Abstract
The coronavirus disease (COVID)-19 pandemic has affected millions worldwide with prevention efforts culminating in the development of a vaccine. An mRNA vaccine, developed by Moderna (Cambridge, MA, USA), mounts an immunologic response leading to antibody neutralization. Commonly reported vaccine side effects include myalgia, fever, and chills, with low reported rates of cardiovascular events. This case demonstrates the development of takotsubo syndrome (TTS) after administration of the COVID-19 vaccine. A 73-year-old woman with recently diagnosed myocardial infarction with no obstructive coronary atherosclerosis (MINOCA) presented with typical chest pain starting less than a day after receiving the Moderna vaccine. She had troponin elevations and new ST-segment abnormalities. Transthoracic echocardiogram (TTE) findings were consistent with mid-ventricular TTS. Treatment included diuretics, beta-blockers, and angiotensin receptor blockers. Prior to discharge, repeat imaging showed improvement in systolic function. This case presents a post-menopausal woman with a recent diagnosis of MINOCA who developed TTS shortly after receiving the COVID-19 vaccine. Risk factors including sex, age, MINOCA, anxiety about the vaccine, and possibly the vaccine itself may have all contributed to the TTS presentation. TTS may occur after COVID-19 vaccination, and appreciation of this potential rare association is important for evaluating vaccine safety and optimizing patient outcomes.
<Learning objective: Takotsubo syndrome (TTS) has been associated with multiple predisposing factors. We present a case of TTS which developed shortly after receipt of an mRNA COVID-19 vaccine. We discuss contributing factors, diagnosis, and treatment of TTS.>
Keywords: Takotsubo cardiomyopathy, Takotsubo syndrome, Stress-induced cardiomyopathy, Moderna, COVID-19, Vaccine, Case report
Introduction
The coronavirus (COVID)-19 pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has negatively affected millions worldwide. To combat the COVID-19 impact, an mRNA vaccine developed by Moderna (Cambridge, MA, USA) mounts an immunologic response which significantly reduces clinical infection rates. Moderna's commonly reported vaccine side effects include muscle pain, fatigue, and headache, with low reported rates of cardiovascular side effects [1]. This case describes the occurrence of takotsubo syndrome (TTS) shortly after receiving the Moderna vaccine in a patient with a prior diagnosis of myocardial infarction with no obstructive coronary atherosclerosis (MINOCA).
Case Report
A 73-year-old woman with history of hypertension and chronic kidney disease presented to our hospital with non-radiating, pressure-like dull chest pain with activity and at rest. Initial evaluation included mild troponin T elevation at 0.1 ng/mL (normal <=0.03 ng/mL) and sinus rhythm without abnormalities on electrocardiogram. Transthoracic echocardiogram (TTE) demonstrated normal left ventricle (LV) wall motion and ejection fraction (EF) of 65%. Coronary angiogram revealed normal coronary arteries, leading to a diagnosis of MINOCA (Fig. 1). She was discharged home on isosorbide mononitrate and atorvastatin with plan for medical management of suspected coronary vasospasm or microvascular dysfunction. Coronary functional testing or provocative testing to evaluate coronary vasospasm was not performed.
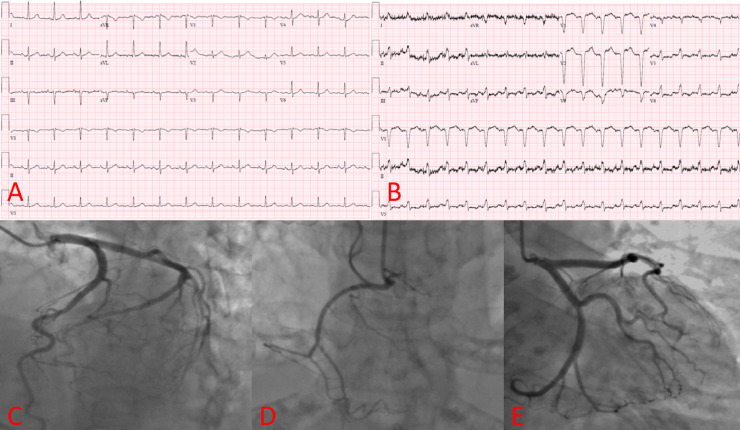
Fig. 1.
Electrocardiogram (ECG) and coronary angiography. ECG (A) noting normal sinus rhythm two months prior to takotsubo syndrome admission in comparison to new ST-T wave changes on ECG (B) depicting inferior and anterolateral infarction. (C-E) Different angiographic views demonstrating no evidence of obstructive coronary artery disease in the left main artery, left anterior descending artery, left circumflex artery, and right coronary artery.
Additional relevant past medical history included rheumatoid arthritis controlled on hydroxychloroquine, acid reflux, asthma, presumed viral pericarditis in 2018 without residual symptoms, and hepatocellular carcinoma with resection in 2017. Other home medications included furosemide, losartan, aspirin, and diltiazem.
She did well until about two months later. At that time, she received the Moderna COVID-19 vaccine and noted acute onset of chest pressure with radiation to the back associated with shortness of breath about 17 h after vaccination. She also had progressive dyspnea on exertion, orthopnea, nausea, vomiting, and fatigue over the next two days. She reported anxiety prior to getting the vaccine. On presentation to the emergency room, vital signs demonstrated a pulse of 118 bpm, blood pressure of 108/57 mmHg, and respiration rate 24 breaths/min; she was afebrile with normal oxygen saturation on room air. Other than jugular venous distention, the remainder of the examination was unremarkable.
Laboratory results revealed troponin elevation of 0.22 ng/mL, baseline creatinine 2.0 mg/dl (ref 0.7–1.3), pro-brain natriuretic peptide of >70000 pg/mL (ref 30–125), and pro-calcitonin 0.61 ug/L (ref 0.00–0.15 ug/L), but with no findings to suspect infection. Electrocardiogram had ST wave changes concerning for infero lateral ischemia and new poor anterior R wave progression (Fig. 1). Differential diagnosis included acute myocardial infarction (MI), coronary artery spasm, acute heart failure exacerbation, acute pulmonary embolism, and TTS. She was admitted for presumptive diagnosis of non-ST elevation myocardial infarction (NSTEMI).
Continuous intravenous heparin for possible NSTEMI and intravenous furosemide for clinical heart failure was initiated. TTE demonstrated mid-ventricular ballooning of the LV, EF 20% with a grade I diastolic dysfunction (E/A: 0.87, E/e': 8.40, tricuspid regurgitation max velocity: 2.20 m/s, right atrial pressure: 15 mmHg, right ventricular systolic pressure or pulmonary arterial systolic pressure: 35 mmHg), and severe right ventricular dysfunction associated with functional severe tricuspid regurgitation (Fig. 2). Only mild mitral regurgitation was seen on TTE.
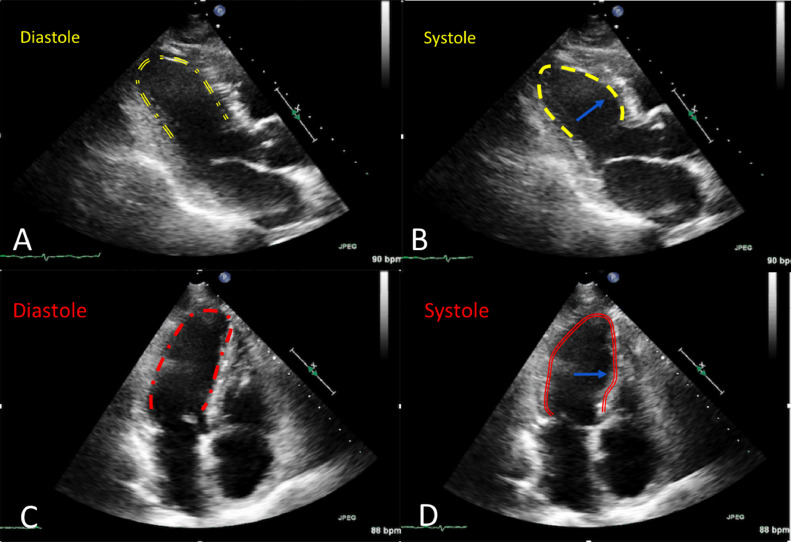
Fig. 2.
Transthoracic echocardiography. Parasternal long-axis 2-chamber views (A,B) and apical 4-chamber views (C,D) showing end-systolic dyskinesis (blue arrow) of the mid-left ventricle consistent with mid-ventricular takotsubo syndrome.
As a result, heparin was discontinued and the patient was treated for acute systolic heart failure from presumed TTS with ongoing intravenous diuresis, metoprolol succinate 25 mg daily, and losartan 25 mg daily. Given recent coronary angiography without evidence of significant coronary artery disease, TTE results not consistent with a coronary distribution, low troponin elevation, elevated creatinine, and clinical presentation, an angiogram was not repeated.
Repeat TTE three days after presentation showed mild improvement in biventricular function with an EF of 35–40%. Cardiac magnetic resonance imaging performed on hospital day seven demonstrated mild LV mid-myocardial hypokinesis without evidence of delayed enhancement or myocarditis (Fig. 3). Overall, her symptoms of heart failure resolved by discharge from the hospital on day eight.
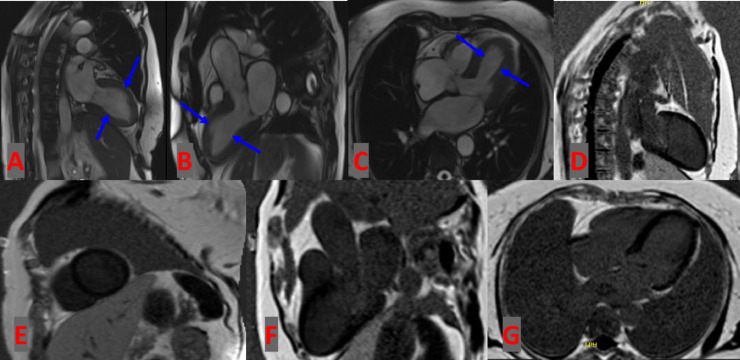
Fig. 3.
Cardiac magnetic resonance imaging. (A-C) Steady-state free precession with 2-, 3-, and 4-chamber cardiac views showing subtle hypokinesis (blue arrow) of the mid-ventricular wall with normal ejection fraction. (D-G) Late gadolinium enhancement with delayed enhancement phase-sensitive inversion recovery imaging showing no evidence of myocardial infarct, consistent with takotsubo syndrome.
Discussion
We present a case of a 73-year-old woman with mid-ventricular wall TTS shortly after receiving the Moderna COVID-19 vaccination in setting of a prior diagnosis of MINOCA due to suspected coronary macro- or microvascular vasospasm with microvascular dysfunction. Although association does not describe causality, this case may increase awareness of a possible correlation between the COVID-19 vaccine and TTS.
TTS typically involves transient wall motion abnormalities of the LV apex, mid-ventricle, or other myocardial distribution not commonly supplied by a single coronary artery. It is a syndrome that disproportionately affects postmenopausal women, attributed to estrogen deficiency, and usually triggered by intense emotional or physical stress [2,3]. The possibility of stress-induced cardiovascular findings in response to the COVID vaccine has been previously described in relation to anxiety towards the vaccine side effects [4]. In the present case, our patient also reported significant anticipatory stress prior to the COVID-19 vaccination.
The etiology of TTS is not well characterized, but the pathogenesis suggests neural activation with norepinephrine and epinephrine release from adrenal glands and sympathetic nerves leading to circulating catecholamine-mediated microvascular dysfunction, as well as myocardial stunning, injury, and elevated cardiac workload [5]. This can occur in the absence of obstructive epicardial coronary artery disease and can mimic other myocardial infarction presentations. MINOCA and TTS may have connection due to overlapping underlying pathophysiology of vasospasm due to endothelial dysfunction or microvascular disease (MVD) and a hyper-reactivity of the vascular smooth muscle cells of the coronary artery [6,7]. Therefore, the patient's previous history of MINOCA either due to vasospasm or MVD may have increased her susceptibility of having TTS mediated via diffuse regional microvascular dysfunction in response to emotional stress and anxiety.
Most patients with TTS experience rapid LV recovery although early clinical course may not be benign due to acute heart failure, thrombotic complications, arrhythmias, and death [8]. Typical management includes treatment of causal contributors and symptom management. Anti-catecholamine therapies such as beta-blockers (BB) have been suggested given the pathophysiology of TTS although there are no prospective studies suggesting optimal medical therapy. Likewise, angiotensin-converting enzyme inhibitors (ACE-i) or angiotensin II type 1 receptor blockers (ARB) may be considered as treatment options in the setting of LV dysfunction, especially since these agents have been postulated to be beneficial in the management of MINOCA [5]. In this case, patient management included IV furosemide given clinical heart failure and initiation of ARB and BB for neuro-hormonal blockade.
ACE2, the receptor for SARS-CoV-2 virus, known to be expressed in myocytes and vascular endothelium, could be an underlying mechanism for direct myocardial injury in COVID-19 disease [9]. However, the Moderna mRNA vaccine would not be expected to have similar cardiovascular effects. In published trials of the Moderna vaccine, less than 0.1% reported severe adverse cardiovascular effects such as congestive heart failure, bradycardia, atrial fibrillation, hypertension, acute myocardial infarction, and acute coronary syndrome [1]. Whether any of these cardiovascular effects potentially represented TTS has not been further reported.
There are limited data regarding the association of other vaccinations with TTS. Prior reports describe a possible association between influenza vaccine and TTS. The vaccine may induce a systemic inflammatory reaction that may cause an increased catecholamine level or an increased myocardial sensitization to catecholamine [10]. In susceptible patients, this response may lead to an imbalance in the cardio-sympathetic system with an adrenergic predominance leading to TTS [10]. While other markers of inflammation were not checked during the presentation, a mildly elevated procalcitonin may represent an inflammatory state. Overall, this patient did not have any other signs or symptoms consistent with systemic inflammation suggestive of infection.
Upon full review, it is possible that a combination of significant risk factors such as female gender, postmenopausal status, anxiety about receiving the vaccine, prior MINOCA diagnosis, and the effects of the COVID-19 vaccination itself all contributed to the development of TTS. In this case, it remains difficult to establish a single causal relationship between these individual contributors, including the vaccine, to the development of TTS. Nevertheless, this case highlights rare adverse clinical consequences following Moderna mRNA vaccination in susceptible patients. While likely extremely rare, TTS may occur after COVID-19 vaccination. Ongoing monitoring of cardiovascular effects possibly associated with vaccination is important for evaluating vaccine safety and optimizing patient outcomes.
Declaration of Competing Interest
The authors declare that there is no conflict of interest.
References
- 1.Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O'Dell S, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurisu S, Kihara Y. Clinical management of takotsubo cardiomyopathy. Circ J. 2014;78:1559–1566. [PubMed] [Google Scholar]
- 3.Amin HZ, Amin LZ, Pradipta A. Takotsubo cardiomyopathy: a brief review. J Med Life. 2020;13:3–7. doi: 10.25122/jml-2018-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakamoto T, Kagawa Y, Endo A, Tanabe K. Intense emotional stress over potential coronavirus disease vaccination side effects leads to takotsubo cardiomyopathy. Circ Rep. 2021;3:476–477. doi: 10.1253/circrep.CR-21-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattar Y, Siew KSW, Connerney M, Ullah W, Alraies MC. Management of takotsubo syndrome: a comprehensive review. Cureus. 2020;12:e6556. doi: 10.7759/cureus.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennan A, Adams H, Galligan J, Whitbourn R. Coronary vasospasm and concurrent takotsubo cardiomyopathy. Br J Cardiol. 2019;26:38–40. [Google Scholar]
- 7.Scalone G, Niccoli G, Crea F. Editor's choice - Pathophysiology, diagnosis and management of MINOCA: an update. Eur Heart J Acute Cardiovasc Care. 2019;8:54–62. doi: 10.1177/2048872618782414. [DOI] [PubMed] [Google Scholar]
- 8.Ghadri JR, Kato K, Cammann VL, Gili S, Jurisic S, Di Vece D, Candreva A, Ding KJ, Micek J, Szawan KA, Bacchi B, Bianchi R, Levinson RA, Wischnewsky M, Seifert B, et al. Long-term prognosis of patients with takotsubo syndrome. J Am Coll Cardiol. 2018;72:874–882. doi: 10.1016/j.jacc.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Shah RM, Shah M, Shah S, Li A, Jauhar S. Takotsubo syndrome and COVID-19: associations and implications. Curr Probl Cardiol. 2021;46 doi: 10.1016/j.cpcardiol.2020.100763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh K, Marinelli T, Horowitz JD. Takotsubo cardiomyopathy after anti-influenza vaccination: catecholaminergic effects of immune system. Am J Emerg Med. 2013;31:1627. doi: 10.1016/j.ajem.2013.06.039. e1-4. [DOI] [PubMed] [Google Scholar]