Abstract
Human mutations and haploinsufficiency of the SHANK family genes are associated with autism spectrum disorders (ASD) and intellectual disability (ID). Complex phenotypes have been also described in all mouse models of Shank mutations and deletions, consistent with the heterogeneity of the human phenotypes. However, the specific role of Shank proteins in synapse and neuronal functions remain to be elucidated. Here, we generated a new mouse model to investigate how simultaneously deletion of Shank1 and Shank3 affects brain development and behavior in mice. Shank1–Shank3 DKO mice showed a low survival rate, a developmental strong reduction in the activation of intracellular signaling pathways involving Akt, S6, ERK1/2, and eEF2 during development and a severe behavioral impairments. Our study suggests that Shank1 and Shank3 proteins are essential to developmentally regulate the activation of Akt and correlated intracellular pathways crucial for mammalian postnatal brain development and synaptic plasticity. Therefore, Akt function might represent a new therapeutic target for enhancing cognitive abilities of syndromic ASD patients.
Subject terms: Neuroscience, Cell biology
Introduction
Shank family members are multidomain scaffold proteins located at the postsynaptic density (PSD) of glutamatergic synapses [1–3] where they interact directly or indirectly with scaffolding and signaling proteins [4, 5]. It has been proposed that through these interactions Shank proteins regulate synapse development, spine structure, and synaptic plasticity [6–8]. Deletions, duplications, and coding mutations in all of the three SHANK genes are presented in several patients with syndromic autism spectrum disorders and intellectual disability (ID) with higher severity when the mutations are present in the SHANK3 and SHANK2 gene compare to SHANK1 [9]. This strongly suggests the importance of Shank proteins in modulating excitatory synaptic signaling that are essential for neuronal functions.
Even if structurally very similar, the three proteins show distinct molecular properties in the modality of postsynaptic targeting and assembly. Indeed the in vitro studies described that Shank2 and Shank3 appear early in forming synapses, and only after a sufficient strengthening of the Shank2/Shank3 scaffold leads to the postsynaptic clustering of Shank1 and maturation of the synaptic contacts [10]. These data suggest that Shank2 and Shank3 play a similar role in forming the glutamatergic synapses while Shank1 seems more important in facilitating synaptic maturation [10].
To clarify the role of Shank proteins in vivo, different mouse models with one gene deleted out of the three genes have been generated and extensively studied. In general, the deletion of Shank1 has a milder phenotype compared to the deletion of either Shank2 or Shank3, suggesting again that Shank2 and Shank3 are more similar to each other compared to Shank1 [11–13].
On the other hand, in the single-gene mutant mice compensation of the remaining genes might cover the major function of Shank proteins at glutamatergic synapses. Ideally, to fully characterize the function of Shank proteins, the three genes should be deleted simultaneously, yet it is a time-consuming experiment and it might result lethal at the embryonic stage. We believe that similar investigations can be unraveled by the deletion of two of the three genes in different combination. Considering the possible different functions between Shank2/3 and Shank1, the best strategy will be to delete Shank2 and Shank3 or one between Shank2 and Shank3 in combination with Shank1. We first decided to delete Shank1 plus Shank3, because deletion of Shank3 results in a stronger phenotype than Shank2.
We found that Shank1–Shank3 double deletion (DKO) strongly impairs postnatal brain development leading to morphological and synaptic defects in cortical and hippocampal neurons. Shank1–3 DKO mice showed a strong developmental reduction in the activation of intracellular signaling pathways involving Akt, S6, ERK1/2, and eEF2 without major changes in the composition of the synaptic glutamate receptors. These developmental molecular alterations are associated to severe behavioral impairment in adults that recapitulates syndromic ASDs. Interestingly we found that cotinine treatment during postnatal day (P) P14-P30 was able to rescue morphological and behavioral defects by restoring Akt phosphorylation.
Materials
Mice generation
Shank1−/−/Shank3−/− double KO (DKO) mice were generated by crossing Shank1 [14] and Shank3Δ11−/− mice [15, 16] single KO mice to obtain double heterozygous mice that were crossed generating Shank1−/−/Shank3−/− DKO. They were housed under constant temperature (22 ± 1 °C) and humidity (50%) conditions with a 12 h light/dark cycle and provided with food and water ad libitum. All animal experiments were performed in compliance with the guidelines of the Italian Ministry of Health and Federal Government of Germany. Every effort was made to minimize the number of mice used and their suffering. PCR genotyping of Shank1−/−/Shank3−/− was performed using the following sets of oligonucleotide primers: Shank1−/− for WT allele forward 5′-AAGGGCCAGCTCATTCCTCC-3′, for mutant allele forward 5′-GCACCGGCTAGCCCAGTCAG-3′, reverse 5′-GGCCAACCTTCACTACATTCTGTC-3′ for both WT and mutant allele; Shank3Δ11−/− for WT allele forward 5′-CAAGTTCATCGCTGTGAAGG-3′, for mutant allele forward 5′-CCTCTAGGCCTGCTAGCTGTT-3′ reverse 5′-AAGAAGCCCCAGAAGTGACA-3′ for both WT and mutant allele. For monitoring the survival following cotinine treatment, separate cohorts of mice weaned into standard vivarium cages were treated or not with cotinine daily (from P15 to P30) and monitored for survival until P30 while daily health surveillance was conducted.
Golgi staining
Mice were perfused transcardially with 0.9% NaCl in distilled water. The brain was taken and immersed in the impregnation solution, made by equal volumes of solution A and solution B (FD Rapid GolgiStainTM Kit, cat PK401) for 1–2 weeks in the dark, at room temperature. The mixture of solution A and B should be prepared at least 24 h before the impregnation and stored in the dark. After the period of impregnation, the brain was immersed in solution C (FD Rapid GolgiStainTM Kit, cat PK401) and stored at 4 °C, in the dark, for at least 72 h. The next day solution C was replaced.
The brain was cut with a vibratome setting frequency at 7.5, speed at 1.5, and slice thickness at 100 μm. Slices were put on gelatinized slides 2% gelatin (Merck), 1% KCr(SO4)12H2O (Carlo Erba). Slices were stained in the dark as described in the manual Kit. Finally, slides were mounted on coverslips with Entellam (Electron Microscopy Sciences). Images were taken with 25X and 63X objectives in a white field. Neurons were analyzed with Neuron Studio software to perform Sholl analysis of branching points and to count spines number. We analyzed neurons from hippocampal CA1 and CA3 regions and from all layers of somatosensory cortex.
Electron Microscopy
Mice were anesthetized by intraperitoneal injection of 10 mg/mL avertine and transcardially perfused with 2.5% glutaraldehyde, 2% paraformaldehyde in 0.15 M sodium cacodylate buffer pH 7.4. Dissected brains were post-fixed for additional 24 h at 4 °C. Coronal sections (100 µm thickness) were obtained with a vibratome (Leica VT1000S), and hippocampi and cortex were manually dissected. After washing, samples were post-fixed with 2% osmium tetroxide, rinsed, stained with 1% uranyl acetate in water for 45 min, dehydrated, and embedded in epoxy resin (Electron Microscopy Science, Hatfield, PA, USA) that was baked for 48 h at 60 °C. Thin sections were obtained with an ultramicrotome (Leica Microsystems, Austria) and observed under a Philips CM10 transmission electron microscopy (TEM) (FEI, Eindhoven, the Netherlands). For quantitative analyses, images were acquired at a final magnification of 25–34 000 using a Morada CDD camera (Olympus, Munster, Germany).
For quantitative analysis of TEM images, the following values were evaluated: length and thickness of the PSDs and number of synapses. Images were analyzed with ImageJ1.47 v (NIH Image) by a blinded independent investigator. All data were presented as mean ± SEM. For each group of data, an Ftest for the comparison of the variances was used before the statistical analysis.
Biochemistry
Cortex and hippocampi were dissected from mouse brain of both sexes. Tissues were homogenized in buffer containing 10 mM Hepes pH 7.4, 2 mM EDTA, protease inhibitors (Roche), and phosphatase inhibitors (Sigma, P8340). Samples were centrifuged at 500 × g for 5 min at 4 °C.
Resulting supernatants were centrifuged at 10,000 × g for 15 min at 4 °C. After the centrifugation, pellets were resuspended in buffer composed by 50 mM Hepes pH 7.4, 2 mM EDTA, 2 mM EGTA, 1% triton-X-100 (Sigma), protease inhibitors, and phosphatase inhibitors and centrifuged at 20,000 × g for 80 min at 4 °C. Finally, pellets were resuspended in buffer containing 50 mM Tris pH 9, 1% sodium deoxicholate (Sigma, D6750). Equal amounts (7 µg) of each sample were separated using SDS-PAGE and subsequently blotted on nitrocellulose membranes according to standard protocols. Incubation with a primary antibody (β-actin, Sigma, cat. A5316; Homer1b/c, Santa Cruz Biotechnology, cat. H-342; mGluR5, Millipore, cat. AB5675; Shank3, Santa Cruz Biothecnology, cat. H-160; β3-tubulin, Sigma, cat. T8578; Arc, Santa Cruz Biotechnology, cat C-8; pERK1/2 Cell Signaling, cat. 9101S; ERK, Cell Signaling, cat. 9102 S; peEF2, Cell Signaling, cat. 2331S; eEF2, Cell Signaling, cat. 2332S; p-AKT, Cell Signaling, cat. 4060S; AKT, Cell Signaling, cat. 2920S; pS6, Cell Signaling, cat. 2215S; S6, Cell Signaling, cat. 22175; PSD-95, Neuromab, cat. 75-028; GluA1, Millipore, cat. AB1504; GluA2, Neuromab, cat. 75-002; GluN1, Neuromab, cat. 75-272; GluN2A, Neuromab, cat. 75-288; GluN2B, Neuromab, cat. 75097; GABA-A-R-alpha1, Neuromab, cat. 75136; GAD65, Synaptic Systems, cat. 198111) was followed by treatment of the membrane with HRP-conjugated secondary antibodies (swine anti-rabbit, 1:5000 or rabbit anti-mouse, 1:5000; both Dako, Hamburg, Germany), and the signal was visualized using Pierce ECL Western Blotting Substrate and further detected using a MicroChemi 4.2 machine. All signals were quantified using Gel analyzer software (www.gelanalyzer.com/) and normalized against the values of the respective signal for β-actin or β3-tubulin.
Behavioral analysis
Animals were housed in groups of four or five individuals of mixed genotypes. All of the tests were conducted during the light portion of the cycle, except for nest building test. To minimize the number of animals each mouse was submitted to a maximum of three tests with an interval of 1 week between two tests. During the test manually recorded the experimenter was blind to genotype and treatment.
Sociability and preference for social novelty tests
The apparatus was a rectangular, three-chamber, transparent polycarbonate box (width = 42.5 cm, height = 22.2 cm, center chamber length = 17.8 cm, and side chamber lengths = 19.1 cm). The test was performed as previously described [15]. For both tests were evaluated a sociability index (time exploring novel mouse 1 − time exploring novel object)/(time exploring novel mouse 1 + time exploring novel object) and a social novelty preference index = (time exploring novel mouse 2 − time exploring familiar mouse)/(time exploring novel mouse 2 + time exploring familiar mouse).
Nest building
The nest building test was performed in the home cage led with 0.5 cm bedding according to Deacon et al. [17]. Each cage was supplied with a “Nestlet”, a 5 cm square of pressed cotton batting, 1 h before the dark phase for four consecutive days. Results were assessed the next morning. The nests were assessed on a 5-point scale. Data were expressed as mean score of 4-day evaluation for each mouse.
Repetitive self grooming
Spontaneous repetitive self-grooming behavior was scored as previously described [18]. Each mouse was individually placed into a standard cylinder (46 cm length × 23.5 cm wide × 20 cm high). Cylinders were empty to eliminate digging in the bedding, which is a potentially competing behavior. The room was illuminated at ~40 lux. A front-mounted CC TV camera (Security Cameras Direct) was placed at ~1 m from the cages to record the sessions. Sessions were videotaped for 20 min. The first 10 min of habituation was not scored. Cumulative time spent grooming all the body regions during the second 10 min of the test session and the total number of grooming episodes was measured.
Rotarod
The rotarod apparatus (Ugo Basile, Biological Research Apparatus, Varese, Italy) was used to measure fore and hindlimb motor coordination and balance. During the training period, each mouse was placed on the rotarod at a constant speed (12 rpm) for a maximum of 120 s, and the latency to fall off the rotarod within this time period was recorded. Mice received four trials per day for 4 consecutive days. The fourth trial of each day was evaluated for statistical analysis.
Balance beam walking
The beam apparatus consisted of 1 m beams with a flat surface of 12 mm or 6 mm width resting 50 cm above the table top on two poles. A black box was placed at the end of the beam as the finish point. Nesting material from home cages was placed in the black box to attract the mouse to the finish point. A lamp (with 60 W light bulb) was used to shine light above the start point and served as an aversive stimulus. A video camera was set on a tripod to record the performance. On training days, each mouse crossed the 12 mm beam three times and then the 6 mm beam three times. The time required to cross to the escape box at the other end (80 cm away) was measured with a stopwatch. The stopwatch started when the nose of the mouse began to cross the beam, and stopped when the animal reaches the escape box. Once the mice are in the safe box, they are allowed some time (~15 s) to rest there. Before the next trial the mice rest for 10 min in their home cages between training sessions on the two beams. On the test day, times to cross each beam were recorded. Two successful trials in which the mouse did not stall on the beam are averaged. The beams and box were cleaned of mouse droppings and wiped with towels soaked with 70% ethanol and then water before the next beam was placed on the apparatus.
Pole test
In the vertical pole task, the mouse was placed on a vertical wire-mesh pole with its head facing upwards. Mice were habituated to the task in two trials per day for 2 days. On test day (third day) mice were subjected to five trials: the total time taken to turn the body and to descend was recorded according to Hickey et al. [19]. A cutoff of 60 s was given. Data were shown as mean of five trials evaluated during the test day.
T-maze test
Mice were deprived of food until they reached 90% of their free-feeding body weight. They were habituated to a black wooden T-maze (with a 41-cm stem section and a 91-cm arms section). Each section was 11 cm wide and had walls that were 19 cm high. The mice were habituated to the T-maze and trained to obtain food within the maze for 5 days as previously described [20]. During the acquisition phase, one arm was designated the reinforcer (Coco Pops; Kellogg’s) in each of ten daily trials. Each mouse was placed at the start of the maze and allowed to freely choose which arm to enter. The number of days required to reach the goal criterion (80% correct for 3 days) was recorded. Each mouse that met the goal for acquisition was then tested using a reversal procedure in which the reinforcer was switched to the opposite arm. A cutoff of 20 days in both acquisition and reversal phase was established.
Morris water maze test
The Morris water maze test was used to analyze changes in the learning and memory abilities of the mice according to the methods described in Morris [21] (adapted for mice). A circular water maze (120 cm in diameter × 50 cm in height) was used. A circular hidden platform with a diameter of 10 cm was placed inside the maze, and its surface was maintained at 0.5 cm below the surface of the water. Floating plastic particles were placed on the surface of the water to hide the platform from sight according to the methods of Zhang et al. [22]. For the habituation trials, the mice were placed in a random area inside the maze and allowed to swim for 60 s. For the acquisition trials, the mice were submitted to 4 trials per day (with 60 min inter-trial intervals) for 4 consecutive days during which each mouse was released into the pool at different starting points and trained to locate a constant platform position. At 24 h after the last trial, a probe test was performed during which the platform was removed. Two days later, a reversal task was performed to assess cognitive flexibility. The platform was placed in the opposite quadrant of the tank, and 4 daily trials were performed for 4 days. On the fifth day, a probe trial was performed that was similar to that in the acquisition phase. The time spent in the target area and the latency to reaching the target zone were evaluated by an experimenter who was blind to the genotypes of the mice.
Novel object recognition test
The novel object recognition test was performed over a 2-day period in an open plastic arena (60 × 50 × 30 cm). Animals were habituated to the test arena for 10 min on the first day. After 1-day habituation, mice were subjected to familiarization (T1) and novel object recognition (T2). During the initial familiarization stage, two identical objects were placed in the center of the arena equidistant from the walls and from each other. Each mouse was placed in the center of the arena between the two objects for ≤20 min or until it had completed 30 s of cumulative object exploration. Object recognition was scored when each animal was within 0.5 cm of an object with its nose toward the object. Exploration was not scored if a mouse reared above the object with its nose in the air or climbed on an object. Mice were returned to the home cage after familiarization and then tested again after different delays (from 5 min to 24 h later). A novel object (never seen before) took the place of one of the two familiars. Scoring of object recognition was performed in the same manner as during the familiarization phase. From mouse to mouse the role (familiar or new object) as well as the relative position of the two objects were counterbalanced and randomly permuted. The objects for mice to discriminate consisted of white plastic cylinders and colored plastic Lego stacks of different shapes. Data were expressed as discrimination index [(time spent exploring novel object − time exploring familiar object)/(time spent exploring novel object + time exploring familiar object)].
Electrophysiology
For hippocampal recordings mice were anesthetized with 2-bromo-2-chloro-1,1,1-trifluoroethane before decapitation. The brains were dissected and immersed in ice-cold artificial cerebrospinal fluid (aCSF) containing (in mol/L): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 1.2 NaH2PO4, 2.4 CaCl2, 10 glucose, and 25 NaHCO3, continuously bubbled with 95% O2 and 5% CO2, pH 7.4. Transverse hippocampal slices (400 μm or 220 μm thick for extracellular or patch-clamp recordings, respectively) were cut by using a vibratome and were allowed to recover in oxygenated aCSF at 30 °C for 30 min, and then at room temperature for another 1–2 h before experimental recordings. Each slice was then transferred into a recording chamber and submerged in aCSF at a constant flow rate (2.9–3 mL/min, T = 29 °C), bubbled with a 95% O2–5% CO2 gas mixture. For extracellular recordings, a stimulating electrode was inserted into the Schaffer collateral fibers or the perforant pathway, and a recording glass electrode filled with a 2 mol/L NaCl solution into the CA1 region or the granule layer of the dentate gyrus of the hippocampal slice. The field excitatory postsynaptic potentials (fEPSP) slope or the population spikes amplitude were evoked every 10 s and measured in the CA1 and DG region, respectively. Paired-pulse ratios at different inter-stimulus interval (50–300 ms) were calculated for each pair of fEPSP, by dividing values of the second postsynaptic response by the first one. Long-term potentiation (LTP) was induced by a high-frequency stimulation protocol (100 Hz) consisting of a 1 s train of stimuli in the CA1 or three trains of stimuli delivered 5 min apart in the DG. Synaptic depotentiation induced after the LTP induction, was obtained by a 10 min low-frequency stimulation protocol (2 Hz). Whole-cell patch-clamp recordings were performed from pyramidal neurons or granule cells, visualized using infrared differential interference contrast microscopy (Olympus) in the CA1 or DG hippocampal regions, respectively. The standard internal solution for patch-clamp recordings contained (in mM): 145 K ± gluconate, 0.1 CaCl2, 2 MgCl2, 0.1 EGTA, 10 HEPES, 0.3 Na-GTP and 2Mg-ATP, adjusted to pH 7.3 with KOH. Pipette resistance ranged from 4 to 7 MΩ, access resistance from 15 to 30 MΩ and the holding potential was −80 and −60 mV for DG granule cells and CA1 pyramidal neurons, respectively. Spontaneous excitatory postsynaptic currents (sEPSCs) were recorded for 5 min in the presence of 50 μM picrotoxin to block GABAA receptor-mediated synaptic transmission, filtered at 0.1 kHz, digitized at 5 kHz using Clampex 10, analyzed offline using automatic detection and subsequently checked manually for accuracy. The Student’s t-test was used for statistical comparisons with significance level established at P < 0.05. All protocols were approved by the Bioethical Committee of the University of Perugia, Perugia, Italy.
Electroencephalographic analysis
Mice were anesthetized with 5% isoflurane. Four screw electrodes (Bilaney Consultants GMBH, Dusseldorf, Germany) were inserted bilaterally through the skull over the cortex (anteroposterior, +2.0–3.0 mm; left–right 2.0 mm from bregma) as previously described [23], a further electrode was placed into the nasal bone as ground. The five electrodes were connected to a pedestal (Bilaney, Dusseldorf, Germany) and fixed with acrylic cement (Palavit, New Galetti and Rossi, Milan, Italy). The animals were allowed to recover for a week from surgery before the experiment. After surgery, EEG activity was recorded in a Faraday chamber using a Power-Lab digital acquisition system (AD Instruments, Bella Vista, Australia; sampling rate 100 Hz, resolution 0.2 Hz) in freely moving awake mice. Basal cerebral activity was recorded continuously for 24 h (from 5 pm to 5 pm). Segments with movement artefacts or electrical noise were excluded from statistical analysis. All EEG tracings were analyzed and scored for the presence of spikes, which were discriminated offline with the spike histogram extension of the software (LabChart v8 Pro Windows). Spikes were defined as having a duration <200 ms with baseline amplitude set to 4.5 times the standard deviation of the EEG signal (determined during inter-spike activity periods, whereas repetitive spiking activity was defined as 3 or more spikes lasting <5 s).
Pharmacological treatment
Cotinine (Sigma-Aldrich C5923) was dissolved in saline. Wild-type (WT) and Shank1−/−/Shank3−/− DKO received an intraperitoneal injection of the cotinine (3 mg/kg)-containing solution or the same volume of vehicle once a day from P14 to P30 or one injection at P14 or at P30.
Data analysis
All of the behavioral, electrophysiological, and imaging experiments and all data analyses were performed under blinded conditions in which the persons performing the experiment and the persons performing the analysis used a random numerical code, which was produced and known by another person, to label the differed samples.
Statistical analyses
Statistical analyses for every experiment are described accordingly in the figure legends. Unless specified, GraphPad Prism version 8 was used to perform most statistical analyses. The sample size for biochemical, morphological imaging, and electrophysiological experiments was determined by empirical evidence accumulated in our laboratory and also on previous literature. For behavioral test the number of mice was chosen to ensure adequate power a on the basis of the program G*power 3.1. Based on the number of comparisons and the pattern of data distribution, appropriate statistical tests were used to analyze the data. Unpaired two-tailed t-test was used to evaluate the difference between two groups; the variances in two groups were similar in all data sets. Two-way analysis of variance followed by post hoc test was used for comparison of multiple samples. Significance was set at Po0.05. All values are presented as mean ± s.e.m.
Results
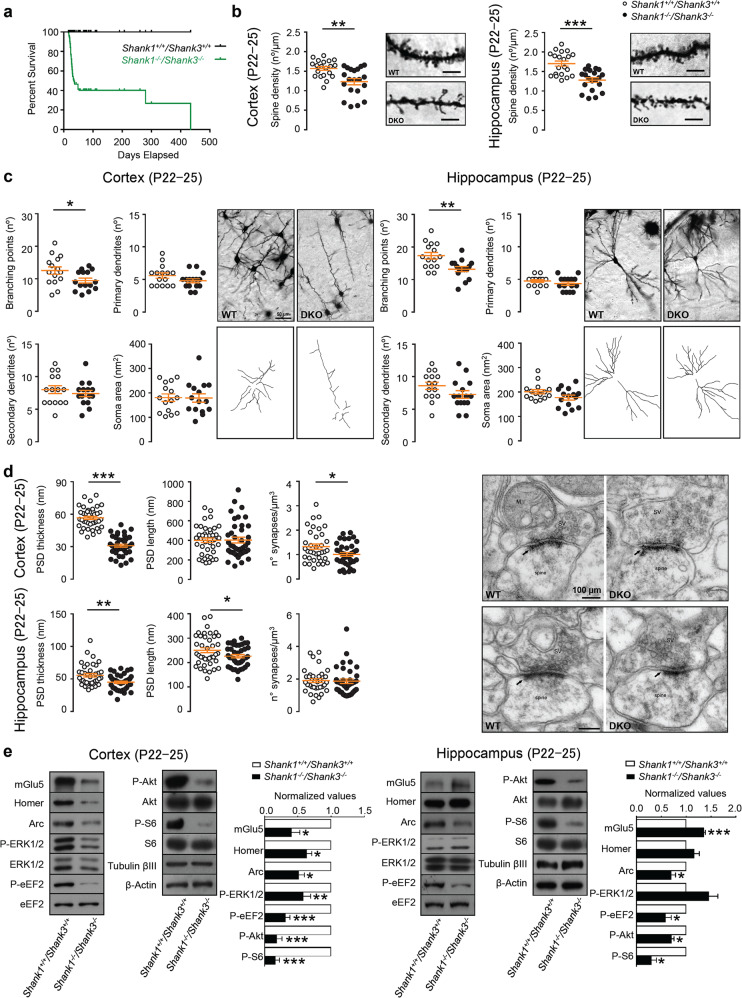
To analyze the role of Shank1 and Shank3 proteins in synaptic and circuitry functions, we generated the Shank1−/−/Shank3−/− DKO mice by crossing Shank1 [14] and Shank3 [15, 16] single KO mice, obtaining double heterozygous mice that were crossed generating DKO mice at the predicted Mendelian frequency. The deletions were verified by PCR (Supplementary Fig. 1a). DKO mutants were viable, but their survival rate was much lower compared to ones with WT littermates and more than 60% of DKO mice died especially around postnatal day (P) P22–P25 (Fig. 1a). Adult mice that survived this critical age window had body and brain sizes that were slightly reduced with respect to WT mice (Supplementary Fig. 1b, c).
Fig. 1. Shank1 and Shank3 double mutation causes severe deficits in young (P22–25) mice.
a Survival curve of the WT and Shank1−/− Shank3−/− DKO mice shows that more than 60% of DKO mice die around postnatal day (P) P22–P25. Comparison of survival curve was made by Log-rank (Mantel-Cox) test; n = 101 animals for each group; ****p < 0.0001. b Representative images and quantification of the spine density in cortical (left panel) and hippocampal neurons (right panel) from P22–25 wild-type and Shank1−/−Shank3−/− DKO mice (Golgi–Cox staining, scale bar: 5 µm). Two-tailed Mann–Whitney test was used for statistical analysis; n = 3 for each group; **p < 0.01; ***p < 0.001. c Quantification of the number of branching points, primary, and secondary dendrites and soma area both in cortex and in hippocampus of Shank1−/− Shank3−/− DKO mice (50 µm). Somatosensory cortex: branching points, secondary dendrites, and soma area were analyzed by unpaired, two-tailed student’s t-test; primary dendrites were analyzed by Two-tailed Mann–Whitney test. Hippocampus CA1 and CA3: secondary dendrites and branching points were analyzed by unpaired, two-tailed student’s t-test; primary dendrites and soma area were analyzed by Two-tailed Mann–Whitney test. Sample size n = 3 for each group; *p < 0.5; **p < 0.01. d Analysis of PSD length, thickness, and number of synapses in cortical (top panels) and hippocampal (bottom panels) neurons from P22–25 of Shank1+/+ Shank3+/+ wild-type (WT) and Shank1−/− Shank3−/− DKO mice. Representative electron microscopy images of cortical and hippocampal synapses from P22–25 wild-type and Shank1−/−Shank3−/− DKO mice (right panels) (100 µm). Somatosensory cortex: PSD thickness and number of synapses were analyzed by unpaired, two-tailed student’s t-test; PSD length was analyzed using two-tailed Mann–Whitney test. Hippocampus CA1: PSD thickness and number of synapses were analyzed by unpaired, two-tailed student’s t-test; PSD length was analyzed using unpaired, two-tailed student’s t-test with Welch’s correction. Analyses are based on a sample size of n = 2 animals for each group (WT and DKO); *p < 0.05; **p < 0.01; ***p < 0.001. e Representative western blots and relative protein quantifications from cortical (left) and hippocampal (right) PSD-enriched fractions derived from P22–-25 wild-type and Shank1−/− Shank3−/− DKO mice. Protein expression was normalized to wild-type levels and plotted as relative change of expression levels. Phosphoprotein levels were normalized against total protein levels. Data were analyzed by one sample t-test; Cortex: mGlu5, Arc n = 9 for each group; Homer1b/c, P-S6 n = 12 for each group; P-ERK1/2 n = 15 for each group; P-eEF2 N = 24 for each group; P-AKT n = 21 for each group. Hippocampus: mGlu5 n = 15 for each group; Homer1b/c, P-ERK1/2, P-AKT, P-S6 n = 9 for each group; Arc, P-eEF2 n = 12 for each group. *p < 0.05; **p < 0.01; ***p < 0.001.
Since the Shank1–3 deletion causes a high mortality rate around P22–P25, a developmental time period crucial to synapse formation and maturation, and ASDs being a neurodevelopmental disorder that manifest in early postnatal life, we assessed synaptic and neuronal morphology in P22–25 DKO and WT mice. We focused the study on two brain areas (the cortex and the hippocampus) that are strongly associated with the pathogenesis of ASD/ID and that express high levels of both, Shank1 and Shank3. Shank1–3 deletion caused a reduction in the number of dendritic spines both in the cortex and in the hippocampus of DKO mice compared to WT mice (Fig. 1b). Moreover, in cortical neurons both in the cortex and in the hippocampus, we found reduced branching points (Fig. 1c). Analysis by electron microscopy of the synaptic ultrastructure revealed a reduction in PSD thickness and synapse number in the cortex (Fig. 1d top panels), whereas, a reduction in PSD length and thickness was found in the hippocampus (Fig. 1d bottom panels) of DKO mice compared to WT littermates.
Overall, these data suggest that Shank1–3 deletion affects spines formation and neuronal maturation both in the cortex and hippocampus. The impact of these deletions on key proteins involved in synaptic function and cellular signaling was then analyzed using PSD-enriched fraction from cortex and hippocampus of P22–25 DKO and WT mice.
As expected, DKO mice did not show signals for the Shank1 and Shank3 deleted isoforms (in the Shank3Δ11−/− the high molecular weight isoforms were deleted, some smaller isoforms remained expressed [15, 16]) neither in cortex nor in hippocampus (Supplementary Fig. 2a) and there was no compensatory increase in Shank2 expression (Supplementary Fig. 2a, b).
Surprisingly, when we analyzed the expression of ionotropic glutamate receptors we did not find any change in the expression levels of AMPA and NMDA receptors (GluN1, GluN2A, GluN2B, GluA1, GluA2) (Supplementary Fig. 2b, c) nor in cortex nor in hippocampus. On the contrary, in cortex of DKO mice there was a significant reduction of phosphorylated Akt, ERK1/2, eEF2, and S6 and reduced levels of Arc, mGlu5, and Homer protein expression (Fig. 1e left panels). In consistence, a major reduction in the phosphorylation of eEF2, Akt, and S6 and reduced expression of Arc was also found in the hippocampus of DKO mice while, mGlu5 expression was significantly increased (Fig. 1e right panels). These altered signaling proteins are known to work in the context of synaptic plasticity and are essential for the formation and stabilization of dendritic spines [24–27]. Interestingly, the dysregulation of their function has been described in different mouse models of ASD and ID [28, 29]. Therefore, the data suggest that Shank1–3 deletion has a strong impact on several intracellular signaling pathways that are important for neuronal activation and synaptic plasticity, eventually leading to defects in neuronal and synapse maturation.
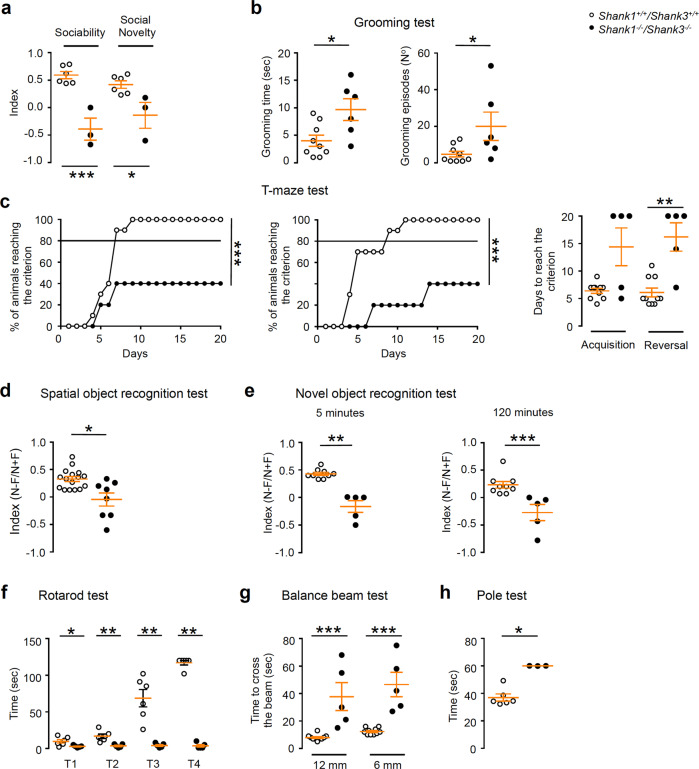
To test the potential effect of Shank1–3 deletion on behavior, we performed a wide set of behavioral tests on P40–P60 old mice. We tested if DKO mice displayed autistic-like impairments in social interaction using a three-chamber test. In comparison to WT mice DKO mice spent more time exploring the compartment containing the empty cage than the compartment containing the stranger mouse. In addition, when a second mouse was placed in the empty cage, DKO mice remained closer to the familiar stranger subject for a longer time suggesting impaired social recognition. Accordingly, the relative sociability index and the social novelty preference index scores were lower in the DKO mice than in their WT littermates (Fig. 2a). Next, we examined nest building behavior, which is relevant to home cage social behaviors [30]. DKO mice were also significantly impaired in this task having an average score less than half compared to WT values (Supplementary Fig. 3a). To test repetitive behavior, a core symptom of ASD, we analyzed self-grooming behavior of DKO mice. Mutant mice showed significantly increased grooming both in terms of total time and number of episodes compared to WT (Fig. 2b). When tested in an elevated plus maze, DKO displayed reduced anxiety-like behavior, spending more time in the open arm and showing increased entries number into the open arm compared to WT littermates (Supplementary Fig. 3b).
Fig. 2. Shank1−/−Shank3−/− double KO mice exhibit syndromic ASDs-like behavior.
a Impaired social interaction of Shank1−/− Shank3−/− DKO in three-chamber assays. Unpaired, two-tailed student’s t-test. was used for statistical analysis; n = 6 WT, n = 3 DKO; *p < 0.05; ***p < 0.001. b Significantly increased repetitive grooming behavior in Shank1−/− Shank3−/− DKO compared to WT mice. Grooming time were analyzed by unpaired, two-tailed student’s t-test; grooming episodes were analyzed by two-tailed Mann–Whitney test; n = 9 WT, n = 6 DKO; *p < 0.05. c, Shank1−/− Shank3−/− DKO mice required more days to achieve the criterion than WT mice both during the acquisition and the reversal phase of the T-maze. The percentage of animals reaching the criterion were analyzed by unpaired, two-tailed student’s t-test with Welch’s correction for the acquisition phase; the reversal phase was analyzed by two-tailed Mann–Whitney test. Days to reach the criterion analyzed by two-tailed Mann–Whitney test; n = 10 WT, n = 5 DKO; **p < 0.01; ***p < 0.001. d Spatial memory is evaluated by determining a discrimination index in the spatial object recognition test. Unpaired, two-tailed student’s t-test with Welch’s correction was used for statistical analysis; n = 16 WT, n = 8 DKO; *p < 0.05. e Shank1−/− Shank3−/− DKO mice show an impairment in novel object recognition at both 5 and 120 min inter-trial intervals compare to WT. Novel object recognition at 5 min was analyzed by unpaired, two-tailed student’s t-test with Welch’s correction; Novel object recognition at 120 min was analyzed by two-tailed Mann–Whitney test; n = 9 WT, n = 5 DKO; **p < 0.01; ***p < 0.001. f Shank1−/− Shank3−/− DKO mice show impaired motor coordination in the rotarod test. All pvalues were derived using two-tailed Mann–Whitney test; n = 6 WT, n = 5 DKO; *p < 0.05; **p < 0.01. g Shank1−/− Shank3−/− DKO are impaired in the balance beam test. Two-tailed Mann–Whitney test was used for the statistical analysis; n = 10 WT, n = 5 DKO; ***p < 0.001. h During the pole test Shank1−/− Shank3−/− DKO spend more time than WT mice to complete the task. Data were analyzed by two-tailed Mann–Whitney test; n = 6 WT, n = 3 DKO; *p < 0.05.
Because Shank1 and Shank3 are highly expressed in the hippocampus, a brain region crucial for learning and memory processes, we analyzed cognitive performances of Shank1–3 DKO mice. Shank1–3 DKO mice showed deficits in spatial learnings, requiring more days to achieve the criterion than WT mice both during the acquisition and the reversal phase of the T-maze test (Fig. 2c). Spatial learning deficits were also found in the Morris water maze test (Supplementary Fig. 3c, d). Moreover, DKO mice showed a significant impairment in the spatial object recognition test where a discrimination index with an inter-trial interval of 120 min was analyzed (Fig. 2d). To test episodic memory, we used the novel object recognition test. Mice were tested after 5 and 120 min delays; DKO mice showed an impairment in novel object recognition at both time points compared to WT mice (Fig. 2e).
Finally, we examined motor behavior of Shank1–3 DKO mice employing the rotarod test and DKO mice showed impairments during all the trials (Fig. 2f). Fine motor coordination and balance were assessed using the beam walking assay. DKO mice spent more time to cross the beam than WT mice (Fig. 2g) and during the pole test DKO mice took longer time to complete the task than WT mice (Fig. 2h).
In brief, the behavioral characterization of DKO mice suggests that Shank1 and Shank3 deletions lead to a severe ASD-like behaviors, causing impairments in sociability and repetitive behavior, the two major ASD core symptoms. In addition, DKO mice displayed deficits in learning and motor behaviors, two co-morbidities associated with ASD.
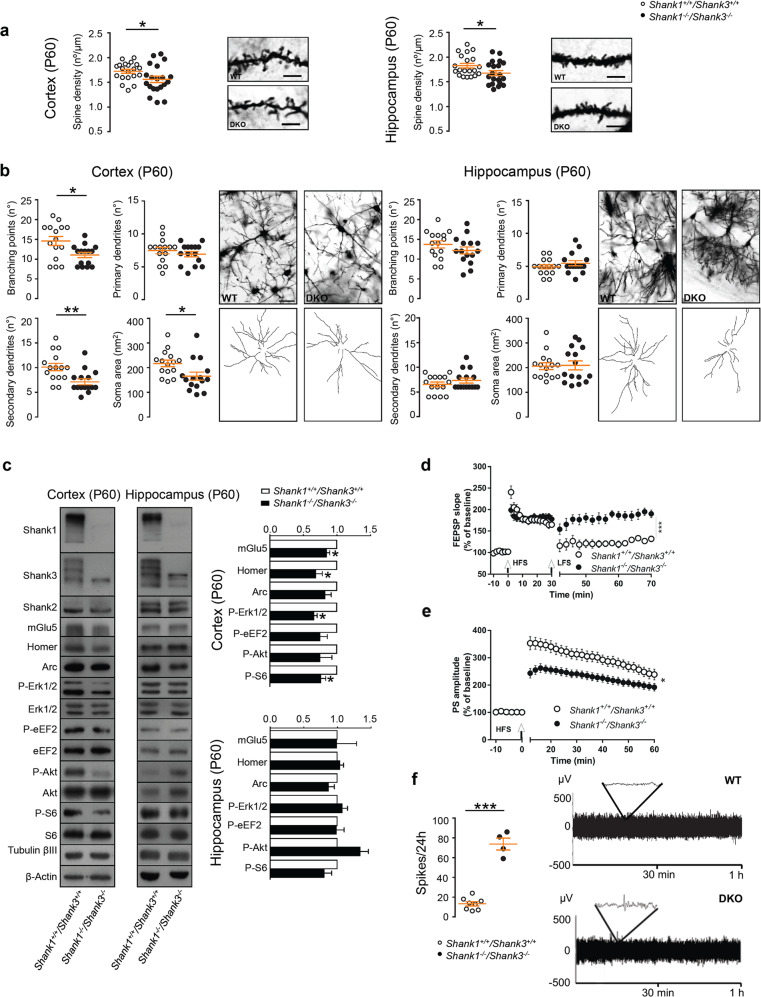
Furthermore, we examined whether the molecular and morphological alterations of Shank1–3 DKO mice that have been identified in P22–25, were also present in adult mice (P60) in which we described strong behavioral deficits.
Surprisingly, we found the extent and the nature of molecular alterations differed between developing and adult mice. Golgi analysis still showed a significant decrease in cortex and hippocampus spine density of Shank1–3 DKO mice compared to WT littermates, but this was less dramatic than in P22–25 DKO mice (Fig. 3a) (indeed the ratio of the mean density DKO/WT was higher at P60 then at P22–25, p < 0.001 for hippocampus, p = 0.1996 in cortex, data not showed). Moreover, cortical neurons of DKO mice were still characterized by reduced dendritic arborizations in terms of number of branching points and secondary dendrites (Fig. 3b left panels), while hippocampal neurons did not present any major defects compared to WT neurons (Fig. 3b right panels). These results were confirmed by biochemical analysis. In PSD-enriched fractions obtained from the cortex of adult DKO mice, we saw the same alterations found in young mice were detected but they were less severe (Fig. 3c). We did not observe any alterations in the protein’s expression and phosphorylation levels of interest in the hippocampus (Fig. 3c).
Fig. 3. Adult (P60) Shank1−/−Shank3−/− double KO mice have morphological and functional deficits.
a Quantification of spine density in cortical (left panel) and hippocampal neurons (right panel) from P60 wild-type and Shank1−/− Shank3−/− DKO mice (5 µm). Cortical spines were analyzed by unpaired, two-tailed student’s t-test; hippocampal neurons were analyzed by two-tailed Mann–Whitney test; n = 3 for each group; *p < 0.05; **p < 0.01. b Analysis of branching points, primary and secondary dendrites and soma area in cortex (left panels) and hippocampus (right panels) of Shank1−/− Shank3−/− DKO and WT mice (50 µm). Branching points were analyzed by unpaired, two-tailed student’s t-test with Welch’s correction; primary dendrites were analyzed by unpaired, two-tailed student’s t-test; secondary dendrites and soma area were analyzed by two-tailed Mann–Whitney test. Hippocampus: branching points and primary dendrites were analyzed by unpaired, two-tailed student’s t-test; secondary dendrites and soma area were analyzed by two-tailed Mann–Whitney test. Sample size n = 3 for each group; *p < 0.05; **p < 0.01. c Quantitative analysis of proteins of PSD-enriched fractions from cortex and hippocampus of P60 WT and Shank1−/− Shank3−/− DKO mice. Protein expression was normalized to wild-type levels and plotted as relative change of expression levels. Phosphoprotein levels were normalized against total protein levels. Data were analyzed by one sample t-test; Cortex: mGlu5, Homer1b/c, Arc, P-S6 n = 4 for each group; P-ERK1/2 n = 3 for each group; P-eEF2, P-AKT n = 5 for each group. Hippocampus: mGlu5, Homer1b/c, P-ERK1/2 n = 3 for each group; Arc, P-AKT, P-S6 n = 4 for each group; P-eEF2 n = 6 for each group. *p < 0.05. d Impaired depotentiation at the Schaffer collaterals-CA1 synapses of Shank1−/− Shank3−/− DKO mice. Data were analyzed by student’s t-test; number of slices, n = 6 WT, n = 8 DKO; ***p < 0.001. e Reduced LTP in the dentate gyrus of Shank1−/− Shank3−/−. Student’s t-test was used for statistical analysis; number of slices, n = 11 WT, n = 20 DKO; *p < 0.05. f Shank1−/− Shank3−/− DKO mice show increase in the number of spikes measured during 24 h compared to WT free-moving mice. Representative 1 h EEG traces (right panels). Data were analyzed using unpaired, two-tailed student’s t-test; n = 8 WT, n = 4 DKO; ***p < 0.001.
However, Shank1–3 mutant mice showed impairment in contextual memory and spatial learning (Fig. 2c–e) suggesting that the hippocampal synaptic plasticity was altered. Thus, we addressed if the hippocampal network of DKO mice showed electrophysiological changes in the excitatory synaptic transmission and long-term synaptic plasticity. sEPSC were recorded from both CA1 pyramidal neurons and DG granule cells. We found that the sEPSCs frequencies, but not the amplitudes, were significantly affected in both CA1 and DG neurons of DKO mice with respect to the currents measured in WT mice (Supplementary Fig. 4). In particular, sEPSCs frequency of DKO neurons was significantly reduced in the CA1 region and increased in the DG region with respect to WT. We also measured the PP facilitation, in the CA1 hippocampal region of DKO mice, and we found that it was unchanged in respect to WT (Supplementary Fig. 4). Overall, these results suggest altered neurotransmission of synaptic network in the hippocampus of DKO mice.
As regards hippocampal long-term synaptic plasticity, we found that LTP, induced at the Schaffer collaterals-CA1 synapses, was normal since no difference was observed between WT and DKO mice (Supplementary Fig. 5). Conversely, DKO mice were unable to express synaptic depotentiation of LTP, as this form of synaptic plasticity was present in CA1 synapses of WT but not of DKO mice (Fig. 3d). Moreover, while LTP was unaffected at CA1 synapses, LTP of the synapses of dentate gyrus of DKO mice was significantly reduced compared to WT littermates (Fig. 3e). Overall these data suggest that, in the hippocampus, Shank1–3 deletion leads to specific impairments of neurotransmission of synaptic network and synaptic plasticity in different hippocampal circuits that might be the cause of the impaired learning and memory found in Shank1–3 mutant mice.
Finally, to investigate if morphological and biochemical defects found in the cortex of Shank1–3 mutant mice were associated with changes in cortical neurons activity we performed continuous recordings of EEG. Here we detected an increase in the number of spikes measured during 24 h (Fig. 3f) confirming circuit dysfunctions also in the cortex of Shank1–3 DKO mice. Moreover, there was a significant increase power in γ frequency band accompanied by a decrease of δ activity as compared to WT mice (Supplementary Fig 6). Interestingly the increase in γ power on EEG is associated with perceptual and cognitive functions that are compromised in autism [31].
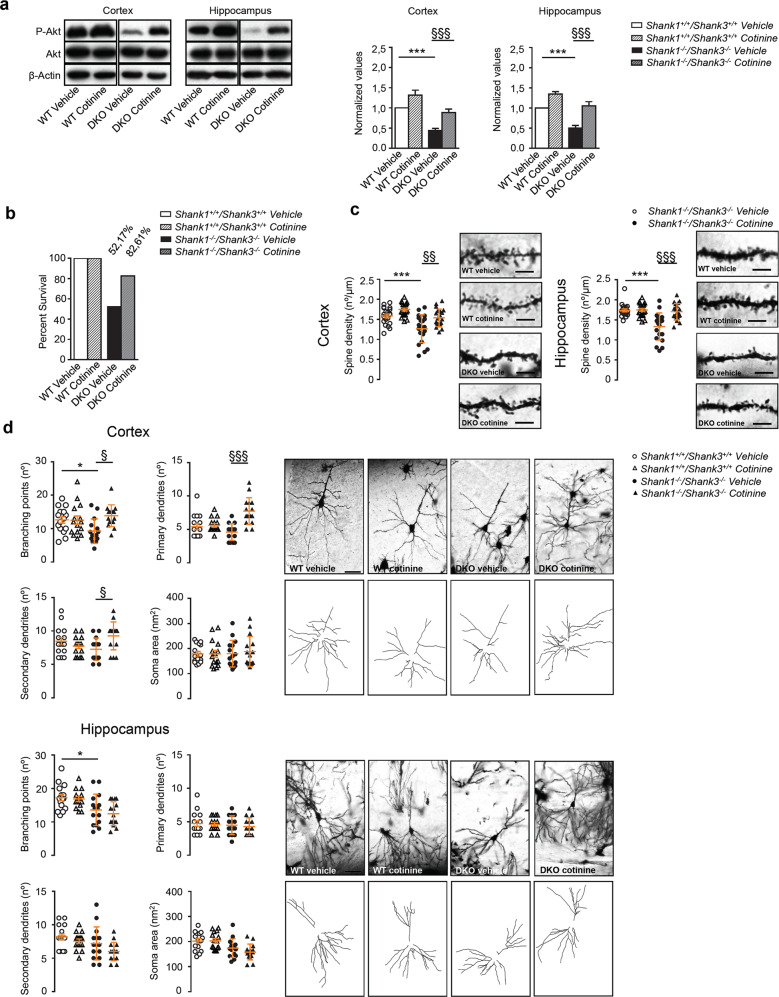
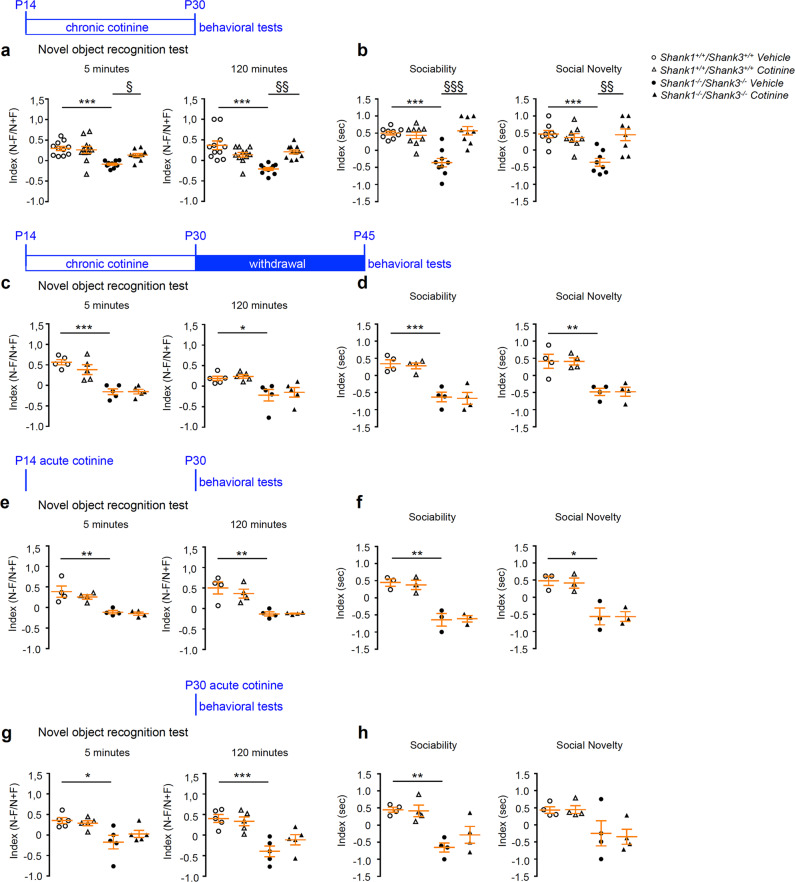
Because Akt phosphorylation was significantly reduced in the hippocampus and the cortex of P22–25 DKO mice, and Akt pathway is also regulated by glutamatergic mGlu5 activity and indirectly regulates the activity of eEF2K the kinase that phosphorylate eEF2 (reduced in the DKO mice), we tested if a pharmacological rescue of Akt phosphorylation from P14 to P30, a critical period extremely important for neuronal maturation and circuits formation, was able to improve survival rate and the morphological defects found in cortex and hippocampus of Shank1–3 DKO mice. Previous studies reported that chronic treatment with cotinine, the major metabolite of nicotine, was able to increase Akt phosphorylation in rodent hippocampi [32, 33] and could improve cognitive performances in mouse and monkey models [34–38]. Our preliminary experiments, performed in slice acutely treated with cotinine, also suggest that cotinine is able to rescue LTP defects in DG of DKO mice (unpublished data). WT and DKO mice were chronically treated with cotinine subcutaneously (3 mg/kg i.p.) or vehicle once a day from P14 to P30. First, we confirmed that chronic treatment with cotinine was indeed able to significantly increase Akt phosphorylation in both cortex and hippocampus of both WT and Shank1–3 DKO mice (Fig. 4a). Chronic cotinine treatment augmented the survival of DKO mice at P30 in comparison to vehicle-treated mice even though it did not completely rescue the DKO mortality to WT levels (Fig. 4b). Moreover, while chronic cotinine treatment does not significantly change the neuronal morphology in the WT mice, the same treatment significantly rescued cortical and hippocampal spine density in DKO mice to the level of WT mice (Fig. 4c). When administered chronically, cotinine also rescued the number of branching points, and increase the number of primary and secondary dendrites in the cortex of DKO mice, while such effect was not observed in the hippocampus (Fig. 4d). Finally, to investigate if cotinine was able to ameliorate memory defects found in Shank1–3 DKO mice, WT and DKO mice were treated with cotinine as described above and 30 min after the last administration of the drug mice were tested using novel object recognition test. The performance of WT mice was unaltered by chronic administration of cotinine; however, chronic cotinine treatment was able to rescue memory impairment of DKO mice after 5 (Fig. 5a left panel) and 120 (Fig. 5a right panel) minutes delay. We also tested the effect of chronic cotinine treatment on reduced sociability found in Shank1–3 DKO mice. As for the memory, the performances of WT mice were not affected nor in terms of sociability nor in terms of social novelty. On the contrary, chronic cotinine treatment fully rescued sociability and social novelty recognition of DKO mice (Fig. 5b). We then evaluated a possible long-lasting rescue effect on behavioral defects by cotinine. Mice chronically treated from P14 to P30 were behavioral tested at P45, after 2 weeks withdrawal of the treatment. Our results clearly showed that after 2 weeks of cotinine withdrawal the mice performed like the untreated ones, meaning that cotinine did not have a long-lasting effect and eventually the mice need to be continuously treated to rescue the behavioral deficits (Fig. 5c, d). To evaluate the effect of a single shot of cotinine on the behavioral impairments, mice were acutely treated at P14 and analyzed for the behavioral phenotype at P30 or acutely treated at P30 and analyzed immediately after the treatment. Similar to the withdrawal effect, a single systemic delivery of cotinine was not able to recover the behavioral defects (Fig. 5e–h).
Fig. 4. Chronic cotinine treatment during brain development rescues neuronal morphology of Shank1−/−Shank3−/− double KO mice.
a Western blot analysis from PSD-enriched fractions after cotinine chronic treatment in cortex (left panel) and hippocampus (right panel) Data were analyzed by two-way ANOVA test; n = 12 for each group; ***p < 0.001 WT vehicle compared with DKO vehicle; §§§, p < 0.001 DKO vehicle compared with DKO cotinine. b Chronic cotinine treatment increased the number of DKO mice that survive at P30. For this analysis we used 23 mice for each genotype and for each treatment (vehicle or cotinine). c Quantification of spine density in cortical (left panel) and hippocampal neurons (right panel) from vehicle-treated and chronically cotinine treated Shank1−/− Shank3−/− DKO mice (5 µm). Two-way ANOVA test was used for statistical analysis; n = 3 for each group; ***p < 0.001. WT vehicle compared with DKO vehicle; §§, p < 0.01; §§§, p < 0.001 DKO vehicle compared with DKO cotinine. d Analysis of neuronal morphology in cortical (left panel) and hippocampal neurons (right panel) from vehicle-treated and chronically cotinine treated Shank1−/− Shank3−/− DKO mice (50 µm). All pvalues were derived using two-way ANOVA test. Sample size n = 3 for each group; Cortex: §, p < 0.05; §§§ p < 0.001 DKO vehicle compared with DKO cotinine; branching points analysis with unpaired, two-tailed student’s t-test showed a significant difference between WT vehicle and DKO vehicle; *p < 0.05. Hippocampus: All pvalues were derived using two-way ANOVA test. *p < 0.05 WT vehicle compared with DKO vehicle.
Fig. 5. Chronic cotinine treatment during brain development rescues behavioral deficits of Shank1−/− Shank3−/− double KO mice.
a Chronic treatment with cotinine, schematically indicated above the panels, rescue novel object recognition memory of Shank1−/− Shank3−/− DKO mice at 5 and 120 min delay. Two-way ANOVA test was used for statistical analysis. Novel object recognition at 5 and 120 min: n = 11 for each group. ***p < 0.0001 WT vehicle compared with DKO vehicle; §, p < 0.05; §§, p < 0.01 DKO vehicle compared with DKO cotinine. b Shank1−/− Shank3−/− DKO mice chronically treated with cotinine showed a rescue of social interaction in three-chamber assay and social novelty. Two-way ANOVA test was used for statistical analysis. Sociability: n = 9 for each group. Social novelty n = 8 for each group. ***p < 0.001 WT vehicle compared with DKO vehicle; §§, p < 0.01; §§§, p < 0.001 DKO vehicle compared with DKO cotinine. c Novel object recognition memory and d Sociability and social novelty were not rescued after chronic treatment with cotinine following by 15 days of drug withdrawal. All pvalues were derived using two-way ANOVA test. Novel object recognition test: n = 5 for each group. Sociability and social novelty: n = 4 for each group. *p < 0.05; **p < 0.001; ***p < 0.0001 WT vehicle compared with DKO vehicle. e Novel object recognition memory and f sociability and social novelty were not rescued in Shank1−/− Shank3−/− DKO mice at P30 after acute cotinine treatment a P14. All pvalues were derived using two-way ANOVA test. Novel object recognition test: n = 4 for each group. Sociability and social novelty: n = 3 for each group. *p < 0.05; **p < 0.001 WT vehicle compared with DKO vehicle. g Novel object recognition memory and h sociability and social novelty were not rescued in Shank1−/− Shank3−/− DKO mice at P30 after acute cotinine treatment a P30. All pvalues were derived using two-way ANOVA test. Novel object recognition test: n = 5 for each group. Sociability and social novelty: n = 4 for each group. *p < 0.05; **p < 0.001; ***p < 0.0001 WT vehicle compared with DKO vehicle.
Discussion
We showed that double deletion of Shank1 and Shank3 affects postnatal brain development by strongly impairing the Akt pathway activation. Moreover, associated intracellular signaling cascades involved in synaptic formation and plasticity were deregulated leading to major morphological and synaptic defects in cortical and hippocampal neurons. These findings were associated with an increase of mortality around P22–P25, a developmental period crucial for synapse formation and maturation. Interestingly, those Shank1–3 DKO mice that survived this critical time window and reached adulthood presented with less significant synaptic defects. In contrast, they showed a severe behavioral phenotype that recapitulates a form of syndromic ASDs. Moreover, we demonstrated that the chronic treatment of Shank1–3 DKO mice with cotinine from P14 to P30 increased Akt phosphorylation, enhanced survival rates and ameliorated cognitive performance and sociability. Overall, our results suggest that Shank proteins are required for Akt and related pathways activation during brain development and synapse formation and that Akt reactivation might represent a new therapeutic target to enhance cognitive functions of ASDs patients.
Considering the proved role of Shank proteins in regulating synapse and circuits functions in the human brain [9] several laboratories have extensively studied all three Shank genes using in vitro approaches and in vivo models. Indeed, Shank1 and Shank3 single KO mice have been described to present some common and peculiar molecular and behavioral alterations.
Shank1 KO mice were first generated by the deletion of exons 14 and 15 leading to the loss of all Shank1 splice variants [14]. Neuronal synapses form Shank1 KO mice displayed a reduced expression of GKAP/SAPAP and Homer, specifically in PSD fractions, dendritic spines from CA1 synapses were less and smaller, and thickness of the PSD was also reduced. Both LTP and long-term depression in the CA1 hippocampus were unaffected. In behavioral experiments, Shank1 KO mice showed some ASD-associated behavioral phenotype, such as a reduction in sniffing during male–female interaction, a reduction in the interaction with novel mice compared with wild-type littermates [39, 40], and increased self-grooming behavior [39–42]. In memory test Shank1 KO mice exhibited an enhanced acquisition of spatial memory but, at the same time, an impaired contextual fear memory [14] and a severe impairment in object recognition memory [43].
Given the complexity of the Shank3 gene, which is transcribed from six intragenic promoters, several Shank3 mutant lines were generated [16, 44–51]. In all these models some Shank3 isoforms are still present and only Wang et al. developed the Shank3 complete KO by the deletion of the protein-coding exons 4–22 [47], however this model showed phenotypes similar with most of the other Shank3 mutant mice. Indeed, all Shank3 mutant lines displayed autistic-like behaviors including increased repetitive behaviors, reduced social interaction and motor coordination deficits. Learning and memory impairments were instead different among individual lines Shank3 mutants. At the molecular level, most of the various Shank3 mutants showed the reduction of AMPA receptor subunits [16, 44–46, 50] and of Homer1b/c at synapses [15, 44, 46, 47].
The Shank1–3 DKO mice exhibit strong impairments in social behavior and severe deficits in learning and memory thus recapitulating major symptoms of syndromic autism.
Our data suggest that the absence of two of Shank proteins causes severe developmental synaptic failure that leads to a strong reduction of signaling pathways involving Akt, S6, ERK1/2 and eEF2, alteration of dendrite formation, dendritic spines and PSD structure ultimately conveying to a reduction in survival rate. Impairment of Akt signaling and reduced S6 phosphorylation, but not ERK1/2 phosphorylation was already demonstrated in one of the Shank3 KO mice in adult, however these mice did not show a reduced viability and were not tested for learning and memory impairments [51]. Instead in the Shank3 full KO mice S6K and ERK1/2 phosphorylation was increased in the striatum and unchanged in the hippocampus [47]. These data, obtained from the single Shank3 KO mice, suggest a specific role for Shank3 in regulating intracellular synaptic signaling possibly by regulating mGlu receptor activation [15, 47]. Instead in vitro experiments indicated that Shank1 is required for fully synaptic maturation [8, 10]. Thus, we can speculate that Shank3 is required for the correct activation of intracellular signaling, while Shank1 for synapse stabilization. Remarkable more than 60% of Shank1–3 DKO mice died especially around postnatal day (P) P22–P25 when a critical period for synapse formation and maturation occurs suggesting that the coordinate action of Shank3 and Shank1 are required for surviving during that critical period. Thus, the major conclusion from our study is that Shank1 and Shank3 work as scaffold proteins that cooperate to activate intracellular pathways essential for neuronal and synaptic maturation and plasticity especially during development because in mice that survive to the critical P22–P25 intracellular pathways are much less impaired. Thus, our data indicate that Shank proteins major function at synapses is to developmentally regulate the correct activation of intracellular signaling. Interestingly we were able to rescue morphological and behavioral alteration by rescuing Akt phosphorylation using the Nicotine metabolite cotinine. Indeed, Bidinosti et al. were able to rescue behavioral defects in the Shank3 KO mice with ATP-competitive CLK2 inhibitor TG003 and Akt activator SC79 [51].
In our study, we decided to use cotinine, the metabolite of nicotine, because it has been proposed to be effective in improving cognitive functions in animal models of psychiatric and neurological diseases [52, 53]. Cotinine works as a positive allosteric of brain nicotine receptors modulating indirectly glutamatergic and serotoninergic synapses [53]. Importantly cotinine has a potential clinical use because it has a longer plasma half-life than nicotine [52, 54] has low toxicity [55], crosses the blood–brain barrier and showed no addictive or cardiovascular effects in humans [53]. Recently cotinine has been used to improves impaired cognition in the mouse model of Fragile X syndrome by increases Akt phosphorylation in the hippocampus of Fmr1 KO mice [33]. Our preliminary experiments, performed in slice acutely treated with cotinine, also suggest that cotinine is able to rescue LTP defects in DG of DKO mice (unpublished data).
In this study, we found that cotinine was able to rescue Akt phosphorylation, some morphological defects, enhanced survival rates, and ameliorated cognitive performance and sociability. Interestingly, our data also indicate that for rescuing the altered behavioral abnormalities the cotinine treatment need to be performed chronically and need to be continued because 2 weeks withdrawal are sufficient to abolish the ability to rescue behavioral alterations in the DKO mice. Thus, our data obtained with cotinine, not only further support the role of Shank proteins in regulating intracellular signaling but also propose cotinine as a possible potent drug to be used for the treatment of syndromic ASD in humans.
Supplementary information
Acknowledgements
This work was supported by the Comitato Telethon Fondazione Onlus (grant no. GGP16131 to CV and GGP17176 to CS) and Regione Lombardia NeOn Progetto "NeOn" POR-FESR 2014–2020, ID 239047, CUP E47F17000000009 to CS and CV and Fondation Jérôme Lejeune (project #1638 to CS and project #1938 to CV). TMB is supported by the DFG (Project-ID 251293561—Collaborative Research Center (CRC) 1149), the Else Kröner Foundation and the project has received funding from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement no. 777394 for the project AIMS-2-TRIALS. This Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation program and EFPIA and AUTISM SPEAKS, Autistica, SFARI. Moreover, funding was received from the Innovative Medicines Initiative 2 Joint Undertaking under grant agreement no. 847818—CANDY.
Author contributions
AM, JP, LP, AT, EV, CC, and SB participated in the execution and analysis of experiments. AM, JP, MF, MS, PC, TMB, CS, and CV participated in the interpretation of the results. CS and CV designed the experiments and wrote the paper.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no confict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Adele Mossa, Jessica Pagano
Contributor Information
Carlo Sala, Email: carlo.sala@in.cnr.it.
Chiara Verpelli, Email: chiara.verpelli@in.cnr.it.
Supplementary information
The online version of this article (10.1038/s41380-020-00979-x) contains supplementary material, which is available to authorized users.
References
- 1.Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, et al. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–82. doi: 10.1016/S0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- 2.Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, et al. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–92. doi: 10.1016/S0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- 3.Boeckers TM, Kreutz MR, Winter C, Zuschratter W, Smalla KH, Sanmarti-Vila L, et al. Proline-rich synapse-associated protein-1/cortactin binding protein 1 (ProSAP1/CortBP1) is a PDZ-domain protein highly enriched in the postsynaptic density. J Neurosci. 1999;19:6506–18. doi: 10.1523/JNEUROSCI.19-15-06506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheng M, Kim E. The Shank family of scaffold proteins. J Cell Sci. 2000;113:1851–6. doi: 10.1242/jcs.113.11.1851. [DOI] [PubMed] [Google Scholar]
- 5.Grabrucker AM, Schmeisser MJ, Schoen M, Boeckers TM. Postsynaptic ProSAP/Shank scaffolds in the cross-hair of synaptopathies. Trends Cell Biol. 2011;21:594–603. doi: 10.1016/j.tcb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Kim E, Sheng M. PDZ domain proteins of synapses. Nat Rev Neurosci. 2004;5:771–81. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi MK, Tang C, Verpelli C, Narayanan R, Stearns MH, Xu R-M, et al. The postsynaptic density proteins homer and shank form a polymeric network structure. Cell. 2009;137:159–71. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sala C, Piech V, Wilson NR, Passafaro M, Liu GS, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–30. doi: 10.1016/S0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- 9.Leblond CS, Nava C, Polge A, Gauthier J, Huguet G, Lumbroso S, et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: a gradient of severity in cognitive impairments. PLoS Genet. 2014;10:e1004580. doi: 10.1371/journal.pgen.1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grabrucker AM, Knight MJ, Proepper C, Bockmann J, Joubert M, Rowan M, et al. Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation. EMBO J. 2011;30:569–81. doi: 10.1038/emboj.2010.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sala C, Vicidomini C, Bigi I, Mossa A, Verpelli C. Shank synaptic scaffold proteins: keys to understanding the pathogenesis of autism and other synaptic disorders. J Neurochem. 2015;135:849–58. doi: 10.1111/jnc.13232. [DOI] [PubMed] [Google Scholar]
- 12.Monteiro P, Feng G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci. 2017;18:147–57. doi: 10.1038/nrn.2016.183. [DOI] [PubMed] [Google Scholar]
- 13.Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78:8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, et al. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vicidomini C, Ponzoni L, Lim D, Schmeisser MJ, Reim D, Morello N, et al. Pharmacological enhancement of mGlu5 receptors rescues behavioral deficits in SHANK3 knock-out mice. Mol Psychiatry. 2017;22:689–702. doi: 10.1038/mp.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–60. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 17.Deacon RM. Assessing nest building in mice. Nat Protoc. 2006;1:1117–9. doi: 10.1038/nprot.2006.170. [DOI] [PubMed] [Google Scholar]
- 18.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 19.Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, et al. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington’s disease mice. Neuroscience. 2008;157:280–95. doi: 10.1016/j.neuroscience.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–82. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Zhang SB, Zhang QQ, Liu M, He XY, Zou Z, et al. Rescue of cAMP response element-binding protein signaling reversed spatial memory retention impairments induced by subanesthetic dose of propofol. CNS Neurosci Ther. 2013;19:484–93. doi: 10.1111/cns.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heise C, Taha E, Murru L, Ponzoni L, Cattaneo A, Guarnieri FC, et al. eEF2K/eEF2 pathway controls the excitation/inhibition balance and susceptibility to epileptic seizures. Cereb Cortex. 2017;27:2226–48. doi: 10.1093/cercor/bhw075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolaienko O, Patil S, Eriksen MS, Bramham CR. Arc protein: a flexible hub for synaptic plasticity and cognition. Semin Cell Dev Biol. 2018;77:33–42. doi: 10.1016/j.semcdb.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Korb E, Finkbeiner S. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 2011;34:591–8. doi: 10.1016/j.tins.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu GY, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–8. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- 27.Verpelli C, Piccoli G, Zanchi A, Gardoni F, Huang K, Brambilla D, et al. Synaptic activity controls dendritic spine morphology by modulating eEF2-dependent BDNF synthesis. J Neurosci. 2010;30:5830–42. doi: 10.1523/JNEUROSCI.0119-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biever A, Valjent E, Puighermanal E. Ribosomal protein S6 phosphorylation in the nervous system: from regulation to function. Front Mol Neurosci. 2015;8:75. doi: 10.3389/fnmol.2015.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkerson JR, Albanesi JP, Huber KM. Roles for Arc in metabotropic glutamate receptor-dependent LTD and synapse elimination: implications in health and disease. Semin Cell Dev Biol. 2018;77:51–62. doi: 10.1016/j.semcdb.2017.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA, et al. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–28. doi: 10.1016/S0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- 31.Rojas DC, Wilson LB. γ-band abnormalities as markers of autism spectrum disorders. Biomark Med. 2014;8:353–68. doi: 10.2217/bmm.14.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, et al. Cotinine reduces amyloid-β aggregation and improves memory in Alzheimer’s disease mice. J Alzheimers Dis. 2011;24:817–35. doi: 10.3233/JAD-2011-102136. [DOI] [PubMed] [Google Scholar]
- 33.Pardo M, Beurel E, Jope RS. Cotinine administration improves impaired cognition in the mouse model of Fragile X syndrome. Eur J Neurosci. 2017;45:490–8. doi: 10.1111/ejn.13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao J, Adam BL, Terry AV. Evaluation of nicotine and cotinine analogs as potential neuroprotective agents for Alzheimer’s disease. Bioorg Med Chem Lett. 2014;24:1472–8. doi: 10.1016/j.bmcl.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grizzell JA, Iarkov A, Holmes R, Mori T, Echeverria V. Cotinine reduces depressive-like behavior, working memory deficits, and synaptic loss associated with chronic stress in mice. Behav Brain Res. 2014;268:55–65. doi: 10.1016/j.bbr.2014.03.047. [DOI] [PubMed] [Google Scholar]
- 36.Patel S, Grizzell JA, Holmes R, Zeitlin R, Solomon R, Sutton TL, et al. Cotinine halts the advance of Alzheimer’s disease-like pathology and associated depressive-like behavior in Tg6799 mice. Front Aging Neurosci. 2014;6:162. doi: 10.3389/fnagi.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terry AV, Buccafusco JJ, Schade RF, Vandenhuerk L, Callahan PM, Beck WD, et al. The nicotine metabolite, cotinine, attenuates glutamate (NMDA) antagonist-related effects on the performance of the five choice serial reaction time task (5C-SRTT) in rats. Biochem Pharm. 2012;83:941–51. doi: 10.1016/j.bcp.2011.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Echeverria V, Zeitlin R. Cotinine: a potential new therapeutic agent against Alzheimer’s disease. CNS Neurosci Ther. 2012;18:517–23. doi: 10.1111/j.1755-5949.2012.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silverman JL, Turner SM, Barkan CL, Tolu SS, Saxena R, Hung AY, et al. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011;1380:120–37. doi: 10.1016/j.brainres.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wöhr M, Roullet FI, Hung AY, Sheng M, Crawley JN. Communication impairments in mice lacking Shank1: reduced levels of ultrasonic vocalizations and scent marking behavior. PLoS One. 2011;6:e20631. doi: 10.1371/journal.pone.0020631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sungur A, Vörckel KJ, Schwarting RK, Wöhr M. Repetitive behaviors in the Shank1 knockout mouse model for autism spectrum disorder: developmental aspects and effects of social context. J Neurosci Methods. 2014;234:92–100. doi: 10.1016/j.jneumeth.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 42.Wöhr M. Ultrasonic vocalizations in Shank mouse models for autism spectrum disorders: detailed spectrographic analyses and developmental profiles. Neurosci Biobehav Rev. 2014;43:199–212. doi: 10.1016/j.neubiorev.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 43.Sungur A, Jochner MCE, Harb H, Kılıç A, Garn H, Schwarting RKW, et al. Aberrant cognitive phenotypes and altered hippocampal BDNF expression related to epigenetic modifications in mice lacking the post-synaptic scaffolding protein SHANK1: implications for autism spectrum disorder. Hippocampus. 2017;27:906–19. doi: 10.1002/hipo.22741. [DOI] [PubMed] [Google Scholar]
- 44.Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, et al. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–42. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, McCoy PA, Rodriguiz RM, Pan Y, Je HS, Roberts AC, et al. Synaptic dysfunction and abnormal behaviors in mice lacking major isoforms of Shank3. Hum Mol Genet. 2011;20:3093–108. doi: 10.1093/hmg/ddr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang X, Bey AL, Katz BM, Badea A, Kim N, David LK, et al. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat Commun. 2016;7:11459. doi: 10.1038/ncomms11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee J, Chung C, Ha S, Lee D, Kim DY, Kim H, et al. Shank3-mutant mice lacking exon 9 show altered excitation/inhibition balance, enhanced rearing, and spatial memory deficit. Front Cell Neurosci. 2015;9:94. doi: 10.3389/fncel.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jaramillo TC, Speed HE, Xuan Z, Reimers JM, Escamilla CO, Weaver TP, et al. Novel Shank3 mutant exhibits behaviors with face validity for autism and altered striatal and hippocampal function. Autism Res. 2017;10:42–65. doi: 10.1002/aur.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kouser M, Speed HE, Dewey CM, Reimers JM, Widman AJ, Gupta N, et al. Loss of predominant Shank3 isoforms results in hippocampus-dependent impairments in behavior and synaptic transmission. J Neurosci. 2013;33:18448–68. doi: 10.1523/JNEUROSCI.3017-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bidinosti M, Botta P, Krüttner S, Proenca CC, Stoehr N, Bernhard M, et al. CLK2 inhibition ameliorates autistic features associated with SHANK3 deficiency. Science. 2016;351:1199–203. doi: 10.1126/science.aad5487. [DOI] [PubMed] [Google Scholar]
- 52.Terry AV, Hernandez CM, Hohnadel EJ, Bouchard KP, Buccafusco JJ. Cotinine, a neuroactive metabolite of nicotine: potential for treating disorders of impaired cognition. CNS Drug Rev. 2005;11:229–52. doi: 10.1111/j.1527-3458.2005.tb00045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moran VE. Cotinine: beyond that expected, more than a biomarker of tobacco consumption. Front Pharm. 2012;3:173. doi: 10.3389/fphar.2012.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buccafusco JJ, Shuster LC, Terry AV. Disconnection between activation and desensitization of autonomic nicotinic receptors by nicotine and cotinine. Neurosci Lett. 2007;413:68–71. doi: 10.1016/j.neulet.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 55.Li P, Beck WD, Callahan PM, Terry AV, Bartlett MG. Pharmacokinetics of cotinine in rats: a potential therapeutic agent for disorders of cognitive function. Pharm Rep. 2015;67:494–500. doi: 10.1016/j.pharep.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.