sustainable health insurance. Faculty of Medicine, University of Amsterdam 2017:197. [Available at: https://hdl.handle.net/11245.1/b294c75f-d7e3-4c5a-ae03-da5011bb01e0]
Alhassan 2019 {published data only}
- Alhassan RK, Nketiah-Amponsah E, Ayanore MA, Afaya A, Salia SM, Milipaak J, et al. Impact of a bottom-up community engagement intervention on maternal and child health services utilization in Ghana: a cluster randomised trial. BMC Public Health 2019;19(1):791. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aliyu 2013 {published data only}
- Aliyu MH, Blevins M, Audet C, Shepherd BE, Hassan A, Onwujekwe O, et al. Optimizing PMTCT service delivery in rural North-Central Nigeria: protocol and design for a cluster randomized study. Contemporary Clinical Trials 2013;36(1):187-97. [DOI: 10.1016/j.cct.2013.06.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2012 {published data only}
- Allen ML, Garcia-Huidobro D, Hurtado GA, Allen R, Davey CS, Forster JL, et al. Immigrant family skills-building to prevent tobacco use in Latino youth: study protocol for a community-based participatory randomized controlled trial. Trials 2012;13:242. [DOI: 10.1186/1745-6215-13-242] [DOI] [PMC free article] [PubMed] [Google Scholar]
Allen 2013 {published data only}
- Allen M, Ghaffar H, Rosas-Lee M, Svetaz MV, Davey C, Palma DM. Collaboration process evaluation of padres informados/jovenes preparados. Journal of Adolescent Health 2013;52(s1):s72. [DOI: 10.1016/j.jadohealth.2012.10.168] [DOI] [Google Scholar]
Allen 2018 {published data only}
- Allen S. Addressing the research to practice gap through co-production & service user leadership in early psychosis intervention research. In: Early intervention in psychiatry. Conference: IEPA 11th international conference on early intervention in mental health - "prevention and early intervention: broadening the scope". United states. Vol. 12 (Supplement 1). 2018:36. [DOI: 10.1111/eip.12722] [DOI]
Allender 2016 {published data only}
- Allender S, Millar L, Hovmand P, Bell C, Moodie M, Carter R, et al. Whole of Systems Trial of Prevention Strategies for Childhood Obesity: WHO STOPS Childhood Obesity. International Journal of Environmental Research and Public Health 2016;13(11):1143. [DOI: 10.3390/ijerph13111143] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarado 1999 {published data only}
- Alvarado R, Zepeda A, Rivero S, Rico N, López S, Díaz S. Integrated maternal and infant health care in the postpartum period in a poor neighborhood in Santiago, Chile. Studies in Family Planning 1999;30(2):133-41. [DOI: 10.1111/j.1728-4465.1999.00133.x] [DOI] [PubMed] [Google Scholar]
Andersson 2015 {published data only}
- Andersson N, Nava-Aguilera E, Arosteguí J, Morales-Perez A, Suazo-Laguna H, Legorreta-Soberanis J, et al. Evidence based community mobilization for dengue prevention in Nicaragua and Mexico (Camino Verde, the Green Way): cluster randomized controlled trial. BMJ 2015;251:h3267. [DOI: 10.1136/bmj.h3267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Andersson 2017 {published data only}
- Andersson N. Camino Verde (The Green Way): evidence-based community mobilisation for dengue control in Nicaragua and Mexico: feasibility study and study protocol for a randomised controlled trial. BMC Public Health 2017;17(Supplement 1):407. [DOI: 10.1186/s12889-017-4289-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Angwenyi 2014 {published data only}
- Angwenyi V, Kamuya D, Mwachiro D, Kalama B, Marsh V, Njuguna P, et al. Complex realities: community engagement for a paediatric randomized controlled malaria vaccine trial in Kilifi, Kenya. Trials 2014;15:65. [DOI: 10.1186/1745-6215-15-65] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2005 {published data only}
- Anonymous. Community based participatory interventions improve neonatal outcomes and birth outcomes for women in rural populations. Evidence-Based Healthcare and Public Health 2005;9(2):137-8. [DOI: 10.1016/j.ehbc.2005.01.018] [DOI] [Google Scholar]
Armstrong 2018 {published data only}
- Armstrong MJ, Mullins CD, Gronseth GS, Gagliardi AR. Impact of patient involvement on clinical practice guideline development: a parallel group study. Implementation Science 2018;13(1):55. [DOI: 10.1186/s13012-018-0745-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arunachalam 2012 {published data only}
- Arunachalam N, Tyagi BK, Samuel M, Krishnamoorthi R, Manavalan R, Tewari SC, et al. Community-based control of Aedes aegypti by adoption of eco-health methods in Chennai City, India. Pathogens and Global Health 2012;106(8):488-96. [DOI: 10.1179/2047773212Y.0000000056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ayles 2013 {published data only}
- Ayles H, Muyoyeta M, Du Toit E, Schaap A, Floyd S, Simwinga M, et al. Effect of household and community interventions on the burden of tuberculosis in southern Africa: the ZAMSTAR community-randomised trial. Lancet 2013;382(9899):1183-94. [DOI: 10.1016/S0140-6736(13)61131-9] [DOI] [PubMed] [Google Scholar]
Azad 2010 {published data only}
- Azad K, Barnett S, Banerjee B, Sanjit S, Khan K, Rego AR, et al. Effect of scaling up women's groups on birth outcomes in three rural districts in Bangladesh: a cluster-randomised controlled trial. Lancet 2010;375(9721):1193-1202. [DOI: 10.1016/S0140-6736(10)60142-0] [DOI] [PubMed] [Google Scholar]
Balcazar 2009 {published data only}
- Balcazar HG, Byrd TL, Ortiz M, Tondapu SR, Chavez M. A randomized community intervention to improve hypertension control among Mexican Americans: using the promotoras de salud community outreach model. Journal of Health Care for the Poor and Underserved 2009;20(4):1079-94. [DOI: 10.1353/hpu.0.0209] [DOI] [PubMed] [Google Scholar]
Balcazar 2010 {published data only}
- Balcázar HG, Heer H, Rosenthal L, Aguirre M, Flores L, Puentes FA, et al. A promotores de salud intervention to reduce cardiovascular disease risk in a high-risk Hispanic border population, 2005-2009. Preventing Chronic Disease 2010;7(2):A28. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Bashir 2019 {published data only}
- Bashir NY, Moore JE, Buckland D, Rodrigues M, Tonelli M Thombs BD. Are patient education materials about cancer screening more effective when co-created with patients? A qualitative interview study and randomized controlled trial. Current Oncology 2019;26(2):15. [DOI: 10.3747/co.26.4621] [DOI] [PMC free article] [PubMed] [Google Scholar]
Batterham 2014 {published data only}
- Batterham RW, Buchbinder R, Beauchamp A, Dodson S, Elsworth GR, Osborne RH. The OPtimising HEalth LIterAcy (Ophelia) process: study protocol for using health literacy profiling and community engagement to create and implement health reform. BMC Public Health 2014;14(1):694. [DOI: 10.1186/1471-2458-14-694] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bedford 2014 {published data only}
- Bedford Russell AR, Passant M, Kitt H. Engaging children and parents in service design and delivery. Archives of Disease in Childhood 2014;99:1158-62. [DOI: 10.1136/archdischild-2013-304869] [DOI] [PubMed] [Google Scholar]
Belin 2008 {published data only}
- Belin TR, Jones A, Tang L, Chung B, Stockdale SE, Jones F, et al. Maintaining internal validity in community partnered participatory research: experience from the community partners in care study. Ethnicity and Disease 2018;28((Supplement 2)):357-64. [DOI: 10.18865/ed.28.S2.357] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beuermann 2018 {published data only}
- Beuermann DW, Amelina M. Does participatory budgeting improve decentralized public service delivery? Experimental evidence from rural Russia. Economics of Governance 2018;19(4):339-79. [DOI: 10.1007/s10101-018-0214-3] [DOI] [Google Scholar]
Bitton 2018 {published data only}
- Bitton A. Finding a Parsimonious Path for Primary Care Practice Transformation. Annals of Family Medicine 2018;16:S16-9. [DOI: 10.1370/afm.2234] [DOI] [PMC free article] [PubMed] [Google Scholar]
Björkman 2009 {published data only}
- Björkman M, Svensson J. Power to the people: evidence from a randomized field experiment on community-based monitoring in Uganda. Quarterly Journal of Economics 2009;124(2):735-69. [www.jstor.org/stable/40506242] [Google Scholar]
Björkman 2010 {published data only}
- Björkman, M and Svensson, J. When is community‐based monitoring effective? Evidence from a randomized experiment in primary health in Uganda. Journal of the European Economic Association 2010;8:571-81. [DOI: 10.1111/j.1542-4774.2010.tb00527.x] [DOI] [Google Scholar]
Björkman Nyqvist 2017 {published data only}
- Björkman Nyqvist M, Walque D, Svensson J. Experimental evidence on the long-run impact of community-based monitoring. American Economic Journal: Applied Economics 2017;9(1):33-69. [DOI: ] [Google Scholar]
Blank Wilson 2014 {published data only}
- Blank Wilson A, Farkas K. Collaborative adaptations in social work intervention research in real-world settings: lessons learned from the field. Journal of evidence-based social work. Journal of Evidence-Based Social Work 2014;11(1-2):183-92. [DOI: 10.1080/15433714.2013.847267] [DOI] [PubMed] [Google Scholar]
Bloom 2014 {published data only}
- Bloom J, Shirazi M, Shirazi A, Stewart S, Popal R. Breast cancer screening in an immigrant population: the Afghan women's breast health program (S18-0116). Symposia. Psycho‐Oncology 2014;23(Supplement 3):53. [DOI: 10.1111/j.1099-1611.2014.3693] [DOI] [Google Scholar]
Bogart 2009 {published data only}
- Bogart LM, Uyeda K. Community-based participatory research: partnering with communities for effective and sustainable behavioral health interventions. Health Psychology 2009;28(4):391-3. [DOI: 10.1037/a0016387] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boivin 2011 {published data only}
- Boivin A, Lehoux P, Lacombe R, Lacasse A, Burgers J, Grol R. Target for improvement: a cluster randomised trial of public involvement in quality-indicator prioritisation (intervention development and study protocol). Implementation Science 2011;6:45. [DOI: 10.1186/1748-5908-6-45] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boivin 2014 {published data only}
- Boivin A, Lehoux P, Burgers J, Grol R. What are the key ingredients for effective public involvement in health care improvement and policy decisions? A randomized trial process evaluation. Milbank Quarterly 2014;92(2):319-50. [DOI: 10.1111/1468-0009.12060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boivin 2014b {published data only}
- Boivin A, Lehoux P, Lacombe R, Burgers J, Grol R. Involving patients in setting priorities for healthcare improvement: a cluster randomized trial. Implementation science 2014;9:24. [DOI: 10.1186/1748-5908-9-24] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boivin 2018 {published data only}
- Boivin A, Richards T, Forsythe L, Gregoire A, L'Esperance A, Abelson J, et al. Evaluating patient and public involvement in research. BMJ 2018;363:k5147. [DOI: 10.1136/bmj.k5147] [DOI] [PubMed] [Google Scholar]
Bos 2007 {published data only}
- Bos AP, Zwieten MCB. Randomized, controlled trials in the emergency setting: a matter of physician-patient relationships, responsibility, and trust. Critical Care Medicine 2007;35(3):979-80. [DOI: 10.1097/01.CCM.0000257366.71279.E8] [DOI] [PubMed] [Google Scholar]
Bower 2015 {published data only}
- Bower P, Roberts C, O'Leary N, Callaghan P, Bee P, Fraser C, et al. A cluster randomised controlled trial and process evaluation of a training programme for mental health professionals to enhance user involvement in care planning in service users with severe mental health issues (EQUIP): study protocol for a randomised controlled trial. Trials 2015;16:348. [DOI: 10.1186/s13063-015-0896-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bramble 2011 {published data only}
- Bramble M, Moyle W, Shum D. A quasi-experimental design trial exploring the effect of a partnership intervention on family and staff well-being in long-term dementia care. Aging & Mental Health 2011;15(8):995-1007. [DOI: 10.1080/13607863.2011.583625] [DOI] [PubMed] [Google Scholar]
Brooker 2005 {published data only}
- Brooker CG, Curran JM, James A, Readhead E. Developing and piloting an audit tool for mental health education and training: the National Mental Health Education Continuous Quality Improvement Tool. Journal of Interprofessional Care 2005;19(3):280-93. [DOI: 10.1080/13561820500053439] [DOI] [PubMed] [Google Scholar]
Brundage 2010 {published data only}
- Brundage MD. Is seeing believing? Journal of Clinical Oncology 2010;28(5):711-3. [DOI: 10.1200/JCO.2009.25.0720] [DOI] [PubMed] [Google Scholar]
Burnell 2015 {published data only}
- Burnell KJ, Selwood A, Sullivan T, Charlesworth GM, Poland F, Orrell M. Involving service users in the development of the Support at Home: interventions to Enhance Life in Dementia Carer Supporter Programme for family carers of people with dementia. Health Expectations 2018;18(1):95-110. [DOI: 10.1111/hex.12012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cabassa 2014 {published data only}
- Cabassa LJ, Gomes AP, Meyreles Q, Capitelli L, Younge R, Dragatsi D, et al. Using the collaborative intervention planning framework to adapt a health-care manager intervention to a new population and provider group to improve the health of people with serious mental illness. Implementation Science 2014;9:178. [DOI: 10.1186/s13012-014-0178-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell‐Scherer 2014 {published data only}
- Campbell-Scherer DL, Asselin J, Osunlana AM, Fielding S, Anderson R, Rueda-Clausen CF, et al. Implementation and evaluation of the 5As framework of obesity management in primary care: design of the 5As Team (5AsT) randomized control trial. Implementation Science 2014;9:78. [DOI: 10.1186/1748-5908-9-78] [DOI] [PMC free article] [PubMed] [Google Scholar]
Capara 2015 {published data only}
- Caprara A, Lima JW, Peixoto AC, Motta CM, Nobre JM, Sommerfeld J, et al. Entomological impact and social participation in dengue control: a cluster randomized trial in Fortaleza, Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 2015;109(2):99-105. [DOI: 10.1093/trstmh/tru187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardoso 2017 {published data only}
- Cardoso G, Papoila A, Tomé G, Killaspy H, King M, Caldas-de-Almeida JM. A cluster randomised controlled trial of a staff-training intervention in residential units for people with long-term mental illness in Portugal: the PromQual trial. Social Psychiatry and Psychiatric Epidemiology 2017;52(11):1435-45. [DOI: 10.1007/s00127-017-1416-7] [DOI] [PubMed] [Google Scholar]
Carmen 2015 {published data only}
- Carman KL, Mallery C, Maurer M, Wang G, Garfinkel S, Yang M, et al. Effectiveness of public deliberation methods for gathering input on issues in healthcare: results from a randomized trial. Social Science & Medicine 2015;133:11-20. [DOI: 10.1016/j.socscimed.2015.03.024] [DOI] [PubMed] [Google Scholar]
Castillo 2018 {published data only}
- Castillo EG, Shaner R, Tang L, Chung B, Jones F, Whittington Y, et al. Improving depression care for adults with serious mental illness in underresourced areas: Community Coalitions versus Technical Support. Psychiatric Services 2018;69(2):195-203. [DOI: 10.1176/appi.ps.201600514] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castro 2012 {published data only}
- Castro M, Sánchez L, Pérez D, Carbonell N, Lefèvre P, Vanlerberghe V, et al. A community empowerment strategy embedded in a routine dengue vector control programme: a cluster randomised controlled trial. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2012;106(5):315-21. [DOI: 10.1016/j.trstmh.2012.01.013] [DOI] [PubMed] [Google Scholar]
Chaney 2011 {published data only}
- Chaney EF, Rubenstein LV, Liu CF, Yano EM, Bolkan C, Lee M, et al. Implementing collaborative care for depression treatment in primary care: a cluster randomized evaluation of a quality improvement practice redesign. Implementation Science 2011;6:121. [DOI: 10.1186/1748-5908-6-121] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2015 {published data only}
- Chang ET, Wells KB, Gilmore J, Tang L, Morgan AU, Sanders S, et al. Comorbid depression and substance abuse among safety-net clients in Los Angeles: a community participatory study. Psychiatric Services 2015;66(3):285-94. [DOI: 10.1176/appi.ps.201300318] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheever 2007 {published data only}
- Cheever LW. Engaging HIV-infected patients in care: their lives depend on it. Clinical Infectious Diseases 2007;44(11):1500-2. [DOI: 10.1086/517534] [DOI] [PubMed] [Google Scholar]
Chiew 2008 {published data only}
- Chiew KS, Shepherd H, Vardy J, Tattersall MHN, Butow PN, Leighl NB. Development and evaluation of a decision aid for patients considering first-line chemotherapy for metastatic breast cancer. Health Expectations 2008;11(111):35-45. [DOI: 10.1111/j.1369-7625.2007.00470.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chin 2007 {published data only}
- Chin MH, Drum ML, Guillen M, Rimington A, Levie JR, Kirchhoff AC, et al. Improving and sustaining diabetes care in community health centers with the health disparities collaboratives. Medical Care 2007;45(12):1135-43. [DOI: 10.1097/MLR.0b013e31812da80e] [DOI] [PubMed] [Google Scholar]
Choi 2011 {published data only}
- Choi WS, Faseru B, Beebe LA, Greiner A, Yeh HW, Shireman T, et al. Culturally-tailored smoking cessation for American Indians: study protocol for a randomized controlled trial. Trials 2011;12:126. [DOI: 10.1186/1745-6215-12-126] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2016 {published data only}
- Choi WS, Beebe LA, Nazir N, Kaur B, Hopkins M, Talawyma M, et al. All Nations Breath of Life: a randomized trial of smoking cessation for American Indians. American Journal of Preventive Medicine 2016;51(5):743-51. [DOI: 10.1016/j.amepre.2016.05.021] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2019 {published data only}
- Choi KR, Sherbourne C, Tang L, Castillo E, Dixon E, Jones A, et al. A comparative effectiveness trial of depression collaborative care: subanalysis of comorbid anxiety. Western Journal of Nursing Research 2019;14(7):1009-31. [DOI: 10.1177/0193945918800333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chomat 2019 {published data only}
- Chomat AM, Menchú AI, Andersson N, Ramirez-Zea M, Pedersen D, Bleile A, et al. Women's circles as a culturally safe psychosocial intervention in Guatemalan indigenous communities: a community-led pilot randomised trial. BMC Womens Health 2019;19(1):53. [DOI: 10.1186/s12905-019-0744-z.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chumbley 2002 {published data only}
- Chumbley GM, Hall GM, Salmon P. Patient‐controlled analgesia: what information does the patient want? Issues and Innovations in Nursing Practice 2002;39(5):459-71. [DOI: 10.1046/j.1365-2648.2002.02311.x] [DOI] [PubMed] [Google Scholar]
Chung 2010 {published data only}
- Chung B, Jones L, Dixon EL, Miranda J, Wells K, Community Partners in Care Steering Council. Using a community partnered participatory research approach to implement a randomized controlled trial: planning community partners in care. Journal of Health Care for the Poor and Underserved 2010;21(3):780-95. [DOI: 10.1353/hpu.0.0345] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2014 {published data only}
- Chung B, Ong M, Ettner SL, Jones F, Gilmore J, McCreary M. 12-month outcomes of community engagement versus technical assistance to implement depression collaborative care: a partnered, cluster, randomized, comparative effectiveness trial. Annals of Internal Medicine 2014;161(Supplement 10):s23-34. [DOI: 10.3122/jabfm.2016.03.150306] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2015 {published data only}
- Chung B, Ngo VK, Ong MK, Pulido E, Jones F, Gilmore J, et al. Participation in training for depression care quality improvement: a randomized trial of community engagement or technical support. Psychiatric Services 2015;66(3):285-94. [DOI: 10.1176/appi.ps.201400099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2014 {published data only}
- Clarke K, Azad K, Kuddus A, Shaha S, Nahar T, Aumon BH, et al. Impact of a participatory intervention with women's groups on psychological distress among mothers in rural Bangladesh: secondary analysis of a cluster-randomised controlled trial. PloS One 2014;9(10):e110697. [DOI: 10.1371/journal.pone.0110697] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clausen 2018 {published data only}
- Clausen J. Involvement of a patient organisation in health technology assessment. Annals of the Rheumatic Diseases; Conference: Annual European Congress of Rheumatology, EULAR 2018 2018;77(Supplement 2):28-9. [DOI: 10.1136/annrheumdis-2018-eular.7623] [DOI] [Google Scholar]
Coker 2016 {published data only}
- Coker TR, Chacon S, Elliott MN, Bruno Y, Chavis T, Biely C, et al. A parent coach model for well-child care among low-income children: a randomized controlled trial. Pediatrics 2016;137(3):e20153013. [DOI: 10.1542/peds.2015-3013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coker 2019 {published data only}
- Coker T, R, Porras-Javier L, Zhang L, Soares N, Park C, Patel A, et al. A telehealth-enhanced referral process in pediatric primary care: a cluster randomized trial. Pediatrics 2019;143(3):e20182738. [DOI: 10.1542/peds.2018-2738] [DOI] [PubMed] [Google Scholar]
Colbourn 2013 {published data only}
- Colbourn T, Nambiar B, Bondo A, Makwenda C, Tsetekani E, Makonda-Ridley A, et al. Effects of quality improvement in health facilities and community mobilization through women's groups on maternal, neonatal and perinatal mortality in three districts of Malawi: maiKhanda, a cluster randomized controlled effectiveness trial. International Health 2013;5(3):180-95. [DOI: 10.1093/inthealth/iht011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2016 {published data only}
- Cooper K, Gillmore C, Hogg L. Experience-based co-design in an adult psychological therapies service. Journal of Mental Health 2016;25(1):36-40. [DOI: 10.3109/09638237.2015.1101423] [DOI] [PubMed] [Google Scholar]
Corrigan 2017 {published data only}
- Corrigan PW, Kraus DJ, Pickett SA, Schmidt A, Sellon E, Hantke E, et al. Using peer navigators to address the integrated health care needs of homeless African Americans with serious mental illness. Psychiatric Services 2017;68(3):264-70. [DOI: 10.1176/appi.ps.201600134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Decat 2013 {published data only}
- Decat P, Nelson E, De Meyer S, Jaruseviciene L, Orozco M, Segura Z, et al. Community embedded reproductive health interventions for adolescents in Latin America: development and evaluation of a complex multi-centre intervention. BMC Public Health 2013;13:31. [DOI: 10.1186/1471-2458-13-31] [DOI] [PMC free article] [PubMed] [Google Scholar]
de la Torre 2013 {published data only}
- la Torre A, Sadeghi B, Green RD, Kaiser LL, Flores YG, Jackson CF, et al. Niños Sanos, Familia Sana: Mexican immigrant study protocol for a multifaceted CBPR intervention to combat childhood obesity in two rural California towns. BMC Public Health 20213;13:1033. [DOI: 10.1186/1471-2458-13-1033] [DOI] [PMC free article] [PubMed] [Google Scholar]
Donato 2019 {published data only}
- Donato K, Mosqueira A, G. Information improves provider behaviour: A replication study of a community-based monitoring programme in Uganda. The Journal of Development Studies 2019;55(5):967-88. [DOI: 10.1080/00220388.2018.1506577 ] [Google Scholar]
Dowrick 2016 {published data only}
- Dowrick C, Bower P, Chew-Graham C, Lovell K, Edwards S, Lamb J, et al. Evaluating a complex model designed to increase access to high quality primary mental health care for under-served groups: a multi-method study. BMC Health Services Research 2016;16:58. [DOI: 10.1186/s12913-016-1298-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Drummond 2014 {published data only}
- Drummond J, Schnirer L, So S, Mayan M, Williamson DL, Bisanz J, et al. The protocol for the Families First Edmonton trial (FFE): a randomized community-based trial to compare four service integration approaches for families with low-income. BMC Health Services Research 2014;14:223. [DOI: 10.1186/1472-6963-14-223] [DOI] [PMC free article] [PubMed] [Google Scholar]
El Ansari 2001 {published data only}
- El Ansari W, Phillips CJ. Interprofessional collaboration: a stakeholder approach to evaluation of voluntary participation in community partnerships. Journal of Interprofessional Care. 2001;15(4):351-68. [DOI: 10.1080/13561820120080481] [DOI] [PubMed] [Google Scholar]
Elstad 2009 {published data only}
- Elstad TA, Eide AH. User participation in community mental health services: exploring the experiences of users and professionals. Scandinavian Journal of Caring Sciences 2009;23(4):674-81. [DOI: 10.1111/j.1471-6712.2008.00660.x] [DOI] [PubMed] [Google Scholar]
Erwin 2018 {published data only}
- Erwin K, Martin MA, Flippin T, Norell S, Shadlyn A, Yang J, et al. Engaging stakeholders to design a comparative effectiveness trial in children with uncontrolled asthma. Journal of Comparative Effectiveness Research 2016;5(1):17-30. [DOI: 10.2217/cer.15.52] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esienumoh 2018 {published data only}
- Esienumoh EE, Allotey J, Waterman H. Empowering members of a rural southern community in Nigeria to plan to take action to prevent maternal mortality: a participatory action research project. Journal of Clinical Nursing 2018;2(7-8):e1600-e11. [DOI: 10.1111/jocn.14244] [DOI] [PubMed] [Google Scholar]
Farmer 2006 {published data only}
- Farmer BC, Croteau KA, Richeson NE, Jones DB. Using pedometers as a strategy to increase the daily steps of older adults with chronic illness: from research to practice. Home Healthcare Curse 2006;24(7):449-56. [DOI: 10.1097/00004045-200607000-00009] [DOI] [PubMed] [Google Scholar]
Fenenga 2015 {published data only}
- Fenenga C. A matter of trust - clients' perspective on healthcare and health insurance services in Ghana. ISSN: 978903677541-0 2015.
Fottrell 2013 {published data only}
- Fottrell E, Azad K, Kuddus A, Younes L, Shaha S, Nahar T, et al. The effect of increased coverage of participatory women’s groups on neonatal mortality in Bangladesh: a cluster randomized trial. JAMA Pediatrics 2013;167(9):816-25. [DOI: 10.1001/jamapedriatrics.2013.2534] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fottrell 2016 {published data only}
- Fottrell E, Jennings H, Kuddus A, Ahmed N, Morrison J, Akter K, et al. The effect of community groups and mobile phone messages on the prevention and control of diabetes in rural Bangladesh: study protocol for a three-arm cluster randomised controlled trial. Trials 2016;17(1):600. [DOI: 10.1186/s13063-016-1738-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Frank 2018 {published data only}
- Frank ES, Basila D, Collyar D, Pinto D, Smith ML, Geirisch J, et al. Abstract P5-17-04: Changing the DCIS conversation: development of an alternative discourse by patient stakeholders in the COMET study. Cancer Research 2018;78(Supplement 4):P5-17-04. [DOI: 10.1158/1538-7445.SABCS17-P5-17-04] [DOI] [Google Scholar]
Fujimori 2014 {published data only}
- Fujimori M, Shirai Y, Asai M, Kubota K, Katsumata N, and Uchitomi Y. Effect of communication skills training program for oncologists based on patient preferences for communication when receiving bad news: a randomized controlled trial. Journal of Clinical Oncology 2014;32(20):2166-72. [DOI: 10.1200/JCO.2013.51.2756] [DOI] [PubMed] [Google Scholar]
Fulkerson 2021 {published data only}
- Fulkerson JA, Horning ML, Barr-Anderson DJ, Linde JA, Sidebottom AC, Lindberg R, et al. Universal childhood obesity prevention in a rural community: Study design, methods and baseline participant characteristics of the NU-HOME randomized controlled trial. Contemporary Clinical Trials 2021;100:106160. [DOI: 10.1016/j.cct.2020.106160] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galiatsatos 2017 {published data only}
- Galiatsatos P, Gurley A, Daniel Hale W. Policy and advocacy for informal caregivers: how state policy influenced a community initiative. Journal of Public Health Policy 2017;38(4):503-8. [DOI: 10.1057/s41271-017-0084-x] [DOI] [PubMed] [Google Scholar]
Gellatly 2018 {published data only}
- Gellatly J, Bee P, Gega L, Bower P, Hunter D, Stewart P, et al. A community-based intervention (Young SMILES) to improve the health-related quality of life of children and young people of parents with serious mental illness: randomised feasibility protocol. Trials 2018;19(1):550. [DOI: 10.1186/s13063-018-2935-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gholipour 2016 {published data only}
- Gholipour K, Tabrizi JS, Asghari-Jafarabadi M, Iezadi S, Farshbaf N, Rahbar-Farzam F, et al. Customer's self-audit to improve the technical quality of maternity care in Tabriz: a community trial. Eastern Mediterranean Health Journal 2016;22(5):309-17. [https://apps.who.int/iris/handle/10665/259967] [DOI] [PubMed] [Google Scholar]
Gitaka 2018 {published data only}
- Gitaka J, Natecho A, Mwambeo HM, Gatungu DM, Githanga D, Abuya T. Evaluating quality neonatal care, call centre service, tele-health and community engagement in reducing newborn morbidity and mortality in Bungoma county, Kenya. BMC Health Services Research 2018;18(1):493. [DOI: 10.1186/s12913-018-3293-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gloppen 2012 {published data only}
- Gloppen KM, Arthur MW, Hawkins JD, Shapiro VB. Sustainability of the Communities That Care prevention system by coalitions participating in the Community Youth Development Study. Journal of Adolescent Health 2012;51(3):259-64. [DOI: 10.1016/j.jadohealth.2011.12.018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glynn 2015 {published data only}
- Glynn SM, Gingerich S, Mueser KT, Cather C, Penn D. The role of family intervention in coordinated specialty care for first episode psychosis. Schizophrenia Bulletin 2015;41(Suppplement 1):S173. [DOI: 10.1093/schbul/sbv010] [DOI] [Google Scholar]
Goldfinger 2012 {published data only}
- Goldfinger JZ, Kronish IM, Fei K, Graciani A, Rosenfeld P, Lorig K, et al. Peer education for secondary stroke prevention in inner-city minorities: design and methods of the prevent recurrence of all inner-city strokes through education randomized controlled trial. Contemporary Clinical Trials 2012;33(5):1065-73. [DOI: 10.1016/j.cct.2012.06.003] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodkind 2017 {published data only}
- Goodkind JR, Amer S, Christian C, Hess JM, Bybee D, Isakson BL, et al. Challenges and innovations in a community-based participatory randomized controlled trial. Health Education & Behavior 2017;44(1):123-30. [10.1177/1090198116639243] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gram 2018 {published data only}
- Gram LZ. Women's empowerment in a complex public health intervention in rural Nepal. Doctoral thesis (Ph.D), UCL (University College London).
Greenfield 2012 {published data only}
- Greenfield D, Hinchcliff R, Westbrook M, Jones D, Low L, Johnston B, et al. An empirical test of accreditation patient journey surveys: randomized trial. International Journal for Quality in Health Care 2012;24(5):495-500. [DOI: 10.1093/intqhc/mzs035] [DOI] [PubMed] [Google Scholar]
Gregory 2011 {published data only}
- Gregory J, Robling M, Bennert K, Channon S, Cohen D, Crowne E, et al. Development and evaluation by a cluster randomised trial of a psychosocial intervention in children and teenagers experiencing diabetes: the DEPICTED study. Health Technology Assessment 2011;15(29):1-202. [DOI: 10.3310/hta15290] [DOI] [PubMed] [Google Scholar]
Groene 2014 {published data only}
- Groene O, Sunol R, Klazinga NS, Wang A, Dersarkissian M, Thompson CA, et al. Involvement of patients or their representatives in quality management functions in EU hospitals: implementation and impact on patient-centred care strategies. International Journal for Quality in Health Care 2014;26(Supplement 1):81-91. [DOI: 10.1093/intqhc/mzu022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Grogan 2012 {published data only}
- Grogan A, Coughlan M, Mahony BO, McKee G. The development of a patient partnership programme and its impact on quality improvements in a comprehensive haemophilia care service. Haemophilia 2012;18(6):875-80. [DOI: 10.1111/j.1365-2516.2012.02885.x] [DOI] [PubMed] [Google Scholar]
Gual 2012 {published data only}
- Gual A. C.12.02 Engaging patients in the treatment of alcohol dependence – The role of reduction of alcohol consumption and “as-needed” dosing. European Neuropsychopharmacology 2012;22(Supplement 2):S450. [Google Scholar]
Gullo 2017 {published data only}
- Gullo S, Galavotti C, Kuhlmann AS, Msiska T, Hastings P, Marti CN. Effects of a social accountability approach, CARE's Community Score Card, on reproductive health-related outcomes in Malawi: a cluster-randomized controlled evaluation. PLoS One 2017;12(2):e0171316. [DOI: 10.1371/journal.pone.0171316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gullo 2018 {published data only}
- Gullo S, Kuhlmann Anne S, Galavotti C, Msiska T, Nathan Marti C, Hastings P. Creating spaces for dialogue: a cluster-randomized evaluation of CARE's Community Score Card on health governance outcomes. BMC Health Services Research 2018;18(1):858. [DOI: 10.1186/s12913-018-3651-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gummersbach 2013 {published data only}
- Gummersbach E, In der Schmitten J, Abholz H-H, Wegscheider K, Pentzek M. Effects of different information brochures on women's decision-making regarding mammography screening: study protocol for a randomized controlled questionnaire study. Trials 2013;14(1):319. [DOI: 10.1186/1745-6215-14-319] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haines 2015 {published data only}
- Haines TP, O'Brien L, Mitchell D, Bowles KA, Haas R, Markham D, et al. Study protocol for two randomized controlled trials examining the effectiveness and safety of current weekend allied health services and a new stakeholder-driven model for acute medical/surgical patients versus no weekend allied health services. Trial 2015;16(1):133. [DOI: 10.1186/s13063-015-0619-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 2017 {published data only}
- Hamilton AB, Brunner J, Cain C, Chuang E, Luger TM, Canelo I, et al. Engaging multilevel stakeholders in an implementation trial of evidence-based quality improvement in VA women's health primary care. Translational Behavioral Medicine 2017;7(36):478-85. [DOI: 10.1007/s13142-017-0501-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanson 2014 {published data only}
- Hanson C, Waiswa P, Marchant T, Marx M, Manzi F, Mbaruku G, et al. Expanded Quality Management Using Information Power (EQUIP): protocol for a quasi-experimental study to improve maternal and newborn health in Tanzania and Uganda. Implementation Science 2014;9(1):41. [DOI: 10.1186/1748-5908-9-41] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harding 2016 {published data only}
- Harding KE, Watts JJ, Karimi L, O'Reilly M, Kent B, Kotis M, et al. Improving access for community health and sub-acute outpatient services: protocol for a stepped wedge cluster randomised controlled trial. BMC Health Services Research 2016;16(a):364. [DOI: 10.1186/s12913-016-1611-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 2013 {published data only}
- Harris S, Paquette-Warren J, Roberts S, Fournie M, Thind A, Ryan BL, et al. Results of a mixed-methods evaluation of partnerships for health: a quality improvement initiative for diabetes care. American Board of Family Medicine 2013;26(6):711-9. [DOI: 10.3122/jabfm.2013.06.120211] [DOI] [PubMed] [Google Scholar]
Henderson 2017 {published data only}
- Henderson JL, Cheung A, Cleverley K, Chaim G, Moretti ME, Oliveira C, et al. Integrated collaborative care teams to enhance service delivery to youth with mental health and substance use challenges: protocol for a pragmatic randomised controlled trial. BMJ Open 2017;7(2):e014080. [DOI: 10.1136/bmjopen-2016-014080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernandez 2013 {published data only}
- Hernandez C, Kim H, Mauleon G, Ruiz A, Robinson JK, Mermelstein RJ. A pilot program in collaboration with community centers to increase awareness and participation in skin cancer screening among Latinos in Chicago. Journal of Cancer Education 2013;28(2):342-5. [DOI: 10.1007/s13187-013-0454-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hobbs 2000 {published data only}
- Hobbs SE, Sodomka PF. Developing partnerships among patients, families, and staff at the Medical College of Georgia Hospital and Clinics. Joint Commission Journal on Quality Improvement. 2000;26(5):268-76. [DOI: 10.1016/S1070-3241(00)26021-5] [DOI] [PubMed] [Google Scholar]
Hoffman 2018 {published data only}
- Hoffman J, Frerichs L, Story M, Jones J, Gaskin K, Apple A, et al. An integrated clinic-community partnership for child obesity treatment: a randomized pilot trial. Pediatrics 2018;141(1):e20171444. [DOI: 10.1542/peds.2017-1444] [DOI] [PubMed] [Google Scholar]
Holt 2014 {published data only}
- Holt CL, Tagai EK, Scheirer MA, Santos SL, Bowie J, Haider M, et al. Translating evidence-based interventions for implementation: experiences from Project HEAL in African American churches. Implementation Science 2014;9:66. [DOI: 10.1186/1748-5908-9-66] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoos 2015 {published data only}
- Hoos A, Anderson J, Boutin M, Dewulf L, Geissler J, Johnston G, et al. Partnering with patients in the development and lifecycle of medicines: a call for action. Therapeutic Innovation and Regulatory Science 2015;49(6):929-39. [DOI: 10.1177/2168479015580384] [DOI] [PMC free article] [PubMed] [Google Scholar]
Houweling 2011 {published data only}
- Houweling TA, Azad K, Younes L, Kuddus A, Shaha S, Haq B, et al. The effect of participatory women’s group on birth outcomes in Bangladesh: does coverage matter? Study protocol for a randomized controlled trial. Trials 2011;12:208. [DOI: 10.1186/1745-6215-12-208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Houweling 2013 {published data only}
- Houweling TAJ, Tripathy P, Nair N, Rath S, Rath S, Gope R, et al. The equity impact of participatory women’s groups to reduce neonatal mortality in India: secondary analysis of a cluster-randomised trial. International Journal of Epidemiology 2013;42(2):520-32. [DOI: 10.1093/ije/dyt012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huppelschoten 2015 {published data only}
- Huppelschoten AG, Nelen WLDM, Westert GP, Golde RJT, Adang EMM, Kremer JAM. Improving patient-centredness in partnership with female patients: a cluster RCT in fertility care. Human Reproduction 2015;30(5):1137-45. [DOI: 10.1093/humrep/dev041] [DOI] [PubMed] [Google Scholar]
Hwang 2012 {published data only}
- Hwang SW, Stergiopoulos V, O'Campo P, Gozdzik A. Ending homelessness among people with mental illness: the At Home/Chez Soi randomized trial of a Housing First intervention in Toronto. BMC Public Health 2012;12:787. [DOI: 10.1186/1471-2458-12-787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hynes 2017 {published data only}
- Hynes DM, Fischer MJ, Schiffer LA, Gallardo R, Chukwudozie IB, Porter A, et al. Evaluating a novel health system intervention for chronic kidney disease care using the RE-AIM framework: insights after two years. Contemporary Clinical Trials. 2017;52:20-6. [10.1016/j.cct.2016.10.003] [DOI] [PubMed] [Google Scholar]
Iezzoni 2018 {published data only}
- Iezzoni LI, Chang Y, Matulewicz H, Heaphy D, Warsett KS, Donelan K. Health plan enrollees with disability informing primary care practices and providers about their quality of care: a randomized trial. Disability and Health Journal 2018;11(4):537-44. [DOI: 10.1016/j.dhjo.2018.05.006] [DOI] [PubMed] [Google Scholar]
Izquierdo 2016 {published data only}
- Izquierdo A, Pulido E, Wells KB, Berkman M, Linski B, Sauer V, et al. Six-month outcomes among older adults of community engagement versus technical assistance to implement depression collaborative care. Journal of General Internal Medicine 2016;28(2 Supplement 1):S93-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Izquierdo 2018 {published data only}
- Izquierdo A, Ong M, Pulido E, Wells KB, Berkman M, Linski B, et al. Community partners in care: 6- and 12-month outcomes of community engagement versus technical assistance to implement depression collaborative care among depressed older adults. Ethnicity & Disease 2018;28(Supplement 2):339-48. [DOI: 10.18865/ed.28.S2.339] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jha 2013 {published data only}
- Jha V, Winterbottom A, Symons J, Thompson Z, Quinton N, Corrado OJ, et al. Patient-led training on patient safety: a pilot study to test the feasibility and acceptability of an educational intervention. Medical Teacher 2013;35(9):e1464-71. [DOI: 10.3109/0142159x.2013.77891] [DOI] [PubMed] [Google Scholar]
Källander 2015 {published data only}
- Källander K, Strachan D, Soremekun S, Hill Z, Lingam R, Tibenderana J, et al. Evaluating the effect of innovative motivation and supervision approaches on community health worker performance and retention in Uganda and Mozambique: study protocol for a randomised controlled trial. Trials 2015;16:157. [DOI: 10.1186/s13063-015-0657-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2017 {published data only}
- Kang Y, Cha S, Yeo S, Christian P. Implementation, utilization and influence of a community-based participatory nutrition promotion programme in rural Ethiopia: programme impact pathway analysis. Public Health Nutrition 2017;20(11):2004-15. [DOI: 10.1017/S1368980017000660] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2010 {published data only}
- Kelly PJ, Lesser J, Cheng AL, Oscos-Sanchez M, Martinez E, Pineda D, et al. A prospective randomized controlled trial of an interpersonal violence prevention program with a Mexican American community. Family & Community Health 2010;33(3):207-15. [10.1097/FCH.0b013e3181e4bc34] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khadyakov 2014 {published data only}
- Khodyakov D, Sharif MZ, Dixon EL, Mendel P, Chung B, Linkski B, et al. An implementation evaluation of the Community Engagement and Planning Intervention in the CPIC Depression Care Improvement Trial. Community Mental Health Journal 2014;50:312-24. [DOI: 10.1007/s10597-012-9586-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khodyakov 2014 {published data only}
- Khodyakov D, Pulido E, Ramos A, Dixon E. Community-Partnered Research Conference Model: the Experience of Community Partners in Care study. Progress in Community Health Partnerships: Research, Education, and Action 2014;8(1):83-97. [DOI: 10.1353/cpr.2014.0008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khodyakov 2017 {published data only}
- Khodyakov D, Kinnett K, Grant S, Lucas A, Martin A, Denger B, et al. Engaging patients and caregivers managing rare diseases to improve the methods of clinical guideline development: a research protocol. Journal of Medical Internet Research, Research Protocols 2017;6(4):e57. [DOI: 10.2196/resprot.6902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khodyakov 2018 {published data only}
- Khodyakov D, Sharif MZ, Jones F, Megan Heller S, Pulido E, Wells KB, et al. Whole person care in under-resourced communities: stakeholder priorities at long-term follow-UP in community partners in care. Ethnicity & Disease 2018;28(Supplement 2):371-80. [DOI: 10.18865/ed.28.S2.371] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014 {published data only}
- Kim H, Suh EE, Lee HJ, Yang HK. The effects of patient participation-based dietary intervention on nutritional and functional status for patients with gastrectomy: a randomized controlled trial. Cancer Nursing 2014;37(2):E10-20. [DOI: 10.1097/NCC.0b013e31829193c8] [DOI] [PubMed] [Google Scholar]
Kim Yeary 2017 {published data only}
- Kim Yeary KH, Long CR, Bursac Z, McElfish PA. Design of a randomized, controlled, comparative-effectiveness trial testing a family model of diabetes self-management education (DSME) vs. standard DSME for Marshallese in the United States. Contemporary Clinical Trials Communications 2017;6:97-104. [DOI: 10.1016/j.conctc.2017.03.007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kneipp 2011 {published data only}
- Kneipp SM, Kairalla JA, Lutz BJ, Pereira D, Hall AG, Flocks J, et al. Public health nursing case management for women receiving temporary assistance for needy families: a randomized controlled trial using community-based participatory research. American Journal of Public Health 2011;101(9):1759-68. [DOI: 10.2105/AJPH.2011.300210] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ko 2015 {published data only}
- Ko N, Bak S, Nelson K, Han A, Bergling E, Castano M, et al. A patient-centered approach to a cancer care delivery innovation for low income patients: medical-legal partnership with patient navigation. Journal of Clinical Oncology 2015;33(15_suppl):e17603-e17603. [DOI: 10.1200/jco.2015.33.15_suppl.e17603] [DOI] [Google Scholar]
Ko 2016 {published data only}
- Ko NY, Bak S, Han A, Nelson K, Gunn C, Bergling E, et al. Abstract C07: A patient-centered approach to a cancer care delivery innovation for low-income patients: Medical-legal partnership with patient navigation. Cancer Epidemiology, Biomarkers & Prevention 2016;25(3 Supplement):Abstract nr C07. [DOI: 10.1158/1538-7755.DISP15-C07] [DOI] [Google Scholar]
Koerner 2014 {published data only}
- Koerner M, Wirtz M, Michaelis M, Ehrhardt H, Steger AK, Zerpies E, et al. A multicentre cluster-randomized controlled study to evaluate a train-the-trainer programme for implementing internal and external participation in medical rehabilitation. Clinical Rehabilitation 2014;28(1):20-35. [DOI: 10.1177/0269215513494874] [DOI] [PubMed] [Google Scholar]
Kogan 2017 {published data only}
- Kogan JN, Schuster J, Nikolajski C, Schake P, Carney T, Morton SC, et al. Challenges encountered in the conduct of Optimal Health: a patient-centered comparative effectiveness study of interventions for adults with serious mental illness. Clinical Trials 2017;14(1):5-16. [DOI: 10.1177/1740774516670895] [DOI] [PubMed] [Google Scholar]
Koniotou 2015 {published data only}
- Koniotou M, Evans BA, Chatters R, Fothergill R, Garnsworthy C, Gaze S, et al. Involving older people in a multi-centre randomised trial of a complex intervention in pre-hospital emergency care: implementation of a collaborative model. Trials 2015;16:298. [DOI: 10.1186/s13063-015-0821-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishnan 2017 {published data only}
- Krishnan J A, Martin MA, Lohff C, Mosnaim GS, Margellos-Anast H, DeLisa JA, et al. Design of a pragmatic trial in minority children presenting to the emergency department with uncontrolled asthma: the CHICAGO Plan. Contemporary Clinical Trials 2017;57:10-22. [DOI: 10.1016/j.cct.2017.03.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krist 2013 {published data only}
- Krist AH, Glenn BA, Glasgow RE, Balasubramanian BA, Chambers DA, Fernandez ME, et al. Designing a valid randomized pragmatic primary care implementation trial: the My Own Health Report (MOHR) project. Implementation Science 2013;8:73. [DOI: 10.1186/1748-5908-8-73] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lalonde 2011 {published data only}
- Lalonde L, Levesque L, Hudon E, Goudreau J, Bareil C, Lussier MT, et al. From priorities to action: improving cardiovascular prevention in primary care. Journal of Population Therapeutics and Clinical Pharmacology 2011;18(2):e181-2. [Google Scholar]
Lam 2016 {published data only}
- Lam CA, Sherbourne C, Tang L, Belin TR, Pluscedia W, Young-Brinn A, et al. The impact of community engagement on health, social, and utilization outcomes in depressed, impoverished populations: secondary findings from a randomized trial. The Journal of the American Board of Family Medicine 2016;29(3):325-38. [DOI: 10.3122/jabfm.2016.03.150306] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lamb 2010 {published data only}
- Lamb G, Havas N, Simpso D, Collaborative S . Developing a curriculum for transitions in care: a novel faculty development program incorporating service learning. Journal of General Internal Medicine 2010;Supplement 3:S441. [DOI: 10.1007/s11606-010-1338-5] [DOI] [Google Scholar]
Lamontagne 2014 {published data only}
- Lamontagne M, Perreault K, Gagnon M. Evaluation of the acceptability, feasibility and effectiveness of two methods of involving patients with disability in developing clinical guidelines: study protocol of a randomized pragmatic pilot trial. Trials 2014;15:118. [DOI: 10.1186/1745-6215-15-118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landry 2017 {published data only}
- Landry CM, Jackson AP, Tang L, Miranda J, Chung B, Jones F, et al. The Effects of Collaborative Care Training on Case Managers' Perceived Depression-Related Services Delivery. Psychiatric Services 2017;68(2):123-30. [DOI: 10.1176/appi.ps.201500550] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawton 2016 {published data only}
- Lawton RJ, Sheard L, O'Hara J. Can patient involvement improve patient safety? A cluster randomized control trial of the patient reporting and action for a safe environment (PRASE) intervention. International Journal for Quality in Health care 2016;28(Supplement 1):21. [DOI: 10.1093/intqhc/mzw104.29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawton 2017 {published data only}
- Lawton R, O'Hara JK, Sheard L, Armitage G, Cocks K, Buckley H, et al. Can patient involvement improve patient safety? A cluster randomised control trial of the Patient Reporting and Action for a Safe Environment (PRASE) intervention. BMJ Quality & Safety 2017;26(8):622-31. [DOI: 10.1136/bmjqs-2016-005570] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewycka 2010 {published data only}
- Lewycka S, Mwansambo C, Kazembe P, Phiri T, Mganga A, Rosato M, et al. A cluster randomised controlled trial of the community effectiveness of two interventions in rural Malawi to improve health care and to reduce maternal, newborn and infant mortality. Trials 2010;11:88. [DOI: 10.1186/1745-6215-11-88] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewycka 2013 {published data only}
- Lewycka S, Mwansambo C, Rosato M, Kazembe P, Phiri T, Mganga A, et al. Effect of women's groups and volunteer peer counselling on rates of mortality, morbidity, and health behaviours in mothers and children in rural Malawi (MaiMwana): a factorial, cluster-randomised controlled trial. Lancet 2013;381(9879):1721-35. [DOI: 10.1016/S0140-6736(12)61959-X] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liddy 2011 {published data only}
- Liddy C, Hogg W, Russell G, Wells G, Armstrong CD, Akbari A, et al. Improved delivery of cardiovascular care (IDOCC) through outreach facilitation: study protocol and implementation details of a cluster randomized controlled trial in primary care. Implementation Science 2011;6:110. [DOI: 10.1186/1748-5908-6-110] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lippeveld 2017 {published data only}
- Lippeveld T. Routine Health Facility and Community Information Systems: creating an Information Use Culture. Global Health-science and Practice 2017;5(3):338-40. [DOI: 10.9745/ghsp-d-17-00319] [DOI] [PMC free article] [PubMed] [Google Scholar]
Livingston 2013 {published data only}
- Livingston JD, Nijdam-Jones A, Lapsley S, Calderwood C, Brink J. Supporting recovery by improving patient engagement in a forensic mental health hospital: results from a demonstration project. Journal of the American Psychiatric Nurses Association 2013;19(3):132-45. [DOI: 10.1177/1078390313489730] [DOI] [PubMed] [Google Scholar]
Lodewijckx 2012 {published data only}
- Lodewijckx C, Decramer M, Sermeus W, Panella M, Deneckere S, Vanhaecht K. Eight-step method to build the clinical content of an evidence-based care pathway: the case for COPD exacerbation. Trials 2012;13:229. [DOI: 10.1186/1745-6215-13-229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loignon 2018 {published data only}
- Loignon C, Dupere S, Fortin M, Ramsden Vivian R, Truchon K. Health literacy - engaging the community in the co-creation of meaningful health navigation services: a study protocol. BMC Health Services Research 2018;18(1):505. [DOI: 10.1186/s12913-018-3315-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovell 2018 {published data only}
- Lovell K, Bee P, Brooks H, Cahoon P, Callaghan P, Carter LA, et al. Embedding shared decision-making in the care of patients with severe and enduring mental health problems: the EQUIP pragmatic cluster randomised trial. PloS One 2018;13(8):e0201533. [DOI: 10.1371/journal.pone.0201533] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luna 2015 {published data only}
- Luna MA, Burt D, Rivera-Andrade AA, Chen D, Gonzalez J, Mendoza C. A cardiovascular disease surveillance study in Santiago Atitlan, Guatemala: a model of community-centered, participatory health research. Annals of Global Health 2015;81(1):97. [Google Scholar]
Ma 2018 {published data only}
- Ma GX, Lee MM, Tan Y, Hanlon AL, Feng Z, Shireman TI, et al. Efficacy of a community-based participatory and multilevel intervention to enhance hepatitis B virus screening and vaccination in underserved Korean Americans. Cancer 2018;124(5):973-82. [DOI: 10.1002/cncr.31134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Machline‐Carrion 2019 {published data only}
- Machline-Carrion MJ, Santucci EV, Damiani LP, Bahit C, Málaga G, Pontes-Neto OM, et al. An international cluster-randomized quality improvement trial to increase the adherence to evidence-based therapies for acute ischemic stroke and transient ischemic attack patients: Rationale and design of the BRIDGE STROKE Trial. American Heart Journal 2019;207:49-57. [DOI: 10.1016/j.ahj.2018.09.009] [DOI] [PubMed] [Google Scholar]
MacLeod 2012 {published data only}
- MacLeod A, Skinner MW, Low E. Supporting hospice volunteers and caregivers through community-based participatory research. Health & Social Care in the Community 2012;20(2):190-8. [DOI: 10.1111/j.1365-2524.2011.01030.x] [DOI] [PubMed] [Google Scholar]
Malfait 2018 {published data only}
- Malfait S, Van Hecke A, De Bodt G, Palsterman N, Eeckloo K. Patient and public involvement in hospital policy-making: identifying key elements for effective participation. Health policy 2018;122(4):380-8. [DOI: 10.1016/j.healthpol.2018.02.007] [DOI] [PubMed] [Google Scholar]
Manandhar 2004 {published data only}
- Manandhar PDS, Osrin D, Prasad Shrestha B, Mesko N, Morrison J, Man Tumbahangphe K, et al. Effect of a participatory intervention with women's groups on birth outcomes in Nepal: cluster-randomised controlled trial. Lancet 2004;361(9438):970-9. [DOI: 10.1016/S0140-6736(04)17021-9] [DOI] [PubMed] [Google Scholar]
Martinez Garcia 2013 {published data only}
- Martínez Garcia JA, Ruiz M, Carmen Vivo M. Using adult children to enhance participation in colorectal cancer screening. Journal of Health Management 2013;15(4):481-500. [DOI: 10.1177/0972063413516219] [DOI] [Google Scholar]
Martínez‐Jaikel 2019 {published data only}
- Martínez-Jaikel T, Frongillo E, Blake C, Fram M, Murillo-Castro A, Esquivel-Solís V. Intervention for women In Costa Rica who have food insecurity and excess body weight: a cluster randomized trial (p04-017-19). Current Developments in Nutrition 2019;3:Supplement 1. [DOI: 10.1093/cdn/nzz051.P04-017-19] [DOI] [Google Scholar]
McCabe 2018 {published data only}
- McCabe MP, Beattie E, Karantzas G, Mellor D, Sanders K, Busija L, et al. A randomized controlled trial to evaluate the effectiveness of a staff training program to implement consumer directed care on resident quality of life in residential aged care. BMC Geriatrics 2018;18(1):187. [DOI: 10.1186/s12877-018-0966-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
McElfish 2017 {published data only}
- McElfish PA, Goulden PA, Bursac Z, Hudson J, Purvis RS, Kim Yeary KH, et al. Engagement practices that join scientific methods with community wisdom: designing a patient‐centered, randomized control trial with a Pacific Islander community. Nursing Inquiry 2017;24:1-11. [DOI: 10.1111/nin.12141] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKay 2011 {published data only}
- McKay MM, Gopalan G, Franco L, Dean-Assael K, Chacko A, Jackson JM, et al. A collaboratively designed child mental health service model: multiple family groups for urban children with conduct difficulties. Research on Social Work Practice 2011;21(6):664-74. [DOI: 10.1177/1049731511406740] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 2017 {published data only}
- Mehta P, Brown A, Chung B, Jones F, Tang L, Gilmore J, et al. Community partners in care: 6-month outcomes of two quality improvement depression care interventions in male participants. Ethnicity & Disease. 2017;27(3):223-32. [DOI: 10.18865/ed.27.3.223] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendel 2011 {published data only}
- Mendel P, Ngo VK, Dixon E, Stockdale S, Jones F, Chung B, et al. Partnered evaluation of a community engagement intervention: use of a kickoff conference in a randomized trial for depression care improvement in underserved communities. Ethnicity & Disease 2011;21(3 Supplement 1):78. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Mendel 2021 {published data only}
- Mendel P, O'Hora J, Zhang L, Stockdale S, Dixon EL, Gilmore J, et al. Engaging community networks to improve depression services: a cluster-randomized trial of a community engagement and planning intervention. Community Mental Health Journal 2021;57(3):457-69. [DOI: 10.1007/s10597-020-00632-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendenhall 2007 {published data only}
- Mendenhall TJ, Doherty WJ. The ANGELS (a neighbor giving encouragement, love, and support): a collaborative project for teens with diabetes. In: The therapist's notebook for family health care: homework, handouts, and activities for individuals, couples, and families coping with illness, loss, and disability (Editors: Linville D, Hertlein KM). Routledge, 2007. [Google Scholar]
Meynard 2011 {published data only}
- Meynard A, Pejic D, Sredic A, Weber CC, Narring F, Sanci L, et al. International partnerships in the field of Adolescent Health research and training: development of youth-friendly family medicine practices in Bosnia and Herzegovina. Journal of Adolescent Health 2011;48(2 Supplement 1):S40-1. [DOI: 10.1016/j.jadohealth.2010.11.091] [DOI] [Google Scholar]
Michaels 2017 {published data only}
- Michaels L, Anastas T, Waddell EN, Fagnan L, Dorr DA. A randomized trial of high-value change using practice facilitation. Journal of the American Board of Family Medicine 2017;30(5):572-82. [DOI: 10.3122/jabfm.2017.05.170013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2017 {published data only}
- Miller MK, Wickliffe J, Humiston SG, Dowd M, Kelly P, DeLurgio S, et al. Adapting an HIV risk reduction curriculum: processes and outcomes.. Health Promotion Practice 2017;18(3):400-9. [DOI: 10.1177/1524839916681990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2020 {published data only}
- Miller LC, Neupane S, Joshi N, Lohani M, Rogers BL, Neupane S, et al. Multisectoral community development in Nepal has greater effects on child growth and diet than nutrition education alone. Public Health Nutrition 2020;23(1):146-61. [DOI: 10.1017/S136898001900260X] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirza 2009 {published data only}
- Mirza M, Hammel J. Consumer-directed goal planning in the delivery of assistive technology services for people who are ageing with intellectual disabilities. Journal of Applied Research in Intellectual Disabilities 2009;22(5):445-57. [DOI: 10.1111/j.1468-3148.2009.00495.x] [DOI] [Google Scholar]
More 2008 {published data only}
- More NS, Bapat U, Das S, Patil S, Porel M, Vaidya L, et al. Cluster-randomised controlled trial of community mobilisation in Mumbai slums to improve care during pregnancy, delivery, postpartum and for the newborn. Trials 2008;9:ISRCTN96256793. [DOI: 10.1186/1745-6215-9-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 2011 {published data only}
- Morrison J, Tumbahangphe KM, Budhathoki B, Neupane R, Sen A, Dahal K, et al. Community mobilisation and health management committee strengthening to increase birth attendance by trained health workers in rural Makwanpur, Nepal: study protocol for a cluster randomised controlled trial. Trials 2011;12:128. [DOI: 10.1186/1745-6215-12-128] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mourad 2011 {published data only}
- Mourad SM, Hermens RP, Liefers J, Akkermans RP, Zielhuis GA, Adang E, et al. A multi-faceted strategy to improve the use of national fertility guidelines; a cluster-randomized controlled trial. Human Reproduction 2011;26(4):817-26. [DOI: 10.1093/humrep/deq299] [DOI] [PubMed] [Google Scholar]
Nahar 2012 {published data only}
- Nahar T, Azad K, Aumon BH, Younes L, Shaha S, Kuddus A, et al. Scaling up community mobilisation through women's groups for maternal and neonatal health: experiences from rural Bangladesh. BMC Pregnancy and Childbirth 2012;12:5. [DOI: 10.1186/1471-2393-12-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nair 2015 {published data only}
- Nair N, Tripathy P, Sachdev HS, Bhattacharyya S, Gope R, Gagrai S, et al. Participatory women's groups and counselling through home visits to improve child growth in rural eastern India: protocol for a cluster randomised controlled trial. BMC Public Health 2015;15:384. [DOI: 10.1186/s12889-015-1655-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Namazzi 2015 {published data only}
- Namazzi G, Waiswa P, Peterson S, Kerber K. Designing for action: adapting and implementing a community-based newborn care package to affect national change in Uganda. Global Health Action 2015;8:1-11. [DOI: 10.3402/gha.v8.24250] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02286193 {published data only}
- Kaiser Permanente, Patient-Centered Outcomes Research Institute. Creating a clinic-community liaison role in primary care: engaging patients and community in health care innovation (LINCC). ClinicalTrials.gov 2014: NCT02286193.
NCT03035474 {published data only}
- NCT03035474. Care optimization through patient and hospital engagement clinical trial for heart failure (CONNECT-HF). ClinicalTrials.gov Identifier: NCT03035474. [DOI] [PubMed]
NCT03044145 {published data only}
- NCT03044145. The cultural formulation interview-engagement aid (CFI-EA). ClinicalTrials.gov Identifier: NCT03044145.
NCT03222466 {published data only}
- St Michael's Hospital, Toronto. Patient educational materials for prostate cancer screening. ClinicalTrials.gov Identifier: NCT03222466.
NCT04514133 {published data only}
- Wake Forest University Health Sciences, and Robert Wood Johnson Foundation. Testing the causal effects of a civic engagement intervention on health and wellbeing among youth (I-ACTED). ClinicalTrials.gov. [NCT04514133]
Newman Owiredu 2017 {published data only}
- Newman Owiredu M, Bellare NB, Chakanyuka Musanhu CC, Oyelade TA, Thom EM, Bigirimana F, et al. Building health system capacity through implementation research: experience of INSPIRE-a multi-country PMTCT implementation research project. Journal of Acquired Immune Deficiency Syndromes 2017;75(Supplement 2):S240-7. [DOI: 10.1097/QAI.0000000000001370] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngo 2016 {published data only}
- Ngo VK, Sherbourne C, Chung B, Tang L, Wright AL, Whittington Y, et al. Community engagement compared with technical assistance to disseminate depression care among low-income, minority women: a randomized controlled effectiveness study. American Journal of Public Health 2016;106:1833-41. [DOI: 10.2105/AJPH.2016.303304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noel 2014 {published data only}
- Noël PH, Romero RL, Robertson M, Parchman ML. Key activities used by community based primary care practices to improve the quality of diabetes care in response to practice facilitation. Quality in Primary Care 2014;22(4):211-9. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Ogbuoji Osondu 2018 {published data only}
- Ogbuoji Osondu RS. Factors Affecting Patient Perceptions of Quality and Health-Seeking Behavior. Doctoral dissertation, Harvard T.H. Chan School of Public Health.
Ojerholm 2016 {published data only}
- Ojerholm E, Halpern SD, Bekelman JE. Default options: opportunities to improve quality and value in oncology. Journal of Clinical Oncology 2016;34(16):1844-7. [DOI: 10.1200/JCO.2015.64.8741] [DOI] [PubMed] [Google Scholar]
Oliver 2009 {published data only}
- Oliver S, Armes DG, Gyte G. Public involvement in setting a national research agenda: a mixed methods evaluation. The Patient: Patient-centered Outcomes Research 2009;2(3):179-90. [DOI: 10.2165/11314860-000000000-00000] [DOI] [PubMed] [Google Scholar]
Ong 2013 {published data only}
- Ong M, Wells KB, Jones L, Chung B, Dixon E, Tang L, et al. Community-partnered cluster-randomized comparative effectiveness trial of community engagement and planning or program technical assistance to address depression disparities. Journal of General Internal Medicine 2013;28:S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ong 2017 {published data only}
- Ong MK, Jones L, Aoki W, Belin TR, Bromley E, Chung B, et al. A community-partnered, participatory, cluster-randomized study of depression care quality improvement: three-year outcomes. Psychiatric Services 2017;68(12):1262-70. [DOI: 10.1176/appi.ps.201600488] [DOI] [PMC free article] [PubMed] [Google Scholar]
Orozco‐Beltran 2013 {published data only}
- Orozco-Beltran D, Ruescas-Escolano E, Navarro-Palazon AI, Cordero A, Gaubert-Tortosa M, Navarro-Perez J, et al. Effectiveness of a new health care organization model in primary care for chronic cardiovascular disease patients based on a multifactorial intervention: the PROPRESE randomized controlled trial. BMC Health Services Research 2013;13:293. [DOI: 10.1186/1472-6963-13-293] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osrin 2003 {published data only}
- Osrin D, Mesko N, Shrestha BP, Shrestha D, Tamang S, Thapa S, et al. Implementing a community-based participatory intervention to improve essential newborn care in rural Nepal. Transactions of the Royal Society of Tropical Medicine and Hygiene 2003;97(1):18-21. [DOI: 10.1016/s0035-9203(03)90008-3] [DOI] [PubMed] [Google Scholar]
Parchman 2013 {published data only}
- Parchman ML, Noel PH, Culler SD, Lanham HJ, Leykum LK, Romero RL, et al. A randomized trial of practice facilitation to improve the delivery of chronic illness care in primary care: initial and sustained effects. Implementation Science 2013;8:93. [DOI: 10.1186/1748-5908-8-93] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2019a {published data only}
- Patel M, Navedal A, Bhattacharya J, Coker TR. Approach to improving cancer care delivery for low-Income and minority patients with cancer. Journal of Community Health 2019;44(5):912-20. [DOI: 10.1007/s10900-019-00632-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2019b {published data only}
- Patel MI, Moore D, Coker TR. End-of-life cancer care redesign: patient and caregiver experiences in a lay health worker-led intervention. The American Journal of Hospice & Palliative Care 2019;36(12):1081-8. [DOI: 10.1177/1049909119847967] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paton 2013 {published data only}
- Paton N, Callander R, Cavill M, Ning L, Weavell W. Collaborative quality improvement: consumers, carers and mental health service providers working together in service co-design. Australasian Psychiatry 2013;21(1):78-9. [DOI: 10.1177/1039856212465347] [DOI] [PubMed] [Google Scholar]
Patzer 2014 {published data only}
- Patzer RE, Gander J, Sauls L, Amamoo MA, Krisher J, Mulloy LL, et al. The RaDIANT community study protocol: community-based participatory research for reducing disparities in access to kidney transplantation. BMC Nephrology 2014;15:171. [DOI: 10.1186/1471-2369-15-171] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peremans 2010 {published data only}
- Peremans L, Rethans JJ, Verhoeven V, Coenen S, Debaene L, Meulemans H, et al. Empowering patients or general practitioners? A randomised clinical trial to improve quality in reproductive health care in Belgium. The European Journal of Contraception & Reproductive Health Care 2010;15(4):280-89. [DOI: 10.3109/13625187.2010.492882] [DOI] [PubMed] [Google Scholar]
Peter 2015 {published data only}
- Peter W, Wees PJ, Verhoef J, Jong Z, Bodegom-Vos L, Hilberdink WK, et al. Effectiveness of an interactive postgraduate educational intervention with patient participation on the adherence to a physiotherapy guideline for hip and knee osteoarthritis: a randomised controlled trial. Disability and Rehabilitation 2015;37(3):274-82. [DOI: 10.3109/09638288.2014.913708] [DOI] [PubMed] [Google Scholar]
Peterson 2018 {published data only}
- Peterson S, Gleason KT, Berger Z, McClenney A, Newman-Toker DE. Partnered (patients assigned to research teams with nurses and er providers to enhance diagnosis). Diagnosis 2018;5(4):eA125-eA6. [DOI: 10.1515/dx-2018-0095] [DOI] [Google Scholar]
Pramanik 2018 {published data only}
- Pramanik S, Ghosh A, Nanda Rituu B, Rouw M, Forth P, Albert S. Impact evaluation of a community engagement intervention in improving childhood immunization coverage: a cluster randomized controlled trial in Assam, India. BMC Public Health 2018;18(1):534. [DOI: 10.1186/s12889-018-5458-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prost 2018 {published data only}
- Prost A, Sanders D, Costello A, Vogel J, Baqui Abdullah H, Nair N, et al. Strengthening the capabilities of families and communities to improve child health in low and middle income countries. BMJ 2018;362:bmj.k2649. [DOI: 10.1136/bmj.k2649] [DOI] [PMC free article] [PubMed] [Google Scholar]
Protik Ali 2018 {published data only}
- Protik Ali E, Nichols-Barrer I, Berman J, Sloan M. Bridging the information gap between citizens and local governments: evidence from a civic participation strengthening program in Rwanda. World Development 2018;108:145-56. [DOI: 10.1016/j.worlddev.2018.03.016] [DOI] [Google Scholar]
Rai 2018 {published data only}
- Rai S, Gurung D, Kaiser BN, Sikkema KJ, Dhakal M, Bhardwaj A, et al. A service user co-facilitated intervention to reduce mental illness stigma among primary healthcare workers: utilizing perspectives of family members and caregivers. Families, Systems, & Health 2018;36(2):198-209. [DOI: 10.1037/fsh0000338] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raynes‐Greenow 2010 {published data only}
- Raynes-Greenow CH, Nassar N, Torvaldsen S, Trevena L, Roberts CL. Assisting informed decision making for labour analgesia: a randomised controlled trial of a decision aid for labour analgesia versus a pamphlet. BMC Pregnancy and Childbirth 2010;10:15. [DOI: 10.1186/1471-2393-10-15] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2013 {published data only}
- Reeves R, West E, Barron D. Facilitated patient experience feedback can improve nursing care: a pilot study for a phase III cluster randomised controlled trial. BMC Health Services Research 2013;13:259. [DOI: 10.1186/1472-6963-13-259] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinhardt 2012 {published data only}
- Reinhardt CH, Noack MJ, Wassmer G, Dumit J, Rolfs A, Klein K. Comparison of three forms of teaching - a prospective randomized pilot trial for the enhancement of adherence. International Journal of Dental Hygiene 2012;10(4):277-83. [DOI: 10.1111/j.1601-5037.2011.00543.x] [DOI] [PubMed] [Google Scholar]
Rogers 2007 {published data only}
- Rogers ES, Teague GB, Lichenstein C, Campbell J, Lyass A, Chen R, et al. Effects of participation in consumer-operated service programs on both personal and organizationally mediated empowerment: results of multisite study. Journal of Rehabilitation Research and Development 2007;44(6):785-99. [DOI] [PubMed] [Google Scholar]
Sarmiento 2018 {published data only}
- Sarmiento I, Paredes-Solis S, Andersson N, Cockcroft A. Safe birth and cultural safety in southern Mexico: study protocol for a randomised controlled trial. Trials 2018;19(1):354. [DOI: 10.1186/s13063-018-2712-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sauers‐Ford 2016 {published data only}
- Sauers-Ford HS, Simmons JM, Shah SS. Strategies to engage stakeholders in research to improve acute care delivery. Journal of Hospital Medicine 2016;11(2):123-5. [DOI: 10.1002/jhm.2492] [DOI] [PubMed] [Google Scholar]
Scholl 2018 {published data only}
- Scholl I, Hahlweg P, Lindig A, Bokemeyer C, Coym A, Hanken H, et al. Evaluation of a program for routine implementation of shared decision-making in cancer care: study protocol of a stepped wedge cluster randomized trial. Implementation Science 2018;131:1. [DOI: 10.1186/s13012-018-0740-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schweitzer 2015 {published data only}
- Schweitzer J. Accountability in the 2015 global strategy for women's, children's and adolescents' health. British Medical Journal 2015;351:h4248. [DOI: 10.1136/bmj.h4248] [DOI] [PubMed] [Google Scholar]
Segal 2010 {published data only}
- Segal SP, Silverman CJ, Temkin TL. Self-help and community mental health agency outcomes: a recovery-focused randomized controlled trial. Psychiatric Services 2010;61(9):905-10. [DOI: 10.1176/ps.2010.61.9.905] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sepuchra 2003 {published data only}
- Sepuchra KR, Belkora JK, Aviv C, Mutchnick S, Esserman LJ. Improving the quality of decision making in breast cancer: consultation planning template and consultation recording template. Oncology Nursing Forum 2003;30:99-106. [DOI: 10.1188/03.ONF.99-106 ] [DOI] [PubMed] [Google Scholar]
Shagi 2008 {published data only}
- Shagi C, Vallely A, Kasindi S, Chiduo B, Desmond N, Soteli S, et al. A model for community representation and participation in HIV prevention trials among women who engage in transactional sex in Africa. AIDS Care 2008;20(9):1039-49. [DOI: 10.1080/09540120701842803 ] [DOI] [PubMed] [Google Scholar]
Sherbourne 2017 {published data only}
- Sherbourne CD, Aoki W, Belin TR, Bromley E, Chung B, Dixon E, et al. Comparative effectiveness of two models of depression services quality improvement in health and community sectors. Psychiatric Services 2017;68(12):1315-20. [DOI: 10.1176/appi.ps.201700170] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shukla 2018 {published data only}
- Shukla M. Impact of a health governance intervention on provincial health system performance in Afghanistan: a quasi-experimental study. Health Systems and Reform 2018;4(3):249-66. [DOI: 10.1080/23288604.2018.1477536] [DOI] [PubMed] [Google Scholar]
Simonsen 2017 {published data only}
- Simonsen SE, Ralls B, Guymon A, Garrett T, Eisenman P, Villalta J, et al. Addressing health disparities from within the community: community-based participatory research and community health worker policy initiatives using a gender-based approach. Women's Health Issues 2017;27(Supplement 2):S46-53. [DOI: 10.1016/j.whi.2017.09.006] [DOI] [PubMed] [Google Scholar]
Singh 2020 {published data only}
- Singh K. Group antenatal care in Rwanda: examining successes and challenges in implementation. Dissertation. University of California, Berkeley 2020.
Sirilak 2013 {published data only}
- Sirilak S, Okanurak K, Wattanagoon Y, Chatchaiyalerk S, Tornee S, Siri S. Community participation of cross-border migrants for primary health care in Thailand. Health Policy and Planning 2013;28(6):658-64. [DOI: 10.1093/heapol/czs105] [DOI] [PubMed] [Google Scholar]
Smiddy 2015 {published data only}
- Smiddy J, Reay J, Peckham S, Williams L, Wilson P. Developing patient reference groups within general practice: a mixed-methods study. British Journal of General Practice 2015;65(632):e177-83. [DOI: 10.3399/bjgp15X683989] [DOI] [PMC free article] [PubMed] [Google Scholar]
Smout 2016 {published data only}
- Smout EM, Enria L, Mooney T, Lees S, Watson-Jones D, Greenwood B, et al. Implementing a novel community engagement system during a clinical trial of a candidate Ebola vaccine within an outbreak setting. International Journal of Infectious Diseases 2016;45(Supplement 1):191. [DOI: 10.1016/j.ijid.2016.02.444] [DOI] [Google Scholar]
Solomon 2017 {published data only}
- Solomon R, Smith C, Kallio J, Fenollosa A, Benerofe B, Jones L, et al. Speaking up: how patient and physician voices shaped a trial to improve goals-of-care eiscussions. The Patient: Patient-centered Outcomes Research 2017;10(4):489-501. [DOI: 10.1007/s40271-017-0226-z] [DOI] [PubMed] [Google Scholar]
South 2016 {published data only}
- South A, Hanley B, Gafos M, Cromarty B, Stephens R, Sturgeon K, et al. Models and impact of patient and public involvement in studies carried out by the Medical Research Council Clinical Trials Unit at University College London: findings from ten case studies. Trials 2016;17:376. [DOI: 10.1186/s13063-016-1488-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2011 {published data only}
- Spencer MS, Rosland AM, Kieffer EC, Sinco BR, Valerio M, Palmisano G, et al. Effectiveness of a community health worker intervention among African American and Latino adults with type 2 diabetes: a randomized controlled trial. American Journal of Public Health 2011;101(12):2263-60. [DOI: 10.2105/AJPH.2010.300106] [DOI] [PMC free article] [PubMed] [Google Scholar]
Springgate 2018 {published data only}
- Springgate BF, Arevian AC, Wennerstrom A, Johnson AJ, Eisenman DP, Sugarman OK, et al. Community resilience learning collaborative and research network (C-LEARN): study protocol with participatory planning for a randomized, comparative effectiveness trial. International Journal of Environmental Research and Public Health 2018;15(8):1683. [DOI: 10.3390/ijerph15081683S2.325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Springgate 2018b {published data only}
- Springgate B, Tang LQ, Ong M, Aoki W, Chung BW, Dixon E, et al. Comparative effectiveness of coalitions versus technical assistance for depression quality improvement in persons with multiple chronic conditions. Ethnicity & Disease 2018;28(Supplement 2):325-38. [DOI: 10.18865/ed.28.S2.325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stanhope 2013 {published data only}
- Stanhope V, Ingoglia C, Schmelter B, Marcus SC. Impact of person-centered planning and collaborative documentation on treatment adherence. Psychiatric Services 2013;64(1):76-9. [DOI: 10.1176/appi.ps.201100489] [DOI] [PubMed] [Google Scholar]
Stephens 2018 {published data only}
- Stephens TJ, Peden CJ, Pearse RM, Shaw SE, Abbott TEF, Jones E, et al. Improving care at scale: process evaluation of a multi-component quality improvement intervention to reduce mortality after emergency abdominal surgery (EPOCH trial). Implementation Science 2018;13(1):142. [DOI: 10.1186/s13012-018-0823-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stockdale 2016 {published data only}
- Stockdale SE, Tang L, Pudilo E, Lucas-Wright A, Chung B, Horta M, et al. Sampling and recruiting community-based programs using community-partnered participation research. Health Promotion Practice 2016;17(2):254-64. [DOI: 10.1177/1524839915605059] [DOI] [PubMed] [Google Scholar]
Storm 2011 {published data only}
- Storm M, Knudsen K, Davidson L, Hausken K, Johannessen Jan O. "Service user involvement in practice": the evaluation of an intervention program for service providers and inpatients in Norwegian Community Mental Health Centers. Psychosis: Psychological, Social and Integrative Approaches 2011;3(1):29-40. [DOI: 10.1080/17522439.2010.501521] [DOI] [Google Scholar]
Svetaz 2016 {published data only}
- Svetaz MV, Sieving R, Allen M, Hager RR, Beckman KJ, Galvan A, et al. "A community based participatory research (CBPR) journey bringing culture and family to the center of an intervention to promote positive youth development and reproductive health: the encuentro project". Journal of adolescent health Conference: 2016 annual meeting of the society for adolescent health and medicine, SAHM 2016 Washington, DC United States 2016;58(2 Supplement 2):S5. [Google Scholar]
Taylor 2017 {published data only}
- Taylor LJ, Rathouz PJ, Berlin A, Brasel KJ, Mosenthal AC, Finlayson E, et al. Navigating high-risk surgery: protocol for a multisite, stepped wedge, cluster-randomised trial of a question prompt list intervention to empower older adults to ask questions that inform treatment decisions. BMJ Open 2017;7(5):e014002. [DOI: 10.1136/bmjopen-2016-014002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thilly 2003 {published data only}
- Thilly N, Briançon S, Juillière Y, Dufay E, Zannad F. Improving ACE inhibitor use in patients hospitalized with systolic heart failure: a cluster randomized controlled trial of clinical practice guideline development and use. Journal of Evaluation in Clinical Practice 2003;9(3):373-82. [DOI: 10.1046/j.1365-2753.2003.00441.x] [DOI] [PubMed] [Google Scholar]
Thornton 2003 {published data only}
- Thornton H, Edwards A, Elwyn G. Evolving the multiple roles of 'patients' in health-care research: reflections after involvement in a trial of shared decision-making. Health Expectations 2003;6:189-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tondora 2010 {published data only}
- Tondora J, O'Connell M, Miller R, Dinzeo T, Bellamy C, Andres-Hyman R, et al. A clinical trial of peer-based culturally responsive person-centered care for psychosis for African Americans and Latinos. Clinical Trials 2010;7(4):368-79. [DOI: 10.1177/1740774510369847] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tran 2004 {published data only}
- Tran AN, Haidet P, Street RL, O'Malley KJ, Martin F, Ashton CM. Empowering communication: a community-based intervention for patients. Patient Education and Counseling. 2004;52:113-21. [DOI: 10.1016/S0738-3991%2803%2900002-8] [DOI] [PubMed] [Google Scholar]
Tran 2018 {published data only}
- Tran NT, Gaffield ME, Seuc A, Landoulsi S, Yamaego WME, Cuzin-Kihl A, et al. Effectiveness of a package of postpartum family planning interventions on the uptake of contraceptive methods until twelve months postpartum in Burkina Faso and the Democratic Republic of Congo: the YAM DAABO study protocol. BMC Health Services Research 2018;18(1):439. [DOI: 10.1186/s12913-018-3199-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Treloar 2015 {published data only}
- Treloar C, Rance J, Bath N, Everingham H, Micallef M, Day C, et al. Evaluation of two community-controlled peer support services for assessment and treatment of hepatitis C virus infection in opioid substitution treatment clinics: the ETHOS study, Australia. International Journal of Drug Policy 2015;2618(101):992-8. [DOI: 10.1016/j.drugpo.2015.01.005] [DOI] [PubMed] [Google Scholar]
Tripathy 2010 {published data only}
- Tripathy P, Nair N, Barnett S, Mahapatra R, Borghi J, Rath S, et al. Effect of a participatory intervention with women's groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomised controlled trial. Lancet 2010;375(9721):1182-92. [DOI: 10.1016/S0140-6736(09)62042-0] [DOI] [PubMed] [Google Scholar]
Tripathy 2011 {published data only}
- Tripathy P, Nair N, Mahapatra R, Rath S, Gope RK, Rath S, et al. Community mobilisation with women's groups facilitated by Accredited Social Health Activists (ASHAs) to improve maternal and newborn health in underserved areas of Jharkhand and Orissa: study protocol for a cluster-randomised controlled trial. Trials 2011;12:182. [DOI: 10.1186/1745-6215-12-182] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tripathy 2016 {published data only}
- Tripathy P, Nair N, Sinha R, Rath S, Gope RK, Rath S, et al. Effect of participatory women's groups facilitated by Accredited Social Health Activists on birth outcomes in rural eastern India: a cluster-randomised controlled trial. Lancet Global Health 2016;4(2):e119-28. [DOI: 10.1016/S2214-109X(15)00287-9] [DOI] [PubMed] [Google Scholar]
Tsianakas 2012 {published data only}
- Tsianakas V, Robert G, Maben J, Richardson A, Dale C, Griffin M, et al. Implementing patient-centred cancer care: using experience-based co-design to improve patient experience in breast and lung cancer services. Supportive Care in Cancer 2012;20(11):2639-47. [DOI: 10.1007/s00520-012-1470-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tumiel‐Berhalter 2011 {published data only}
- Tumiel-Berhalter LM, Kahn L, Watkins R, Goehle M, Meyer C. The implementation of Good For The Neighborhood: a participatory community health program model in four minority underserved communities. Journal of Community Health. 2011;36(4):669-74. [DOI: 10.1007/s10900-011-9358-6] [DOI] [PubMed] [Google Scholar]
Uding 2007 {published data only}
- Uding N, Sety M, Kieckhefer Gail M. Family involvement in health care research: the "Building on Family Strengths" case study. Families, Systems, & Health 2007;25(3):307-22. [DOI: 10.1037/1091-7527.25.3.307] [DOI] [Google Scholar]
Uding 2009 {published data only}
- Uding N, Kieckhefer GM, Trahms CM. Parent and community participation in program design. Clinical Nursing Research 2009;18(1):68-79. [DOI: 10.1177/1054773808330096] [DOI] [PubMed] [Google Scholar]
Vale 2018 {published data only}
- Vale CL, Cragg WJ, Cromarty B, Hanley B, South A, Stephens R, et al. When participants get involved: reconsidering patient and public involvement in clinical trials at the MRC Clinical Trials Unit at UCL. Trials 2018;19(1):95. [DOI: 10.1186/s13063-018-2471-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanderboom 2014 {published data only}
- Vanderboom CE, Holland DE, Lohse CM, Targonski PV, Madigan EA. Enhancing patient-centered care. Western Journal of Nursing Research 2014;36(1):47-65. [DOI: 10.1177/0193945913490841] [DOI] [PubMed] [Google Scholar]
Van Malderen 2017 {published data only}
- Van Malderen L, De Vriendt P, Mets T, Verté D, Gous E. Experiences and effects of structurally involving residents in the nursing home by means of participatory action research: a mixed method study. Journal of the American Medical Directors Association 2017;18(6):495-502. [DOI: 10.1016/j.jamda.2016.12.072] [DOI] [PubMed] [Google Scholar]
Van Malderen 2017a {published data only}
- Van Malderen L, De Vriendt P, Mets T. Active ageing in the nursing home: could participatory action research provide the answer? Action Research 2017;15(3):239-57. [DOI: 10.1177/1476750316636668] [DOI] [Google Scholar]
von dem Knesebeck 2002 {published data only}
- dem Knesebeck O, Joksimovic L, Badura B, Siegrist J. Evaluation of a community-level health policy intervention. Health Policy 2002;61:111-22. [DOI: 10.1016/S0168-8510(01)00221-4] [DOI] [PubMed] [Google Scholar]
Waiswa 2016 {published data only}
- Waiswa P, O'Connell T, Bagenda D, Mullachery P, Mpanga F, Henriksson DK, et al. Community and District Empowerment for Scale-up (CODES): a complex district-level management intervention to improve child survival in Uganda: study protocol for a randomized controlled trial. Trials 2016;17(1):135. [DOI: 10.1186/s13063-016-1241-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wathne 2018 {published data only}
- Wathne JS, Kleppe LKS, Harthug S, Blix HS, Nilsen RM, Charani E. The effect of antibiotic stewardship interventions with stakeholder involvement in hospital settings: a multicentre, cluster randomized controlled intervention study. Antimicrobial Resistance and Infection Control 2018;7:109. [DOI: 10.1186/s13756-018-0400-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 2001 {published data only}
- Watson MR, Horowitz AM, Garcia I, Canto MT. A community participatory oral health promotion program in an inner-city Latino community. Journal of Public Health Dentistry 2001;61(1):34-41. [DOI: 10.1111/j.1752-7325.2001.tb03353.x] [DOI] [PubMed] [Google Scholar]
Weinstein 2006 {published data only}
- Weinstein J. Involving mental health service users in quality assurance. Health Expectations 2006;9(2):98-109. [DOI: 10.1111/j.1369-7625.2006.00377.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wells 2013 {published data only}
- Wells KB, Jones L, Chung B, Dixon EL, Tang L, Gilmore J, et al. Community-partnered cluster-randomized comparative effectiveness trial of community engagement and planning or resources for services to address depression disparities. Journal of General Internal Medicine 2013;28(10):1268-78. [DOI: 10.1007/s11606-013-2484-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Werner 2019 {published data only}
- Werner RM, Kanter GP, Polsky D. Association of physician group participation in Accountable Care Organizations with patient social and clinical characteristics. JAMA Network Open 2019;2(1):e187220. [DOI: 10.1001/jamanetworkopen.2018.7220] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wheatley 2002 {published data only}
- Wheatley K, Djulbegovic B, Glasmacher A. Priority-setting decisions for new cancer drugs. Lancet 2002;359(9316):1525. [DOI: 10.1016/S0140-6736(02)08452-0] [DOI] [PubMed] [Google Scholar]
Williams 2013 {published data only}
- Williams AB, Honghong W, Burgess J, Xianhong L, Danvers K. Cultural adaptation of an evidence-based nursing intervention to improve medication adherence among people living with HIV/AIDS (PLWHA) in China. International Journal of Nursing Studies 2013;50(4):487-94. [DOI: 10.1016/j.ijnurstu.2012.08.018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winters 2016 {published data only}
- Winters E. The design and implementation of a team-based care model in an ambulatory BMT clinic. Biology of Blood and Marrow Transplantation 2016;22(3 Supplement 22):S461. [Google Scholar]
Wolf 2008 {published data only}
- Wolf DM, Lehman L, Quinlin R, Zullo T, Hoffman L. Effect of patient-centered care on patient satisfaction and quality of care. Journal of Nursing Care Quality 2008;23(4):316-21. [DOI: 10.1097/01.NCQ.0000336672.02725.a5] [DOI] [PubMed] [Google Scholar]
Wolfe 2019 {published data only}
- Wolfe I, Lemer C, Heys M, Ingrassia A, Newham J, Forman J, et al. Implementing and Evaluating the CYPHP Evelina London new care model to improve health, healthcare quality, and patterns of service use among children and young people. International Journal of Integrated Care 2019;19(4):253. [DOI: 10.5334/ijic.s3253] [DOI] [Google Scholar]
Wood Dauphinee 2011 {published data only}
- Wood Dauphinee SL, Bayley M, Mokry J, Salbach N, Thorpe K. Stroke Canada optimization of rehabilitation by evidence: implementation trial (SCORE: iT). Cerebrovascular Diseases 2011;31(Supplement 2):43. [DOI: 10.1159/000329448] [DOI] [Google Scholar]
Yano 2016 {published data only}
- Yano EM, Darling JE, Hamilton AB, Canelo I, Chuang E, Meredith LS, et al. Cluster randomized trial of a multilevel evidence-based quality improvement approach to tailoring VA Patient Aligned Care Teams to the needs of women Veterans. Implementation Science 2016;11(1):1-14. [DOI: 10.1186/s13012-016-0461-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Younes 2012 {published data only}
- Younes L, Houweling TA, Azad K, Costello A, Fottrell E. Estimating coverage of a women’s group intervention among a population of pregnant women in rural Bangladesh. BMC Pregnancy Childbirth 2012;12:60. [DOI: 10.1186/1471-2393-12-60] [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2005 {published data only}
- Young AS, Chinman M, Forquer SL, Knight EL, Vogel H, Miller A, et al. Use of a consumer-led intervention to improve provider competencies. Psychiatric Services 2005;56(8):967-75. [DOI: 10.1176/appi.ps.56.8.967] [DOI] [PubMed] [Google Scholar]
Zhang 2020 {published data only}
- Zhang Y. Young, low-income mothers' involvement in doula home visiting services. Dissertation.The University of Chicago School of Social Service Administration 2020.
Zimmerman 2017 {published data only}
- Zimmerman RK, Brown AE, Pavlik VN, Moehling KK, Raviotta JM, Lin CJ, et al. Using the 4 Pillars Practice Transformation Program to increase pneumococcal immunizations for older adults: a Cluster-Randomized Trial. Journal of the American Geriatrics Society 2017;6556(1):114-22. [DOI: 10.1111/jgs.14451] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwar 2008 {published data only}
- Zwar N, Hermiz O, Hasan I, Comino E, Middleton S, Vagholkar S, et al. A cluster randomised controlled trial of nurse and GP partnership for care of chronic obstructive pulmonary disease. BMC Pulmonary Medicine 2008;8:8. [DOI: 10.1186/1471-2466-8-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
English 2018 {published data only}
- Dickinson WP, Nease DE, Rhyne RL, Knierim KE, Fernald DH, la Cerda DR, et al. Practice transformation and patient engagement to improve cardiovascular care: from EvidenceNOW Southwest (ENSW). The Journal of the American Board of Family Medicine 2020;33(5):675-86. [DOI: 10.3122/jabgm.2020.05.190375] [DOI] [PMC free article] [PubMed] [Google Scholar]
- English AF, Dickinson LM, Zittleman L, Nease Jr DE, Herrick A, Westfall JM, et al. A community engagement method to design patient engagement materials for cardiovascular health. Annals of Family Medicine 2018;16:s58-64. [DOI: 10.1370/afm.2173] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald DH, Mullen R, Hall T, Bienstock A, Kirchner S, Knierim K, et al. Exemplary practices in cardiovascular care: results on clinical quality measures from the EvidenceNOW Southwest Cooperative. Journal of General Internal Medicine 2020;35(11):3197-3204. [DOI: 10.1007/s11606-020-06094-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall TL, Knierim KE, Nease DE, Staton EW, Nkouaga C, Dickinson LM, et al. Primary care practices' implementation of Patient Team-Partnership: findings from Evidence NOW Southwest. The Journal of the American Board of Family Medicine 2019;32(4):490-504. [DOI: 10.3122/jabfm.2019.04.180361] [DOI] [PubMed] [Google Scholar]
- NCT02515578. ClinicalTrials.gov.
Gai 2019 {published data only}
- Gai Tobe R, Islam MT, Yoshimaru Y, Hossain J. Strengthening the community support group to improve maternal and neonatal health seeking behaviours: a cluster-randomized controlled trial in Satkhira District, Bangladesh. PLoS ONE 2019;14(2):e0212847. [DOI: 10.1371/journal.pone.0212847] [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2013 {published data only}
- James AS, Richardson V, Wang JS, Proctor EK, Colditz GA. Systems intervention to promote colon cancer screening in safety net settings: protocol for a community-based participatory randomized controlled trial. Implementation Science 2013;8:58. [DOI: 10.1186/1748-5908-8-58] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathukrishnan M, Sufcliffe S, Hunleth JM, Wang JS, Colditz GA, James AS. Conducting a randomized trial in rural and urban safety-net health centres: added value of community-based participatory research. Comtenporary Clinical Trials Communications 2018;10:29-35. [DOI: 10.1016/j.conctc.2018.02.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindquist 2020 {published data only}
- Lindquist L, Ramirez-Zohfeld V, Wong N, Forcucci C, Beverly R. Leveraging patient partners to disseminate a Long-Term Support Services (LTSS) planning tool, PlanYourLifespan.org. Journal of the American Medical Directors Association 2020;21:B31-2. [DOI: 10.1016/j.jamda.2020.01.087] [DOI] [Google Scholar]
Morrison 2020 {published data only}
- Morrison J, Tumbahangphe K, Sen A, Gram L, Budhathoki B, Neupane R, et al. Health management committee strengthening and community mobilisation through women's groups to improve trained health worker attendance at birth in rural Nepal: a cluster randomised controlled trial. BMC Pregnancy Childbirth 2020;20(1):268. [DOI: 10.1186/s12884-020-02960-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmer 2015 {published data only}
- Dimopoulos-Bick T, Dawda P, Maher L, Verma R, Palmer V. Experience-Based Co-Design: tackling common challenges. The Journal of Health Design 2018;3(1):86-93. [DOI: 10.21853/JHD.2018.46] [DOI] [Google Scholar]
- Palmer V, Piper D, Richard L, Furler J, Herrman H, Cameron J, et al. Getting to the CORE of the links between engagement, experience and recovery outcomes. NewParadigm 2015;Summer:41-5. [Google Scholar]
- Palmer V. The engagement and psychosocial recover nexus: testing an experience-based co-design methodology in community mental health services. In: Australian and New Zealand Journal of Psychiatry. Congerence: Royal Australian and New Zealand College of Psychiatrists, RANZCP annual congress Australia. Vol. 51 Supplement 1. Netherlands: SAGE Publications, 2017.
- Palmer VJ, Chondros P, Piper D, Callander R, Weavell W, Godbee K, et al. The CORE study protocol: a stepped wedge cluster randomised controlled trial to test a co-design technique to optimise psychosocial recovery outcomes for people affected by mental illness in the community mental health setting. BMJ Open 2015;5(3):e006688. [DOI: 10.1136/bmjopen-2014-006688] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer VJ, Piper D, Richard L, Furler J, Herrman H, Cameron J, et al. Balancing opposing forces - a nested process evaluation study protocol for a stepped wedge designed cluster randomised controlled trial: the CORE study. International Journal of Qualitative Methods 2016;15(1):1-10. [DOI: 10.1177/1609406916672216] [DOI] [Google Scholar]
- Palmer WJ, Weavell W, Callander R, Piper D, Richard L, Maher L, et al. The Participatory Zeitgeist: an explanatory theoretical model of change in an era of coproduction and codesign in healthcare improvement. Medical Humanities 2019;45:247-57. [DOI: 10.1136/medhum-2017-011398] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard L, Piper D, Weavell W, Callander R, Iedema R, Furler J. Advancing engagement methods for trials: the CORE study relational model of engagement for a stepped wedge cluster randomised controlled trial of experience-based co-design for people living with severe mental illnesses. Trials 2017;18(1):169. [DOI: 10.1186/s13063-017-1878-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shrestha 2011 {published data only}
- Amano S, Shrestha BP, Chaube SS, Higuchi M, Manandhar DS, Osrin D, et al. Effectiveness of female community health volunteers in the detection and management of low-birth-weight in Nepal. Rural Remote Health 2014;14(1):2508. [PMC free article] [PubMed] [Google Scholar]
- Shrestha BP, Bhandari B, Manandhar DS, Osrin D, Costello A, Saville N. Community interventions to reduce child mortality in Dhanusha, Nepal: study protocol for a cluster randomized controlled trial. Trials 2011;12:136. [DOI: 10.1186/1745-6215-12-136] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Kjellström 2019 {published data only}
- Kjellström S, Areskoug-Josefsson K, Andersson GB, Andersson AC, Ockander M, Käll J, et al. Exploring, measuring and enhancing the coproduction of health and well-being at the national, regional and local levels through comparative case studies in Sweden and England: the ‘Samskapa’ research programme protocol. BMJ Open 2019;9:e029723. [DOI: 10.1136/bmjopen-2019-029723] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawtell 2018 {published data only}
- Sawtell M, Sweeney L, Wiggins M, Salisbury C, Eldridge S, Greenberg L, et al. Evaluation of community-level interventions to increase early initiation of antenatal care in pregnancy: protocol for the Community REACH study, a cluster randomised controlled trial with integrated process and economic evaluations. Trials 2018;19(1):163. [DOI: 10.1186/s13063-018-2526-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACSQHC 2008
- Australian Commission on Safety and Quality in Health Care. Australian Charter of Healthcare Rights. www.safetyandquality.gov.au/national-priorities/charter-of-healthcare-rights/ (accessed 24 July 2018).
ACSQHC 2011
- Australian Commission on Safety and Quality in Health Care. Patient-centred care: improving quality and safety through partnerships with patients and consumers. www.safetyandquality.gov.au/our-work/partnering-consumers/person-centred-care (accessed 24 July 2018).
ACSQHC 2017
- Australian Commission on Safety and Quality in Health Care. National Safety and Quality Health Service Standards. 2nd ed. www.safetyandquality.gov.au/publications-and-resources/resource-library/national-safety-and-quality-health-service-standards-1 (accessed 2 July 2019).
ACSQHC 2018
- Australian Commission on Safety and Quality in Health Care. Review of key attributes of high-performing person-centred healthcare organisations. www.safetyandquality.gov.au/our-work/partnering-consumers/person-centred-healthcare-organisations (accessed 2 July 2019).
Arnstein 1969
- Arnstein SR. A ladder of citizen participation. Journal of the American Institute of Planners 1969;35:216-24. [DOI: 10.1080/01944366908977225] [DOI] [Google Scholar]
Bardes 2012
- Bardes CL. Defining ‘patient-centered medicine'. New England Journal of Medicine 2012;66(9):782-3. [DOI: 10.1056/NEJMp1200070] [DOI] [PubMed] [Google Scholar]
Batalden 2016
- Batalden M, Batalden P, Margolis P, Seid M, Armstrong G, Opipari-Arrigan L, et al. Coproduction of healthcare service. BMJ Quality & Safety 2016;25(7):509-17. [DOI: 10.1136/bmjqs-2015-004315] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berwick 2009
- Berwick DM. What ‘patient-centered' should mean: confessions of an extremist. Health Affairs 2009;28(4):w555-65. [DOI: 10.1377/hlthaff.28.4.w555] [DOI] [PubMed] [Google Scholar]
Bombard 2018
- Bombard Y, Baker GR, Orlando E, Fancott C, Bhatia P, Casalino S, et al. Engaging patients to improve quality of care: a systematic review. Implementation Science 2018;13:98. [DOI: 10.1186/s13012-018-0784-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Byrne 2020
- Byrne AL, Balwin A, Harvey C. Whose centre is it anyway? Defining personcentredcare in nursing: An integrative review. PLoS One 2020;15(3):e0229923. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conklin 2015
- Conklin A, Morris Z, Nolte E. What is the evidence base for public involvement in health-care policy?: results of a systematic scoping review. Health Expectations 2012;18:155-65. [DOI: 10.1111/hex.12038] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooke 2016
- Cooke J, Langley J, Wolstenholme D, Hampshaw S. ‘Seeing' the difference: the importance of visibility and action as a mark of ‘authenticity' in co-production. Comment on ‘Collaboration and co-production of knowledge in healthcare: opportunities and challenges'. International Journal of Health Policy and Management 2016;6(6):345-8. [DOI: 10.15171/ijhpm.2016.136] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coulter 2011
- Coulter A, Collins A. Making shared decision making a reality: no decision about me, without me. www.kingsfund.org.uk/publications/making-shared-decision-making-reality (accessed 24 July 2018).
Coulter 2015
- Coulter A, Entwistle VA, Eccles A, Ryan S, Shepperd S, Perera R. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD010523. [DOI: 10.1002/14651858.CD010523.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2002
- Crawford MJ, Rutter D, Manley C, Weaver T, Bhui K, Fulop N, et al. Systematic review of involving patients in the planning and development of health care. BMJ 2002;325(7375):1263. [DOI: 10.1136/bmj.325.7375.1263] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalton 2016
- Dalton J, Chambers D, Harden M, Street A, Parker G, Eastwood A. Service user engagement in health service reconfiguration: a rapid evidence synthesis. Journal of Health Services Research & Policy 2016;21(3):195-205. [DOI: 10.1177/1355819615623305] [DOI] [PubMed] [Google Scholar]
Delaney 2018
- Delaney LJ. Patient-centred care as an approach to improving health care in Australia. Collegian 2018;25(1):119-23. [DOI: 10.1016/j.colegn.2017.02.005] [DOI] [Google Scholar]
Delbanco 2001
- Delbanco T, Berwick DM, Boufford JI, Edgman‐Levitan S, Ollenschläger G, Plamping D, et al. Healthcare in a land called People Power: nothing about me without me. Health Expectations 2001;4(3):144-50. [DOI: 10.1046/j.1369-6513.2001.00145.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2013
- Doyle C, Lennox L, Bell D. A systematic review of evidence on the links between patient experience and clinical safety and effectiveness. BMJ Open 2013;3(1):e001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dwamena 2012
- Dwamena F, Holmes-Rovner M, Gaulden CM, Jorgenson S, Sadigh G, Sikorskii A, et al. Interventions for providers to promote a patient-centred approach in clinical consultations. Cochrane Database of Systematic Reviews 2012, Issue 12. Art. No: CD003267. [DOI: 10.1002/14651858.CD003267.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edgman‐Levitan 2013
- Edgman-Levitan S, Brady C, Howitt P. Report of the Patient and Family Engagement Working Group 2013: Partnering with Patients,Families, and Communities for Health: A Global Imperative (2013). WISH Patient and Family Engagement Report 2013; Available from www.wish.org.qa/summits/wish-2013/forums-research-chairs/patient-engagement/.
Esmail 2015
- Esmail L, Moore, E, Rein A. Evaluating patient and stakeholder engagement in research: moving from theory to practice. Journal of Comparative Effectiveness Research 2015;4(2):133-45. [DOI: 10.2217/cer.14.79] [DOI] [PubMed] [Google Scholar]
Flemming 2019
- Flemming K, Booth A, Garside R, Tunçalp Ö, Noyes J. Qualitative evidence synthesis for complex interventions and guideline development: clarification of the purpose, designs and relevant methods. BMJ Global Health 2019;4:e000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fucile 2017
- Fucile B, Bridge E, Duliban C, Law MP. Experience-based co-design: a method for patient and family engagement in system-level quality improvement. Patient Experience Journal 2017;4(2):53-60. [Google Scholar]
Gilligan 2021
- Gilligan C, Powell M, Lynagh MC, Ward BM, Lonsdale C, Harvey P, et al. Interventions for improving medical students' interpersonal communication in medical consultations. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD012418. [DOI: 10.1002/14651858.CD012418.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed prior to 19 November 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Gray‐Burrows 2018
- Gray-Burrows KA, Willis TA, Foy R, Rathfelder M, Bland P, Chin A, et al. Role of patient and public involvement in implementation research: a consensus study. BMJ Quality & Safety 2018;27(10):858-64. [DOI: doi:10.1136/ bmjqs-2017-006954] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greene 2012
- Greene SM, Tuzzio L, Cherkin D. A framework for making patient-centered care front and center. Permanente Journal 2012;16(3):49-53. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haldane 2019
- Haldane V, Chuah FLH, Srivastava A, Singh SR, Koh GCH, et al. Community participation in health services development, implementation, and evaluation: a systematic review of empowerment, health, community, and process outcomes. PLOS ONE 2019;14(5):e0216112. [DOI: 10.1371/journal.pone.0216112] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harden 2018
- Harden E, Thomas J, Cargo M, Harris J, Pantoja T, Flemming K, et al. Cochrane Qualitative and Implementation Methods Group guidance series – paper 5: methods for integrating qualitative and implementation evidence within intervention effectiveness reviews. Journal of Clinical Epidemiology 2018;97:39-48. [DOI: 10.1016/j.jclinepi.2017.10.023] [DOI] [PubMed] [Google Scholar]
Hart 2004
- Hart D. Patients' rights and patients' participation. Individual and collective involvement: partnership and participation in health law. European Journal of Health Law 2004;11(1):17-28. [DOI: 10.1163/157180904323042308] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hubbard 2007
- Hubbard G, Kidd L, Donaghy E, McDonald C, Kearney N. A review of literature about involving people affected by cancer in research, policy and planning and practice. Patient Education and Counseling 2007;65(1):21-33. [DOI: 10.1016/j.pec.2006.02.009] [DOI] [PubMed] [Google Scholar]
IPFCC 2012
- Institute for Patient- and Family-Centered Care. What is PFCC? www.ipfcc.org/about/pfcc.html (accessed 24 July 2018).
Jun 2018
- Jun GT, Canham A, Altuna-Palacios A, Ward JR, Bhamra R, Rogers S, et al. A participatory systems approach to design for safer integrated medicine management. Ergonomics 2018;61(1):48-68. [DOI: 10.1080/00140139.2017.1329939] [DOI] [PubMed] [Google Scholar]
Kaufman 2011
- Kaufman J. Infographics. cccrg.cochrane.org/Infographics (accessed 2 July 2019).
Klein 1999
- Klein S, Tracy D, Kitchener HC, Walker LG. The effects of the participation of patients with cancer in teaching communication skills to medical undergraduates: a randomised study with follow-up after 2 years. European Journal of Cancer 1999;35(10):1448-56. [DOI: 10.1016/S0959-8049(99)00153-7] [DOI] [PubMed] [Google Scholar]
Legaré 2014
- Legaré F, Stacey D, Turcotte S, Cossi MJ, Kryworuchko J, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2014, Issue 9. Art. No: CD006732. [DOI: 10.1002/14651858.CD006732.pub3] [DOI] [PubMed] [Google Scholar]
Leyshon 2015
- Leyshon S, McAdam S. Scene setter: the importance of taking a systems approach to person-centred care. BMJ 2015;350:h985. [DOI: 10.1136/bmj.h985] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luxford 2011
- Luxford K, Safran DG, Delbanco T. Promoting patient-centered care: a qualitative study of facilitators and barriers in healthcare organizations with a reputation for improving the patient experience. International Journal for Quality in Health Care 2011;23(5):510-5. [DOI: 10.1093/intqhc/mzr024] [DOI] [PubMed] [Google Scholar]
Mackintosh 2020
- Mackintosh NJ, Davis RE, Easter A, Rayment-Jones H, Sevdalis N, Wilson S, et al. Interventions to increase patient and family involvement in escalation of care for acute life-threatening illness in community health and hospital settings. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD012829. [DOI: 10.1002/14651858.CD012829.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKenzie 2017
- McKenzie E, Potestio ML, Boyd JM, Niven DJ, Brundin-Mather R, Bagshaw SM, et al. Reconciling patient and provider priorities for improving the care of critically ill patients: a consensus method and qualitative analysis of decision making. Health Expectations 2017;20(6):1367-74. [DOI: 10.1111/hex.12576] [DOI] [PMC free article] [PubMed] [Google Scholar]
McMillan 2013
- McMillan SS, Kendall E, Sav A, King MA, Whitty JA, Kelly F, et al. Patient-centered approaches to health care: a systematic review of randomized controlled trials. Medical Care Research and Review 2013;70(6):567-96. [DOI: 10.1177/1077558713496318] [DOI] [PubMed] [Google Scholar]
Merner 2019
- Merner B, Hill S, Colombo C, Xafis V, Gaulden CM, Graham-Wisener L, et al. Consumers and health providers working in partnership for the promotion of person-centred health services: a co-produced qualitative evidence synthesis. Cochrane Database of Systematic Reviews 2019, Issue 2. Art. No: CD013274. [DOI: 10.1002/14651858.CD013274] [DOI] [Google Scholar]
Milton 2012
- Milton B, Attree P, French B, Povall S, Whitehead M, Popay J. The impact of community engagement on health and social outcomes: a systematic review. Community Development Journal 2012;47(3):316-34. [DOI: 10.1093/cdj/bsr043] [DOI] [Google Scholar]
Mockford 2012
- Mockford C, Staniszewska S, Griffiths F, Herron-Marx S. The impact of patient and public involvement on UK NHS health care: a systematic review. International Journal of Qualilty in Health Care 2012;24(1):28-38. [DOI] [PubMed] [Google Scholar]
Moore 2017
- Moore L, Britten N, Lydahl D, Naldemirci O, Elam M, Wolf A. Barriers and facilitators to the implementation of person-centred care in different healthcare contexts. Scandinavian Journal of Caring Sciences 2017;31(4):662-73. [DOI: 10.1111/scs.12376] [DOI] [PMC free article] [PubMed] [Google Scholar]
National Patient Safety Foundation 2014
- National Patient Safety Foundation’s Lucian Leape Institute. Safety Is Personal: Partnering with Patients and Families for the Safest Care. National Patient Safety Foundation; Boston, USA (2014). Available from www.ihi.org/resources/Pages/Publications/Safety-Is-Personal-Partnering-with-Patients-and-Families-for-the-Safest-Care.aspx.
Nelson 1998
- Nelson G, Ochocka J, Griffin K, Lord J. ‘Nothing about me, without me': participatory action research with self-help/mutual aid organizations for psychiatric consumer/survivors. American Journal of Community Psychology 1998;26(6):881-912. [DOI: 10.1023/A:1022298129812] [DOI] [PubMed] [Google Scholar]
Nilsen 2006
- Nilsen ES, Myrhaug HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database of Systematic Reviews 2006, Issue 3. Art. No: CD004563. [DOI: 10.1002/14651858.CD004563.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ocloo 2016
- Ocloo J, Matthews R. From tokenism to empowerment: progressing patient and public involvement in healthcare improvement. BMJ Quality & Safety 2016;25(8):626-32. [DOI: 10.1136/bmjqs-2015-004839] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ocloo 2021
- Ocloo J, Garfield S, Dean Franklin B, Dawson S. Exploring the theory, barriers and enablers for patient and public involvement across health, social care and patient safety: a systematic review of reviews. Health Res Policy Systems 2021;19(1):8. [DOI: 10.1186/s12961-020-00644-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ogden 2017
- Ogden K, Barr J, Greenfield D. Determining requirements for patient-centred care: a participatory concept mapping study. BMC Health Services Research 2017;17(1):780. [DOI: 10.1186/s12913-017-2741-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ottmann 2011
- Ottmann G, Laragy C, Allen J, Feldman P. Coproduction in practice: participatory action research to develop a model of community aged care. Systemic Practice and Action Research 2011;24(5):413-27. [DOI: 10.1007/s11213-011-9192-x] [DOI] [Google Scholar]
Palmer 2016
- Palmer VJ, Piper D, Richard L, Furler J, Herrman H, Cameron J, et al. Balancing opposing forces – a nested process evaluation study protocol for a stepped wedge designed cluster randomized controlled trial of an experience based codesign intervention: the CORE study. International Journal of Qualitative Methods 2016;15(1):1609406916672216. [DOI: 10.1177/1609406916672216] [DOI] [Google Scholar]
Pelletier 2011
- Pelletier J-F, Lesage A, Delorme A, Macaulay A C, Salsberg J, Valle C & Davidson L. User-led research: a global and person-centered initiative. International Journal of Mental Health Promotion 2011;13(1):4-12. [DOI: 10.1080/14623730.2011.9715645] [DOI] [Google Scholar]
Picker Institute 1987
- Picker Institute. Principles of Patient-Centred Care. Bethesda (MD): Picker Institute, 1987. [Google Scholar]
Rathert 2012
- Rathert C, Wyrwich MD, Boren SA. Patient-centered care and outcomes: a systematic review of the literature. Medical Care Research Reviews 2012;70(4):351-79. [DOI] [PubMed] [Google Scholar]
Repper 2007
- Repper J, Breeze J. User and carer involvement in the training and education of health professionals: a review of the literature. International Journal of Nursing Studies 2007;44(3):511-9. [DOI: 10.1016/j.ijnurstu.2006.05.013] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ryan 2013
- Ryan R, Hill S, Prictor M, McKenzie J, Cochrane Consumers and Communication Group. Study quality guide. cccrg.cochrane.org/author-resources (accessed 24 July 2018).
Ryan 2016
- Ryan R, Horey D, Oliver S, McKenzie J, Prictor M, Santesso N, et al. Cochrane Consumers and Communication Group standard protocol text and additional guidance for review authors. cccrg.cochrane.org/author-resources (accessed 24 July 2018).
Sanders 2008
- Sanders EBN, Stappers PJ. Co-creation and the new landscapes of design. CoDesign 2008;4(1):5-18. [DOI: 10.1080/15710880701875068] [DOI] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sharma 2015
- Sharma T, Bamford M, Dodman D. Person-centred care: an overview of reviews. Contemporary Nurse 2015;51(2-3):107-20. [DOI: 10.1080/10376178.2016.1150192] [DOI] [PubMed] [Google Scholar]
Shields 2012
- Shields L, Zhou H, Pratt J, Taylor M, Hunter J, Pascoe E. Family-centred care for hospitalised children aged 0 to 12 years. Cochrane Database of Systematic Reviews 2012, Issue 10. Art. No: CD004811. [DOI: 10.1002/14651858.CD004811.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stacey 2017
- Stacey D, Legaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD001431. [DOI: 10.1002/14651858.CD001431.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2008
- Stone S. A retrospective evaluation of the impact of the Planetree patient-centered model of care on inpatient quality outcomes. Health Environments Research & Design 2008;1(4):55–69. [DOI] [PubMed] [Google Scholar]
Synnot 2018
- Synnot A, Bragge P, Lowe D, Nunn J, O'Sullivan M, Horvat L, et al. Research priorities in health communication and participation: international survey of consumers and other stakeholders. BMJ Open 2018;8:e019481. [DOI: 10.1136/bmjopen-2017-019481] [DOI] [PMC free article] [PubMed] [Google Scholar]
Synnot 2019
- Synnot A, Tong A, Bragge P, Lowe D, Nunn JS, O'Sullivan M, et al. Selecting, refining and identifying priority Cochrane Reviews in health communication and participation in partnership with consumers and other stakeholders. Health Research Policy and Systems 2019;17(1). [DOI: 10.1186/s12961-019-0444-z] [DOI] [PMC free article] [PubMed]
Tempfer 2011
- Tempfer C, Nowak P. Consumer participation and organizational development in health care: a systematic review. Wiener Klinische Wochenschrift 2011;123:408-14. [DOI: 10.1007/s00508-011-0008-x] [DOI] [PubMed] [Google Scholar]
Tritter 2003
- Tritter J, Barley V, Daykin N, Evans S, McNeill J, Rimmer J, et al. Divided care and the third way: user involvement in statutory and voluntary sector cancer services. Sociology of Health & Illness 2003;25:429-56. [DOI: 10.1111/1467-9566.00353] [DOI] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization (WHO). Building a working definition of a partnership. www.who.int/patientsafety/implementation/apps/resources/defining_partnerships-apps.pdf (accessed 24 July 2018).
Wildridge 2004
- Wildridge V, Childs S, Cawthra L, Madge B. How to create successful partnerships – a review of the literature. Health Information and Libraries Journal 2004;21(Suppl 1):3-19. [DOI: 10.1111/j.1740-3324.2004.00497.x] [DOI] [PubMed] [Google Scholar]
Wolf 2017
- Wolf A, Moore L, Lydahl D, Naldemirci O, Elam M, Britten N. The realities of partnership in person-centred care: a qualitative interview study with patients and professionals. BMJ Open 2017;7(7):e016491. [DOI: 10.1136/bmjopen-2017-016491] [DOI] [PMC free article] [PubMed] [Google Scholar]